Abstract
Pharmacogenomics is the study of genetic variants that impact drug effects through changes in a drug’s pharmacokinetics and pharmacodynamics. Pharmacogenomics is being integrated into clinical pain management practice because variants in individual genes can be predictive of how a patient may respond to a drug treatment. Pain is subjective and is considered challenging to treat. Furthermore, pain patients do not respond to treatments in the same way, which makes it hard to issue a consistent treatment regimen for all pain conditions. Pharmacogenomics would bring consistency to the subjective nature of pain and could revolutionize the field of pain management by providing personalized medical care tailored to each patient based on their gene variants. Additionally, pharmacogenomics offers a solution to the opioid crisis by identifying potentially opioid-vulnerable patients who could be recommended a nonopioid treatment for their pain condition. The integration of pharmacogenomics into clinical practice creates better and safer healthcare practices for patients. In this article, we provide a comprehensive history of pharmacogenomics and pain management, and focus on up to date information on the pharmacogenomics of pain management, describing genes involved in pain, genes that may reduce or guard against pain and discuss specific pain management drugs and their genetic correlations.
Introduction
Pain affects approximately 100 million Americans and furthermore costs the US approximately $600 billion per year.Citation1 According to the International Association for the Study of Pain, pain is defined as “an unpleasant sensory and emotional experience that is associated with actual or potential tissue damage or described in terms of such damage”.Citation2 Pain can be acute or chronic; somatic or visceral; nociceptive, neuropathic, or inflammatory in nature. Nociceptive pain refers to the response to noxious stimuli and continues only in the maintained presence of noxious stimuli.Citation3 Inflammatory pain results from injury of tissues and subsequent activation of inflammatory markers, which sensitize nociceptive pathways resulting in patients experiencing pain with otherwise innocuous stimuli and heightens sensitivity of pain perception. Neuropathic pain is the maladaptive response of the nervous system to damage.Citation4 Lower back pain is the primary cause of disability worldwide and has a prevalence of 9.4%, which increases with age. Approximately 20% of US adults have chronic pain and it is one of the most common reasons adults seek medical care.Citation5,Citation6 Chronic pain is particularly debilitating because it affects all aspects of life including physical, mental, social, occupational, and family environments.Citation7 Additionally,psychiatric disorders are common among patients with chronic pain and include depression (2–83%), anxiety (1–65%) and substance use disorders (1–25%).Citation8
There are several options available to treat pain, including nonpharmacologic measures, pharmacotherapy, and surgical interventions. Opioids are commonly used to treat pain conditions however, they have the potential to be abused and to cause addiction. Misuse of prescription opioids (including abuse, dependence, and overdose) is a serious public health problem and costs approximately $78 billion per year in the US.Citation9 The Centers for Disease Control and Prevention (CDC) officially declared fatal prescription drug overdose as an epidemic in 2012.Citation10 More recently in 2017, approximately 70,237 people died due to drug overdose (including illicit drugs and prescription drugs) of which, 25% were due to prescription opioids. And overall from 1999–2017, 218,000 people have died in the US from opioid overdoses. Taken together, opioids are clearly a potential danger to certain patients, but until recently, pain management physicians had no way to decipher which patients could become addicted. Pharmacogenomics can be a powerful tool to tackle the opioid epidemic, by which physicians could elucidate individual genetic variants in patients, and thus, patient potential for drug abuse.
Pharmacogenomics is the study of the role of the genome in drug response. It focuses on the genetic variants that impact drug effects through changes in a drug’s pharmacokinetics (the study of how the organism affects the drug, ie, absorption, distribution, metabolism, elimination) or pharmacodynamics (the study of how a drug affects an organism, ie, physiologic, biochemical, and molecular effects of drugs on the body and involves receptor binding and chemical interactions).Citation11 In 1959, Friedrich Vogel first created the term “pharmacogenetics”, however, the origins of pharmacogenomics can be traced back to 510 BC. Pythagoras observed that some individuals ingesting fava beans experienced potentially fatal hemolytic anemia, whereas others did not. It was later found out to be due to an inherited deficiency of glucose-6-phosphate dehydrogenase (G6PD).Citation12 Elliott Vesell and George Page showed that monozygotic twins displayed significantly less variability in antipyrine pharmacokinetics.Citation13 In the 1960s, Price Evans et al observed a considerable variation of isoniazid metabolism among certain individuals and believed it to be due to genetic factors.Citation14 Decades later, Blum et al showed that the genetic polymorphism in isoniazid acetylation was due to inherited variants in the gene encoding N-acetyltransferase 2 (NAT2).Citation15 Subsequent studies showed the pattern of inheritance for many drug effects, and in 1987 CYP2D6 (the hepatic cytochrome P450 2D6), the first polymorphic human drug metabolizing gene to be cloned.Citation16 For an explanation of gene nomenclature, see . Since then, hundreds of CYP2D6 alleles have been identified. CYP2D6 is highly polymorphic; it accounts only for 2–5% of the total hepatic P450 enzymes; however, it is involved in the metabolism of 25% of all drugs used in clinical practice.Citation17 Several commonly used opioids, including codeine, tramadol, hydrocodone, oxycodone are metabolized by CYP2D6. The analgesic effect of codeine stems from its conversion to morphine; and, the amount of morphine produced from the parent drug codeine can be highly variable between individuals depending on rate of metabolism of codeine, which is in turn dependent on the CYP2D6 polymorphism of the individual. Individuals with certain gene alleles can be classified into metabolic categories: ultrarapid metabolizer (UM) with higher than normal function of the enzyme, extensive metabolizer (EM), intermediate metabolizer (IM), and poor metabolizer (PM) where an individual has little or no enzymatic action.Citation18 These differences reinforce the fact that individual patients vary significantly in their response to the “universal” doses of opioids that are used in practice where one dose of medicine can be ineffective to one person and lethal to another.
Table 1 Gene nomenclature explanation
Several candidate genes involved in the metabolism of opioids (pharmacokinetic related candidate genes such as CYP2D6, CYP3A4/A5, UGT2B7, ABCB1, ABCC3, SLC22A1) and pharmacodynamic related candidate genes such as OPRM1, COMT, KCNJ6) are under investigation and seem promising for clinical use in the future.Citation19 Knowledge of pharmacogenomics is slowly being integrated into mainstream clinical practice. For example, Vanderbilt university is carrying out panel-based pharmacogenomic testing through the Vanderbilt Pharmacogenomic Resource for Enhanced Decisions in Care and Treatment (PREDICT) program and has enrolled more than 10,000 patients.Citation20 StJude’s Children’s Research Hospital has developed the PG4KDS protocol which aims to use the pharmacogenetic tests in the electronic health record (EHR) to preemptively guide prescribing.Citation21 In 2016, ACPE (Accreditation Council for Pharmacy Education) incorporated pharmacogenomics into pharmacy education and pharmacogenomics is included as one of the factors that should be emphasized in the evidence-based clinical decision-making and medication therapy management aspects of clinical practice.Citation22
Pharmacogenomics is rapidly evolving, and numerous efforts are in the pipeline to apply the knowledge of pharmacogenomics into clinical practice so as to offer better and safer healthcare to patients. In this article, we focus on the pharmacogenomics of pain management, describing genes involved in pain, genes that may reduce or guard from pain and discuss specific pain management drugs and their genetic correlation.
