Abstract
Adenine triphosphate (ATP)-binding cassette (ABC) transporter proteins, such as ABCB1/P-glycoprotein (P-gp) and ABCG2/breast cancer resistance protein (BCRP), transport various structurally unrelated compounds out of cells. ABCG2/BCRP is referred to as a “half-type” ABC transporter, functioning as a homodimer, and transports anticancer agents such as irinotecan, 7-ethyl-10-hydroxycamptothecin (SN-38), gefitinib, imatinib, methotrexate, and mitoxantrone from cells. The expression of ABCG2/BCRP can confer a multidrug-resistant phenotype on cancer cells and affect drug absorption, distribution, metabolism, and excretion in normal tissues, thus modulating the in vivo efficacy of chemotherapeutic agents. Clarification of the substrate preferences and structural relationships of ABCG2/BCRP is essential for our understanding of the molecular mechanisms underlying its effects in vivo during chemotherapy. Its single-nucleotide polymorphisms are also involved in determining the efficacy of chemotherapeutics, and those that reduce the functional activity of ABCG2/BCRP might be associated with unexpected adverse effects from normal doses of anticancer drugs that are ABCG2/BCRP substrates. Importantly, many recently developed molecular-targeted cancer drugs, such as the tyrosine kinase inhisbitors, imatinib mesylate, gefitinib, and others, can also interact with ABCG2/BCRP. Both functional single-nucleotide polymorphisms and inhibitory agents of ABCG2/BCRP modulate the in vivo pharmacokinetics and pharmacodynamics of these molecular cancer treatments, so the pharmacogenetics of ABCG2/BCRP is an important consideration in the application of molecular-targeted chemotherapies.
Introduction
Various mechanisms are involved in the multidrug resistance of cancer cells, including reduced drug uptake, the efflux of intracellular drugs, the activation of deoxyribonucleic acid (DNA) repair pathways, and the induction of the antiapoptotic machinery.Citation1 The adenine triphosphate (ATP)-binding cassette (ABC) transporter proteins, particularly P-glycoprotein (P-gp) (P-gp/MDR1 [multidrug resistance 1]/ABCB1), multidrug resistance protein 1 (MRP1) (MRP1/ABCC1), and breast cancer resistance protein (BCRP) (BCRP/MXR [mitoxantrone resistance]/ABCP/ABCG2), function as key molecules in the multidrug-resistant phenotype of cancer cells.Citation2–Citation4 They mediate the ATP-dependent unidirectional efflux of various compounds, both endogenous and exogenous, from cells.Citation2–Citation5
ABCG2/BCRP, a “half transporter” member of the ABCG subfamily,Citation6 has been found in cancer-drug-resistant human cancer cell lines isolated by in vitro selection.Citation7–Citation9 The overexpression of ABCG2/BCRP confers resistance to various chemotherapeutic drugs, such as the topoisomerase I inhibitor topotecan and the antifolate agent methotrexate,Citation9,Citation10 and ABCG2/BCRP is probably associated with clinical drug resistance, including that in patients with acute myelogenous leukemia or acute lymphocytic leukemia.Citation11–Citation17 Because ABCB1/P-gp is quite an important factor in the drug resistance observed in clinical leukemia, a large-scale analysis that assesses both ABCG2/BCRP and ABCB1/P-gp is essential to properly delineating the contribution of ABCG2/BCRP to drug resistance in cancer patients.
ABCG2/BCRP structure and activity
ABCG2/BCRP is a 655 amino acid, 72 kDa protein with a single ABC signature domain within the nucleotide-binding domain and six transmembrane domains, as shown in . Structural and functional studies of ABCG2/BCRP have provided valuable insight into the molecular mechanisms of ABCG2/BCRP-mediated transport. The cloning of ABCG2/BCRP cDNAs (complementary DNAs) from drug-selected clone cells and normal tissues has revealed functional variations in the amino acid substitutions in the ABCG2/BCRP protein and altered substrate preferences. ABCG2/BCRP proteins in drug-selected cells, such as the S1-M1-80 and MCF7/AdVp3000 cell lines, are mutant forms, and unique mutations in ABCG2/BCRP have been identified at amino acid position 482.Citation18 MCF7/AdVp3000 and S1-M1-80 cells express R482T and R482G variants of ABCG2/BCRP, respectively, and are highly resistant to both mitoxantrone and doxorubicin. Anthracyline resistance and a rhodamine efflux capacity are also unique phenotypes of these two ABCG2/BCRP-overexpressing cell lines.Citation18,Citation19 The substitution of Arg at position 482 in ABCG2/BCRP with Gly or Thr confers additional efflux activity for rhodamine 123, doxorubicin, and other anthracyclines, which are usually not good substrates of wild-type ABCG2/BCRP.Citation18,Citation20 In contrast, the ABCG2/BCRP variants R482G and R482T lose their methotrexate-transporting activity but, at the same time, confer increased mitoxantrone resistance.Citation19,Citation21–Citation23 The COOH terminus of the transmembrane 3 region, in close proximity to position 482, is involved in the substrate-binding pocket of ABCG2/BCRP, and the Arg residue at position 482 affects drug–ABCG2/BCRP interactions.Citation23–Citation25 Moreover, 13 variant ABCG2/BCRP proteins with substitutions at R482 (R482N, C, M, S, T, V, A, G, E, W, D, Q, and H, but not Y or K) confer strong resistance to doxorubicin and mitoxantrone in PA317 cells, as in S1-M1-80 and MCF7/AdVp3000 cells.Citation26 Thus, structural variations in ABCG2/BCRP affect its drug efflux activity. In addition, mutations in ABCG2/BCRP at N557 and H630 severely affect this resistant phenotype. Cells expressing either N557D or H630E mutant ABCG2/BCRP displayed lower resistance to SN-38 (7-ethyl-10-hydroxycamptothecin), although the mitoxantrone resistance of these cells was similar to that of cells expressing wild-type ABCG2/BCRP.Citation26 Structural studies using three-dimensional homology modeling of ABCG2/BCRP have also indicated that the transmembrane domains of ABCG2/BCRP function as drug-recognition interfacesCitation24,Citation25,Citation27 and suggest that R482 is located in the central cavity of the protein, with its side chain pointing toward the drug translocation pathway.Citation28 Therefore, the drug efflux activity of ABCG2/BCRP is influenced by various mutations, which will necessarily affect the clinical efficacy of ABCG2/BCRP-transportable anticancer drugs.
