Abstract
Glioblastoma multiforme (GBM) is the most frequent and most devastating of the primary central nervous system tumors, with few patients living beyond 2 years postdiagnosis. The damage caused by the disease and our treatments for the patients often leave them physically and cognitively debilitated. Generally, GBMs appear after very short clinical histories and are discovered by imaging (using magnetic resonance imaging [MRI]), and the diagnosis is validated by pathology, following surgical resection. The treatment response and diagnosis of tumor recurrence are also tracked by MRI, but there are numerous problems encountered with these monitoring modalities, such as ambiguous interpretation and forms of pseudoprogression. Diagnostic, prognostic, and predictive biomarkers would be an immense boon in following treatment schemes and in determining recurrence, which often requires an invasive intracranial biopsy to verify imaging data. Extracellular vesicles (EVs) are stable, membrane-enclosed, virus-sized particles released from either the cell surface or from endosomal pathways that lead to the systemic release of EVs into accessible biofluids, such as serum/plasma, urine, cerebrospinal fluid, and saliva. EVs carry a wide variety of proteins, nucleic acids, lipids, and other metabolites, with many common features but with enough individuality to be able to identify the cell of origin of the vesicles. These components, if properly interrogated, could allow for the identification of tumor-derived EVs in biofluids, indicating tumor progression, relapse, or treatment failure. That knowledge would allow clinicians to continue with treatment regimens that were actually effective or to change course if the therapies were failing. Here, we review the features of GBM EVs, in terms of EV content and activities that may lead to the use of EVs as serially accessible biomarkers for diagnosis and treatment response in neuro-oncology.
Introduction
Glioblastoma multiforme (GBM; World Health Organization [WHO] grade IV glioma) is the most common primary malignant brain tumor in the United States, striking some three in 100,000 people. Patients with GBM are faced with a poor prognosis as 5-year survival rates are less than 5% for all age groups (of those ≥75 years of age, virtually none are alive by that time).Citation1 Roughly 17,000 Americans are diagnosed with GBM each year, and the disease claims ~13,000 lives, with most succumbing in 1–2 years, despite gross total surgical resection, external beam radiation treatment, and the latest in multimodal chemotherapy.Citation2 These therapies are relatively nonspecific and come at significant cognitive and physiopsychological costs to the patient, as the brain is an organ that can not tolerate much collateral damage. Despite the relatively short time course of the disease, the financial burdens at a familial level, are staggering,Citation3 and at a national level, the therapies may represent the most expensive (ie, ineffective) treatments per life-year saved when factoring in quality-of-life adjustments.Citation4,Citation5 Thus, at the individual, familial, and a national level, GBMs take a horrendous toll.
Glioblastoma: surgical and other therapeutic limitations
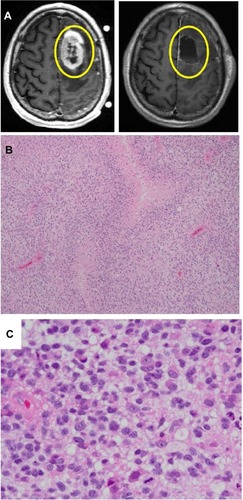
GBMs are highly invasive malignant neoplasms of glial cells within the central nervous system that carry a very poor prognosis, despite advancements in surgical and oncological modalities. Neurosurgical resection of GBMs has failed to offer patients with a curative option as even radiological imaging with contrast enhancement of disease inadequately correlates with actual neoplastic disease burden (). Thus, gross total resection, using the radiological image of the apparent tumor margins, fails to address the microinfiltrative disease beyond the borders of the radiological depiction (which can be identified upon histological representation).Citation6–Citation8 Histologic characterization of GBMs commonly shows small areas of central necrosis, with a distinct pseudopalisading rim of anaplastic glial cells and a hyperplastic, hyperpermeable vasculature, which differentiates these tumors from lower-grade astrocytomas ( and ). Examination of the pseudopalisading network of neoplastic glial cells is remarkable for disease heterogeneity. The neoplastic heterogeneity observed in GBM poses significant challenges in disease treatment as well-known oncological therapeutics fail to target the entire disease burden and may actually select for inherently resistant neoplastic cells.Citation9–Citation12 Further histological investigation of the brain parenchyma beyond the location of the radiographical depiction of the disease often reveals significant microinfiltrative tumor cells, which are thought to be a major contributor to the dismal prognosis of GBM.Citation6,Citation9 The inability to cure GBM via neurosurgical resection and the failure of our current therapeutics contribute to the urgent need for further GBM tumor characterization.
Figure 1 Radiographic (MRI) images and histology of GBMs.
Abbreviations: GBM, glioblastoma multiforme; MRI, magnetic resonance imaging.
Characterization of glioblastoma
There have been remarkable strides made recently in the molecular genetic characterizations of GBMs, which have been valuable in the initial understanding of the detrimental effects of GBM and may prove useful in the stratifcation of patients by therapeutic response and/or in clinical trials.Citation13 However, these types of tumor identifiers are obviously evident only after the tumor is present and accessible. For the purposes of serial monitoring during the course of treatment or the early detection of recurrent tumors, biofluid-based biomarkers will be essential tools. Virtually all biofluids (eg, serum/plasma, urine, cerebrospinal fluid, breast milk, semen, colostrum, bronchial lavage fluid, ascites fluid, saliva, and synovial fluid) have one set of common components – extracellular vesicles, consisting of exosomes and microvesicles.Citation14,Citation15
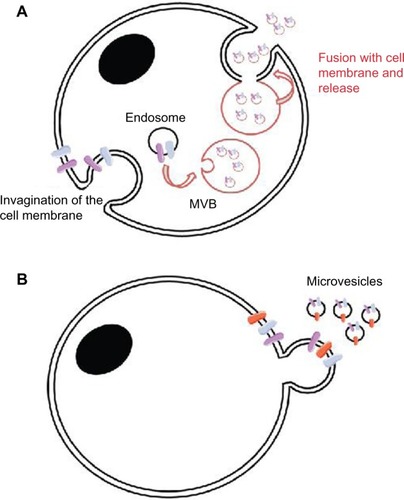
Extracellular vesicles (EVs) are membrane-enclosed, virus-sized (30–1,000 nm) nanovesicles that are released extracellularly from cells, either directly from the cell surface (called “microvesicles,” “microparticles,” or “ectosomes”) by membrane “blebbing” or via the formation in an endocytic route, with fusion of a late endosome/multivesicular body with the plasma membrane (“exosomes”)Citation14,Citation15 (). Extracellular release deposits EVs into systemic or circulating fluids, with the potential for proximal and distal effects. These vesicles contain numerous cellular constituents, such as proteins, nucleic acids, lipids, and metabolites, that have common denominators in terms of the biogenesis of vesicle formation and cargo loading, but the constituents also have sufficient specificity that they can identify the cell of origin of their release. EVs are stable and protect the constituents from nucleases, proteases, and the other means of degradation found in the biologically harsh extracellular environments.Citation16 EVs also have physicochemical properties that enable their purification via standard procedures.Citation17,Citation18 Based on these features, EVs appear to be veritable repositories of potential biomarkers for health and disease,Citation19 and this is clearly evident in oncology as well,Citation20 especially in neuro-oncology, where the needs for diagnostic, prognostic, and predicative biomarkers are urgent.Citation21
Figure 2 Two main modes of EV formation.