Types of pain
Acute pain
Acute pain is characterized by pain that has an inciting event, is sudden in onset, is time-limited, and has the potential to develop into a pathological condition.Citation23 By providing information about the location and magnitude of harmful stimuli, acute pain heightens vigilance and promotes appropriate responses to address the stimuli.Citation24 In this way, acute pain is characterized as having a useful biological purpose. Acute pain typically lasts less than 3 months and its treatment involves interrupting painful nociceptive signals and addressing their underlying cause.Citation25,Citation26
Chronic pain
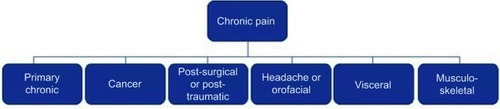
In contrast to acute pain, chronic pain is a disease state that serves no biological purpose, lacks a recognizable endpoint, and if related to disease or injury, extends beyond the time period expected for healing.Citation25 Chronic pain usually lasts longer than 3–6 months and is considered a unique clinical entity.Citation26 Many patients with chronic pain additionally experience changes involving emotion, behavior, and affect.Citation24 Chronic pain can be subdivided into several etiologies (see ).Citation26 Treatment of chronic pain involves a multidisciplinary approach and the utilization of various therapeutic strategies.Citation25
Inflammatory pain
Inflammatory pain is due to the excitability of peripheral nociceptive fibers due to anti-inflammatory mediators such as cytokines, chemokines, bradykinin, prostaglandins, and proteases.Citation27 These mediators are released by injured tissues and activated immune cells in response to harmful stimuli and interact with one of the following categories of receptors: G-protein coupled receptors, tyrosine kinase receptors, and ionotropic receptors. Regardless of the receptor utilized, the result of these receptor-ligand interactions is the lowering of action potential threshold via depolarization and subsequent hyperexcitability of sensory neurons.Citation27
Both acute and chronic pain are characterized by regulation of gene expression within the sensory neuron, which includes modification in the expression of receptors implicated in pain sensation. Changes in receptor transcription during acute inflammation involves local changes at the site of inflammation. In contrast, chronic inflammation prompts transcriptional changes at the level of the dorsal root ganglion.Citation27
Neuropathic pain
Neuropathic pain is caused by malfunction of the somatosensory nervous system and is characterized by both positive and negative symptoms such as sensations of burning and evoked pain.Citation28,Citation29 Conditions associated with neuropathic pain include diabetes, HIV infection, alcohol abuse, vitamin or mineral deficiencies, and vasculitis.Citation29 Neuropathic pain is relatively common and occurs in 25% of diabetic patients and 35% of HIV-positive patients.Citation28 Clinically, neuropathic pain must be differentiated from nociceptive pain because management differs between the two.Citation29 Current first-line medications for the treatment of neuropathic pain include serotonin norepinephrine reuptake inhibitors, tricyclic antidepressants, gabapentin, and pregabalin. Second-line options include transdermal patches of capsaicin or lidocaine. Opioids are reserved as third-line agents.Citation28
Cancer pain
Up to 80% of patients with invasive cancer experience cancer pain, and the number of cancer diagnoses is estimated to reach 20 million by the year 2025.Citation30,Citation31 Unfortunately, up to 50% of cancer patients have inadequate pain control and 25% actually die in pain.Citation30 As such, cancer pain is a significant clinical problem and optimizing its management is important. Although the exact pathophysiology is not fully understood, cancer pain is thought of as a distinct pain entity resulting from complicated interactions between neoplastic cells and cells of the patient’s immune and neurological systems. Opioids are the most effective pharmacologic agents in the treatment of cancer pain.Citation31
Other factors that result in individual pain differences
Environmental factors
Environmental factors likely contribute to the multifactorial causes of individual pain symptom differences among patients. Associations between pain severity and demographic factors such as race, ethnicity, preferred language, sex, and age have been reported.Citation32 An inverse relationship between socioeconomic status and chronic widespread pain prevalence has also been described.Citation33 For instance, lower levels of education were found to be significantly and inversely related to more severe pain and functional impairment in women with chronic pelvic pain.Citation34 Additionally, living in less affluent areas is associated with frequent analgesic use and increased morbidity of chronic noninflammatory musculoskeletal pain when compared to patients living in more affluent areas.Citation35
The relationship between stress, illness, and pain is complex. While short-term stress has several positive effects, such as enhanced immune system activity, chronic stress may contribute to illness and variances in pain perception.Citation36 In adolescents, perceived stress may be related to variation in pain intensity and probability of reporting pain.Citation37 Stress is also thought to potentiate pain in patients with fibromyalgia.Citation38 Along with other environmental and psychological factors, stress has also been described as a risk factor for the development of tension headaches.Citation39
The interplay between pharmacologic agents may also contribute to individual pain differences amongst patients. The use of certain psychoactive drugs such as benzodiazepines or selective serotonin reuptake inhibitors in patients undergoing surgery has been associated with significantly higher utilization of morphine postoperatively when compared to patients who did not take such drugs preoperatively.Citation40
Biological factors
Age and gender also contribute to differences in pain management and pain perception. Age-dependent differences in distribution, metabolism, and elimination of various medications make age a critical consideration for medication dose requirements. For example, advanced age has been associated with increased sensitivity to the analgesic effects of morphine.Citation40 Studies examining sex-related differences in opiate utilization in the postoperative period have found that men consume more morphine postoperatively than women.Citation41
Psychological factors
The interaction between depression and pain symptoms is a growing area of study, and numerous reviews have described associations between depression and pain.Citation33,Citation35,Citation42 Both the prevalence of pain in depressed cohorts and the prevalence of depression in pain cohorts are higher than when these two conditions are examined in isolation.Citation43 Psychological factors such as anxiety and depression have been described as risk factors for the development of tension headaches.Citation39 Additionally, it has been suggested that psychological factors may contribute to the observation that low socioeconomic status is inversely associated with higher levels of chronic pain. After controlling for psychologic factors, the strength of this inverse relationship is lessened.Citation33 Thus while it is generally accepted that that pain symptoms and psychological factors such as depression are common comorbidities, a thorough understanding of the interplay between the two is complicated and not fully understood.Citation43
Genetic factors
Individual responses to opioids have been examined using twin studies, which estimate that 24–60% of the variances in cold-pressor pain and heat pain are attributable to genetic factors.Citation44 Epigenetic mechanisms are thought to play a role in both the expression of pronociceptive genes and the evolution of acute pain into chronic pain.Citation45 Interestingly, single nucleotide polymorphisms (SNPs) within specific genes (caspase 9, interleukin 16) increase the rate of self-reporting of pain by patients without interfering with the progression of underlying disease processes.Citation45 Additionally, polymorphisms involving the serotonin 5-HT2A receptor, serotonin transporter, and dopamine-4 receptor are more common in patients with fibromyalgia.Citation45 Harnessing the genetic polymorphisms in transporters, receptors, drug-metabolizing enzymes, and other drug targets linked to individual differences in the efficacy and the toxicity of drugs can revolutionize the field of pain management.
Ethnic factors
There is considerable evidence suggesting ethnicity is also a factor in pain differences.Citation46
For example, African Americans report greater pain and suffering compared to Caucasians for conditions such as glaucoma, AIDS, migraine, jaw pain, headache, postoperative pain, angina pectoris, joint pain, arthritis, myofascial pain.Citation47 Furthermore, 27% of African Americans and 28% of Hispanics over 50 years old report having severe pain most of the time, compared to only 17% of non-Hispanic white individuals.Citation48 African Americans also have lower thresholds for pain, cold, heat, pressure, and ischemia compared to Caucasions.Citation49 American Indians, Alaska Natives and Aboriginal people of Canada also have a higher prevalence of pain symptoms and painful conditions compared to the general US population.Citation50 Individuals from Singapore and individuals of Malayan descent have a lower pain severity compared to Chinese individuals, whereas Indian individuals report greater pain severity compared to Malayan and Chinese individuals.Citation51 Australian women also rate menstrual pain as more intense compared to Chinese women.Citation52 Swedes report more frequent pain in lower back, neck, shoulders, hands, and elbows compared to Sami (northern Scandinavian indigenous individuals) men and women.Citation53 There are also intra-ethnic differences that should be accounted for. For example, one study examining European individuals found significant differences in the way in which pain was expressed by individuals from different European countries, yet there were no reported differences in pain perception.Citation54 This same group also reported different emotional responses to chronic pain and pain intensity within a group of individuals classified as white.Citation55 This group included Hispanic, old American (third-generation US born non-Hispanic Caucasian), Irish, Italian, French-Canadian and Polish heritages. The Hispanic group reported significantly higher pain intensity ratings, followed by Italians. Overall, there is a wide variety of individual differences in pain, especially when considering ethnicity. Health-care providers should take these differences into account when treating patients for pain.Citation81,Citation82,Citation120 See .
Table 2 Ethnic differences in CYP2D6 activity
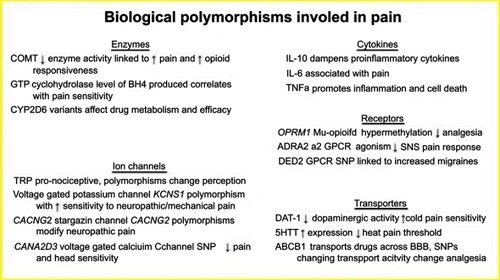
Biological polymorphisms involved in pain
Cytokines
Pathological pain involves the release of pro-inflammatory cytokines from activated macrophages in response to stress or injury. In contrast, some cytokines produced, such as IL-10, have anti-inflammatory properties that act in opposition to the pro-inflammatory cytokines.