Figure 1 Schematic diagram of ABCG2/BCRP protein and its amino acid variations. Amino acid variations by single-nucleotide polymorphisms are marked with stars. Transmembrane domains are shown as cylinders.
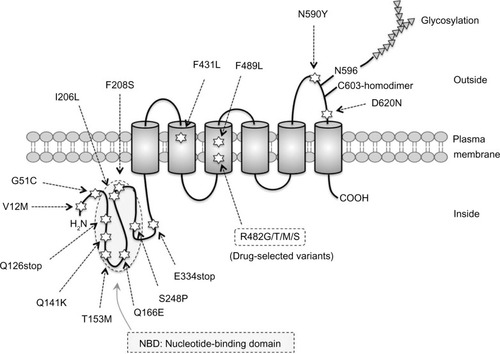
ABCG2/BCRP functions as a homodimer.Citation29,Citation30 A disulfide bridge at C603 is involved in homodimer formation,Citation31 and crystallization studies suggest a tetrameric complex composed of four ABCG2/BCRP homodimers.Citation32 ABC transporter research has also demonstrated that the activity of these proteins is affected by both posttranslational regulationCitation33,Citation34 and genetic polymorphisms.Citation35–Citation37 ABCG2/BCRP is targeted to the plasma membrane via the endoplasmic reticulum–Golgi pathway, and during its passage, undergoes N-linked glycosylation at N596Citation38,Citation39 and the formation of an intramolecular disulfide bond between C592 and C608, which affects its stability.Citation40 Oligomeric glycosylated ABCG2/BCRP is degraded in the lysosome, whereas underglycosylated, misfolded ABCG2/BCRP lacking the intramolecular disulfide bond is targeted to the proteasomal system for degradation.Citation39
Single-nucleotide polymorphisms (SNPs) of ABCG2/BCRP
Primary structural variations of ABCG2/BCRP are associated with its drug-transporter function, as described above. Numerous germ-line mutations in the ABCG2/BCRP gene have been found in ethnically diverse populations,Citation36,Citation41–Citation43 as shown in . Therefore, SNPs in the ABCG2/BCRP gene would influence the pharmacological effects of ABCG2/BCRP differently in different patients.
421C>A (Q141K) ABCG2/BCRP SNP
Japanese population and human cancer cell lines have been shown to have three variant ABCG2/BCRP cDNAs with the substitutions: 34G>A (V12M), 421C>A (Q141K), and a nucleotide 944–949 deletion, removing A315 and T316 (Δ315–316).Citation44 The 34G>A and 421C>A variants are SNPs. The frequency of the 421C>A SNP in a normal Japanese population demonstrated that 57 of 124 samples had the A421 allele, and nine of these were homozygous for this polymorphism.Citation36,Citation45 These data suggest that some Japanese individuals probably express low levels of ABCG2/BCRP. The 421C>A SNP appears to be very common in Asian populations, with reported allelic frequencies between 27% and 34%,Citation35,Citation44,Citation46 whereas this SNP is rare in sub-Saharan African and African-American populations, with frequencies of <5%.Citation47 Its frequency in Caucasian populations is approximately 10%.Citation48 The physiological significance of the 421C>A ABCG2/BCRP SNP has been analyzed in relation to the pharmacokinetics of diflomotecan, a new camptothecin derivative anticancer agent, during a Phase I study.Citation49 This study showed that five patients who were heterozygous for the A421 allele had much higher plasma levels of diflomotecan after its intravenous administration than 15 homozygous wild-type individuals (mean values of 138 ng h/mL/mg versus 46.1 ng h/mL/mg, respectively). Consistent with this, 421C>A ABCG2/BCRP-transfected murine fibroblast PA317 (PA/Q141K) cells expressed less exogenous ABCG2/BCRP protein levels than wild-type ABCG2/BCRP-transfected cells.Citation44 Drug accumulation was higher in PA/Q141K cells than in other ABCG2/BCRP transfectants, suggesting that the SNP 421C>A (Q141K) reduces ABCG2/BCRP function. These observations from laboratory and clinical studies suggest that the levels and functions of ABCG2/BCRP expressed from the 421C>A ABCG2/BCRP allele are reduced compared with those of the wild-type protein. The Q141K mutation is located within the functionally important ATP-binding region between the Walker A and B motifs of ABCG2/BCRP and thus is likely to affect the ATPase activity of the protein.Citation35,Citation50
In terms of the genetic polymorphisms of ABCG2/BCRP and the expression of the protein, other non-synonymous SNPs, F208S and S441N, have been shown to affect ABCG2/BCRP protein levels at the plasma membrane, indicating that these non-synonymous SNPs reduce the stability of ABCG2/BCRP by enhancing its ubiquitin-mediated proteasomal proteolysis, resulting in reduced ABCG2/BCRP function.Citation51
376C>T (Q126stop) ABCG2/BCRP SNP
Another SNP within the ABCG2/BCRP gene, 376C>T, is present at low frequencies in healthy Japanese samples as a heterozygosity (reported frequencies of 3/124 and 2/120 in two studies).Citation44,Citation47 The frequency of the T376 allele of ABCG2/BCRP is low and is not observed in Caucasian or African-American groups. A combination of the 376C>T and 421C>A SNPs would be expected to occur in a considerable proportion of the Japanese population. Because these SNPs would have negative effects on ABCG2/BCRP activity, the combined 376C>T 421T/C>A variant is expected to show severely reduced ABCG2/BCRP activity.
