Abstract
Trastuzumab (Herceptin), a monoclonal antibody directed against the human epidermal growth-factor receptor 2 (HER2), is the poster child for antibody-based targeted therapy in breast cancer. Pertuzumab, another humanized monoclonal antibody, binds to a different domain of HER2 and prevents the formation of HER2:HER3 dimers, which is the most potent heterodimer in the HER family. The combination of trastuzumab and pertuzumab has synergistic activity, and is associated with improved clinical outcomes. The US Food and Drug Administration (FDA) approved pertuzumab in combination with trastuzumab-based chemotherapy originally as first-line therapy for metastatic HER2-positive breast cancer in 2012, and more recently as neoadjuvant therapy for localized disease in 2013. Pertuzumab is the first neoadjuvant drug to receive accelerated approval by the FDA based on pathological complete response as the primary end point. In this article, we review the mechanism of action, pharmacokinetics, clinical efficacy, safety, and current role of pertuzumab in the management of breast cancer, as well as ongoing clinical trials and future directions regarding the utility of pertuzumab as a personalized therapeutic option for HER2-positive breast cancer. In the coming years, we anticipate increased utilization of neoadjuvant trials for drug development, biomarker discovery, and validation, and envision conduct of personalized breast cancer clinics in which therapies will be routinely selected based on genetic alterations in the tumor. Regardless of the targeted therapy combinations employed based on tumor genomic profile, trastuzumab and pertuzumab will likely continue to form the backbone of the personalized regimen for HER2-positive breast cancer.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Breast cancer is the most common noncutaneous cancer in the US, accounting for nearly one in three cancers diagnosed in females. It is estimated that 232,340 women in the US will get diagnosed with breast cancer in 2013.Citation1 Human epidermal growth-factor receptor 2 (HER2) is amplified in approximately one-quarter of breast cancers, and HER2-positive tumors represent an aggressive subtype of breast cancer.Citation2
HER2 was discovered in the mid-1980s as a transmembrane tyrosine-kinase receptor with a primary sequence very similar to that of the human epidermal growth-factor 1, and hence was termed HER2.Citation3,Citation4 Its location on chromosomal 17 and the 185 kD oncogene product of HER2 was coincident with the mouse neu oncogene, suggesting that the two genes were identical, and thus the term HER2-neu was coined.Citation2,Citation5 Subsequently, it was reported that HER2 gene amplification occurs in 20%–30% of human breast cancers and HER2 overexpression was a significant and independent predictor of time to relapse and survival among patients with breast cancer.Citation6–Citation9
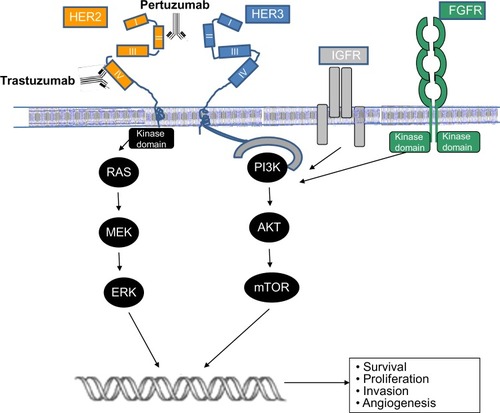
HER2 is a member of the epidermal growth-factor receptor family, all members of which have four distinct domains in their molecular structure: an extracellular ligand-binding site, a transmembrane region, an intracellular kinase, and an intracellular C-terminal tail used for downstream signaling. In order to initiate this signaling, a ligand must bind to a receptor (HER1, HER3, or HER4), and secondary dimerization must occur. Once two family members dimerize, the kinase domain of the first receptor becomes active and phosphorylates the C-terminal tail of the other, which can then recruit scaffold and adaptor proteins for further downstream signaling.Citation10 The HER2 receptor is the preferred dimerization partner for the other family members, and is unique in that it lacks a known ligand, but exists in a conformation that is always available to heterodimerize with other family members (HER1, HER3, and HER4).
When overexpressed, HER2 can homodimerize and initiate ligand-independent constitutive signaling.Citation11 HER3 receptors lack a kinase domain of their own, and thus are incapable of forming homodimers. However, HER3 can form heterodimers with other HER members, and its C-terminal tail contains phosphotyrosine sites that bind with key protein domains.Citation12 HER3 can lead to activation of multiple downstream signaling pathways, making HER2:HER3 heterodimers extremely potent signaling initiators.Citation12,Citation13 The formation of dimers initiates downstream signaling to several pathways, particularly the phosphoinositide 3-kinase (PI3K) and the mitogen-activated protein kinase pathways.Citation13 Other members of the growth-factor family, such as fibroblast growth-factor receptor and insulin growth-factor receptor, can also induce intracellular signaling.Citation14 The net consequence of these signaling events is an increase in cellular survival, proliferation, invasiveness, and angiogenesis, as outlined in .Citation11
Figure 1 Schema outlining the activation of the human epidermal growth-factor receptor 2 pathway and antibody blockade by trastuzumab and pertuzumab.