IL-6 is a pro-inflammatory cytokine linked to responses to nerve injury and has effects on regeneration and feelings of neuropathic pain. A study examining IL-6 infusions intrathecally revealed IL-6 causes increased neuronal responses and hyperalgesia to temperature changes.Citation56 Another study analyzing inflammatory cytokines and pain reported that following primary total knee arthroplasty, there is a positive correlation between serum IL-6 concentrations and the degree of pain reported postoperatively.Citation57
Tumor necrosis factor alpha (TNFα) is a pro-inflammatory cytokine that mediates its effects via the surface receptors TNFR1 and TNFR2 and activation of NFkB. TNFα promotes regulation of pathways of apoptosis, pain, and inflammatory responses. Neurons and nociceptors contain TNFα receptors, and TNFα injections into nerves lead to degeneration of the nerve distal to the point of injection and hyperalgesia. Both effects are eliminated with administration of systemic TNF binding protein, which blocks the effects of TNFα.Citation57
IL-10 is an anti-inflammatory cytokine that dampens the activity of the pro-inflammatory cytokines by preventing their release from macrophages. IL-10 also diminishes the effects of IL-6 and TNFα through receptor blockade. Some clinical studies have found evidence suggesting that low levels of serum IL-10 contribute to chronic pain states or patients with chronic, diffuse inflammation.Citation57 Another study found that administration of IL-10 postmyocardial infarction reduced inflammation enough within the ventricle to promote and facilitate healing of the damaged myocardium.Citation58
Enzymes
Catechol O-methyl transferase (COMT) is an enzyme present at nerve terminals and is responsible for the degradation of the catecholamines dopamine, epinephrine, and norepinephrine. Different haplotypes of COMT enzyme expression are associated with variations in pain sensitivity through differences in pain processing. A study involving patients with diagnoses of chronic pain conditions associated with the Val(158)Met polymorphism with a poorly functioning COMT enzyme, causing increased sensitivity to perioperative pain and fibromyalgia. Reduced levels of functioning COMT enzyme was also linked to increased responsiveness to opioids for the treatment of chronic pain.Citation59
The enzyme GTP cyclohydrolase (GTPCH) is encoded by the GCH1 gene and is involved in the pathway leading to the production of tetrahydrobiopterin (BH4). The level of BH4 is positively correlated with pain sensitivity following injury to sensory neurons. SNPs within the GCH1 gene are linked to pain sensitivity, with some variations being protective from pain and others exacerbating it. This concept has been primarily studied in African Americans with sickle cell disease, with the presence of variant rs8007267 being protective from chronic pain and variant rs3783641 being associated with increased crisis pain.Citation60
CYP2D6 is a well-studied cytochrome P450 enzyme responsible for the metabolism of many opioid analgesics. There are more than 80 unique alleles of CYP2D6, and variations in expression affect drug metabolism and alter the efficacy of these drugs, see .Citation61 Extensive metabolizers have two wild-type alleles, which allow regular enzyme activity and predictable drug responses. In one extreme are the poor metabolizers, with two nonfunctional alleles, leading to near absent enzyme activity. Conversely, some individuals inherit either multiple copies of the functional allele, or an overactive promoter that results in increased gene transcription and elevated enzyme activity. Intermediate metabolizers have an allele with reduced functionality and impaired enzymatic activity.Citation61 Studies show that 7–10% of the Caucasian population may be poor CYP2D6 metabolizers, meaning that these individuals are at risk for failure of drug therapy in prodrugs that require activation by this enzyme, or they are at risk for toxicity in drugs that require breakdown by this enzyme.Citation62 See .
Table 3 Phenotypic effects of SNPs on drug metabolism
Ion channels
Transient receptor potential (TRP) channels are found throughout peripheral afferent nerves and are responsive to many intra- and extracellular mechanical, chemical, osmotic, and thermal stimuli.Citation63 TRP channels are pronociceptive, responding mostly to noxious inflammatory stimuli. Therefore polymorphisms that make TRP less sensitive, such as Ile585Val in TRPV1, have been shown to decrease the experience of pain in patients with knee osteoarthritis.Citation64 Additionally, TRP channel polymorphisms can change the somatosensory nature of pain—different people will have varying heat, cold, or mechanical pain thresholds from polymorphisms in TRP channels.Citation65
Voltage-gated potassium channels play a significant role in nociceptive signaling and regulation of pain pathways. KCNS1 is a genetic polymorphism in the voltage-gated potassium channels that leaves individuals susceptible to pain associated with HIV, back pain, and phantom limb pain following amputation. KCNS1 is expressed in tissues all throughout the body. The role of this ion channel was confirmed with KCNS-knockout mice exhibiting a predisposition to neuropathic pain and enhanced sensitivity to mechanical pain.Citation66
CACNG2 encodes for the protein stargazin that is needed for trafficking and ion flow through glutamatergic AMPA receptors in the nervous system. Polymorphisms in CACNG2 have been linked to susceptibility to neuropathic pain. A study investigating neuropathic pain in breast cancer patients following mastectomy found that a specific 3 SNP haplotype of CACNG2 was linked to the tendency to develop phantom breast pain, confirming CACNG2 as a modifier of neuropathic pain.Citation67
CACNA2D3 encodes the alpha-2/delta protein in voltage-gated Ca2+ channels and has been documented to contribute to nociceptive pain response to noxious heat. In humans, a specific SNP has been located that leads to reduced pain sensitivity to heat and chronic back pain postsurgically. Interestingly, when studied in mice, mutant CACNA2D3 exhibited impaired pain and heat sensitivity, but had intense activation of sensory brain regions including sight, smell, and hearing, showing impaired transmission of signals between high-order pain pathways with possible cross-activation of other sensory pathways in the brain.Citation68
Receptors
OPRM1 is the human mu-opioid receptor gene that is known to play a role in the analgesic effects of opioids. Inadequate pain management in cancer patients has been linked to hypermethylation of OPRM1, yielding a decreased response to the analgesic effects of opioids. Chronic or high-dose use of opioids was correlated with this hypermethylation and downregulation of receptors, confirming a mechanism of tolerance.Citation69 Furthermore, the presence of a specific A118G polymorphism in OPRM1 leads to a less active receptor that has been linked to individuals with reduced analgesic responses to morphine postoperatively.Citation70
ADRA2 is an alpha-2-adrenergic G protein-coupled receptor that plays a significant role in the regulation and release of sympathetic nervous system neurotransmitters. Pharmacologically, this receptor is the target of pain control therapies, such as central acting alpha-2 agonists, that reduce the release of sympathetic neurotransmitters and relieve symptoms of opioid withdrawal. Alpha-2 adrenergic receptors located in the spinal column dorsal horns, when activated with an agonist, inhibit the release of substance P and the activity of the nociceptive neurons, leading to analgesia. This is illustrated by the clinical use of clonidine, an alpha-2 agonist, in the treatment of chronic pain as well as opioid withdrawal symptoms.Citation71
Dopamine receptor D2 (DRD2) is a G-protein coupled receptor that inhibits adenylyl cyclase. This receptor is well-known as the site of action of many antipsychotic drugs, but it has also been studied in migraine headaches. A specific SNP rs1800497 was linked to increased prevalence of migraine headaches in Chinese females, leading to the proposal of the possible use of DRD2 antagonists in the prevention of treatment of migraine headaches.Citation72
Transporters
DAT-1 is a transporter that regulates the reuptake of dopamine into the presynaptic neuron from the synaptic cleft. Polymorphisms in this transporter have been linked to individual differences in cold pain tolerance in healthy subjects. The specific polymorphism studied was a 40 base-pair repeat in the transporter within the 3ʹ untranslated region that led to decreased transport and low activity of dopamine, suggesting that low dopaminergic activity yields higher sensitivity to pain.Citation73
The serotonin transporter (5HTT) is responsible for serotonin’s reuptake from the synaptic cleft. Specific polymorphisms known as the 5-HT transporter-linked polymorphic region (5-HTTLPR) has been studied intensely in patients with chronic pain, looking at high-, intermediate-, and low-expressing groups. A study analyzing the variations of the triallelic 5-HTTLPR and perception of heat pain found that individuals with high levels of serotonin transporter expression had lower pain thresholds for heat pain than those with intermediate levels of expression, highlighting the complex role of serotonin in pain modulation.Citation74
ABCB1 is an ATP-binding cassette transporter responsible for transport of various molecules across membranes, including the blood–brain barrier, and is a member of the multidrug resistance family of transporters. ABCB1 transports various opioid analgesics across the blood–brain barrier, and specific polymorphisms are linked to changes in analgesic effects of these drugs. In a study of lung cancer patients undergoing radical operations, patients possessing homozygous rs2032582 and rs1128503 loci consumed significantly higher doses of sulfentanil postoperatively to achieve adequate analgesic effects, supporting that these specific SNPs cause decreased transport activity.Citation75 See .