Additional ABCG2/BCRP SNPs
Other ABCG2/BCRP SNPs, such as 34G>A, 151G>T, 376C>T, 421C>A, 458C>T, 496C>G, 616A>C, 623T>C, 742T>C, 1000G>T, 1291T>C, 1465T>C, 1768A>T, and 1858G>A, cause amino acid substitutions. Synonymous mutations, such as 114T>C, 369C>T, 474C>T, 564A>G, 1098G>A, and 1425A>G, have been identified in the coding region of ABCG2/BCRP. The highest allele frequency for the 34G>A SNP is observed in Mexican-Indians, and there are significant differences in the frequencies of this SNP in Caucasian, Japanese, and Swedish populations.Citation52,Citation53 Transfection studies of ABCG2/BCRP (V12M) encoding the 34G>A SNP showed that the expression levels and drug-resistance of this variant are similar to those of wild-type ABCG2/BCRP, suggesting that V12M does not affect ABCG2/BCRP protein activity.Citation44 However, a recent report suggested a possible association between this polymorphism and the alternative splicing of ABCG2/BCRP mRNA (messenger ribonucleic acid), particularly the liver-specific splicing of polymorphic exon 2 in these transcripts.Citation54 ABCG2/BCRP mRNA expression was significantly lower in Hispanic livers with the 34G>A variant genotype, and individuals with the 34G>A allele displayed reduced ABCG2/BCRP expression in their liver cells. Although these observations suggest that chemotherapy regimens containing ABCG2/BCRP-substrate anticancer drugs may have increased efficacy in these patients because the expression of ABCG2/BCRP is reduced, another study has shown that Chinese acute myelogenous leukemia patients with the wild-type 34GG genotype had longer disease-free survival and longer overall survival than those with the 34GA/AA genotypes.Citation55 The reason for the unpredictable effects of the 32G>A SNP on ABCG2/BCRP is unknown. The polymorphic and differential expression of another splicing variant of ABCG2/BCRP mRNA, involving exon 1b in the liver, also seems to be associated with lower ABCG2/BCRP expression.Citation56 Approximately 90% of patients with the 34G>A SNP of ABCG2/BCRP show exon 2 skipping in the liver, and the lower level of ABCG2/BCRP mRNA in the liver may be associated with this SNP in the Hispanic population.Citation54
ABCG2/BCRP expression and physiological functions
ABCG2/BCRP is widely expressed in the placenta, blood–brain barrier, gastrointestinal tract, liver, kidney, testis, and lactating breast. ABCG2/BCRP localizes apically in the epithelia of the small intestine and colon,Citation57 on the luminal surfaces of the endothelial cells of the human brain,Citation58 and on the luminal surfaces of the kidney tubules.Citation59 Experimental inhibition of ABCG2/BCRP activity affected drug distribution in various in vivo experiments.Citation60,Citation61 These observations suggest a possible role for ABCG2/BCRP in controlling the absorption/distribution of its substrate compounds. Various cancers also contain subpopulations of stem cells that are characterized by the expression of ABCG2/BCRP and other ABC transporters.Citation62 These transporters have been suggested to play an important role in the multidrug resistance of these cancer stem-like cells during chemotherapy.Citation63 Indeed, the experimental inhibition of ABCG2/BCRP has been shown to suppress the proliferation of side population cells in cancer cell lines.Citation64
ABCG2/BCRP extrudes various types of compounds among its functional substrates, including sulfated hormone metabolites, the chlorophyll metabolite pheophorbide A, fluorescent dyes such as Hoechst 33342 and BODIPY (boron-dipyrromethene)-prazosin, cimetidine, various flavonoids, and some antibiotics.Citation30,Citation65 Porphyrin/heme was the first-identified endogenous ABCG2/BCRP substrate,Citation66 and ABCG2/BCRP regulates heme homeostasis under hypoxic conditions.Citation67 ABCG2/BCRP also transports endogenous folates, such as the mono-, di-, and triglutamates of folic acid, and participates in the energy-dependent efflux of certain folates and antifolates.Citation22 Recently, genome-wide screening for the genetic determinants of gout found that an SNP of ABCG2/BCRP (Q141K) is associated with high uric acid levels, and demonstrated that uric acid is a natural substrate of ABCG2/BCRP.Citation68,Citation69
ABCG2/BCRP also appears to play a protective role against xenobiotics and their metabolites.Citation30,Citation70 The typical ABCG2/BCRP substrates, irinotecan and SN-38, are detoxified by glucuronidation with uridine-diphosphate–glucuronyltransferase, and ABCG2/BCRP can extrude SN-38–glucuronide.Citation71 Interestingly, ABCG2/BCRP can transport another of the glucuronide conjugates, 17-β-estradiol 17-(β-D-glucuronide), and the sulfated conjugates estrone-3-sulfate and dehydroepiandrosterone are also substrates of ABCG2/BCRP.Citation22,Citation70 The apical localization of ABCG2/BCRP in the intestinal epithelium and the bile canalicular membrane also suggests the intestinal absorption and hepatobiliary excretion of ABCG2/BCRP substrates.Citation57,Citation72–Citation74 ABCG2/BCRP may also play a protective role by transporting dietary carcinogens. A study by van Herwaarden et alCitation72 showed that BCRP1 effectively restricts the exposure of mice to the ingested food carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine by reducing its uptake from the gut lumen and by mediating its hepatobiliary and intestinal elimination. Similarly, ABCG2/BCRP limits the intestinal uptake of the carcinogens 2-amino-3-methylimidazo(4,5-f)quinoline, 3-amino-1,4-dimethyl-5H-pyrido(4,3-b)indole, and aflatoxin B1 in a mouse model.Citation75 Overall, ABCG2/BCRP may restrict the bioavailability of orally administered ABCG2/BCRP-substrate anticancer agents, such as topotecan (and its metabolite SN-38), irinotecan, camptothecin derivatives, methotrexate, flavopiridol, and others.Citation65 Therefore, the functional activity of ABCG2/BCRP is an important consideration in ABCG2/BCRP-transportable drug absorption, distribution, metabolism, and excretion in patients.