While HER2-positive tumors represent an aggressive subtype of breast cancer, the prognosis of HER2-positive breast cancer has changed dramatically since the introduction of trastuzumab (Herceptin®), a monoclonal antibody against HER2.Citation15 Multiple large randomized clinical trials have demonstrated that trastuzumab-based chemotherapy is associated with significant improvement in response rate, time to disease progression, and overall survival in metastatic breast cancer, as well as improved disease-free survival and overall survival in localized breast cancer.Citation16–Citation19
Despite the effectiveness of trastuzumab, a number of patients develop primary and secondary resistance. The mechanisms of trastuzumab resistance include incomplete HER2 blockade from trastuzumab alone, heterodimeric signaling from other growth factors, alteration in downstream signaling due to oncogene mutations, and loss of HER2 extracellular domain.Citation20,Citation21 Several therapeutic strategies to combat this issue have emerged. A common approach is to initiate dual HER2 blockade by combining two different drugs against HER2, such as pertuzumab (Perjeta®, Genentech, South San Francisco, CA, USA) and lapatinib (Tykerb®, Genentech). Trastuzumab emtansine, an antibody drug conjugate, is another drug that has demonstrated efficacy in trastuzumab-resistant breast cancer.Citation13,Citation22 The clinical success of these therapies highlights the fact that HER2 tumors that have become resistant to trastuzumab continue to rely on HER2 signaling, and rational targeting of the pathway can overcome trastuzumab resistance.
In this review, we focus on pertuzumab and examine its 1) mechanism of action and rationale for development, 2) clinical efficacy in various settings, 3) pharmacokinetics, 4) dose and regimen, 5) safety and tolerability, and 6) current role in the management of breast cancer. We also review ongoing clinical trials and future directions regarding the utility of pertuzumab as a personalized therapeutic option for HER2-positive breast cancer.
Pertuzumab
Mechanism of action and rationale for development
As demonstrated in , trastuzumab and pertuzumab bind to different epitopes of HER2. Pertuzumab is a humanized monoclonal antibody that binds to HER2 at subdomain II, which is the extracellular epitope of the receptor involved in the dimerization of HER2.Citation23 Pertuzumab prevents HER2:HER3 dimer formation and subsequent HER3-mediated signaling, whereas trastuzumab binds to subdomain IV of the HER2 extracellular domain, preventing HER2 cleavage.Citation24 The theoretically complementary mechanisms of pertuzumab and trastuzumab imply a greater efficacy when used together, as they would provide a more complete block of downstream signaling. Both of the antibodies share common mechanisms of action, such as antibody-dependent cellular cytotoxicity.
In preclinical models, the combination of pertuzumab with trastuzumab was found to reduce intracellular signaling and proliferation in breast cancer cell lines.Citation25,Citation26 In addition, the combination induced greater responses in tumor xenografts than either antibody alone.Citation25 Pertuzumab has been also shown to have inhibitory effects in other tumor models, including prostate, ovarian, and lung cancer.Citation27–Citation29 The in vitro effects for both combination and monotherapies set the stage for evaluation of pertuzumab in clinical trials. We focus on breast cancer trials in this review.
Clinical efficacy
Metastatic breast cancer
Pertuzumab was initially explored in combination with trastuzumab as treatment for HER2-positive metastatic breast cancer in patients who had progressed on trastuzumab therapy. In a Phase II clinical trial that included advanced HER2-positive breast cancer patients with up to three lines of prior cytotoxic therapies, or those who had progressed during trastuzumab-based therapy as last treatment (n=66), the pertuzumab-and-trastuzumab combination induced a response rate of 24.2%, with a median progression-free survival of 5.5 months.Citation30 The combination was well tolerated, and adverse events were minimal.