Opioid receptor polymorphisms
Variations in the mu, kappa, and delta opioid receptors also play a significant role in the opioid response. Over 100 variants of the opioid receptor mu 1 gene (OPRM1) have been identified. The most studied allele, 118 A>G polymorphism (rs1799971), is prevalent in 2–48% of the population.Citation76 This particular mutation results in an increased binding of beta-endorphins to the mu opioid receptor, and is hypothesized provide higher pain relief in homozygotes, and thus decreased daily requirements for morphine.Citation77,Citation78
Polymorphisms with similar clinical relevance have been found for the OPRK1 and OPRD1 genes as well. κ-opioid receptor activation is responsible for spinal analgesia and is responsible for similar adverse effects as the μ-receptor (respiratory depression, sedation, and dysphoria), while the δ-opioid receptor is implicated in dysphoria and psychomimetic effects.Citation76 These receptors, particularly the δ-receptor, have importance with regards to addiction as mentioned in the buprenorphine section, and thus serve as potential future targets of targeted addiction therapy.Citation79,Citation80
Genetic polymorphisms associated with drugs used to treat pain
Morphine
Morphine is metabolized through glucuronidation via UGT2B7 and UGT1A1, forming the active metabolite morphine 6-glucuronide as well as the inactive metabolite morphine 3-glucuronide. UGT2B7 is the primary enzyme involved in biotransformation, accounting for 60% of metabolite formation.Citation83 While numerous studies detail the effects of genetic polymorphisms on morphine’s biotransformation, efficacy to treatment, and potential for toxicity, guidelines to support dose selection of morphine are lacking. Genotypes related to morphine’s ability to treat pain, such as the GG genotype for OPRM1, may help inform appropriate dose selection. In one study, patients with the GG genotype often require higher daily doses of morphine to achieve appropriate levels of analgesia, in comparison to the wild-type A allele (225+143 mg/day vs 97+89 mg/day in those with the A allele for OPRM1, P=0.006).Citation84 Another study showed variability for the development of respiratory depression in individuals with polymorphisms for the p-glycoprotein transporter ABCB1, resulting in extended hospital stay.Citation85
Codeine
Codeine is a prodrug metabolized by O-demethylation to the active analgesic morphine via the CYP2D6 pathway. This mechanism accounts for about 10% of the overall elimination of codeine. CYP2D6 activity can vary considerably among individuals, as there are approximately 100 different variants of the genes that have been identified to date.Citation76 The most clinically significant polymorphisms arise from those harboring two loss of function variants, also known as poor metabolizers, and those harboring at least three normal function variants, also known as ultrarapid metabolizers. Poor metabolizers produce low plasma concentrations of the active morphine metabolite, and thus are less likely to achieve adequate pain control with codeine. Ultrarapid metabolizers, however,run the risk of reaching supratherapeutic levels of morphine. Furthermore, there is a relationship between the metabolism of codeine and morphine when the drugs are adiministered at the same time. Genotypic differences in UGT2B7, which is responsible for metabolizing morphine into morphine-6-glucuronide and morphine-3-glucuronide, can impact codeine’s therapeutic effect. In particular, the UGT2B7*2/*2 genotype, which results in a reduced function of the enzyme, has been associated with higher toxicity. Several pharmacokinetic studies have illustrated the effects of these phenotypes on metabolite formation. In one study, a single dose of 30 mg codeine was administered to 12 UM individuals in comparison to 11 EMs and three PMs.Citation86 Significant differences were detected between EM and UM groups for areas under the plasma concentration versus time curves (AUCs) for morphine with a median (range) AUC of 11 (5–17) μg*h*L−1 in EMs and 16 (10–24) μg*h*L−1 in UMs relative to individuals with the PM phenotype (0.5 μg*h*L−1, P=0.02).
The codeine-CYP2D6 interaction is the only opioid-gene interaction that currently has an actionable Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetic-based recommendations, primarily due to the severe nature the toxicities related to rapid metabolism phenotypes in young users, such as respiratory depression.Citation87 On the basis of these guidelines, it is strongly recommended that UMs and PMs should avoid codeine due to potential for toxicity and lack of efficacy, respectively. Recent updates by the US Food and Drug Administration (FDA) to the label for both codeine and tramadol also contraindicate the use of codeine for pain or cough in patients younger than 12 years of age.Citation88 Furthermore, the FDA warns against the use of codeine in obese adolescents and those with obstructive sleep apnea or severe lung disease.
Tramadol
Tramadol, like codeine, is also a prodrug bioactivated by the CYP2D6 enzyme, through which it is metabolized to its active metabolite O-desmethyltramadol (ODT). Similar to codeine, tramadol’s effects are impacted by individuals with UM and PM phenotypes. In one study, tramadol failed to provide adequate pain relief at 48 hours following surgery in patients with the CYP2D6 polymorphism (P<0.001).Citation89 In UMs, respiratory depression may occur, and has been described in at least one case report to date.Citation89 The same cautions were made by the FDA in response to tramadol as were made for codeine in April 2017, namely a contraindication for the use of tramadol in individuals less than 18 years of age following tonsillectomy or adenoidectomy.Citation88 Although there is no CPIC guideline for tramadol, its metabolism by CYP2D6 would make it an unsuitable alternative to codeine and thus be treated in a similar fashion to codeine with regards to cautions and recommendations for use.
Hydrocodone
Hydrocodone, a semisynthetic opioid, follows metabolism via CYP2D6 and CYP3A4 to form hydromorphone and norhydrocodone respectively. These are further conjugated by UGT enzymes into water soluble metabolites that are excreted by the kidneys. Importantly, hydromorphone’s affinity for the μ-opioid receptor is far higher than that of hydrocodone, up to 100-fold greater. Like tramadol and codeine, variations in CYP2D6 have an impact on analgesia from hydrocodone.Citation87 At least one study determined that patients who underwent cesarean section delivery and were determined to be UMs of CYP2D6 had approximately a 10-fold increase in hydromorphone plasma concentration compared to those with PM phenotype.Citation90 Based on this profile, hydrocodone may not be a good alternative to codeine or tramadol based on the CPIC guidelines for codeine. This recommendation is based mainly on hydrocodone’s role as a substrate for CYP2D6, rather than from robust evidence. Given this status, CPIC currently recommends more research for this particular drug with respect to the impact of genetic polymorphisms. Recent studies have shown an opportunity to use hydrocodone pharmacogenetics to tailor responses to therapy, while at the same time assessing conversion of hydrocodone to hydromorphone in the body.Citation91
Oxycodone and oxymorphone
Oxycodone and oxymorphone are metabolized by CYP450 and to a lesser extent, UDP-glucuronosyltransferases (UGT), specifically UGT2B7.Citation92 The UGTs are a secondary metabolizing system responsible for the formation of glucuronides. Geneticvariability in the enzyme 2B7 exisits, however, the in vitro and in vivo functional significance of these allele variants are not well defined.Citation93 Like hydrocodone, oxycodone is metabolized via the specific enzymes CYP3A4 and CYP2D6 into noroxycodone and oxymorphone, respectively. Unlike hydrocodone and tramadol, however, the parent drug for oxycodone exhibits some analgesic effect, and the drug also undergoes a more complex metabolic pathway than the previous two. CYP3A4 mediates the primary metabolic pathway, accounting for more than 50% of the overall conversion of oxycodone. In similar fashion to codeine and tramadol, PMs of CYP2D6 exhibit lower conversion into its active metabolites and thus exhibit a lower analgesic response as well as a lower potential for adverse effects in comparison to extensive metabolizers.Citation94 Moreover, UMs tend to have a much higher response and potential for side effects to doses of oxycodone. One study of cancer patients noted that differences in CYP3A impacted patient response to oxycodone.Citation95 Given the complex interplay between oxycodone metabolism and its associated pharmacogenetics, current CPIC guidelines recommend further studies before definitive treatment guidance can be given.Citation87
Diamorphine
Diamorphine, more commonly known by the street name “heroin” is metabolized into 6-monoacetylmorphine (6-MAM) primarily via the enzymes hCE-1 and partly by hCE-2.Citation96 Additionally, variations in loci for the kappa and delta opioid receptor genes OPRK1 and OPRD1 have been connected to the potential for addiction and dependence to diamorphine. Additional research involving these interactions could elucidate their role as targets for addiction therapy.Citation80
Fentanyl
Fentanyl is metabolized by the enzymes CYP3A4 and CYP3A5. Variations in CYP enzymes have been demonstrated to impact plasma concentrations of fentanyl. In one study of 60 adult patients with cancer receiving transdermal fentanyl, the plasma concentration of fentanyl was shown to be twice as high in CYP3A5*3 homozygotes compared to CYP3A5*1 carriers.Citation97 The same study also showed that polymorphisms in the gene ABCB1 can lead to significant changes in fentanyl plasma concentrations, with the ABCB1 1236TT variant being associated with a lower need for rescue medication. To date there have been no statistically significant findings for fentanyl-related adverse effects, in the previous study or current body of literature. As such, more research is needed before clinical adoption of fentanyl pharmacogenetics would be useful.