Pharmacological interaction of ABCG2/BCRP with molecular-targeted drugs
ABCG2/BCRP and anticancer kinase inhibitors
A growing number of small-molecule inhibitors of oncogenic kinases have come into clinical use and have shown great potential as anticancer drugs.Citation76,Citation77 Imatinib mesylate, which targets BCR-ABL (B-cell receptor-Abelson), was the first approved protein kinase inhibitor.Citation78 Imatinib is very effective against chronic myeloid leukemia and other cancers associated with the deregulation of these kinase pathways. Resistance to this drug is typically conferred by mutations in the target kinase within the region of the drug–kinase interaction,Citation79–Citation82 but another mechanism leading to imatinib resistance correlates with ABCB1/P-gp expression.Citation83,Citation84 As shown in , a number of recent studies have indicated possible interactions between many kinase inhibitors and ABC transporters, including ABCB1/P-gp and ABCG2/BCRP.Citation85–Citation90 ABCG2/BCRP has a potent ability to interact with numerous clinically important kinase inhibitors, including imatinib,Citation87–Citation89,Citation91–Citation99 nilotinib,Citation95 dasatinib,Citation96 lapatinib,Citation97 gefitinib,Citation42,Citation98–Citation105 canertinib,Citation106 erlotinib,Citation107–Citation109 sorafenib,Citation110 pazopanib,Citation111 vandetanib,Citation112 vemurafenib,Citation113 axitinib,Citation114 and ponatinib.Citation115 In contrast, bosutinib is unlikely to be a substrate of ABCG2/BCRP but probably inhibits it.Citation116 A recently developed anaplastic lymphoma kinase inhibitor, crizotinib, is also unlikely to interact with ABCG2/BCRP, although it is a good substrate of ABCB1/P-gp.Citation117
Table 1 Interactions between tyrosine kinase inhibitors and ABCG2/BCRP
A variety of kinase-inhibiting compounds appear to interact with ABC transporters.Citation85,Citation118–Citation124 These protein kinase inhibitors are designed to compete with ATP in the kinase domain and thus show competitive suppressive effects.Citation125 Therefore, most ABCG2/BCRP-interactive kinase inhibitors used at higher concentration were initially suspected to block the ATPase activity of this protein. However, Saito et alCitation126 demonstrated that gefitinib binds to ATP-bound ABCG2/BCRP, indicating that the as yet undetermined gefitinib-binding site in ABCG2/BCRP is not in the ATP-binding domain. Using photoaffinity labeling technique with 125I-labeled iodoarylazidoprazosin, a typical substrate of ABCB1/P-gp and ABCG2/BCRP, Brendel et alCitation95 demonstrated that imatinib and nilotinib bind to ABCG2/BCRP at the substrate-interaction site, whereas Shi et alCitation107 showed that erlotinib does not compete with iodoarylazidoprazosin at the substrate-binding sites on ABCG2/BCRP or ABCB1/P-gp. Some interesting studies have proposed the presence of multiple drug-binding sites in this ABC transporter,Citation127,Citation128 suggesting that kinase inhibitors may target these substrate-binding pockets. Further studies are required to properly clarify the modes of interaction between kinase inhibitors and ABCG2/BCRP.
Pharmacological interactions affected by ABCG2/BCRP polymorphisms
Studies of the pharmacological interactions between ABCG2/BCRP and molecular-targeted kinase inhibitors have revealed that clinically used kinase inhibitors are in vivo substrates and/or inhibitors of this ABC transporter. Gefitinib is an orally administered, active, selective epidermal growth factor receptor tyrosine kinase inhibitor (TKI) used to treat patients with advanced non-small-cell lung cancer (NSCLC),Citation129,Citation130 and ABCG2/BCRP is expressed in intestinal epithelial cells and at the blood–brain and blood–cerebrospinal barriers, where it restricts the penetration of the brain by xenobiotics.Citation65,Citation131 Stewart et alCitation98 showed that the oral bioavailability of irinotecan, a good substrate of ABCG2/BCRP, is affected by the oral administration of gefitinib. Zhuang et alCitation132 have also shown that the oral administration of gefitinib increases the penetration of topotecan across the brain extracellular fluid but, conversely, reduces its penetration of the ventricular cerebrospinal fluid.
Importantly, recent studies have tentatively implicated some ABCG2/BCRP polymorphisms in the pharmacokinetics of molecular-targeted drugs,Citation103 as shown in . A common functional SNP in the ABCG2/BCRP gene, 421C>A, was shown to be associated with diarrhea in 124 patients treated with oral gefitinib (250 mg once daily); 44% of the patients with a heterozygous 421C>A allele developed diarrhea, whereas 12% of the other patients were homozygous for the wild-type allele. Other polymorphisms at the ABCG2/BCRP locus appear to affect gefitinib-induced diarrhea. A significant number of patients carrying the ABCG2 (−15622C/T) polymorphism and the ABCG2 (1143C/T, −15622C/T) haplotype developed gefitinib-dependent moderate-to-severe diarrhea.Citation133 These studies suggest that patients with reduced ABCG2/BCRP activity arising from a genetic variation might be at increased risk of gefitinib-induced diarrhea, and these genetic markers should be considered in the optimization of NSCLC treatments with gefitinib. However, no clear association was reported between the 421C.A SNP of ABCG2/BCRP and a susceptibility to gefitinib-induced adverse effects in a Japanese population.Citation134 The skin toxicity of gefitinib is also reported to be unrelated to this SNP.Citation103 More large-scale analyses are required to resolve these discrepancies.
Table 2 Single-nucleotide polymorphisms of the ABCG2/BCRP gene
Like gefitinib, erlotinib has been shown to interact with ABCG2/BCRP.Citation107,Citation135 Two polymorphic loci identified in the ABCG2/BCRP promoter and intron, −15622C/T and 1143C/T, which reduce the protein’s expression, were reported to be associated with modulation of pharmacokinetic parameters for erlotinib.Citation108 The area under the curve and maximum observed concentration (Cmax) were higher for the ABCG2 1143 C/T or T/T (lower expression) genotype, and Cmax was higher in patients with the −15622 C/T or T/T (lower expression) genotype than in those with the C/C genotype. These observations indicate that ABCG2/BCRP recognizes erlotinib as a substrate in vivo.