Subsequently, pertuzumab was examined as part of a first-line therapeutic combination in the pivotal CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab) trial, a randomized, double-blind, placebo-controlled study consisting of 808 patients with HER2-positive metastatic breast cancer who were assigned to trastuzumab and docetaxel with either placebo or pertuzumab.Citation31,Citation32 The pertuzumab-based combination was associated with a significant increase in median progression-free survival from 12.4 to 18.5 months, and follow-up analysis indicated a significant increase in overall survival, with no significant difference in cardiac toxicity.Citation32,Citation33 Subgroup analysis of CLEOPATRA indicated that the magnitude of efficacy of dual HER2 blockade with pertuzumab and trastuzumab was comparable, irrespective of whether or not patients were trastuzumab-naïve.Citation34 Additionally, analysis of biomarkers for prognosis or predictive value indicated that PIK3CA mutation and HER2/HER3 levels were significantly associated with a poor prognosis in both arms.Citation35 A significant limitation of this study was the limited number of patients who had prior exposure to trastuzumab (about 11%). This was primarily due to a lack of use of trastuzumab in the neoadjuvant or adjuvant setting at the time of patient enrollment. It is expected that ongoing trials, in particular the PHEREXA (A Study of a Combination of Trastuzumab and Capecitabine with or Without Pertuzumab in Patients with HER2-Positive Metastatic Breast Cancer) trial (), will bring greater clarity to the magnitude of benefit in the second-line setting.
Table 1 Key randomized clinical trials evaluating pertuzumab therapy for HER2-positive breast cancer
Pertuzumab, like other HER2-directed therapies, has minimal efficacy in HER2-negative breast cancer. In a Phase II randomized trial among women with metastatic HER2-negative breast cancer (n=79), pertuzumab was found to have limited efficacy, with only 7.7% of patients achieving either a partial response or greater than 6 months of stable disease.Citation36 In another trial among women with trastuzumab-resistant metastatic breast cancer, patients (n=29) received pertuzumab monotherapy, and at the time of progression, 17 received dual blockade with pertuzumab and trastuzumab. The dual-combination therapy arm had significantly higher progression-free survival compared to monotherapy (17.4 weeks versus 7.1 weeks), highlighting the clinical effectiveness of dual blockade.Citation37
Neoadjuvant trials
Neoadjuvant (preoperative) therapy refers to administration of systemic therapy before surgery. Potential advantages of neoadjuvant over adjuvant therapy include downstaging of tumors to increase the likelihood of breast-conserving therapy, early initiation of systemic therapy to prevent distant metastasis, and the ability to assess in vivo tumor response to therapy by assessing pathological complete response (pCR), a surrogate marker of survival.Citation38 Traditionally, single-agent trastuzumab-based neoadjuvant therapy has been considered the standard of care. However, a number of clinical trials have evaluated the clinical utility of dual HER2-directed therapy, and the resulting data suggest targeting multiple mechanisms may increase efficacy. A recent meta-analysis reported that dual HER2 blockade compared to trastuzumab alone did not improve breast-conserving surgery rate (relative risk [RR] 1.03, P=0.84), but significantly increased rates of pCR overall (RR 1.39, P<0.00001), with no increase in grade 3/4 toxicity (RR 1.13, P=0.16).Citation39 Higher pCR was associated with improved disease-free survival as well as overall survival, which is further evidence of pCR as a surrogate end point for survival in HER2-positive breast cancers.Citation40
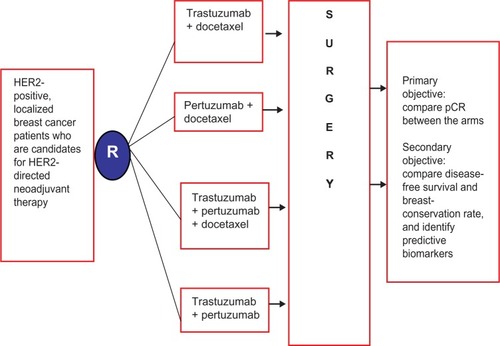
Two pertuzumab-containing neoadjuvant regimens merit particular attention. NeoSphere (Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation) enrolled 417 breast cancer patients with locally advanced, inflammatory, or early HER2-positive breast cancers >2 cm to pertuzumab, trastuzumab, both with docetaxel, or both without docetaxel.Citation41 The schema of the NeoSphere trial is outlined in . Patients treated with dual HER2 blockade plus docetaxel had significantly improved pCR at 45.8%, compared with trastuzumab plus docetaxel, with pCR of 29%. Of note, those treated with dual HER2 therapy without chemotherapy had a pCR of 16.8%, suggesting a subset of breast cancers might respond to dual HER2 blockade without chemotherapy. There were no significant differences in tolerability between the arms that contained chemotherapy; however, antibody-only therapy had significantly fewer serious adverse events. TRYPHAENA (Neoadjuvant Pertuzumab and Trastuzumab Concurrent or Sequential with an Anthracycline-Containing or Concurrent with an Anthracycline-Free Standard Regimen: A Randomized Phase II Study), another Phase II clinical trial, was conducted in the neoadjuvant setting to evaluate the toxicity of HER2 blockade with the chemotherapeutic agents anthracycline and carboplatin.Citation42 The study enrolled 225 patients with locally advanced and inflammatory HER2-positive breast cancer. Pertuzumab and trastuzumab were administered, either sequentially or concurrently with an anthracycline-containing regimen or concurrently with an anthracycline-free regimen, to determine the safety profile. Regardless of the chemotherapy chosen, the combination of pertuzumab with trastuzumab in the neoadjuvant setting resulted in high pCR rates (45.3%–66%). These two neoadjuvant trials led to an accelerated approval of pertuzumab by the US Food and Drug Administration (FDA) as a neoadjuvant therapy (see the “Current role in management of breast cancer” section).