Abbreviations: EV, extracellular vesicle; MVB, multivesicular body.
Biomarkers – general considerations
The term “biomarker” (as in “biological marker”) seems straightforward, but some definitions are applicable. Typically a biomarker refers to “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”Citation22 This is a broad statement but encompasses most aspects. There are three recognized sub-categories of biomarkers: 1) diagnostic markers that identify the presence of and specific type of disease; 2) prognostic markers used to imply outcomes independently of therapy; and 3) predictive markers that can inform in the context of particular treatment response. For gliomas, we currently know of nothing truly useful as an early-stage diagnostic biomarker as most GBMs present as full-blown tumors with little or no prior clinical evidence of their existence.Citation23 There are certain molecular features of GBMs that aid in and verify diagnoses, and some of those are also prognostic and may be predictive (study of this is ongoing).Citation24 The current line of thought in this area is that recurrent genetic abnormalities are able to subcategorize GBMs into groups, defined as Classical, Mesenychmal, Proneural, and Neural.Citation25 The main features of these designations are shown in . The gene expression patterns and genomic alterations associated with the GBM categories may eventually lead to better therapeutic strategy design, but for now, the most disturbing correlation is that only patients with the Proneural subtype show any trend towards longer survival compared with patients in the other three categories. Nonetheless, that prolonged survival is not statistically significantly different compared with the other groups and in general, it is still less than 2 years postdiagnosis.
Table 1 Subclassifications of glioblastomas (based on TCGA)Citation25
It is important to realize that the molecular signatures of GBMs are based on analyses of actual tumor specimens or biopsies from them. Thus, the use of those molecules in the ongoing monitoring of patient progress or response to therapies may be limited, suggesting that biofluid-based biomarkers are likely to be of far more practical benefit. Circulating biomarkers may be serially collected, which is essential for therapeutic assessment, and the (usually) minimally invasive nature of collection (compared with the substantial intracranial interventions at surgery or biopsy) is also attractive.
As mentioned above, most GBMs present acutely, with relatively few clear indications of the oncoming pathogenesis (usually, headache, seizures, intracranial pressure, vision/hearing issues, and even personality changes), making the window for early disease screening almost nonexistent. Imaging techniques, such as magnetic resonance imaging (MRI)Citation26/magnetic resonance spectroscopy (MRS)Citation27 are standard brain tumor diagnostics; these are clearly valuable but are limited to use after a tumor is suspected due to the aforementioned symptoms. Also, these techniques are extremely expensive, as is the entire brain tumor affliction.Citation28 Such imaging modalities generally offer little histologic or prognostic information without employing specialized technologies such as various forms of positron emission tomagraphy, vessel architectural imaging, or stimulated Raman scattering. Beyond that, the only acceptable diagnoses are made, intra- or postoperatively, by pathology, which offers prognostic evaluations using immunohistochemistry or genetic techniques.Citation29,Citation30 Obviously, those tests require tumor access from surgery or biopsy, both intracranially invasive procedures. This is especially true for recurrent tumors (which occur with nearly all patients with high-grade gliomas), where imaging distinctions for remnant tumor, regrowth, or postradiation necrosis are difficult,Citation31 and “pseudoprogression” (radiographic indications – usually, gadolinium enhancement – that appear as progressively growing tumor following radiation but later resolve or devolve into radiation necrosis) is another common problem.Citation32,Citation33 Tumor diagnostic/biomarker capabilities from an accessible compartment (blood/sera, urine, or saliva) would be a major advance for neuro-oncology, especially since there seem to be no circulating exfoliated brain tumor cellsCitation34 (cerebral spinal fluid offers mixed resultsCitation35,Citation36). Thus, most biomarker efforts for GBM are geared towards the determination of recurrent disease, either in the context of standard of care treatment or in a clinical trial setting, with an emphasis on clinical-level assessments.
Circulating biomarkers in glioblastoma – current state
Proteins
Most of the proteins previously identified as putative GBM biomarkers in blood may be more closely associated with the GBM and its treatment as markers of neurotrauma rather than being unequivocally related to the tumor itself.Citation34 These proteins include glial fibrillary acidic protein (GFAP), vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), basic fibroblast growth factor (bFGF), chitinase-3-like protein (CHI3L1) or YKL-40, and matrix metalloproteinase 9 (MMP9). Other factors that are known to be secreted by tumors or by immune cells following encounters with GBMs include transforming growth factor beta (TGF-β) family membersCitation37 as well as interleukin 10 (IL-10).Citation38 So far, there has been no clinical validation of these serum-borne molecules as glioma biomarkers, but such research is ongoing.Citation21
Lipids
There is little mention of circulating lipid species as putative biomarkers for GBMs, but 24S-hydroxycholesterol is among the lipids described as brain-specific that have been identified in the systemic blood circulation.Citation39 However, hydroxycholesterol seems only to appear at high levels in serum following severe central nervous system (CNS) trauma and not necessarily in response to brain tumors.Citation40
Nucleic acids
The levels of free nucleic acids in circulation is low and are difficult to detect due to the lack of protection from nucleases that are present in the circulating system. However, some potential biomarker candidates have been proposed. The circulating nucleic acid content in the serum or plasma of GBM patients consists of both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) species and was summarized in a review by Holdhoff et al.Citation34 Briefy, the potential circulating tumor DNA biomarkers include methylated promoter regions of O-6-methyl-guanine-DNA methyltransferase (MGMT), cyclin-dependent kinase inhibitor 2A (p16), death-associated protein kinase 1 (DAPK), and Ras association (RalGDS/AF-6) domain family member 1 (RASSF1A). Others have also shown methylation of promoter regions in MGMT and phosphatase and tensin homolog (PTEN); however, detection rates of these epigenetic changes were low in serum samples, or not detectable in the case of PTEN, compared with the astrocytic tumor samples.Citation41 Additionally, the authors analyzed the loss of heterozygosity (LOH) of chromosomes 10q, 19q, and 1p in oligodendroglial tumors and reported a higher percentage of LOH in the tumor tissue compared with the serum samples.
Different RNA species have also been detected in the circulation. EGFRvIII message was detected in platelets isolated from glioma patients.Citation42 Circulating tumor microRNA (miRNA) has also been detected in the blood of GBM patients.Citation34,Citation43 In these studies, miRNA 21 (miR-21) and miRNA 128 (miR-128) were shown to be upregulated in the blood of GBM patients. The cohorts in these studies were relatively small, less than 20 patients at most, and an analysis for these molecules should be performed on a higher number of patients to provide more evidence of the use of these molecules as biomarkers. Additionally, it is unclear how small miRNAs are present in serum without some form of proteinaceous or membrane-enclosed protection. EVs may provide that protection.