Sunitinib, an oral multitargeted TKI for vascular endothelial growth factor receptors 1, 2, and 3, platelet-derived growth factor receptor α and β, c-Kit, Fms-like tyrosine kinase 3 receptor, and the receptor encoded by the RET proto-oncogene, is used as a first-line treatment for metastatic renal cell carcinoma and imatinib-resistant metastatic gastrointestinal stromal tumors. The haplotype (−15622C/T, 1143C/T) at the ABCG2 locus that has been associated with gefitinib-associated adverse effects and increased erlotinib exposure, is also related to the development of increased sunitinib toxicity. The prevalence of toxicity higher than grade 2 increased when one or two copies of TT were present in the ABCG2 (−15622C/T, 1143C/T) haplotype.Citation136 Another SNP in ABCG2/BCRP has also been shown to be associated with adverse effects similar to those attributed to sunitinib-related toxicity. Among 12 different genetic polymorphisms examined, the ABCG2/BCRP 421AA genotype correlated with the development of grade 3 or grade 4 thrombocytopenia and neutropenia in Korean patients suffering from metastatic renal cell carcinoma, and may be predominantly associated with the risk of sunitinib-related toxicity in those patients.Citation137 Intriguingly, the 421C>A SNP of ABCG2/BCRP may have another effect on tumor lysis syndrome with hyperuricemia during TKI-based molecular-targeted therapy. Several studies have reported renal failure and tumor lysis syndrome during TKI-based molecular-targeted therapies, including with sorafenib for hepatocellular carcinoma, with imatinib for chronic myelogenous leukemia, and with flavopiridol for chronic lymphocytic leukemia.Citation138–Citation141 Because the Q141K variant of ABCG2/BCRP corresponding to SNP 421C>A reduces uric acid transportCitation68 and those TKIs are substrates/inhibitors of ABCG2/BCRP, TKI therapy in patients with the SNP 421C>A (Q141K) may be at higher risk of tumor lysis syndrome.Citation5
A defect in the pharmacological interaction has been suggested between sunitinib and another ABCG2/BCRP germ-line mutant allele, 1291T>C.Citation109 Remarkably, sunitinib reversed wild-type ABCG2/BCRP-mediated drug resistance and competitively inhibited ABCG2/BCRP-mediated estrone 3-sulfate transport and the binding of 125I-iodoarylazidoprazosin to ABCG2/BCRP. The F431L variant of ABCG2/BCRP, which is expressed from a germ-line mutant allele 1291T>C, was insensitive to sunitinib-mediated inhibition. Thus, residue F431 of ABCG2/BCRP may have impact on the pharmacological interaction with sunitinib.
Perspectives
Molecular analyses of the functional interactions between novel molecular targeted drugs and the ABC transporter ABCG2/BCRP have demonstrable utility as indicators of the clinical efficacy of these anticancer agents in individual patients. Such pharmacological interactions might be influenced by personal genotypes, and the increased risk of adverse effects from putative ABCG2/BCRP substrates should be evaluated when considering combinations of protein kinase inhibitors, even at their clinically used doses.
Acknowledgments
Owing to space limitations, we apologize that we could not cite the excellent work of many investigators. This work was supported by a Grant-in-Aid for Cancer Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Disclosure
The authors have no conflicts of interest to disclose.
References
- TsuruoTNaitoMTomidaAMolecular targeting therapy of cancer: drug resistance, apoptosis and survival signalCancer Sci200394152112708468
- AmbudkarSVDeySHrycynaCARamachandraMPastanIGottesmanMMBiochemical, cellular, and pharmacological aspects of the multidrug transporterAnnu Re Pharmacol Toxicol199939361398
- SchinkelAHJonkerJWMammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overviewAdv Drug Deliv Rev20035532912535572
- GottesmanMMLingVThe molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein researchFEBS Lett2006580998100916405967
- RobeyRWIeranoCZhanZBatesSEThe challenge of exploiting ABCG2 in the clinicCurr Pharm Biotechnol20101259560821118093
- AllenJDSchinkelAHMultidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2)Mol Cancer Ther2002142743412477055
- DoyleLAYangWAbruzzoLVA multidrug resistance transporter from human MCF-7 breast cancer cellsProc Natl Acad Sci U S A19989515665156709861027
- AllikmetsRSchrimlLMHutchinsonARomano-SpicaVDeanMA human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistanceCancer Res199858533753399850061
- BrangiMLitmanTCiottiMCamptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cellsCancer Res1999595938594610606239
- MaliepaardMvan GastelenMAde JongLAOverexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell lineCancer Res1999594559456310493507
- RossDDKarpJEChenTTDoyleLAExpression of breast cancer resistance protein in blast cells from patients with acute leukemiaBlood20009636536810891476
- SteinbachDSellWVoigtAHermannJZintlFSauerbreyABCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemiaLeukemia2002161443144712145683
- van den Heuvel-EibrinkMMWiemerEAPrinsAIncreased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML)Leukemia20021683383911986944
- BenderraZFaussatAMSayadaLBreast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemiasClin Cancer Res2004107896790215585622
- PlasschaertSLVan Der KolkDMDe BontESVellengaEKampsWADe VriesEGBreast cancer resistance protein (BCRP) in acute leukemiaLeuk Lymphoma20044564965415160935
- SuvannasankhaAMindermanHO’LoughlinKLBreast cancer resistance protein (BCRP/MXR/ABCG2) in acute myeloid leukemia: discordance between expression and functionLeukemia2004181252125715208643
- UgglaBStahlEWagsaterDBCRP mRNA expression v. clinical outcome in 40 adult AML patientsLeuk Res20052914114615607361
- HonjoYHrycynaCAYanQWAcquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cellsCancer Res2001616635663911559526
- VolkELFarleyKMWuYLiFRobeyRWSchneiderEOverexpression of wild-type breast cancer resistance protein mediates methotrexate resistanceCancer Res2002625035504012208758
- AllenJDJacksonSCSchinkelAHA mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistanceCancer Res2002622294229911956086
- OzvegyCVaradiASarkadiBCharacterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutationJ Biol Chem2002277479804799012374800
- ChenZSRobeyRWBelinskyMGTransport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transportCancer Res2003634048405412874005
- EjendalKFDiopNKSchweigerLCHrycynaCAThe nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate bindingProtein Sci2006151597160716815914