Buprenorphine
Buprenorphine, a semisynthetic opioid, is metabolized via CYP3A4. Used mainly for treating opioid addiction, the drug exerts its effects at the OPRD1 receptor. Most notably, the connection between specific SNPs such as rs58111 and rs529520 have been predictive of outcomes for the use of buprenorphine in treating opioid dependence, with the rs58111 SNP being associated with a more favorable response to buprenorphine.Citation98
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Patient variability to NSAIDs is impacted by variations in select CYP enzymes, namely CYP2C9, which metabolizes many NSAIDs. Two allelic variants of CYP2C9, CYP2C9*2 and CYP2C9*3, result in reduced inactivation of NSAID substrates by 50% and 15% respectively. This lower metabolism results in prolonged action of the drugs, and thus higher of side effects such as GI bleed.Citation99 A prospective multicenter, study identified a higher rate of acute upper GI bleeding related to use of nonaspirin NSAIDs in patients with the CYP2C9*3 variant when compared to patients receiving aspirin, indicating the potential risk associated with certain NSAIDs in patients with the CYP2C9*3 loss-of-function allele.Citation100 Additionally, variability of the prostaglandin-endoperoxidase synthase 1 and 2 genes (PTGS1 and PTGS2) influences response to particular NSAIDs.Citation99 PTGS1 encodes for COX1, and PGTS2 codes for COX2. Mutations resulting in a higher number of COX2 over COX1 were determined to respond better to selective agents such as rofecoxib, while agents with COX1 effects such as ibuprofen showed better pain response in individuals expressing more PTGS1.
Ketamine
Ketamine metabolism occurs via N-demythylation by CYP3A4, CYP2B6, and CYP2C, with significant analgesic and sedative activity achieved through antagonism of the NMDA receptor. Despite there being several cytochrome enzymes involved in ketamine’s metabolism, strong correlates between polymorphisms and clinical importance have yet to be identified.Citation101
Lidocaine
Lidocaine, a local anesthetic with activity at sodium channels, is metabolized via CYP3A4, and thus is theoretically impacted by influence of inducers and inhibitors of CYP3A4. The most pronounced effects on variation in clinical efficacy, however, are due to mutations in the sodium channel gene SCN9A.Citation78 One invitro study showed that the 395N>K mutation in this gene produces greater resistance to lidocaine, and another showed that individuals with phenotypes associated with red hair in the melanocortin-1 receptor gene (MCR1) had reduced efficacy to subcutaneous lidocaine.Citation102
Remifentanil
Remifentanil is a synthetic opioid analgesic drug that is potent and short-acting. It is used during surgery to treat pain and as an adjunct to anaesthetics. Remifentanil is a specific mu-type-opioid receptor agonist. There is evience to suggest increased pain sensitivity in Met158 individuals following treatment with remifentanil. As previsouly mentioned, the COMT gene has variations that can affect opioid drug metabolism, specifically at the 158 codon where either Val or Met can be present. A 2006 study suggested that individuals with Val alleles show increased COMT activity and have decreased prefrontal extracellular dopamine compared to those with the Met substitution.Citation103 Val158 alleles may also be associated with an advantage in the processing of aversive stimuli, or pain. Individuals who are homozygous for the Met158 allele show increased pain sensitivity, likely through a lower-functioning μ-opioid system response to prolonged pain.Citation104,Citation105 A cohort study investigating repeated thermal-pain stimulation both before and after one single dose of opiate in caucasions showed that individuals with the Val158 genotype did not respond to either the initial noxious stimulus or the analgesic response to remifentanil.Citation106 Yet, reported pain in Met15 individuals were higher following repeated heat stimulation and postremifentanil treatment, suggesting that the initial pain response is not mediated by COMT and may only present after the endogenous pain response is challenged. This reaction could be produced by an increased susceptibility of these individuals to opioid-induced hyperalgesia.Citation102
Escitalopram
Escitalopram is an SSRI used to treat autism spectrum disorder, but it can also be used to treat neuropathic pain. CYP2C19 is the enzyme responsible for metabolizing escitalopram and individuals can carry up to 30 different alleles. The majority of patients will carry the CYP2C19*1, *2, or *17 alleles, where *17 allele is an ultrarapid metabolizer. A normal functioning enzyme is indicated by CYP2C19*1, while CYP2C19*2 and CYP2C19*3 are the most common non-functioning alleles.Citation107 Ultrarapid metabolizers should be administered an alternative drug that is not metabolized by CYP2D19. Extensive and intermediate metabolizers should be administered medication normally. Poor metabolizers should have a 50% reduction in the recommended starting dose with a titration to the response dose or should be given a drug that is not metabolized by CYP2D19.Citation107
See for an overview of drugs, their clinical utility, associated polymorphisms and phenotypic effect of the genetic variant.
Table 4 A list of drugs, their clinical utility, associated polymorphisms and phenotypic effect of the genetic variant
Pharmacogenomics improving pain management
Pain-associated genetic factors vary with pain classification, but all classifications have some genetic component. The effect of polymorphisms in the OPRM1 and COMT genes, which transcribe opioid receptor mu 1 and catechol-O-methyltransferase respectively, are relatively well categorized in their effect on acute postoperative, cancer-related, and chronic pain.Citation109,Citation110 When patients are homozygous for the common amino acid substitution val158met, they require a dose of morphine that is significantly higher than homozygous met/met patients.Citation109 Similarly, cancer patients with a 118GG polymorphism in the OPRM1 gene need a higher morphine dose than patients with 118AA (1,2,3). Other genes, such as CREB1, GIRK2, and CACNA1E, have similar consequences on the pain relieving effects of opioids.Citation111
Improving pain management necessitates expanding upon the clinical features analyzed when making treatment decisions.Citation111 Pharmacogenomics can provide valuable information to guide drug choice and dosing for more effective, safer treatments.Citation111,Citation112 CYP2D6 tests—including full sequence analysis and targeted variant analyses utilizing PCR—are available to determine a patient’s metabolizer level of codeine.Citation18,Citation111 DNA is gathered via a buccal swab which prevents unnecessary burden on the patient while providing the clinician with a powerful tool to improve the patient’s treatmentCitation112 Instead of analyzing a specific gene in relation to one treatment as in the case of codeine and CYP2D6, creating a pain-related gene panel would allow more informed and effective initial treatment by the clinician.Citation112 Panels already exist in this regard for laboratory experimentation, however, they are not FDA approved, nor are they ready to be implemented in the clinic.Citation113 Interpretation of pain is an incredibly complex pathophysiological process, which can only be partially explained by genetics, but studying and understanding variability in adverse effects and treatment effectiveness based on pharmacogenetics can benefit patient outcomes.Citation111
An recent breakthrough study suggests a direct benefit of personalized patient medical care. Smith et al investigated CYP2D6-guided opioid therapy as a possible way to improve patient pain control. They found that, in fact, CYP2D6 does improve pain control in CYP2D6 intermediate and poor metabolizers. Patients experiencing chronic pain from seven different clinics were enrolled in the study. The patients were randomly assigned to either a CYP2D6-guided care group or a usual care group.