Extracellular vesicles in neuro-oncology: biology and potential biomarkers
The term “exosome” was used to describe exfoliated microvesicles from the rat C-6 glioma line over 30 years ago;Citation44 the vesicle sizes (500–1,000 nm) are what we would describe today as microvesicles, and their origin appears to be from the cell surface rather than endosomal. In those and other earlier studiesCitation45,Citation46 it is unclear whether any controls were used for the fetal calf serum supplementing the cell culture medium as it is now known that the fetal bovine sera frequently used in cell culture contain extracellular vesicles.Citation47 Those vesicles may be cleared by centrifugation (ie, >100,000× g) prior to inclusion of the sera in culture medium. We may have been the first to describe exosomes in brain tumor cell lines from both human and murine sources,Citation48 and our studies were soon followed by important publications from Al-Nedawi et alCitation49 and Skog et al.Citation50 The former demonstrated the presence of glioma-specific mutant EGFR variant III (EGFRvIII) in EVs from both cell lines transfected with the mutant construct and from EVs in the sera of mice growing those EGFRvIII-expressing tumors. Incubation of glioma cells that did not express the mutant receptor with EVs from cells that did express it showed the transfer of EGFRvIII to the nonexpressing cells, with the concomitant activation of tumor-promoting signaling pathways associated with EGFRvIII signaling (extracellular signal–regulated kinase [ERK]/protein kinase B [AKT] phosphorylation, VEGF release). The latter paper demonstrated the presence of mRNAs and miRNAs in the sera of patients with GBMs that may be of diagnostic utility. A major advantage of nucleic acid technologies in regard to EVs is that they have standard preparation/handling conditions, and one has the ability to amplify signals from small amounts of starting material, which is not possible with other biologics (eg, proteins and lipids). The paper also showed that there was a differential distribution of specific messenger (m)RNAs in EVs compared with the relative amounts in the cells of origin and that a model mRNA could be passaged from cell to cell by EVs, resulting in translation of the mRNA to protein in the recipient cells. Among the mRNAs found in at least some patient serum EVs, was that encoding EGFRvIII as a tumor-specific marker. Finally, the EVs had angiogenic-promoting activities in human brain microvascular endothelial cells.
Our group later showedCitation51 that EGFRvIII appeared on the surfaces of exosomes from EGFRvIII-expressing cells (along with heat shock proteins [HSPs] 70 and 27) and that EGFRvIII protein could be identified on EVs from the sera of patients with GBM; however, EGFR seemed to be present in sera EVs from both patients and healthy donors. Exosomes from a murine brain tumor line served as potent anticancer vaccines in a prophylactic setting but were largely ineffectual in a stringent therapeutic setting, suggesting that immune responses to tumor exosomes may be strongly context-dependent. We also performed the first proteomic survey of brain tumor exosomes in that study, identifying 36 distinct proteins, including endogenous murine retroviral Gag polyproteins. Those proteins are indicative of a retrovirus known to be involved in the progression of murine tumors by subversion of immune surveillance.Citation52 This paper noted the immune dichotomy of brain tumor exosomes, demonstrating the presence of putative tumor antigens as well as a number of immunosuppressive entities.
Other articles of interest have shown that various brain tumor cell EVs (from GBMs, oligodendroglioma cells, and medulloblastoma cells) have activities and impacts on recipient cells that range from transformation of those cellsCitation53 to induction of apoptosis,Citation54 along with growth promotion and migratory-driving activities.Citation55 That paperCitation55 again showed the immune dichotomy of medulloblastoma exosomes in both immunosuppressive and immunostimulatory functions and identified almost 150 proteins in proteomic analyses. Furthermore, in other studies,Citation56,Citation57 exosomes/EVs isloated from hypoxic GBM cells enhanced GBM bioactivity and induced epithelial cells to secrete tumor-promotion factors, resulting in increased tumor growth, migration, and vascularization and angiogenesis. This involved paracrine signaling via tissue factor associated with EVs. Thus, exosomes/EVs can serve as transfer mechanisms for tumor survival and progression.Citation56,Citation57 Curiously, coagulopathies are part of the comorbidities associated with brain tumors, and tissue factor likely plays a significant part. While its presence on EVs is well known, the role of tissue factor in the context of EVs remains unclearCitation56,Citation58 and may represent an area into which therapeutic intervention can expand.Citation59
There have been some extremely interesting publications describing the role of EVs in the biology of GBM, but the attempts to find clear and utilizable biomarkers are at early stages, and the field faces the same (lack of) outcomes that have plagued the cancer biomarker field for years – ie, large numbers of putative biomarkers on input, few approved entities on output.Citation60,Citation61 The development of useful, robust, and clinically viable biomarkers follows a complex, multiphase pathway with harsh go/no-go decisions and is fraught with difficulties that involve biology, bioinformatics, and current plus developing (and currently unforeseen) biotechnologies.Citation62 However, as biomarkers are tied to clinical drug development platforms in the form of companion diagnostics, and as multiplexed systems may be necessary to validate drug efficacy,Citation63 EVs will likely play vital roles as reservoirs of multiple types of biologic materials suitable for biomarker assays.
As alluded to previously, EVs contain proteins, nucleic acids, lipids, and other metabolites that reflect their cells of origin; individual or multiple entities amongst those biomaterials may prove to be the identifiers of cancer cells in the host. As EVs are present in (and purifiable from) essentially all routinely obtainable biofluids, a distinct advantage in this scenario is that EVs bundle multiple potential biomarkers in one package. Thus, multiplexed assays that evaluate more than one biomaterial (eg, RNA and protein) could theoretically be performed on the same sample. The concept of “liquid biopsy” is often applied to circulating tumor cellsCitation64 and more recently to circulating cell-free (cf)-DNA,Citation65 but the numbers of circulating tumor cells are often low (or even nonexistent, in the cases of patients with GBM), and cf-DNA is largely nontumor in origin. EVs overcome these barriers in liquid biopsy – EVs are known to occur in elevated quantities in the blood of cancer patients,Citation66–Citation68 and the protein and nucleic acid contents can be tumor-specific.Citation50,Citation51,Citation55,Citation69,Citation70 With this background, we will explore what is known and what may be possible concerning the use of EVs as biomarkers for GBM.
Glioblastoma extracellular vesicle protein content
Perhaps the best known and most specific protein marker for GBMs is EGFRvIII; this has been identified on both cell line and patient serum EVs.Citation49–Citation51 It is a tumor-specific mutation (also found in several other cancer types that overexpress EGFR)Citation71 that deletes 267 amino acids from the extracellular domain, resulting in a truncated receptor that has no clear ligands but nonetheless signals constitutively.Citation72,Citation73 There are some discrepancies concerning the frequency of the mutant protein in GBM patient samples, perhaps resulting from differences in techniques and reagents,Citation71 but estimates range from 25% to over 60%. Thus, EGFRvIII seems to be an excellent putative biomarker from a diagnostic perspective, and its loss of expression may be a predictive biomarker if the protein is directly targeted.Citation74 However, it is likely that evaluation of the protein status would be the most direct assessment, but access to antibody reagents is limited, resulting in the use of nucleic acid techniques for detection.Citation75 There remains concern over the lack of correlation between polymerase chain reaction (PCR)-based detection of EGFRvIII and the actual protein expression levels,Citation71 so specificity and sensitivity issues may depend on the techniques employed.