- HazaiEBikadiZHomology modeling of breast cancer resistance protein (ABCG2)J Struct Biol2008162637418249138
- LiYFPolgarOOkadaMEsserLBatesSEXiaDTowards understanding the mechanism of action of the multidrug resistance-linked half-ABC transporter ABCG2: a molecular modeling studyJ Mol Graph Model20072583785117027309
- MiwaMTsukaharaSIshikawaEAsadaSImaiYSugimotoYSingle amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectantsInt J Cancer200310775776314566825
- RosenbergMFBikadiZChanJThe human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantroneStructure20101848249320399185
- CaiXBikadiZNiZRole of basic residues within or near the predicted transmembrane helix 2 of the human breast cancer resistance protein in drug transportJ Pharmacol Exp Ther201033367068120203106
- KageKTsukaharaSSugiyamaTDominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerizationInt J Cancer20029762663011807788
- DoyleLARossDDMultidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2)Oncogene2003227340735814576842
- HenriksenUFogJULitmanTGetherUIdentification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2J Biol Chem2005280369263693416107343
- McDevittCACollinsRFConwayMPurification and 3D structural analysis of oligomeric human multidrug transporter ABCG2Structure2006141623163217098188
- XieYXuKLinnDEThe 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cellsJ Biol Chem20082833349335618056989
- TakadaTSuzukiHGotohYSugiyamaYRegulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cellsDrug Metab Dispos20053390590915843490
- KondoCSuzukiHItodaMFunctional analysis of SNPs variants of BCRP/ABCG2Pharm Res2004211895190315553238
- YanaseKTsukaharaSMitsuhashiJSugimotoYFunctional SNPs of the breast cancer resistance protein-therapeutic effects and inhibitor developmentCancer Lett2006234738016303243
- TamuraAWakabayashiKOnishiYRe-evaluation and functional classification of non-synonymous single nucleotide polymorphisms of the human ATP-binding cassette transporter ABCG2Cancer Sci20079823123917297656
- DiopNKHrycynaCAN-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membraneBiochemistry2005445420542915807535
- Wakabayashi-NakaoKTamuraAFurukawaTNakagawaHIshikawaTQuality control of human ABCG2 protein in the endoplasmic reticulum: ubiquitination and proteasomal degradationAdv Drug Deliv Rev200961667219111842
- WakabayashiKNakagawaHTamuraAIntramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 proteinJ Biol Chem2007282278412784617686774
- HonjoYMorisakiKHuffLMSingle-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1)Cancer Biol Ther2002169670212642696
- SugimotoYTsukaharaSIshikawaEMitsuhashiJBreast cancer resistance protein: molecular target for anticancer drug resistance and pharmacokinetics/pharmacodynamicsCancer Sci20059645746516108826
- CascorbiIRole of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugsPharmacol Ther200611245747316766035
- ImaiYNakaneMKageKC421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistanceMol Cancer Ther2002161161612479221
- YoshiokaSKatayamaKOkawaCThe identification of two germ-line mutations in the human breast cancer resistance protein gene that result in the expression of a low/non-functional proteinPharm Res2007241108111717373578
- ItodaMSaitoYShiraoKEight novel single nucleotide polymorphisms in ABCG2/BCRP in Japanese cancer patients administered irinotacanDrug Metab Pharmacokinet20031821221715618737
- KobayashiDIeiriIHirotaTFunctional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placentaDrug Metab Dispos2005339410115475413
- de JongFAMarshSMathijssenRHABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan dispositionClin Cancer Res2004105889589415355921
- SparreboomAGelderblomHMarshSDiflomotecan pharmacokinetics in relation to ABCG2 421C>A genotypeClin Pharmacol Ther200476384415229462
- MizuaraiSAozasaNKotaniHSingle nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2Int J Cancer200410923824614750175
- NakagawaHTamuraAWakabayashiKUbiquitin-mediated proteasomal degradation of non-synonymous SNP variants of human ABC transporter ABCG2Biochem J200841162363118237272
- ZamberCPLambaJKYasudaKNatural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestinePharmacogenetics200313192812544509
- BackstromGTaipalensuuJMelhusHGenetic variation in the ATP-binding cassette transporter gene ABCG2 (BCRP) in a Swedish populationEur J Pharm Sci20031835936412694888
- PoonkuzhaliBLambaJStromSAssociation of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphismsDrug Metab Dispos20083678079518180275
- WangFLiangYJWuXPPrognostic value of the multidrug resistance transporter ABCG2 gene polymorphisms in Chinese patients with de novo acute leukaemiaEur J Cancer2011471990199921531129
- NakanishiTBailey-DellKJHasselBANovel 5′ untranslated region variants of BCRP mRNA are differentially expressed in drug-selected cancer cells and in normal human tissues: implications for drug resistance, tissue-specific expression, and alternative promoter usageCancer Res2006665007501116707421
- MaliepaardMSchefferGLFaneyteIFSubcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissuesCancer Res2001613458346411309308
- AronicaEGorterJARedekerSLocalization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brainEpilepsia20054684985715946326
- FetschPAAbatiALitmanTLocalization of the ABCG2 mitoxantrone resistance-associated protein in normal tissuesCancer Lett2006235849215990223
- MarchettiSde VriesNABuckleTEffect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/-(triple-knockout) and wild-type miceMol Cancer Ther200872280228718723475
- FurmanWLNavidFDawNCTyrosine kinase inhibitor enhances the bioavailability of oral irinotecan in pediatric patients with refractory solid tumorsJ Clin Oncol2009274599460419687340
- DingXWWuJHJiangCPABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapyLife Sci20108663163720159023
- ZhouSMorrisJJBarnesYLanLSchuetzJDSorrentinoBPBcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivoProc Natl Acad Sci U S A200299123391234412218177
- KatayamaRKoikeSSatoSSugimotoYTsuruoTFujitaNDofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug exportCancer Sci20091002060206819673889
- SarkadiBHomolyaLSzakacsGVaradiAHuman multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense systemPhysiol Rev2006861179123617015488
- JonkerJWBuitelaarMWagenaarEThe breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyriaProc Natl Acad Sci U S A200299156491565412429862