Future directions
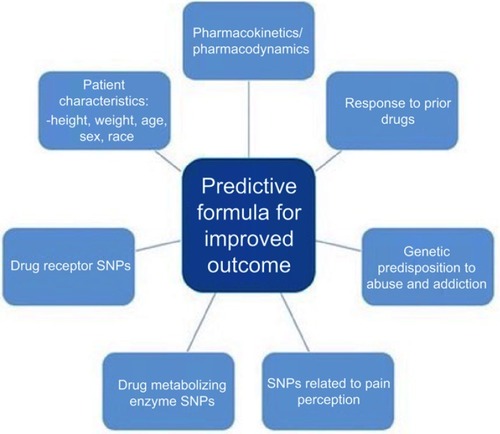
There are several major challenges for the future of pharmacogenomics in pain management. Primarily, the cost associated with implementing a pharmacogenomics program is high and this can be a deterrent to health-care institutions. To address this, identifying patients who, based on the pharmacogenetics, may not respond to drug treatments, have a high risk of adverse events, or a high risk of drug abuse and addiction, could improve costs associated with chronic pain management and patient outcomes.Citation113 By developing predictive formulas for patient outcomes, physicians could utilize patient characteristics (height, weight, age, etc), genotypes from pharmacogenetic testing, and drug pharmacokinetics/pharmacodynamics along with additional predictive markers to better treat patients, see . Yoshida et al used this approach to develop a predictive formula for postoperative fentanyl dose requirement using patients’ SNP profile for several genes involved in pain relieving effects of opioids.Citation111 The precision of the developed formula proved to be low, but promising nonetheless.Citation111 Further study and identification of SNPs related to analgesics dosing, effectiveness, metabolism, and adverse effects will allow for new variables to be included in similar formulas to expand their predictive power.Citation101
Furthermore, Next-Generation Sequencing (NGS), Illumina, Inc., San Diego, CA, USA is the new standard for sequencing technologies and is vital to understanding pharmacogenetics. The costs associated with NextGen Sequencing are decreasing, which will improve the affordability of genetic testing to the individual patient.Citation114 Testing time is another major challenge associated with pharmacogenomics in pain management and cutting down on this testing time is crucial to the widespread acceptance of this system.Citation101 Patients in severe pain cannot afford to wait for results to return before receiving treatment, therefore, protocols should be in place to immediately help the patient while waiting for test results before a transition to the best long-term treatment option. Another challenge mentioned earlier, suggests that further research is needed to understand the pharmacogenetics behind both pain perception and pain management, especially to move beyond studying dosing and toxicity to investigate the efficacy of employing one pain relief medication versus another in an individual patient.Citation101,Citation114 For example, studies exploring ethnicity-based genetic polymorphisms associated with metabolizer type have a variety of categorized results, even in recent studies. outlines several studies ranging from 2002–2018 which have overlapping polymorphisms associated with each category of metabolizer. More research is needed to make the results of these studies more consistent with one another. If these major challenges are addressed there is an increased likelihood that pharmacogenomics in the field of pain management will have a more widespread acceptance.
Table 5 Evidence for correlations between ethnicity or gene polymorphisms based on metabolizer status
Conclusion
A universal approach to health care, especially related to pain management, is no longer an option. Since all patients have different responses to medications and pharmacogenomics now allows us to explain these differences in response, it is clear that individualized medicine tailored to each individual patient is the future of patient care. This should, however, be viewed with promise, as individualized patient-specific care can save money, improve patient experiences and improve patient outcomes. Monitoring the DNA polymorphisms in each patient can allow health-care providers to predict how a patient will respond to a drug and could potentially save a patient’s life. This is of particular importance when considering the relationship between pain management and drug addiction. There are established genetic polymorphisms in individuals who abuse drugs, yet a clinician currently assesses the potential for abuse by interacting with the patient and reports from those close to the patient, thereby missing potentially crucial genetic predispositions to abuse.Citation113 Therefore, a health-care provider could prevent the possibility of a patient to abuse a drug based on their polymorphisms and suggest an alternate treatment regimen. By applying the knowledge of pharmacogenomics into clinical practice health-care providers can offer safer comprehensive health care to patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- Institute of Medicine (US) Committee on Advancing Pain Research, Care and E. Relieving pain in America: a blueprint for transforming prevention, care, education, and research - PubMed - NCBI. Natl Acad Collect Rep Funded Natl Inst Heal. 2011. doi:10.7205/MILMED-D-16-00012
- IASP Group. Pain terms: a list with definitions and notes on usage. Recommended by the IASP subcommitee on taxonomy. Pain. 1979. doi:10.1016/0304-3959(79)90175-1
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009. doi:10.1146/annurev.neuro.051508.135531
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999. doi:10.1016/S0140-6736(99)01307-0
- Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018. doi:10.15585/mmwr.mm6736a2
- Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat. 2006;13 Available from: http://europepmc.org/abstract/MED/16471269.
- Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016. doi:10.2147/JPR.S105892
- Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc. 2016. doi:10.1016/j.mayocp.2016.04.029
- Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016. doi:10.1097/MLR.0000000000000625
- Paulozzi L, Baldwin G, Franklin G, et al. CDC grand rounds: prescription drug overdoses - a U.S. Epidemic. Morb Mortal Wkly Rep. Vol 61. 2012;[pii].
- Vogel F. Moderne Probleme der Humangenetik. Ergeb Inn Med Kinderheilkd. 1959. doi:10.1007/978-3-642-94744-5_2
- Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–350. doi:10.1038/nature1581726469045
- Vesell ES, Page JG. Genetic control of drug levels in man: antipyrine. Science (80-). 1968. doi:10.1126/science.161.3836.72
- Price Evans DA, Manley KA, McKusick VA. Genetic control of isoniazid metabolism in man. Br Med J. 1960. doi:10.1136/bmj.2.5197.485
- Blum M, Demierre A, Grant DM, Heim M, Meyer UA. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci. 1991. doi:10.1073/pnas.88.12.5237
- Gonzalez FJ, Skodat RC, Kimura S, et al. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988. doi:10.1038/331442a0
- Ingelman-Sundberg M, Evans WE. Unravelling the functional genomics of the human CYP2D6 gene locus. Pharmacogenetics. 2001. doi:10.1097/00008571-200110000-00002
- Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95(4):376–382. doi:10.1038/clpt.2013.25424458010
- Matic M, De Wildt SN, Tibboel D, Van Schaik RHN. Analgesia and opioids: a pharmacogenetics shortlist for implementation in clinical practice. Clin Chem. 2017. doi:10.1373/clinchem.2016.264986
- Van Driest SL, Shi Y, Bowton E, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014. doi:10.1038/clpt.2013.229
- Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: A model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet Part C Semin Med Genet. 2014. doi:10.1002/ajmg.c.31391
- Weitzel KW, Aquilante CL, Johnson S, Kisor DF, Empey PE. Educational strategies to enable expansion of pharmacogenomics-based care. Am J Heal Pharm. 2016. doi:10.2146/ajhp160104
- Tighe P, Buckenmaier CC, Boezaart AP, et al. Acute pain medicine in the United States: a status report. Pain Med (United States). 2015;16(9):1806–1826. doi:10.1111/pme.12760
- Bonica JJ. Neurophysiologic and pathologic aspects of acute and chronic pain. Arch Surg. 1977;112(6):750–761. doi:10.1001/archsurg.1977.0137006008201416580
- Grichnik KP, Ferrante FM. The difference between acute and chronic pain. Mt Sinai J Med. (58):217–220. 1991.1875958
- Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1. doi:10.1097/j.pain.000000000000016025599292
- Linley JE, Rose K, Ooi L, Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch Eur J Physiol. 2010;459(5):657–669. doi:10.1007/s00424-010-0784-620162302
- Murnion BP. Neuropathic pain: current definition and review of drug treatment. Aust Prescr. 2018;41(3):60–63. doi:10.18773/austprescr.2018.02229921999
- Gierthmühlen J, Baron R. Neuropathic pain. Semin Neurol. 2016;36(5):462–468. doi:10.1055/s-0036-158495027704502
- Nersesyan H, Slavin KV. Current aproach to cancer pain management: availability and implications of different treatment options. Ther Clin Risk Manag. 2007;3(3):381–400. doi:10.1073/pnas.070575910418488078
- Berg D, Gerlach H. Recent advances in understanding and managing sepsis. F1000Research. 2018;7:1570. doi:10.12688/f1000research.15758.1