Gene amplification of EGFR is common in GBMs, as is overexpression of the protein (and with EGFRvIII expression in a subset of the EGFR-amplified tumors).Citation76 WeCitation51 and othersCitation49,Citation77 have identified EGFR in EVs from GBM cells and patient sera, but it also appears to be in EVs from healthy donorsCitation51 and from nontumor cell lines,Citation78,Citation79 which begs the question of specificity. However, it may be a valuable biomarker tool as part of a multiparameter screening assay.Citation77 In a very limited study, we also identified EGFR2/v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ErbB2)/human epidermal growth factor receptor 2 (HER2) as a relatively specific marker for EVs, from patients with medulloblastoma.Citation55 Perhaps 17% (or more) of GBMs probed express HER2,Citation80,Citation81 so this protein may also be a useful biomarker, but it is unclear whether it is incorporated into EVs from healthy donors; much larger sample sizes are necessary to establish that correlation.
Of the other proteins listed above as putative circulating protein biomarkers for GBM, all but CHI3L/YKL-40 have been found in EVs (and the mRNA for YKL-40 was identified).Citation50 We had identified GFAP in medulloblastoma, but it has also been identified in healthy donor human plasma EVs,Citation82 suggesting that it may not be tumor-specific. VEGF-A protein was identified in human GBM cell line EVs by antibody array,Citation50 and so far, there appear to be no other normal cell EVs carrying it, but it is obviously not a tumor-specific marker. Li et al have identified 112 proteins in a proteomic analysis of U251MG cell line-derived EVs;Citation83 of those, all but one (myoferlin) had been previously identified in other EVs. Our group found bFGF (also called FGF2) in medulloblastoma EVs,Citation55 suggesting that it may be a tumor marker, but again, it is not tumor-specific, as is true of MMP9, TGF- β, and IL-10. MMP9 mRNA was found in GBM EVs,Citation50 and the protein is present and active in ovarian cancer EVs.Citation84 MMPs and other extracellular proteases play important roles in modifying the tumor microenvironment for angiogenesis, migration, and invasion, implicating EVs as major players in this important function.
GBMs leave their hosts profoundly immunosuppressed,Citation85,Citation86 and those effects are often linked to TGF-β and IL-10.Citation38 Both of these cytokines are produced by immune system cells but have been identified as EV components from both cancer and normal cell types.Citation51,Citation82 There may be a question regarding the normal background amounts of those cytokines in EVs as IL-10 was identified coming from mesenchymal stem cell EVs,Citation82 and active TGF-β from EVs of thymus cell origin may drive regulatory T cell phenotypes.Citation87 Curiously, we saw what appeared to be latent forms of TGF-β on serum EVs from patients with GBMs.Citation51 Since active TGF-β1 has a serum half-life of <2 minutes,Citation88 transport of it in blood may rely on EVs.
Other tumor-specific mutations identified in brain tumors are in the isocitrate dehydrogenases 1 (IDH1) and 2 (IDH2). These mutations are far more prevalent in lower-grade gliomas (and secondary gliomas) than in the high grades (such as GBMs).Citation89 While we identified IDH1 in the proteome of medulloblastoma EVs,Citation55 the peptide sequence coverage did not include the potentially mutated regions. However, using an antibody specific for the IDH1 R132H mutant protein, Shao et alCitation77 included the enzyme as part of a four-protein GBM molecular signature to interrogate GBM cell line and patient sera EVs. This was part of a micro nuclear magnetic resonance (micro-NMR) device study that indicated such a molecular signature, coupled with an extremely sensitive instrument, could accurately type responders vs nonresponders in both animal models and patients receiving therapy for GBMs. This, again, strongly suggests that effective biomarkers may come in packages (such as EVs), utilizable as a panel, rather than as individual entities.Citation90,Citation91 It also suggests that despite the nanoscale of EVs, microfabrication of devices capable of interrogating such vesicles for clinical applications is truly possible.
Glioblastoma extracellular vesicle lipid content
While relatively little is known about the lipid components of EVs,Citation92 we are learning more all the time.Citation93 Nonetheless, almost nothing has been reported on the lipid contents of GBM EVs. 24S-hydroxycholesterol in serum has been associated with CNS traumaCitation94 but not so with brain tumors;Citation40 still, it is unclear whether the methods for extraction and identification of 24S-hydroxycholesterol would have encompassed a significant population of extracellular vesicles,Citation95 and cholesterol is one of the highly enriched lipids in extracellular vesicles.Citation93 Thus, there may be CNS-enriched (or specific) lipid markers on the EVs from CNS tumors, but their discovery is pending.
Glioblastoma extracellular vesicle nucleic acid content
The nucleic acid content of EVs is quite diverse. Skog et alCitation50 were the first to report on the mRNA and miRNA content of GBM EVs. Balaj et al found that EVs from different types of CNS tumors contained single-stranded DNA, and both complementary (c)DNA and genomic DNA as well as retrotransposable elements.Citation69 Li et al reported that an abundance of miRNAs and small (presumably noncoding) RNAs are packaged in glioma EVs.Citation83 miRNAs may be very useful sources of biomarker potential in GBMs, given the differences in the miRNA signature for healthy subjects and GBM patients.Citation83,Citation96,Citation97
Although the nucleic acid content of EVs is diverse, there are only a few potential biomarkers that have been suggested to date. As with the protein content of GBM-derived EVs, the EGFRvIII mRNA is also one of the most specific and well-known markers that has been detected in EVs. In their work, Skog et alCitation50 purified EVs from the serum of GBM patients and detected EGFRvIII mRNA in 28% of samples tested using PCR-based methods. The percentage of EGFRvIII mRNA compared with total RNA in EVs is consistent with the percentage of GBM patients who are positive for this particular mutation, ie, about 30% of GBM patients have the EGFRvIII mutation. The detection of EGFRvIII RNA in glioma EVs was also reported by Nilsson et al, and they found that EVs are used to transfer the message to recipient cells.Citation42 The work of Chen et al is promising in its potential to use the detection of the IDH1 mutation in CSF as a biomarker, especially considering the sensitivity of this technique.Citation70 The authors used very sensitive PCR methods to detect and quantify wild-type and mutant IDH1 transcripts in EVs isolated from the CSF of glioma patients and found higher levels of mutant IDH1 in the CSF of patients with gliomas compared with healthy controls. The sensitivity of the technique is evident considering that IDH1 mutants are relatively rare in patients with GBM.Citation98 However, the source of material (CSF) is not a prime candidate for serial evaluation of biomarker status.
As mentioned above, Li et alCitation83 detected several different types of RNA in glioma EVs. Of particular interest, is their finding that in the U251 cell line, derived EV miR-21 was detected in high levels, consistent with Skog et al.Citation50 This finding complements the results from Skog et al, also showing a high expression of miR-21 in serum EVs from patients with GBM.Citation40 The results of both of these groups are consistent with reports that miR-21 is highly overex-pressed in glioma tissue and suggest that miR-21 has clear potential as a biomarker in GBMs,Citation97,Citation99 particularly in the context of other putative markers. It should be pointed out that miR-21 is not specific for GBMs and is also found in the EVs of healthy donors,Citation100 so “context and community” will likely be important to determine the utility of miRNAs as biomarkers. As sophisticated, sensitive PCR methodologies become robust and more widely usable in a diagnostic setting, it is conceivable that the detection of other highly abundant GBM-specific miRNAs in biofluid EVs will be possible and may provide additional biomarker candidates.