- KrishnamurthyPRossDDNakanishiTThe stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with hemeJ Biol Chem2004279242182422515044468
- WoodwardOMKottgenACoreshJBoerwinkleEGugginoWBKottgenMIdentification of a urate transporter, ABCG2, with a common functional polymorphism causing goutProc Natl Acad Sci U S A2009106103381034219506252
- MatsuoHTakadaTIchidaKCommon defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese populationSci Transl Med200915ra11
- SuzukiMSuzukiHSugimotoYSugiyamaYABCG2 transports sulfated conjugates of steroids and xenobioticsJ Biol Chem2003278226442264912682043
- NakatomiKYoshikawaMOkaMTransport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cellsBiochem Biophys Res Commun200128882783211688982
- van HerwaardenAEJonkerJWWagenaarEThe breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridineCancer Res2003636447645214559835
- HiranoMMaedaKMatsushimaSNozakiYKusuharaHSugiyamaYInvolvement of BCRP (ABCG2) in the biliary excretion of pitavastatinMol Pharmacol20056880080715955871
- van HerwaardenAESchinkelAHThe function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxinsTrends Pharmacol Sci200627101616337280
- van HerwaardenAEWagenaarEKarnekampBMerinoGJonkerJWSchinkelAHBreast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milkCarcinogenesis20062712313016000399
- SawyersCTargeted cancer therapyNature200443229429715549090
- KrauseDSVan EttenRATyrosine kinases as targets for cancer therapyN Engl J Med200535317218716014887
- CapdevilleRBuchdungerEZimmermannJMatterAGlivec (STI571, imatinib), a rationally developed, targeted anticancer drugNat Rev Drug Discov2002149350212120256
- GorreMEMohammedMEllwoodKClinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplificationScience200129387688011423618
- ShannonKMResistance in the land of molecular cancer therapeuticsCancer Cell200229910212204529
- AzamMLatekRRDaleyGQMechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABLCell200311283184312654249
- DaubHSpechtKUllrichAStrategies to overcome resistance to targeted protein kinase inhibitorsNat Rev Drug Discov200431001101015573099
- MahonFXBellocFLagardeVMDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line modelsBlood20031012368237312609962
- IllmerTSchaichMPlatzbeckerUP-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylateLeukemia20041840140814724652
- HegedusTOrfiLSeprodiAVaradiASarkadiBKeriGInteraction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1Biochim Biophys Acta2002158731832512084474
- BurgerHNooterKPharmacokinetic resistance to imatinib mesylate: role of the ABC drug pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the oral bioavailability of imatinibCell Cycle200431502150515611623
- Ozvegy-LaczkaCHegedusTVaradyGHigh-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporterMol Pharmacol2004651485149515155841
- HoughtonPJGermainGSHarwoodFCImatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitroCancer Res2004642333233715059881
- BreedveldPPluimDCiprianiGThe effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patientsCancer Res2005652577258215805252
- LemosCJansenGPetersGJDrug transporters: recent advances concerning BCRP and tyrosine kinase inhibitorsBr J Cancer20089885786218253130
- BurgerHvan TolHBoersmaAWImatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pumpBlood20041042940294215251980
- ThomasJWangLClarkREPirmohamedMActive transport of imatinib into and out of cells: implications for drug resistanceBlood20041043739374515315971
- JordanidesNEJorgensenHGHolyoakeTLMountfordJCFunctional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylateBlood20061081370137316627755
- LiuWBaerMRBowmanMJThe tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2Clin Cancer Res2007132463247017438106
- BrendelCScharenbergCDohseMImatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cellsLeukemia2007211267127517519960
- HiwaseDKSaundersVHewettDDasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implicationsClin Cancer Res2008143881388818559609
- PolliJWHumphreysJEHarmonKAThe role of efflux and uptake transporters in N-{3-chloro-4-[(3-fluorobenzyl)oxy] phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino} methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactionsDrug Metab Dispos20083669570118216274
- StewartCFLeggasMSchuetzJDGefitinib enhances the antitumor activity and oral bioavailability of irinotecan in miceCancer Res2004647491749915492275
- YanaseKTsukaharaSAsadaSIshikawaEImaiYSugimotoYGefitinib reverses breast cancer resistance protein-mediated drug resistanceMol Cancer Ther200431119112515367706
- CarterTAWodickaLMShahNPInhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinasesProc Natl Acad Sci U S A2005102110111101616046538
- ElkindNBSzentpeteryZApatiAMultidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib)Cancer Res2005651770177715753373
- NakamuraYOkaMSodaHGefitinib (“Iressa”, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistanceCancer Res2005651541154615735043
- CusatisGGregorcVLiJPharmacogenetics of ABCG2 and adverse reactions to gefitinibJ Natl Cancer Inst2006981739174217148776
- LeggasMPanettaJCZhuangYGefitinib modulates the function of multiple ATP-binding cassette transporters in vivoCancer Res2006664802480716651435
- KatayamaKShibataKMitsuhashiJNoguchiKSugimotoYPharmacological interplay between breast cancer resistance protein and gefitinib in epidermal growth factor receptor signalingAnticancer Res2009291059106519414346
- ErlichmanCBoernerSAHallgrenCGThe HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug effluxCancer Res20016173974811212277
- ShiZPengXXKimIWErlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistanceCancer Res200767110121102018006847
- RudinCMLiuWDesaiAPharmacogenomic and pharmacokinetic determinants of erlotinib toxicityJ Clin Oncol2008261119112718309947
- KawaharaHNoguchiKKatayamaKMitsuhashiJSugimotoYPharmacological interaction with sunitinib is abolished by a germ-line mutation (1291T>C) of BCRP/ABCG2 geneCancer Sci20101011493150020345483
- HuSChenZFrankeRInteraction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transportersClin Cancer Res2009156062606919773380
- MinochaMKhuranaVQinBPalDMitraAKEnhanced brain accumulation of pazopanib by modulating P-gp and Bcrp1 mediated efflux with canertinib or erlotinibInt J Pharm201243612713422688250
- HegedusCTruta-FelesKAntalffyGInteraction of the EGFR inhibitors gefitinib, vandetanib, pelitinib and neratinib with the ABCG2 multidrug transporter: implications for the emergence and reversal of cancer drug resistanceBiochem Pharmacol20128426026722548830