Adjuvant trials
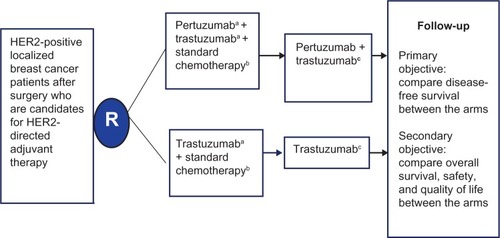
While trastuzumab has an established role in the adjuvant therapy of HER2-positive patients, the role of adjuvant pertuzumab is not clear.Citation16,Citation43 The ongoing APHINITY (Adjuvant Pertuzumab and Herceptin in Initial Therapy of Breast Cancer) trial will evaluate the benefit of dual blockade in adjuvant therapy with trastuzumab and chemotherapy in patients who have undergone resection for HER2-positive breast cancer.Citation44 A schema of the APHINITY trial design is shown in . The primary outcome is disease free survival among the targeted 3,806 participants, with secondary outcomes including overall survival, quality of life, and safety, including cardiac safety. The initial results from this trial are anticipated in 2016 and will be pivotal in clarifying the role and long term safety of pertuzumab in the adjuvant setting.
Figure 3 Schema of the APHINITY clinical trial evaluating efficacy of adjuvant pertuzumab based therapy in localized HER2-positive breast cancer.
Abbreviations: HER, human epidermal growth-factor receptor; IV, intravenous; q3 weeks, every 3 weeks; APHINITY, Adjuvant Pertuzumab and Herceptin in Initial Therapy of Breast Cancer.
Review of pharmacokinetics
Pertuzumab shows a pharmacokinetic profile similar to most immunoglobulin G antibodies, including trastuzumab. It has a mean half-life of about 3 weeks, and is well tolerated up to a dose of 25 mg/kg.Citation45 Some degree of individual variability and linear pharmacokinetics has been observed. Current dosing recommendations consist of infusing a loading dose of 840 mg and administering 420 mg every 3 weeks. The pharmacokinetics are affected only slightly by body weight or body surface area and are unaltered in the elderly, and thus dose adjustment is not required.Citation46
Safety and tolerability
Adverse effects
In general, pertuzumab is safe and well tolerated; however, it is recommended to observe patients for a period of 60 minutes after the first infusion and 30 minutes after subsequent infusions. While less than 1% of the infusion reactions in the CLEOPATRA trial were grade 3–4, if one of these serious reactions does occur, the infusion should be interrupted and appropriate rescue therapy administered. The patient should be monitored closely until they return to baseline, and providers should consider permanent discontinuation of pertuzumab therapy. For patients randomized to the pertuzumab-based regimen within the trial, mild day 1 infusion reactions occurred in up to 13.0% in the pertuzumab treated group versus 9.8% in control group. Overall, the most common (>30%) adverse reactions were diarrhea, alopecia, neutropenia, nausea, fatigue, rash, and peripheral neuropathy, while the most common grade 3–4 adverse reactions (2%) were febrile neutropenia, neutropenia, leukopenia, diarrhea, peripheral neuropathy, anemia, asthenia, and fatigue.Citation47 More serious adverse reactions are rare, and include anaphylactic reactions, drop in left ventricular ejection fraction (EF), and negative effects on fetal development in utero. Adverse reactions resulted in discontinuation of therapy in 6.1% of the patients in the pertuzumab arm versus 5.3% in control arm.Citation32
Pregnancy
Pertuzumab use is not recommended for women who are pregnant or breastfeeding. It is imperative to verify status of pregnancy prior to starting pertuzumab, and contraception is recommended during and for 6 months after therapy. Preclinical studies in animals have resulted in oligohydramnios, delayed renal development, and even fetal death. If pertuzumab is unknowingly administered during pregnancy, or if a patient becomes pregnant while receiving pertuzumab, that information should be immediately reported to the Genentech Adverse Event Line. In addition, those women should be persuaded to enroll in the MotHER Pregnancy Registry.Citation48
Immunogenicity
In general, monoclonal antibodies have a potential for immunogenic responses in the form of antibody production. Anti-pertuzumab antibodies have been noted in a small proportion of patients; however, the pharmacokinetic impact or clinical significance of these antibodies is not known. In the CLEOPATRA trial, approximately 2.8% of patients in the pertuzumab group and 6.2% of patients in the placebo-treated group tested positive for anti-pertuzumab antibodies.Citation32 The presence of pertuzumab antibodies despite no pertuzumab being administered is not well understood, though it is possible the assay is detecting antibodies to trastuzumab. The presence of antibodies did not correlate with infusion reactions.