Other aspects of extracellular vesicles relatable to biomarkers
EV surfaces as interrogable landscapes
The surfaces of EVs contain a great deal of information relative to the sizes of these tiny vesicles and considering the relatively limited internal volumes of them. Various aspects of EV surfaces may reveal much about their abilities to differentially represent the presence of tumor vs normal cells, or the changes in tumor cells due to treatment.
Glycome
The surfaces of cancer cells are often differentially glycosylated compared with their normal cell counterparts,Citation101 thus suggesting that tumor glycomes may distinguish tumor cells from normal cells. Technologies for carbohydrate analyses are progressing, including better separation and mass spectrometry identification, as well as microarray-style high-throughput methods.Citation102 The glycosylated surfaces of EVs, either in the form of glycoproteins, glycolipids, or surface-bound carbohydrates, are ripe for this type of probing.
Recently, Batista et al utilized a lectin array to study the glycomes of EVs harvested from several different sources.Citation103 They showed differences between the cell surface glycomes and the resulting EV glycomes, which tended to be conserved, although there were distinguishing features in the gross glycomic compositions. Urinary exosome surface glycosylation has also been proven to distinguish between EVs from healthy donors vs those with polycystic kidney disease,Citation104 suggesting that these surface phenomena may harbor carbohydrate biomarkers. We recently reported that particular antibodies that recognize the brain tumor-specific gangliosides 3′-isoLM1 and/or 3′,6′-isoLD1Citation105 would bind to glioma exosomes,Citation106 demonstrating that such antigens could be present on circulating EVs as biomarkers. This is a relatively unexplored area in EV biology, but it may hold great promise as a means for identifying pathology-related circulating EVs.
Proteome
The EV surface proteome is likely to contain proteins (in particular, glycoproteins) that reflect the cancerous state. GBM “stem cells” were used to profile cell surface glycoproteins whose expression was upregulated on the “neurospheres” stem cells compared with classic, long-term adherent cultures.Citation107 Receptor-type tyrosine protein phosphatase ζ (phosphacan), tenascin-C, chondroitin sulfate proteoglycan NG2 (CSPG4), podocalyxin-like protein 1, and cluster of differentiation (CD)90 were upregulated (and CD44 was comparatively downregulated). Of those glycoproteins, tenascin C, CSPG4, and CD44 have been identified in EVs,Citation82 and there is an antibody (81C6) that specifically recognizes brain tumor tenascin CCitation108 as well as a potential therapeutic antibody against CSPG4.Citation109 Thus, the stem cell portions of GBMs may have surface markers that could be released in EVs; due to the association of stem cells with tumor therapeutic resistance, these may be valuable markers, as harbingers of impending recurrence.
As mentioned above, our group identified EGFRvIII on glioma cell EV surfaces, along with HSPs 27 and 70,Citation51 and later, we showed HSP90 on medulloblastoma EV surfaces.Citation55 The presence of HSPs on brain tumor EV surfaces fits with the demonstration of the HSPs and other chaperones on the surfaces of a variety of brain tumor cells.Citation48,Citation110 As these proteins are typically regarded as intracellularly localized, these data suggest that location may relate to biomarker status, with tumors altering the “standard” localizations of proteins. Newer technologies, such as nanoparticle tracking analysis (NTA) with fluorescent (usually antibody) staining, allows for the specific quantification of labeled vesicles within the overall population of vesicles present, thus allowing for both the detection and enumeration of putative tumor or pathologic EVs in a mixed field of vesicles (eg, such as from serumCitation111 or from urineCitation112). Another use of antibody detection for both surface and internal EV content was mentioned previously, where using a microfluidic NMR chip, GBM EVs were labeled (or lysed and internal materials labeled) with targeted magnetic nanoparticles against a four-protein signature (EGFR, EGFRvIII, podoplanin [PDPN], and IDH1 mutant).Citation77 This rendered the EVs superparamagnetic and yielded faster proton decay rates for enhanced NMR signal. While none of the markers individually had high sensitivity (but overall good specificity), the combination of the four markers resulted in both high sensitivity and specificity, and the ability to quantify EVs at several logs better than current technologies. A major message here is that multiple biomarkers may be able to overcome the limitations of one or a few.
Surface antigens
One interesting host response to tumor EVs is the antibody response against presumed surface antigens. This has been shown with model or known antigens “loaded” into EVs,Citation113,Citation114 but we have also shown high-titer murine responses against naturally occurring brain tumor antigens;Citation51,Citation106 these responses occur very quickly and in the absence of adjuvant. Conversely, tumor antigens (eg, HER2) on the surfaces of circulating tumor-derived EVs have the capacity to bind and effectively titrate out therapeutic antibodies, such as trastuzumab, potentially reducing the effectiveness of such drugs.Citation115 There appear to be natural antibodies that recognize reticulocyte EVs, with potential involvement in the elimination of apoptotic bodies.Citation116 We have shown that circulating EVs from the sera of patients with GBMs carry substantial amounts of bound antibody; those antibodies may be “eluted” from the EVs, and at least some fraction of the antibodies recognize tumor antigens from GBM cell lines, in Western blots.Citation106 This raises the possibility that antibodies on circulating tumor EVs may be able to reveal the presence of tumor antigens, which may produce a signature on high-density protein or peptide arrays alerting of tumor presence. From a predictive perspective, one issue would be our lack of knowledge of the clearance or half-lives of such antibodies, so there may be more value in them from an initial diagnostic perspective.
Glioblastoma extracellular vesicle biologic activities with biomarker potential
One should consider the concept that particular molecular entities of EVs may be difficult to identify and ascertain as recognizably different between tumors and normal cell types. However, the collection of materials may have endemic, measurable activities that could distinguish between pathologic and normal states; in a further connection of tumorigenicity to a measurable readout, EVs may be able induce responses in model cell types that could reveal the presence of tumor and perhaps even, in some situations, an “aggressiveness” measure of the tumor. The following are some possible areas of assay development.
Metabolic
Biologic/metabolic enzymes represent about 25% of the proteins found in brain tumor exosomesCitation51,Citation55 and glycolytic enzymes are among the top ten proteins identified in EVs.Citation117 Ronquist et al recently demonstrated that prostasomes (EVs released from the prostate gland into seminal fluid) can functionally generate adenosine triphosphate (ATP) from substrates such as glucose or fructose,Citation118 indicating that EVs have intrinsic metabolic capacity. We recently showed that EVs from the sera of patients with GBM possessed the tumor-associated enzyme pyruvate kinase M2 (PKM2) necessary for the Warburg effect and that the addition of EVs from GBM cells increased the expression of various metabolic enzymes in those recipient cells.Citation106 One potential effect of the increased enzyme quantities could be increased measurable metabolic output; for instance, the addition of GBM EVs to GBM (or other reporter) cells might drive lactate release, suggesting that such assays may reveal the presence of tumor EVs by surrogate cellular-based assays.
Attractant
We previously demonstrated that medulloblastoma cells migrated towards EVs used as attractants in Boyden chamber/transwell migration assays,Citation55 and this has proven true for EVs of other tumor types.Citation119 While this likely has more biologic than diagnostic significance, certain elements of EV activity (eg, extracellular proteolytic activity of MMP9) may have useful, measurable outputs in the appropriate assays.