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. doi:10.1016/j.jpain.2015.05.00226028573
- Davies KA, Silman AJ, Macfarlane GJ, et al. The association between neighbourhood socio-economic status and the onset of chronic widespread pain: results from the EPIFUND study. Eur J Pain. 2009;13(6):635–640. doi:10.1016/j.ejpain.2008.07.00318782674
- Roth RS, Punch MR, Bachman JE. Educational achievement and pain disability among women with chronic pelvic pain. J Psychosom Res. 2001;51(4):563–569. doi:10.1016/S0022-3999(01)00242-211595244
- Brekke M, Hjortdahl P, Kvien TK. Severity of musculoskeletal pain: relations to socioeconomic inequality. Soc Sci Med. 2002;54(2):221–228. doi:10.1016/S0277-9536(01)00018-111824927
- Wu MT, Pan HB, Lai PH, Chang JM, Tsai SH, Wu CW. CT of gastritis cystica polyposa. Abdom Imaging. 1994;19(1):8–10. doi:10.1007/BF021658528161914
- Østerås B, Sigmundsson H, Haga M. Perceived stress and musculoskeletal pain are prevalent and significantly associated in adolescents: an epidemiological cross-sectional study chronic disease epidemiology. BMC Public Health. 2015;15(1):1–10. doi:10.1186/s12889-015-2414-x25563658
- Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K, Nater UM. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome-The role of cortisol and alpha-amylase. Psychoneuroendocrinology. 2016;63:68–77. doi:10.1016/j.psyneuen.2015.09.01826431802
- Song TJ, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Anxiety and depression in tension-type headache: a population-based study. PLoS One. 2016;11(10):1–12. doi:10.1371/journal.pone.0165316
- Coulbault L, Beaussier M, Verstuyft C, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79(4):316–324. doi:10.1016/j.clpt.2006.01.00716580900
- Periasamy S, Poovathai R, Pondiyadanar S. Influences of gender on postoperative morphine consumption. J Clin Diagnostic Res. 2014;8(12):GC04–GC07. doi:10.7860/JCDR/2014/10770.5319
- Elsbernd A. Zum Verh??ltnis von pflegerischem Wissen, pflegerischer Handlungsfreiheit und den Grenzen des Gehorsams der individuellen Pflegeperson. Pflege. 1994;7(2):105–116. doi:10.1002/npr2.120038018806
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity. Arch Intern Med. 2003;163(20):2433. doi:10.1001/archinte.163.20.243314609780
- Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136(1–2):21–29. doi:10.1016/j.pain.2007.06.00817692462
- James S. Human pain and genetics: some basics. Br J Pain. 2013;7(4):171–178. doi:10.1177/204946371350640826516521
- Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012;2(3):219–230. doi:10.2217/pmt.12.723687518
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–294. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12974827. Accessed May 1, 2019. doi:10.1046/j.1526-4637.2003.03034.x12974827
- Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: non-Hispanic whites, non-Hispanic blacks, and Hispanics. J Pain. 2007;8(1):75–84. doi:10.1016/j.jpain.2006.06.00216949874
- Mossey JM. Defining racial and ethnic disparities in pain management. Clin Orthop Relat Res. 2011;469(7):1859–1870. doi:10.1007/s11999-011-1770-921249483
- Jimenez N, Garroutte E, Kundu A, Morales L, Buchwald D. A review of the experience, epidemiology, and management of pain among American Indian, Alaska Native, and Aboriginal Canadian peoples. J Pain. 2011;12(5):511–522. doi:10.1016/j.jpain.2010.12.00221330217
- Chan A, Malhotra C, Do YK, Malhotra R, Østbye T. Self reported pain severity among multiethnic older Singaporeans: does adjusting for reporting heterogeneity matter? Eur J Pain. 2011;15(10):1094–1099. doi:10.1016/j.ejpain.2011.05.00621646030
- Zhu X, Wong F, Bensoussan A, Lo SK, Zhou C, Yu J. Are there any cross-ethnic differences in menstrual profiles? A pilot comparative study on Australian and Chinese women with primary dysmenorrhea. J Obstet Gynaecol Res. 2010;36(5):1093–1101. doi:10.1111/j.1447-0756.2010.01250.x20846252
- Sjölander P. What is known about the health and living conditions of the indigenous people of northern Scandinavia, the Sami? Glob Health Action. 2011;4(1):8457. doi:10.3402/gha.v4i0.8457
- Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105–2116. doi:10.1016/S0140-6736(17)30638-428456509
- Bates MS, Rankin-Hill L. Control, culture and chronic pain. Soc Sci Med. 1994;39(5):629–645. Accessed May 1, 2019. doi:10.1016/0277-9536(94)90020-5.7973863
- Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007. doi:10.1097/AIA.0b013e318034194e
- Si HB, Yang TM, Zeng Y, et al. Correlations between inflammatory cytokines, muscle damage markers and acute postoperative pain following primary total knee arthroplasty. BMC Musculoskelet Disord. 2017. doi:10.1186/s12891-017-1597-y
- Jung M, Ma Y, Iyer RP, et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017. doi:10.1007/s00395-017-0622-5
- Tammimäki A, Männistö PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012. doi:10.1097/FPC.0b013e3283560c46
- Sadhu N, Jhun EH, Yao Y, et al. Genetic variants of GCH1 associate with chronic and acute crisis pain in African Americans with sickle cell disease. Exp Hematol. 2018. doi:10.1016/j.exphem.2018.07.004
- Muneer S. Utilizing pharmacogenomics when selecting personalized medicine for patients with chronic pain. American Health and Drug Benefits. Faculty Perspectives in Chronic Pain. 2016.
- Světlík S, Hronová K, Bakhouche H, Matoušková O, Slanař O. Pharmacogenetics of chronic pain and its treatment. Mediators Inflamm. 2013. doi:10.1155/2013/864319
- Jara-Oseguera A, Simon SA, Rosenbaum T. TRPV1: on the road to pain relief. Curr Mol Pharmacol. 2008;1(3):255–269.20021438
- Valdes AM, De WG, Doherty SA, et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann Rheum Dis. 2011;70(9):1556–1561. doi:10.1136/ARD.2010.14812221616913
- Binder A, May D, Baron R, et al. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. Gaetano C, ed PLoS One. 2011;6(3):e17387. doi:10.1371/journal.pone.001738721468319
- Tsantoulas C, Denk F, Signore M, Nassar MA, Futai K, McMahon SB. Mice lacking Kcns1 in peripheral neurons show increased basal and neuropathic pain sensitivity. Pain. 2018. doi:10.1097/j.pain.0000000000001255
- Nissenbaum J. From mouse to humans: discovery of the CACNG2 pain susceptibility gene. Clin Genet. 2012. doi:10.1111/j.1399-0004.2012.01924.x
- Neely GG, Hess A, Costigan M, et al. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell. 2010. doi:10.1016/j.cell.2010.09.047
- Viet CT, Dang D, Aouizerat BE, et al. OPRM1 methylation contributes to opioid tolerance in cancer patients. J Pain. 2017. doi:10.1016/j.jpain.2017.04.001
- Mahmoud S, Thorsell A, Sommer WH, et al. Pharmacological consequence of the A118G μ opioid receptor polymorphism on morphine-and fentanyl-mediated modulation of Ca2+ channels in humanized mouse sensory neurons. Anesthesiology. 2011. doi:10.1097/ALN.0b013e318231fc11
- Giovannitti JA, Thoms SM, Crawford JJ. Alpha-2 Adrenergic Receptor Agonists: a Review of Current Clinical Applications. Anesth Prog. 2015. doi:10.2344/0003-3006-62.1.31
- Deng Y, Huang J, Zhang H, Zhu X, Gong Q. Association of expression of DRD2 rs1800497 polymorphism with migraine risk in Han Chinese individuals. J Pain Res. 2018. doi:10.2147/JPR.S151350
- Treister R, Pud D, Ebstein RP, et al. Associations between polymorphisms in dopamine neurotransmitter pathway genes and pain response in healthy humans. Pain. 2009. doi:10.1016/j.pain.2009.09.001
- Hooten WM, Hartman WR, Black JL, Laures HJ, Walker DL. Associations between serotonin transporter gene polymorphisms and heat pain perception in adults with chronic pain. BMC Med Genet. 2013. doi:10.1186/1471-2350-14-78
- Zhao Z, Lv B, Zhao XZ, Zhang Y. Effects of OPRM1 and ABCB1 gene polymorphisms on the analgesic effect and dose of sufentanil after thoracoscopic-assisted radical resection of lung cancer. Biosci Rep. 2019;39(1). doi:10.1042/BSR20181211
- Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37(9):1105–1121. doi:10.1002/phar.198628699646
- Nielsen LM, Olesen AE, Branford R, Christrup LL, Sato H, Drewes AM. Association between human pain-related genotypes and variability in opioid analgesia: an updated review. Pain Pract. 2015;15(6):580–594. doi:10.1111/papr.1223225201705
- Cohen M, Sadhasivam S, Vinks AA. Pharmacogenetics in perioperative medicine. Curr Opin Anaesthesiol. 2012;25(4):419–427. doi:10.1097/ACO.0b013e328355612922673786
- Crist RC, Ambrose-Lanci LM, Vaswani M, et al. Case-control association analysis of polymorphisms in the δ-opioid receptor, OPRD1, with cocaine and opioid addicted populations. Drug Alcohol Depend. 2013;127(1–3):122–128. doi:10.1016/j.drugalcdep.2012.06.02322795689
- Butelman ER, Yuferov V, Kreek MJ. κ-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35(10):587–596. doi:10.1016/j.tins.2012.05.00522709632
- Horn JR, Hansten PD. Get to know an enzyme: CYP2D6. Pharm Times. Available from: https://www.pharmacytimes.com/publications/issue/2008/2008-07/2008-07-8624. Published 2008. Accessed April 24, 2019.
