Abstract
Diabetes and its microvascular complications in patients poses a significant challenge and constitutes a major health problem. When it comes to manifestations in the eye, each case of diabetic retinopathy (DR) is unique, in terms of the phenotype, genotype, and, more importantly, the therapeutic response. It is therefore important to identify factors that distinguish one patient from another. Personalized therapy in DR is a new trend aimed at achieving maximum therapeutic response in patients by identifying genotypic and phenotypic factors that may result in less than optimal response to conventional therapy, and consequently, lead to poorer outcome. With advances in the identification of these genetic markers, such as gene polymorphisms and human leucocyte antigen associations, as well as development of drugs that can target their effects, the future of personalized medicine in DR is promising. In this comprehensive review, data from various studies have been analyzed to present what has been achieved in the field of pharmacogenomics thus far. An insight into future research is also provided.
Introduction
Diabetes is a major health problem that is frequently associated with long-term microvascular complications. The number of people suffering from diabetes (approximately 366 million in 2011 worldwide) is expected to reach approximately 552 million by the year 2030. Diabetic retinopathy (DR) and its complications are responsible for legal blindness in as many as 2.6% of the population worldwide, in patients diagnosed with both type I and II diabetes.Citation1,Citation2
There is large variation in the incidence and severity of visual loss and other complications associated with DR. For example, the incidence of vitreous hemorrhage in patients with DR ranges from 17% to 63%.Citation3 Similarly, the prevalence of diabetic macular edema (DME) ranges from 0.85% to 12.3% among patients with diabetes.Citation4 The current standard of care, ie, anti-vascular endothelial growth factor (anti-VEGF) therapy, has shown a significant improvement (≥3 lines visual acuity) in only 44.8% with 0.3 mg ranibizumab in the RISE study,Citation5 while 55.2% patients receiving the same therapy failed to show a similar response. Multiple factors contributing to this variability in response have been studied. It has been proposed that, in addition to environmental factors, genetic makeup of patients may play a role in such variability.
In this review, we explore the developments in the field of pharmacogenomics concerning DR. The analysis may enable clinician-scientists to understand the possible mechanisms behind individual variations in response to standard therapeutic interventions in patients with DR.
Methods
A systematic review of the literature was performed using the United States National Library of Medicine (PubMed), Ovid search engine, and the Medline database to retrieve the literature on the phenotypes and genotypes in DR, genetic basis of patient-to-patient variation in response, and pharmacogenomics. The keywords and MESH headings used were as follows: diabetic retinopathy, genotype, phenotype, polymorphism, linkage, mutation, and responder. Additional articles were also obtained by studying the reference list of these articles. Only articles published in English were included for the review.
In the literature review, both prospective and retrospective studies were included. Studies focusing on patients developing DR as a microvascular complication in both type 1 and type 2 diabetes were included. Studies including various ethnic groups were included in order to ensure comprehensive data analysis. Data from larger clinical trials were also obtained.
Results
Established therapy for diabetic retinopathy
Systemic control of the underlying risk factors, ie, hyperglycemia, hypertension, and hyperlipidemia, has been shown to reduce the risk of progression of DR.Citation6 Local therapies, which include laser photocoagulation, intravitreal or periocular steroids, and anti-VEGF, on the other hand, are mainly used for the management of DME.Citation7 Currently, the use of anti-VEGF agents is gaining popularity and becoming the standard of care for management of DME owing to its favorable outcome.Citation8 Thus, the mainstay of therapy for DR that is manifested by DME, among other complications, is periodic intravitreal injections of anti-VEGF.Citation9 Such therapy is aimed at antagonizing VEGF and its downstream effects.
Among the various pharmacologic agents used, bevacizumab is a humanized murine monoclonal antibody that binds to all isoforms of VEGF-A. Ranibizumab is a smaller molecule with high binding affinity to VEGF-ACitation10 and is approved for treatment of DME by the United States Food and Drug Administration (FDA). Pegaptanib sodium is an aptamer specifically inhibiting the VEGF-A 165 isoform, and aflibercept is a human fusion protein incorporating ligand-binding elements from VEGF receptors and the Fc region of an IgG1 molecule. Aflibercept has been recently approved for the treatment of DME by the FDA. Aflibercept offers similar efficacy as the other anti-VEGF agents with the potential advantage of less frequent dosing.Citation11
In addition to anti-VEGF therapy, intravitreal and periocular injections of corticosteroids and laser photocoagulation have been used to treat DR as well.