Signaling
EVs are known to transport phosphoproteins with putative signaling potential,Citation120,Citation121 and GBM EVs can alter signaling pathways within recipient cells.Citation122 In similar assays, our unpublished data show that EVs from “stressed” GBM cells (ie, those induced to undergo the unfolded protein response)Citation123 drive more extensive changes in receptor tyrosine kinase signaling pathways on recipient GBM cells than do those EVs from “unstressed” cells (not shown). One could imagine a standard cell line assay, where EVs from patient body fluids were incubated with the model cell line, which was then lysed and assayed for specific (ie, limited) changes in phosphoprotein status indicative of tumor EV-driven phenotypic alterations. It is conceivable that EVs themselves could be lysed and exposed to phosphoantibody arrays that might reveal the presence of high levels of tumor-associated phosphoproteins. Other EV protein posttranslational modifications that may indicate tumor presence may include glycosylation (see above), ubiquitination,Citation124 citrullination,Citation125 acylation,Citation126 and other lipid modifications. We are likely just scratching the surface in understanding posttranslational modifications and their impacts on cargo loading into EVs, and potential downstream biologic significance.
Discussion
EVs show great promise as reservoirs of biomarkers for patients with glioblastoma. The presence of EVs in every biofluid allows for theoretically easy access in minimally or noninvasive fashion, and the assortment of biomolecules surely provides a signature for the presence of disease or of change in the disease state. In few cases are the components of that signature truly tumor-specific (in the cases of GBMs, perhaps EGFRvIII and IDH mutants are tumor-restricted). Thus, we may need to quantify potential (combinations of) biomarkers, and possibly their locations in or on EVs, to distinguish the signature as distinct from normal tissue EVs. That signature may be only readable through a translation via bioassays rather than by the direct identification of “letters” or “words” in the signature. There is a great need for biomarker development in neuro-oncology since our current imaging systems often fail to provide adequate information concerning recurrent tumor growth or current biological status of either the primary or recurrent tumor. This paucity of information tends to drive a fairly uniform treatment regimen for every patient and thus, fails to account for a personalized approach to monitoring disease and individual responses to therapy. It may be that these circulating “fat balls” can provide that window into the presence and status of the tumor that will give us a therapeutic edge in the treatment of these devastating diseases.
Acknowledgments
The authors wish to thank Dr Bette K Kleinschmidt- DeMasters, from the Department of Pathology at the University Of Colorado School Of Medicine, for her histological contributions to this paper. This work was supported by an award from the National Institutes of Health 1R01EB016378 – 01A1 and the Cancer League of Colorado (to MWG).
Disclosure
The authors report no conflicts of interest in this work.
References
- DolecekTAProppJMStroupNEKruchkoCCBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009Neuro Oncol201214Suppl 5v1v4923095881
- GrossmanSAYeXPiantadosiSNABTT CNS ConsortiumSurvival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United StatesClin Cancer Res20101682443244920371685
- BradleySSherwoodPRDonovanHSI could lose everything: understanding the cost of a brain tumorJ Neurooncol200785332933817581698
- PickardJDBaileySSandersonHReesMGarfieldJSSteps towards cost-benefit analysis of regional neurosurgical careBMJ199030167536296352121302
- HeimbergerABSampsonJHThe PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patientsExpert Opin Biol Ther2009981087109819591631
- DasSMarsdenPAAngiogenesis in glioblastomaN Engl J Med2013369161561156324131182
- DeaNFournier-GosselinMPMathieuDGoffauxPFortinDDoes extent of resection impact survival in patients bearing glioblastoma?Can J Neurol Sci201239563263722931705
- LacroixMAbi-SaidDFourneyDRA multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survivalJ Neurosurg200195219019811780887
- CloughesyTFCaveneeWKMischelPSGlioblastoma: from molecular pathology to targeted treatmentAnnu Rev PatholEpub 852013
- DenysenkoTGenneroLJuenemannCHeterogeneous phenotype of human glioblastoma. In vitro studyCell Biochem FunctEpub July82013
- ThomasRPXuLWLoberRMLiGNagpalSThe incidence and significance of multiple lesions in glioblastomaJ Neurooncol20131121919723354652
- ArakawaYMizowakiTMurataDRetrospective analysis of bevacizumab in combination with Ifosfamide, Carboplatin, and Etoposide in patients with second recurrence of glioblastomaNeurol Med Chir (Tokyo)2013531177978524140770
- TanakaSLouisDNCurryWTBatchelorTTDietrichJDiagnostic and therapeutic avenues for glioblastoma: no longer a dead end?Nat Rev Clin Oncol2013101142623183634
- GranerMWBrain tumor exosomes and microvesicles: pleiotropic effects from tiny cellular surrogatesGaramiMMolecular Targets of CNS TumorsRijekaInTech2012
- RaposoGStoorvogelWExtracellular vesicles: exosomes, microvesicles, and friendsJ Cell Biol2013200437338323420871
- MartinsVRDiasMSHainautPTumor-cell-derived microvesicles as carriers of molecular information in cancerCurr Opin Oncol2013251667523165142
- LässerCEldhMLötvallJIsolation and characterization of RNA-containing exosomesJ Vis Exp201259e303722257828
- ThéryCAmigorenaSRaposoGClaytonAIsolation and characterization of exosomes from cell culture supernatants and biological fluidsCurr Protoc Cell Biol2006Chapter 3Unit 3.2218228490
- ProperziFLogozziMFaisSExosomes: the future of biomarkers in medicineBiomark Med20137576977824044569
- RakJExtracellular vesicles – biomarkers and effectors of the cellular interactome in cancerFront Pharmacol201342123508692
- Farias-EisnerGBankAMHwangBYGlioblastoma biomarkers from bench to bedside: advances and challengesBr J Neurosurg201226218919422176646
- Biomarkers Definitions Working GroupBiomarkers and surrogate endpoints: preferred definitions and conceptual frameworkClin Pharmacol Ther2001693899511240971
- OhgakiHKleihuesPGenetic pathways to primary and secondary glioblastomaAm J Pathol200717051445145317456751
- JohnsonDRGalanisEIncorporation of prognostic and predictive factors into glioma clinical trialsCurr Oncol Rep2013151566323125011
- VerhaakRGHoadleyKAPurdomECancer Genome Atlas Research NetworkIntegrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1Cancer Cell20101719811020129251
- PriceSJThe role of advanced MR imaging in understanding brain tumour pathologyBr J Neurosurg200721656257518071983
- ShahNSattarABenantiMHollanderSCheuckLMagnetic resonance spectroscopy as an imaging tool for cancer: a review of the literatureJ Am Osteopath Assoc20061061232716428685
- BradleyCJYabroffKRDahmanBFeuerEJMariottoABrownMLProductivity costs of cancer mortality in the United States: 2000–2020J Natl Cancer Inst2008100241763177019066273