Cardiac toxicity
Cardiac toxicity is associated with anti-HER2 therapies, particularly trastuzumab, which occurs due to a toxic effect on cardiac myocytes; however, pertuzumab appears to have lower cardiac toxicity than trastuzumab.Citation49,Citation50 In the CLEOPATRA study, left ventricular dysfunction was documented in 4.4% of patients in the pertuzumab-treated group versus 8.3% of patients in the placebo group. Grade 3 left ventricular systolic dysfunction occurred in 1.2% of patients in the pertuzumab treated group versus 2.8% of patients in the placebo group.Citation32 It is thought that patients with prior anthracycline use or chest radiation may be at higher risk for decline of EF; therefore, it is recommended that patients should have echocardiography at baseline and every 3 months while on pertuzumab therapy. If a patient experiences a drop in EF to <40%, or EF of 40%–45% with a 10% absolute decrease from baseline, the provider should hold pertuzumab (and trastuzumab) for at least 3 weeks. Therapy can be reinitiated if the left ventricular EF on repeat echocardiography at 3 weeks recovers to >45%, or 40%–45% if associated with a less than 10% absolute decrease from baseline. If recovery is not demonstrated, discontinuation of therapy should be considered. It is also important to note that clinical trials to date have not included patients with a baseline EF of <50%, history of congestive heart failure, drop in EF to <50% on trastuzumab, or conditions that impair LV function (hypertension, myocardial infarction, arrhythmia), and thus the safety of pertuzumab-based therapy for such patients is not known.
Current role in management of breast cancer
Pertuzumab (Perjeta) was FDA-approved in 2012, with a primary indication of first-line use in metastatic breast cancer in combination with trastuzumab and docetaxel. This approval was based primarily on results from the CLEOPATRA study. In September 2013, based on the results from neoadjuvant trials (), the FDA granted accelerated approval to neoadjuvant pertuzumab in combination with trastuzumab-based chemotherapy for patients with localized, locally advanced, inflammatory, or early (>2 cm tumor size or lymph node-positive) HER2-positive breast cancer.Citation51
The pertuzumab-based regimens, as per the current FDA prescribing label, are listed in .Citation52 The recommended loading dose of pertuzumab is 840 mg given as an intravenous infusion over 60 minutes. Subsequent doses are 420 mg given as an intravenous infusion over 30–60 minutes, and there are no recommended dose reductions.Citation47 It is important to note that the safety of neoadjuvant pertuzumab for more than six cycles in early breast cancer, or as part of a doxorubicin-containing regimen, has not been established. Also, cost issues need to be balanced against potential benefits.Citation53
Table 2 Pertuzumab-based chemotherapy regimens as per the US Food and Drug Administration prescribing label (updated September 2013)
Pertuzumab is the first neoadjuvant drug to receive accelerated approved by the FDA based on pCR as the primary end point. The full approval is contingent on the final results of APHINITY, the confirmatory study detailed in both and in the “Neoadjuvant trials” section of this review. The FDA reserves the right to withdraw approval for pertuzumab in the neoadjuvant setting if the results of APHINITY are negative. This accelerated process represents a paradigm shift in FDA approval, and may replace the long-standing model of drug development in oncology, which is protracted, inefficient, and costly. The neoadjuvant trial model offers a platform for rapid and efficient triage of drugs, as well as biomarker discovery to facilitate development of personalized therapy for breast cancer, and is reviewed in the next section.
Future directions
In the current era of targeted and personalized therapeutics in cancer, the classic approach of “one size fits all” cancer therapy is increasingly becoming obsolete. Since the remarkable successes of trastuzumab for HER2 breast cancer, there have been great strides in the development of targeted therapies. The field of breast cancer, particularly HER2-positive breast cancer, is now expanding with the addition of multiple active targeted therapies. In the future, we anticipate the routine use of genomics to identify biomarkers and accordingly select patients who will optimally benefit from particular anti-HER2 agents or therapy combinations. In addition, we expect increased utilization of neoadjuvant trials for drug development, biomarker discovery, and validation. These issues are discussed in the following sections.