Concerns with established therapy for diabetic retinopathy
Clinical trials have demonstrated marked variations in response to anti-VEGF therapy in DME. For instance, with 0.3 mg ranibizumab, 44.8% of patients gained >3 lines on the EDTRS chart at 24 months, with the rest of the study subjects showing less than optimal response in the RISE study, whereas 34% patients receiving 0.3 mg ranibizumab demonstrated an improvement of >3 lines on ETDRS chart in the RIDE study.Citation5 In both trials, there were few patients that showed loss of >15 letters, ie, six out of 250 patients in RISE and seven out of 252 patients in RIDE study. Similarly, trials have also documented the efficacy of bevacizumab in patients with DME. The bevacizumab or laser therapy (BOLT) study demonstrated that, in patients receiving intravitreal injections of bevacizumab, there was >3 EDTRS lines improvement in visual acuity in 39% of patients.Citation12 In the DA VINCI study, 34% of the patients showed a significant level of visual acuity improvement following therapy with aflibercept.Citation13 Variation of treatment response with anti-VEGF therapy has also been demonstrated in other retinal diseases such as age-related macular degeneration. In the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT), 34.2% and 31.3% patients showed an improvement of >15 letters following treatment with monthly ranibizumab and bevacizumab, respectively.Citation14
The results of these major clinical trials indicate that, among patients with DR and DME, there are asymmetric responses. There is large variation in the clinical response, and many patients do not demonstrate satisfactory levels of visual acuity improvement ().
Table 1 Visual outcome analysis in patients with diabetic retinopathy enrolled in various clinical trials.
Phenotype variation in diabetic retinopathy
Available data suggest that phenotypic variation occurs in patients with DR. Multimodal imaging has demonstrated that some patients may present with certain features on biomicroscopy and indirect ophthalmoscopy, such as rates of microaneurysm accumulation and foveal avascular zone alterations that may be associated with worse visual prognosis.Citation21 In this 3-year follow-up study performed on 14 eyes with type 2 diabetes, the level of DR was graded by the Wisconsin Card-Sorting Test. Areas of abnormally increased hyperfluorescence were analyzed at baseline and follow-up visits after stabilization of mean HbA1c levels. Based on the intensity and persistence of the leakage sites, a genetic basis for this phenotypic variation in DR was suggested by the authors.
Studies with larger cohorts were subsequently performed to validate the results of the previous study.Citation22,Citation23 A prospective studyCitation22 including 410 patients with type 2 diabetes was conducted to follow up the patients for 2 years or until the time they developed macular edema. The results of this study demonstrated the presence of three distinct phenotypes of DR. Phenotype A was identified with lowest microaneurysm turnover and central subfoveal thickness. Phenotype B was identified with higher central subfield retinal thickness, whereas phenotype C had higher microaneurysms turnover but variable macular thickness. A study involving patients with type 1 diabetes suggested that the genetic makeup may affect the breakdown of the blood–retinal barrier or pericyte apoptosis, resulting in different phenotypes of DR.Citation24
As an example, shows the differences in progression of DR in two patients. In the first patient (panels A and B), there is an increased turnover of microaneurysms over time, whereas the second patient (panels C and D) has an increased leakage on fluorescein angiography and thickness over a period of 3 months without much microaneurysm turnover.
Figure 1 Characteristic differences in the phenotype of diabetic retinopathy (DR) in two patients.
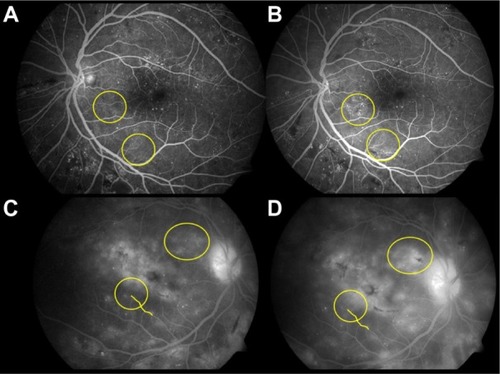
The FIND-Eye studyCitation25 in 2008 suggested increased severity of DR in individuals predisposed genetically to more microvascular complications, including diabetic nephropathy. A number of ethnic groups were included in this study, such as American Indians, European Americans, African Americans, and Mexican Americans. Hereditability of the severity of retinopathy was assessed by sibling analysis. Another study that retrospectively reviewed charts of both Black and Latino diabetic patients demonstrated that Latinos may be at a greater risk for a specific retinopathy phenotype characterized by extravasation of intraretinal hemorrhages and poorer prognosis.Citation26 Conclusive data that identify specific complication risks for each ethnic group are not available.
Morphometric analysis of eyes of patients with DR have shown that certain features on spectral domain optical coherence tomography imaging, such as disruption of photoreceptor inner segment–outer segment junction, can significantly decrease retinal sensitivity. Further studies are required to correlate genetic basis of this phenotype observation.Citation27
There is increasing evidence for the genetic basis for phenotypic variations in patients with DR.Citation28 Differences in phenotypes may result in increased risk of progression of the disease and visual loss due to higher levels of ischemia and VEGF release.Citation16,Citation29 With advancement in clinical examination techniques and imaging modalities, it may be possible to identify more phenotypic variations in DR. Early identification of such a cohort of patients may enable clinicians to prepare for more aggressive therapy in order to salvage vision.
Genotype variation in diabetic retinopathy
Data from a number of studies suggest the presence of various genetic polymorphisms in susceptibility to DR. There are a number of studies that provide evidence to the presence of various pathogenetic factors, including activity of aldose reductase (AR) and protein kinase C as well as processes such as glycation, platelet adhesion, and aggregation.