- TakeiHBhattacharjeeMBRiveraADancerYPowellSZNew immunohistochemical markers in the evaluation of central nervous system tumors: a review of 7 selected adult and pediatric brain tumorsArch Pathol Lab Med2007131223424117284108
- NakamuraMShimadaKIshidaENakaseHKonishiNGenetic analysis to complement histopathological diagnosis of brain tumorsHistol Histopathol200722332733517163407
- CzernickiTSzeszkowskiWMarchelAGołebiowskiMSpectral changes in postoperative MRS in high-grade gliomas and their effect on patient prognosisFolia Neuropathol2009471434919353433
- Van MieghemEWozniakAGeussensYDefining pseudoprogression in glioblastoma multiformeEur J Neurol201320101335134123679051
- TranDKJensenRLTreatment-related brain tumor imaging changes: So-called “pseudoprogression” vs tumor progression: Review and future research opportunitiesSurg Neurol Int20134Suppl 3S129S13523682339
- HoldhoffMYovinoSGBoaduOGrossmanSABlood-based biomarkers for malignant gliomasJ Neurooncol2013113334535223670054
- QianXGoumnerovaLCDe GirolamiUCibasESCerebrospinal fluid cytology in patients with ependymoma: a bi-institutional retrospective studyCancer2008114530731418698591
- KajikawaHOhtaTOhshiroHHaradaKIshikawaSCerebrospinal fluid cytology in patients with brain tumors; a simple method using the cell culture techniqueActa Cytol1977211162167264751
- KaminskaBKocykMKijewskaMTGF beta signaling and its role in glioma pathogenesisAdv Exp Med Biol201398617118722879069
- RolleCESenguptaSLesniakMSMechanisms of immune evasion by gliomasAdv Exp Med Biol2012746537622639159
- BjörkhemILütjohannDDiczfalusyUStåhleLAhlborgGWahrenJCholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulationJ Lipid Res1998398159416009717719
- BretillonLSidénAWahlundLOPlasma levels of 24S-hydroxycholesterol in patients with neurological diseasesNeurosci Lett20002932879011027840
- LavonIRefaelMZelikovitchBShalomESiegalTSerum DNA can define tumor-specific genetic and epigenetic markers in gliomas of various gradesNeuro Oncol201012217318020150384
- NilssonRJBalajLHullemanEBlood platelets contain tumor-derived RNA biomarkersBlood2011118133680368321832279
- RothPWischhusenJHappoldCA specific miRNA signature in the peripheral blood of glioblastoma patientsJ Neurochem2011118344945721561454
- TramsEGLauterCJSalemNJrHeineUExfoliation of membrane ecto-enzymes in the form of micro-vesiclesBiochim Biophys Acta1981645163706266476
- BastidaEOrdinasAEscolarGJamiesonGATissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesisBlood19846411771846733271
- KassisSLauterCJStojanovMSalemNExfoliation of the beta-adrenergic receptor and the regulatory components of adenylate cyclase by cultured rat glioma C6 cellsBiochim Biophys Acta198688634744822871868
- MenonSIsenbergDAFetal calf serum in growth medium obscures the detection of early anticardiolipin antibody secreting clonesJ Immunol Methods1995186165707561149
- GranerMWCummingRIBignerDDThe heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequencesJ Neurosci20072742112141122717942716
- Al-NedawiKMeehanBMicallefJIntercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cellsNat Cell Biol200810561962418425114
- SkogJWürdingerTvan RijnSGlioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkersNat Cell Biol200810121470147619011622
- GranerMWAlzateODechkovskaiaAMProteomic and immunologic analyses of brain tumor exosomesFASEB J20092351541155719109410
- MangeneyMPothlichetJRenardMDucosBHeidmannTEndogenous retrovirus expression is required for murine melanoma tumor growth in vivoCancer Res20056572588259115805254
- AntonyakMALiBBoroughsLKCancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fbronectin to recipient cellsProc Natl Acad Sci U S A2011108124852485721368175
- Lo CiceroASchieraGProiaPOligodendroglioma cells shed microvesicles which contain TRAIL as well as molecular chaperones and induce cell death in astrocytesInt J Oncol20113961353135721842121
- EppleLMGriffithsSGDechkovskaiaAMMedulloblastoma exosome proteomics yield functional roles for extracellular vesiclesPLoS One201277e4206422848702
- SvenssonKJKucharzewskaPChristiansonHCHypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cellsProc Natl Acad Sci U S A201110832131471315221788507
- KucharzewskaPChristiansonHCWelchJEExosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor developmentProc Natl Acad Sci U S A2013110187312731723589885
- MagnusND’AstiEGarnierDMeehanBRakJBrain neoplasms and coagulationSemin Thromb Hemost201339888189524108471
- MitroulisIKambasKAnyfantiPDoumasMRitisKThe multivalent activity of the tissue factor-thrombin pathway in thrombotic and non-thrombotic disorders as a target for therapeutic interventionExpert Opin Ther Targets2011151758921062231
- SrivastavaSCancer biomarker discovery and development in gastrointestinal cancers: early detection research network-a collaborative approachGastrointest Cancer Res200714 Suppl 2S60S6319360150
- SawyersCLThe cancer biomarker problemNature2008452718754855218385728
- PrensnerJRChinnaiyanAMSrivastavaSSystematic, evidence-based discovery of biomarkers at the NCIClin Exp Metastasis201229764565222868876
- JørgensenJTA changing landscape for companion diagnosticsExpert Rev Mol Diagn201313766766924063394
- Alix-PanabièresCPantelKCirculating tumor cells: liquid biopsy of cancerClin Chem201359111011823014601
- CrowleyEDi NicolantonioFLoupakisFBardelliALiquid biopsy: monitoring cancer-genetics in the bloodNat Rev Clin Oncol201310847248423836314
- TaylorDDGercel-TaylorCMicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancerGynecol Oncol20081101132118589210
- JansaRSustarVFrankMNumber of microvesicles in peripheral blood and ability of plasma to induce adhesion between phospholipid membranes in 19 patients with gastrointestinal diseasesBlood Cells Mol Dis200841112413218387323
- Galindo-HernandezOVillegas-ComonfortSCandanedoFElevated concentration of microvesicles isolated from peripheral blood in breast cancer patientsArch Med Res201344320821423506723
- BalajLLessardRDaiLTumour microvesicles contain retrotransposon elements and amplified oncogene sequencesNat Commun2011218021285958
- ChenWWBalajLLiauLMBEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesiclesMol Ther Nucleic Acids20132e10923881452
- GanHKCvrljevicANJohnsTGThe epidermal growth factor receptor variant III (EGFRvIII): where wild things are alteredFEBS J2013280215350537023777544
- BatraSKCastelino-PrabhuSWikstrandCJEpidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII geneCell Growth Differ1995610125112598845302
- WikstrandCJReistCJArcherGEZalutskyMRBignerDDThe class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic targetJ Neurovirol1998421481589584952
- SampsonJHHeimbergerABArcherGEImmunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastomaJ Clin Oncol201028314722472920921459
- YoshimotoKDangJZhuSDevelopment of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samplesClin Cancer Res200814248849318223223
- HegiMERajakannuPWellerMEpidermal growth factor receptor: a re-emerging target in glioblastomaCurr Opin Neurol201225677477923007009
- ShaoHChungJBalajLProtein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapyNat Med201218121835184023142818