Role of biomarkers and genome sequencing
Genome sequencing of tumor specimens is gaining increased traction in routine clinical practice.Citation54 We anticipate that over the coming years, next-generation sequencing will be utilized routinely for discovery of genomic alterations in tumors. While promising, it should be noted that routine tumor sequencing does pose a number of challenges. Besides the ethical and regulatory issues, tumor sequencing generates an enormous amount of data, with the consequent challenge of unscrambling “driver” mutations from “passenger” mutations. As we move in the genomic era, it will be particularly important to focus on driver mutations that are “actionable”, ie, modulated by a targeted therapy, and design biomarker-stratified clinical trials appropriately.Citation55
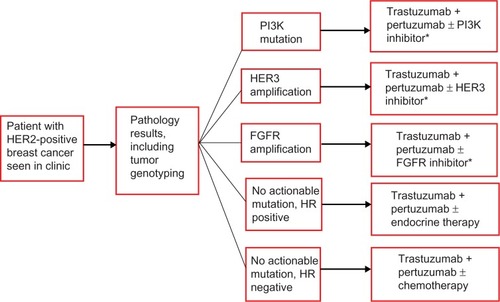
For HER2-positive breast cancer, it will be particularly important to identify oncogene mutations in the PI3K pathway, not only because PI3K mutations predict poor therapeutic response to trastuzumab and pertuzumab but also that tumors harboring a PI3K mutation can be subject to inhibition by PI3K inhibitors.Citation56,Citation57 Similarly, overexpression of HER3 is a biomarker that is associated with poor prognosis and lower response to HER2 therapies, including pertuzumab, and combination with anti-HER3 therapies would be potentially important for this subgroup.Citation58–Citation60 Obviously, these biomarker-driven hypotheses will need to be investigated and validated before they can be incorporated into clinical practice. We anticipate that neoadjuvant trials will increasingly be utilized for this purpose, as discussed in the next section.
Role of neoadjuvant trials
Neoadjuvant trials utilizing pCR, a validated surrogate end point for survival, as the primary end point provide an attractive platform for rapid triage of drug efficacy, biomarker identification, and validation.Citation61 The FDA approval of neoadjuvant pertuzumab based on pCR as the primary end point serves as a validation of this drug-development model.Citation62 Over the coming years, there will be a marked increase in the utilization of neoadjuvant trials in breast clinics, not only for drug approval of novel agents but also to compare different combinations for synergistic HER2 blockade, to identify predictive biomarkers for individual therapies, and to determine the optimal sequencing of HER2-blocking agents.Citation63 Neoadjuvant trials testing combination therapies that have the potential of overcoming treatment resistance by inhibiting compensatory cross talk between pathways, such as the combination of HER2 directed therapies with PI3K inhibitors, or anti-HER3 therapies, will be particularly valuable. Similarly, for HER2-positive tumors that are also hormone receptor-positive, it would be important to incorporate therapies that also target the estrogen receptor, since the combination of neoadjuvant endocrine and HER2 therapy can have additive therapeutic efficacy.Citation64 Safety is a key issue for neoadjuvant trials, and it will be important to consider carefully the amount of safety data present, avoid drugs with significant overlapping toxicities, and utilize innovative protocol development like the adaptive study design.Citation65
In the coming years, we envision personalized breast cancer clinics in which therapies will be routinely selected based on genetic alterations in tumors. A potential example of such a personalized therapy selection for HER2-positive breast cancer is outlined in . Trastuzumab and pertuzumab will likely form the backbone of the various targeted therapy combinations employed in the personalized regimen.
Figure 4 Schema of personalized therapy selection based on molecular profiling of breast cancer.