- MittapalliRKVaidhyanathanSSaneRElmquistWFImpact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032)J Pharmacol Exp Ther2012342334022454535
- PollerBIusufDSparidansRWWagenaarEBeijnenJHSchinkelAHDifferential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokineticsDrug Metab Dispos20113972973521282407
- SenRNatarajanKBhullarJThe novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2Mol Cancer Ther2012112033204422778153
- HegedusCOzvegy-LaczkaCApatiAInteraction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological propertiesBr J Pharmacol20091581153116419785662
- TangSCNguyenLNSparidansRWWagenaarEBeijnenJHSchinkelAHIncreased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridarInt J Cancer20141341484149424037730
- HooijbergJHBroxtermanHJSchefferGLPotent interaction of flavopiridol with MRP1Br J Cancer19998126927610496352
- RobeyRWMedina-PerezWYNishiyamaKOverexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cellsClin Cancer Res2001714515211205902
- BoernerSATourneMEKaufmannSHBibleKCEffect of P-glycoprotein on flavopiridol sensitivityBr J Cancer2001841391139611355953
- JagerWGehringEHagenauerBAustSSenderowiczAThalhammerTThe role of hepatic Mrp2 in the interaction of flavopiridol and bilirubin: impact on therapyInt J Clin Pharmacol Ther20034161061114692715
- SeamonJARuggCAEmanuelSRole of the ABCG2 drug transporter in the resistance and oral bioavailability of a potent cyclin-dependent kinase/Aurora kinase inhibitorMol Cancer Ther200652459246717041089
- YokotaAKimuraSMasudaSINNO-406, a novel BCR-ABL/Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph+ leukemia cells in the central nervous system, and cyclosporine A augments its in vivo activityBlood200710930631416954504
- RobeyRWShuklaSSteadmanKInhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structureMol Cancer Ther200761877188517575116
- NobleMEEndicottJAJohnsonLNProtein kinase inhibitors: insights into drug design from structureScience20043031800180515031492
- SaitoHHiranoHNakagawaHA new strategy of high-speed screening and quantitative structure-activity relationship analysis to evaluate human ATP-binding cassette transporter ABCG2-drug interactionsJ Pharmacol Exp Ther20063171114112416489126
- EjendalKFHrycynaCADifferential sensitivities of the human ATP-binding cassette transporters ABCG2 and P-glycoprotein to cyclosporin AMol Pharmacol20056790291115598974
- ClarkRKerrIDCallaghanRMultiple drugbinding sites on the R482G isoform of the ABCG2 transporterBr J Pharmacol200614950651516981002
- HerbstRSFukuokaMBaselgaJGefitinib – a novel targeted approach to treating cancerNat Rev Cancer2004495696515573117
- CappuzzoFLigorioCJannePAProspective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trialJ Clin Oncol2007252248225517538169
- RobeyRWPolgarODeekenJToKWBatesSEABCG2: determining its relevance in clinical drug resistanceCancer Metastasis Rev200726395717323127
- ZhuangYFragaCHHubbardKETopotecan central nervous system penetration is altered by a tyrosine kinase inhibitorCancer Res200666113051131317145877
- LemosCGiovannettiEZucaliPAImpact of ABCG2 polymorphisms on the clinical outcome and toxicity of gefitinib in non-small-cell lung cancer patientsPharmacogenomics20111215917021332310
- AkasakaKKaburagiTYasudaSImpact of functional ABCG2 polymorphisms on the adverse effects of gefitinib in Japanese patients with non-small-cell lung cancerCancer Chemother Pharmacol20096669169820035425
- LiJCusatisGBrahmerJAssociation of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patientsCancer Biol Ther2007643243817312388
- van ErpNPEechouteKvan der VeldtAAPharmacogenetic pathway analysis for determination of sunitinib-induced toxicityJ Clin Oncol2009274406441219667267
- KimHRParkHSKwonWSPharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patientsCancer Chemother Pharmacol20137282583524013576
- Gafter-GviliARamRGafterUShpilbergORaananiPRenal failure associated with tyrosine kinase inhibitors – case report and review of the literatureLeuk Res20093412312719640584
- LinTSRuppertASJohnsonAJPhase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk diseaseJ Clin Oncol2009276012601819826119
- HuangWSYangCHSorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patientWorld J Gastroenterol2009154464446619764104
- Al-KaliAFarooqSTfayliATumor lysis syndrome after starting treatment with Gleevec in a patient with chronic myelogenous leukemiaJ Clin Pharm Ther20093460761019744017
- DohseMScharenbergCShuklaSComparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinibDrug Metab Dispos2010381371138020423956
- DaiCLTiwariAKWuCPLapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2Cancer Res2008687905791418829547
- ShuklaSRobeyRWBatesSEAmbudkarSVSunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2Drug Metab Dispos20093735936518971320
- ZhengLSWangFLiYHVandetanib (Zactima, ZD6474) antagonizes ABCC1- and ABCG2-mediated multidrug resistance by inhibition of their transport functionPLoS One20094e517219390592
- JoveletCBenardJForestierFFarinottiRBidartJMGilSInhibition of P-glycoprotein functionality by vandetanib may reverse cancer cell resistance to doxorubicinEur J Pharm Sci20124648449122484209
- ReynerELSevidalSWestMAIn vitro characterization of axitinib interactions with human efflux and hepatic uptake transporters: implications for disposition and drug interactionsDrug Metab Dispos2013411575158323729661
- LagasJSvan WaterschootRASparidansRWWagenaarEBeijnenJHSchinkelAHBreast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulationMol Cancer Ther2010931932620103600
- DurmusSSparidansRWWagenaarEBeijnenJHSchinkelAHOral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridarMol Pharm201293236324523020847
- WuCPSimHMHuangYHOverexpression of ATP-binding cassette transporter ABCG2 as a potential mechanism of acquired resistance to vemurafenib in BRAF(V600E) mutant cancer cellsBiochem Pharmacol20128532533423153455
- SparreboomALoosWJBurgerHEffect of ABCG2 genotype on the oral bioavailability of topotecanCancer Biol Ther2005465065815908806
- ZamboniWCRamanathanRKMcLeodHLDisposition of 9-nitrocamptothecin and its 9-aminocamptothecin metabolite in relation to ABC transporter genotypesInvest New Drugs20062439340116505951
- VolkELSchneiderEWild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporterCancer Res2003635538554314500392
- WangXFurukawaTNitandaTBreast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitorsMol Pharmacol200363657212488537