- SandersonMPKellerSAlonsoARiedleSDempseyPJAltevogtPGeneration of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretionJ Cell Biochem200810361783179717910038
- KimHSChoiDYYunSJProteomic analysis of microvesicles derived from human mesenchymal stem cellsJ Proteome Res201211283984922148876
- ForseenSEPottiAKokaVKochMFraimanGLevittRIdentification and relationship of HER-2/neu overexpression to short-term mortality in primary malignant brain tumorsAnticancer Res20022231599160212168843
- MineoJFBordronAQuintin-RouéIRecombinant humanised anti-HER2/neu antibody (Herceptin) induces cellular death of glioblastomasBr J Cancer20049161195119915328518
- microvesicles.org [homepage on the Internet]Vesiclepedia2013 Available from: microvesicles.orgAccessed December 13, 2013
- LiCCEatonSAYoungPEGlioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cellsRNA Biol20131081333134423807490
- GiustiID’AscenzoSMillimaggiDCathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesiclesNeoplasia200810548148818472965
- FecciPEMitchellDAWhitesidesJFIncreased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant gliomaCancer Res20066663294330216540683
- WaziriAGlioblastoma-derived mechanisms of systemic immunosuppressionNeurosurg Clin N Am2010211314219944964
- WangGJLiuYQinAThymus exosomes-like particles induce regulatory T cellsJ Immunol200818185242524818832678
- PhilipAO’Connor-McCourtMDInteraction of transforming growth factor-beta 1 with alpha 2-macroglobulin. Role in transforming growth factor-beta 1 clearanceJ Biol Chem19912663322290222961718991
- CohenALHolmenSLColmanHIDH1 and IDH2 mutations in gliomasCurr Neurol Neurosci Rep201313534523532369
- XiaoZPrietoDConradsTPVeenstraTDIssaqHJProteomic patterns: their potential for disease diagnosisMol Cell Endocrinol20052301–29510615664456
- YurkovetskyZRLinkovFYE MalehornDLokshinAEMultiple biomarker panels for early detection of ovarian cancerFuture Oncol20062673374117155900
- PilzerDGasserOMoskovichOSchifferliJAFishelsonZEmission of membrane vesicles: roles in complement resistance, immunity and cancerSpringer Semin Immunopathol200527337538716189651
- LlorenteASkotlandTSylvänneTMolecular lipidomics of exosomes released by PC-3 prostate cancer cellsBiochim Biophys Acta2013183171302130924046871
- WeinerMFVegaGLDiaz-ArrastiaRPlasma 24S- hydroxycholesterol and other oxysterols in acute closed head injuryBrain Inj2008227–861161518568715
- DzeletovicSBreuerOLundEDiczfalusyUDetermination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometryAnal Biochem1995225173807778789
- MizoguchiMGuanYYoshimotoKMicroRNAs in human malignant gliomasJ Oncol2012201273287422848219
- ContiAAguennouzMLa TorreDmiR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumorsJ Neurooncol200993332533219159078
- GuptaRWebb-MyersRFlanaganSBucklandMEIsocitrate dehydrogenase mutations in diffuse gliomas: clinical and aetiological implicationsJ Clin Pathol2011641083584421752797
- HermansenSKDahlrotRHNielsenBSHansenSKristensenBWMiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomasJ Neurooncol20131111718123104517
- TanakaYKamoharaHKinoshitaKClinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinomaCancer201311961159116723224754
- AbbottKLPierceJMLectin-based glycoproteomic techniques for the enrichment and identification of potential biomarkersMethods Enzymol201048046147620816222
- RakusJFMahalLKNew technologies for glycomic analysis: toward a systematic understanding of the glycomeAnnu Rev Anal Chem (Palo Alto Calif)2011436739221456971
- BatistaBSEngWSPilobelloKTHendricks-MuñozKDMahalLKIdentification of a conserved glycan signature for microvesiclesJ Proteome Res201110104624463321859146
- GerlachJQKrügerAGalloglySSurface glycosylation profiles of urine extracellular vesiclesPLoS One201389e7480124069349
- WikstrandCJFredmanPSvennerholmLBignerDDDetection of glioma-associated gangliosides GM2, GD2, GD3, 3′-isoLM1 3′,6′-isoLD1 in central nervous system tumors in vitro and in vivo using epitope-defined monoclonal antibodiesProg Brain Res19941012132237518092
- GranerMWEppleLMDustoNLCirculating exosomes as new biomarkers for brain disease and injuryProceedings of SPIE 8723 Sensing Technologies for Global Health, Military Medicine, and Environmental Monitoring IIIMay 29, 2013Baltimore, MD
- HeJLiuYXieXIdentification of cell surface glycoprotein markers for glioblastoma-derived stem-like cells using a lectin microarray and LC-MS/MS approachJ Proteome Res2010952565257220235609
- KurpadSNZhaoXGWikstrandCJBatraSKMcLendonREBignerDDTumor antigens in astrocytic gliomasGlia19951532442568586461
- PoliAWangJDominguesOTargeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survivalOncotarget2013491527154624127551
- GranerMWRaynesDABignerDDGuerrieroVHeat shock protein 70-binding protein 1 is highly expressed in high-grade gliomas, interacts with multiple heat shock protein 70 family members, and specifically binds brain tumor cell surfacesCancer Sci2009100101870187919659607
- Gercel-TaylorCAtaySTullisRHKesimerMTaylorDDNanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patientsAnal Biochem20124281445322691960
- OosthuyzenWSimeNEIvyJRQuantification of human urinary exosomes by nanoparticle tracking analysisJ Physiol2013591Pt 235833584224060994
- KuateSCinatlJDoerrHWUberlaKExosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodiesVirology20073621263717258782
- RountreeRBMandlSJNachtweyJMExosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacyCancer Res201171155235524421670078
- BattkeCRuissRWelschUTumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCCCancer Immunol Immunother201160563964821293856
- BlancLBarresCBette-BobilloPVidalMReticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2Blood200711093407341617666570
- MathivananSSimpsonRJExoCarta: A compendium of exosomal proteins and RNAProteomics20099214997500019810033
- RonquistKGEkBStavreus-EversALarssonARonquistGHuman prostasomes express glycolytic enzymes with capacity for ATP productionAm J Physiol Endocrinol Metab20133046E576E58223341497
- O’BrienKRaniSCorcoranCExosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cellsEur J Cancer20134981845185923453937
- AlcazarOHawkridgeAMCollierTSProteomics characterization of cell membrane blebs in human retinal pigment epithelium cellsMol Cell Proteomics20098102201221119567368
- BiasuttoLChiechiACouchRLiottaLAEspinaVRetinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stressExp Cell Res2013319132113212323669273
- SvenssonKJChristiansonHCWittrupAExosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1J Biol Chem201328824177131772423653359
- GranerMWThe unfolded protein response in glioblastomas: passing the stress testCNS Oncology201326469472 Available from: http://www.futuremedicine.com/toc/cns/2/6Accessed January 10, 2014
- StahlPDBarbieriMAMultivesicular bodies and multivesicular endosomes: the ins and outs of endosomal trafficSci STKE20022002141pe3212122203
- SkrinerKAdolphKJungblutPRBurmesterGRAssociation of citrullinated proteins with synovial exosomesArthritis Rheum200654123809381417133577
- ShenBWuNYangJMGouldSJProtein targeting to exosomes/microvesicles by plasma membrane anchorsJ Biol Chem201128616143831439521300796