- Ting S, Schug S. The pharmacogenomics of pain management: prospects for personalized medicine. J Pain Res. 2016;9:49–56.26929662
- Holthe M, Klepstad P, Zahlsen K, et al. Morphine glucuronide-to-morphine plasma ratios are unaffected by the UGT2B7 H268Y and UGT1A1*28 polymorphisms in cancer patients on chronic morphine therapy. Eur J Clin Pharmacol. 2002;58(5):353–356. doi:10.1007/s00228-002-0490-112185559
- Klepstad P, Rakvag TT, Kaasa S, et al. The 118 A>G polymorphism in the human u-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48(10):1232–1239. doi:10.1111/j.1399-6576.2004.00517.x15504181
- Sadhasivam S, Chidambaran V, Zhang X, et al. Opioid-induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharmacogenomics J. 2015;15(2):119–126. doi:10.1038/tpj.2014.5625311385
- Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7:257–265. doi:10.1038/sj.tpj.650040616819548
- Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for Cytochrome P450 2D6 Genotype and Codeine Therapy: 2014 Update. Clin. Pharmacol. Ther. 2014;95(4):376–382.
- US Food and Drug Administration. FDA Drug Safety Communication: FDA Requires Labeling Changes for Prescription Opioid Cough and Cold Medicines to Limit Their Use to Adults 18 Years and Older. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-labeling-changes-prescription-opioid-cough-and-cold. Accessed May 30, 2019.
- Stamer UM, Stüber F, Muders T, Musshoff F. Respiratory depression with tramadol in a patient with renal impairment and CYP2D6 gene duplication. Anesth Analg. 2008;107(3):926–929. doi:10.1213/ane.0b013e31817b796e18713907
- Stauble ME, Moore AW, Langman LJ, et al. Hydrocodone in postoperative personalized pain management: pro-drug or drug? Clin Chim Acta. 2014;429:26–29. doi:10.1016/j.cca.2013.11.01524269714
- Linares OA, Fudin J, Daly AL, Boston RC. Individualized hydrocodone therapy based on phenotype,pharmacogenetics, and pharmacokinetic dosing. Clin J Pain. 2015;31(12):1026–1035. doi:10.1097/AJP.000000000000021425621429
- Romand S, Spaggiari D, Marsousi N, et al. Characterization of oxycodone in vitro metabolism by human cytochromes P450 and UDP-glucuronosyltransferases. J Pharm Biomed Anal. 2017;144:129–137. doi:10.1016/j.jpba.2016.09.02427692933
- Holmquist GL. Opioid metabolism and effects of cytochrome P450. Pain Med. 2009;10(suppl1):S20–S29. doi:10.1111/j.1526-4637.2009.00596.x
- Zwisler ST, Enggaard TP, Mikkelsen S, Brosen K, Sindrup SH. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol Scand. 2010;54(2):232–240. doi:10.1111/j.1399-6576.2009.02104.x19719813
- Naito T, Takashina Y, Yamamoto K, et al. CYP3A5*3 affects plasma disposition of noroxycodone and dose escalation in cancer patients receiving oxycodone. J Clin Pharmacol. 2011;51(11):1529–1538. doi:10.1177/009127001038803321209234
- Bencharit S, Morton CL, Xue Y, Potter PM, Redinbo MR. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat Struct Biol. 2003;10(5):349–356. doi:10.1038/nsb91912679808
- Takashina Y, Naito T, Mino Y, Yagi T, Ohnishi K, Kawakami J. Impact of CYP3A5 and ABCB1 gene polymorphisms on fentanyl pharmacokinetics and clinical responses in cancer patients undergoing conversion to a transdermal system. Drug Metab Pharmacokinet. 2012;27(4):414–421.22277678
- Clarke T-K, Crist RC, Ang A, et al. Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharmacogenomics J. 2014;14(3):303–308. doi:10.1038/tpj.2013.3024126707
- Kapur BM, Lala PK, Shaw JLV. Pharmacogenetics of chronic pain management. Clin Biochem. 2014;47(13–14):1169–1187. doi:10.1016/j.clinbiochem.2014.05.06524912048
- Carbonell N, Verstuyft C, Massard J, et al. CYP2C9*3 loss-of-function allele is associated with acute upper gastrointestinal bleeding related to the use of NSAIDs other than aspirin. Clin Pharmacol Ther. 2010;87(6):693–698. doi:10.1038/clpt.2010.3320445534
- Saba R, Kaye AD, Urman RD. Pharmacogenomics in pain management. Anesthesiol Clin. 2017;35(2):295–304. doi:10.1016/j.anclin.2017.01.01528526150
- Liem EB, Teresa VJ, Tsueda K, Sessler DI. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiology. 2005;102(3):509–514.15731586
- Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11(10):745–748. Accessed May 4, 2019. doi:10.1017/S1092852900014863.17008817
- Diatchenko L, Nackley AG, Slade GD, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125(3):216–224. doi:10.1016/j.pain.2006.05.02416837133
- Vuilleumier PH, Stamer UM, Landau R. Pharmacogenomic considerations in opioid analgesia. Pharmgenomics Pers Med. 2012;5:73–87. doi:10.2147/PGPM.S2342223226064
- Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val158met Polymorphism. Toland AE, ed PLoS One. 2009;4(6):e6016. doi:10.1371/journal.pone.000601619547755
- Hicks J, Bishop J, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. doi:10.1002/cpt.14725974703
- Obeng AO, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37(9):1105–1121. doi:10.1002/phar.198628699646
- Reyes-Gibby CC, Shete S, Rakvåg T, et al. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130(1–2):25–30. doi:10.1016/j.pain.2006.10.02317156920
- James S. Human pain and genetics: some basics. Br J Pain. 2013;7(4):171–178. doi:10.1177/204946371350640826516521
- Yoshida K, Nishizawa D, Ide S, Ichinohe T, Fukuda K, Ikeda K. A pharmacogenetics approach to pain management. Neuropsychopharmacol Rep. 2018;38(1):2–8. doi:10.1002/npr2.1200330106264
- Purchase A, Marschler M, Webster L. Pharmacogenomics in pain management personalized pain therapy. doi:10.1016/j.cll.2016.05.007 Available from: https://prahs.com/resources/whitepapers/Pharmacogenomics-in-Pain-Management.pdf
- Belfer I. Personalized Pain Medicine: Pharmacogenetic Testing for Pain and Opioid Addiction PA I N M E D I C I N E N E W S • S E P T E M B E R 2 0 1 5. Available from: https://www.painmedicinenews.com/Review-Articles/Article/09-15/Personalized-Pain-Medicine-Pharmacogenetic-Testing-for-Pain-and-Opioid-Addiction/33480/ses=ogst?%20target=
- Ko T-M, Wong C-S, Wu J-Y, Chen Y-T. Pharmacogenomics for personalized pain medicine. Acta Anaesthesiol Taiwanica. 2016;54(1):24–30. doi:10.1016/J.AAT.2016.02.001
- Bijl MJ, Visser LE, Hofman A, et al. Influence of the CYP2D6*4 polymorphism on dose, switching and discontinuation of antidepressants. Br J Clin Pharmacol. 2008;65(4):558–564. doi:10.1111/j.1365-2125.2007.03052.x18070221
- Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist. 2006;11(2):126–135. doi:10.1634/theoncologist.11-2-12616476833
- Zhou S-F, Liu J-P, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41(2):89–295. doi:10.1080/0360253090284348319514967
- Dean L. Codeine Therapy and CYP2D6 Genotype. Bethesda (MD): National Center for Biotechnology Information (US); 2012 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28520350. Accessed 430, 2019.
- Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS. Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med. 2017;19(1):69–76. doi:10.1038/gim.2016.8027388693
- Del Tredici AL, Malhotra A, Dedek M, et al. Frequency of CYP2D6 alleles including structural variants in the United States. Front Pharmacol. 2018;9:305. doi:10.3389/fphar.2018.0030529674966