Abbreviations: PI3K, phosphoinositide 3-kinase; HER, human epidermal growth-factor receptor; FGFR, fibroblast growth-factor receptor; HR, hormone receptor.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts and Figures: 2013–2014AtlantaAmerican Cancer Society2013
- SlamonDJClarkGMWongSGLevinWJUllrichAMcGuireWLHuman breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogeneScience198723547851771823798106
- CoussensLYang-FengTLLiaoYCTyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogeneScience19852304730113211392999974
- KingCRKrausMHAaronsonSAAmplification of a novel v-erbB-related gene in a human mammary carcinomaScience198522947179749762992089
- SchechterALSternDFVaidyanathanLThe neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigenNature198431259945135166095109
- TandonAKClarkGMChamnessGCUllrichAMcGuireWLHER-2/neu oncogene protein and prognosis in breast cancerJ Clin Oncol198978112011282569032
- ToikkanenSHelinHIsolaJJoensuuHPrognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-upJ Clin Oncol1992107104410481351537
- SeshadriRFirgairaFAHorsfallDJMcCaulKSetlurVKitchenPClinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study GroupJ Clin Oncol19931110193619428105035
- MarksJRHumphreyPAWuKOverexpression of p53 and HER-2/neu proteins as prognostic markers in early stage breast cancerAnn Surg199421943323417909221
- PegramMDKonecnyGSlamonDThe molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancerCancer Treat Res2000103577510948442
- ArteagaCLSliwkowskiMXOsborneCKPerezEAPuglisiFGianniLTreatment of HER2-positive breast cancer: current status and future perspectivesNat Rev Clin Onc2012911632
- AminDNCampbellMRMoasserMMThe role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeuticsSemin Cell Dev Biol201021994495020816829
- BaselgaJSwainSMNovel anticancer targets: revisiting ERBB2 and discovering ERBB3Nat Rev Cancer20099746347519536107
- LuYZiXZhaoYMascarenhasDPollakMInsulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin)J Natl Cancer Inst200193241852185711752009
- SlamonDJLeyland-JonesBShakSUse of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med20013441178379211248153
- Piccart-GebhartMJProcterMLeyland-JonesBTrastuzumab after adjuvant chemotherapy in HER2-positive breast cancerN Engl J Med2005353161659167216236737
- SlamonDEiermannWRobertNAdjuvant trastuzumab in HER2-positive breast cancerN Engl J Med2011365141273128321991949
- McArthurHLMahoneyKMMorrisPGAdjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancerCancer2011117245461546821681735
- RodriguesMJPeronJFrénelJSBenefit of adjuvant trastuzumab-based chemotherapy in T1ab node-negative HER2-overexpressing breast carcinomas: a multicenter retrospective seriesAnn Oncol201324491692423104720
- Mohd SharialMSCrownJHennessyBTOvercoming resistance and restoring sensitivity to HER2-targeted therapies in breast cancerAnn Oncol201223123007301622865781
- NahtaREstevaFJHerceptin: mechanisms of action and resistanceCancer Lett2006232212313816458110
- VermaSMilesDGianniLTrastuzumab emtansine for HER2-positive advanced breast cancerN Engl J Med2012367191783179123020162
- FranklinMCCareyKDVajdosFFLeahyDJde VosAMSliwkowskiMXInsights into ErbB signaling from the structure of the ErbB2-pertuzumab complexCancer Cell20045431732815093539
- KristjansdottirKDizonDHER-dimerization inhibitors: evaluating pertuzumab in women’s cancersExpert Opin Biol Ther201010224325020001562
- ScheuerWFriessTBurtscherHBossenmaierBEndlJHasmannMStrongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor modelsCancer Res200969249330933619934333
- NahtaRHungMCEstevaFJThe HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cellsCancer Res20046472343234615059883
- AgusDBAkitaRWFoxWDTargeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growthCancer Cell20022212713712204533
- LangdonSPFaratianDNagumoYMullenPHarrisonDJPertuzumab for the treatment of ovarian cancerExpert Opin Biol Ther20101071113112020465533
- HerbstRSDaviesAMNataleRBEfficacy and safety of single-agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancerClin Cancer Res200713206175618117947484
- BaselgaJGelmonKAVermaSPhase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapyJ Clin Oncol20102871138114420124182
- BaselgaJSwainSMCLEOPATRA: a phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancerClin Breast Cancer201010648949121147694
- BaselgaJCortésJKimSBPertuzumab plus trastuzumab plus docetaxel for metastatic breast cancerN Engl J Med2012366210911922149875
- SwainSMKimSBCortésJPertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 studyLancet Oncol201314646147123602601
- Ciruelos GilEMBrufskyAImYHEfficacy and safety of first-line (1L) pertuzumab (P), trastuzumab (T), and docetaxel (D) in HER2-positive MBC (CLEOPATRA) in patients previously exposed to trastuzumabJ Clin Oncol2013Suppl 31600
- BaselgaJCortésJImSABiomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in HER-2-positive, first-line metastatic breast cancer (MBC)Cancer Res201272Suppl 24S5S11
- GianniLLladóABianchiGOpen-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancerJ Clin Oncol20102871131113720124183
- CortésJFumoleauPBianchiGVPertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancerJ Clin Oncol201230141594160022393084
- BardiaABaselgaJPreoperative chemotherapy for operable breast cancerLippmanMHarrisJMonica MorrowMOsborneKDiseases of the BreastPhiladelphiaLippincott Williams & Wilkins2013
- ReynoldsKLBhatiaAChengXNeoadjuvant single and dual HER2 blockade among patients with localized HER2-positive breast cancerJ Clin Oncol201331Suppl 26147
- BardiaAGreenupRMoyBPathological complete remission after neoadjuvant chemotherapy predicts improved survival in the various breast cancer subtypes: Systematic review and meta-analysesPoster presented at: AACR Advances in Breast Cancer ResearchOctober 12–15, 2011San Francisco, CA
- GianniLPienkowskiTImYHEfficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trialLancet Oncol2012131253222153890
- SchneeweissAChiaSHickishTNeoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline-containing or concurrent with an anthracycline-free standard regimen: a randomized phase II study (TRYPHAENA)Cancer Res201171Suppl 24S5S6
- GianniLDafniUGelberRDTreatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trialLancet Oncol201112323624421354370
- von MinckwitzGBaselgaJBradburyIOT1-02-04: adjuvant pertuzumab and herceptin in initial therapy of breast cancer: APHINITY (BIG 4-11/BO25126/TOC4939g)Cancer Res201171Suppl 24OT1-02-04
- AgusDBGordonMSTaylorCPhase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancerJ Clin Oncol200523112534254315699478
- NgCMLumBLGimenezVKelseySAllisonDRationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysisPharm Res20062361275128416715358
- Genentech Perjeta [full prescribing information]San FranciscoGenentech2012
- MotHER Pregnancy Registry Available from: http://www.herceptinpregnancyregistry.comAccessed January 16, 2014
- CroneSAZhaoYYFanLErbB2 is essential in the prevention of dilated cardiomyopathyNat Med20028545946511984589
- PerezEACardiac toxicity of ErbB2-targeted therapies: what do we know?Clin Breast Cancer20088Suppl 3S114S12018777950
- US Food Drug AdministrationFDA approves Perjeta for neoadjuvant breast cancer treatment [press release]Silver Spring, MDFDA9302013 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm370393.htmAccessed October 5, 2013
- US Food Drug AdministrationPertuzumab [prescribing information] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125409s051lbl.pdf?et_cid=32583475&et_rid=463654371&linkid=+http%3a%2f%2fwww.accessdata.fda.gov%2fdrugsatfda_docs%2flabel%2f2013%2f125409s051lbl.pdfAccessed on October 5, 2013
- ChernewMENewcomerLNSwainSMTreatment and cost implications of pertuzumabAm J Manag Care2012184SP151SP15323009230
- BanerjiSCibulskisKRangel-EscarenoCSequence analysis of mutations and translocations across breast cancer subtypesNature2012486740340540922722202
- SleijferSBogaertsJSiuLLDesigning transformative clinical trials in the cancer genome eraJ Clin Oncol201331151834184123589555
- EstevaFJGuoHZhangSPTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancerAm J Pathol201017741647165620813970
- HankerABPfefferleADBalkoJMMutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapiesProc Natl Acad Sci U S A201311035143721437723940356
- OcanaAVera-BadilloFSerugaBTempletonAPandiellaAAmirEHER3 overexpression and survival in solid tumors: a meta-analysisJ Natl Cancer Inst2013105426627323221996
- MakhijaSAmlerLCGlennDClinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancerJ Clin Oncol20102871215122319901115
- BerghoffASBago-HorvathZPreusserMCo-expression of HER3 in HER2-positive metastatic breast cancer patients is an independent predictor of impaired prognosisPoster presented at: European CongressSeptember 27–October 1, 2013Amsterdam, Netherlands
- BardiaABaselgaJNeoadjuvant therapy as a platform for drug development and approval in breast cancerClin Cancer Res201319236360637024298066
- ProwellTMPazdurRPathological complete response and accelerated drug approval in early breast cancerN Engl J Med2012366262438244122646508
- UntchMvon MinckwitzGNeoadjuvant chemotherapy: early response as a guide for further treatment: clinical, radiological, and biologicalJ Natl Cancer Inst Monogr201120114313814122043061
- RimawiMFMayerIAForeroAMulticenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006J Clin Oncol201331141726173123569315
- DeMicheleABerryDAZujewskiJDeveloping safety criteria for introducing new agents into neoadjuvant trialsClin Cancer Res201319112817282323470967
- RocheHoffmann-LaA Study of Pertuzumab in Addition to Chemotherapy and Herceptin (Trastuzumab) as Adjuvant Therapy in Patients With HER2-Positive Primary Breast Cancer Available from: http://clinicaltrials.gov/show/NCT01358877 NLM identifier: NCT01358877Accessed December 10, 2013
- RocheHoffmann-LaA Study of Trastuzumab Emtansine (T-DM1) Plus Pertuzumab/Pertuzumab Placebo Versus Trastuzumab [Herceptin] Plus a Taxane in Patients With Metastatic Breast Cancer (MARIANNE) Available from: http://clinicaltrials.gov/show/NCT01120184 NLM identifier: NCT01120184Accessed December 10, 2013
- RocheHoffmann-LaA Study of a Combination of Trastuzumab and Capecitabine With or Without Pertuzumab in Patients With HER2-positive Metastatic Breast Cancer (PHEREXA) Available from: http://clinicaltrials.gov/show/NCT01026142 NLM identifier: NCT01026142Accessed December 10, 2013