Genetic basis of morphological variation in diabetic retinopathy
Results from twin studiesCitation30 and various clinical trials such as the Diabetic Control and Complications Trial (DCCT) suggest genetic polymorphisms that may play an important role in manifestations of DR, such as familial clustering of proliferative DR.Citation31 It has been shown that only about half the number of patients diagnosed with nonproliferative DR progress to the proliferative disease.Citation30,Citation31 Further investigations into the genetic factors that influence the manifestations of retinopathy have revealed human leucocyte antigen (HLA) associations and single nucleotide polymorphisms (SNPs), which may contribute to the initiation or promotion of inflammatory cascade.Citation32
Genome-wide association studies
One of the strategies to identify genetic predisposition to severe DR is genome-wide association studies (GWAS). Using this data, millions of common polymorphisms can be identified in the human genome. Following the successful GWAS for age-related macular degeneration, similar studies have been performed for various populations for DR.
Two large cohorts including patients with type 1 diabetes, the Epidemiology of Diabetes Intervention and Control Trial and the Genetics of Kidney in Diabetes studies, did not find any genome-wide association except a relationship between severe DR and the SNP rs476141.Citation32 This SNP is located between the AKT3 and ZNF238 genes that may play a role in cell survival, insulin signaling, and angiogenesis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) found a new potential SNP rs4865047 located in the CEP125 gene associated with severe DR.Citation33
In the Taiwanese population, five loci have been identified in patients who are susceptible for the development of DR. These loci include PLXDC2 and ARHGAP22, which play a role in capillary endothelial proliferation and permeability.Citation34 In a study with Mexican-American population, 32 SNPs were identified from 11 regions in patients with DR with modest association. These SNPs belonged to loci from genes that are usually not associated with DR.Citation35
A large collaborative study with European-American population, the Candidate-gene Association Resource (CARe), comprised more than 2,500 subjects with type 2 diabetes. In this study, polymorphisms associated with DR identified included certain SNPs in the P-selectin (SELP) gene. These polymorphisms were associated with increased severity of DR. However, the results of this study were not replicable in studies involving other ethnic and racial groups.Citation33 Thus, polymorphisms involving the selectin genes have not conclusively been shown to be associated with severe DR.
More studies that can validate data and include larger cohorts have been possible because of rapid advances in technology and means to identify genetic variations. However, presently, GWAS have various limitations. These include replicability of data due to ethnic variations in polymorphisms. The commercial arrays can identify only rare, low-frequency variants. Larger studies with different ethnic groups may be required to provide stronger statistical support for confirming association.Citation32
Linkage studies and HLA association
Linkage analysis helps in the identification of alleles that may predispose to the development of more aggressive form of DR. Linkage analysis was carried out in Pima Indians to identify susceptibility genes for DR.Citation36 In this study, a region close to angiotensin II receptor gene AGTR1 located on chromosome 3 was identified to be associated with retinopathy. Familial aggregation of severe DR was linked to chromosome 1 in another study.Citation37 However, further studies are required to conclusively establish any association.
Several studies have also attempted to identify associations between HLA and severity of DR. Literature supports the association between HLA DR4 and DR3 phenotypes to be associated with proliferative DR. The odds of developing proliferative retinopathy among individuals with HLA-DR 3/0 are 3.74 times the control arm, with the true population effect being 1.8–7.8 times.Citation38,Citation39
Candidate gene studies
Candidate genes encoding proteins that may play an important role in the pathogenesis of DR have been studied extensively in order to identify their polymorphisms as potential pharmacogenetic markers. An attempt to identify these markers in DR in individuals with diabetes has been made and involved various population subgroups. Certain polymorphisms belong to pathways that are distinct from VEGF.
Apart from VEGF, certain candidate genes such as aldose reductase (AR),Citation40 endothelial nitric oxide synthase (eNOS),Citation41 receptor for advanced glycation end products (RAGE),Citation42 and erythropoietin (EPO)Citation43 genes have been studied extensively in various ethnic groups. Polymorphisms in these candidate genes can potentially affect various metabolic pathways, thereby increasing the risk of severe DR. For example, mutations in the methylenetetrahydrofolate reductase (MTHFR) gene are associated with high plasma homocysteine levels,Citation44,Citation45 increasing the risk of proliferative DR.Citation46–Citation48 There is, however, no clear consensus on the exact role of various candidate gene polymorphisms, and further research in this direction is warranted.
lists the important potential pharmacogenetic markers of severe DR based on evidences from various population case–control trials. Data from meta-analysis have also been included, where available. With more research, our knowledge of genetic markers is going to expand.
Table 2 Possible pharmacogenetic markers for diabetic retinopathy
VEGF pathway and VEGF gene polymorphisms
VEGF expression plays a central role in the pathogenesis of DR. The upregulation of VEGF in the eyes of patients with diabetes is associated with breakdown of the blood–retinal barrier and increased vascular permeability.Citation68 This leads to clinical manifestations of retinopathy. Higher levels of VEGF in the vitreous are correlated with increased severity of macular edema and DR.Citation69,Citation70
SNPs in the VEGF gene and its polymorphisms are of particular interest because therapy of DR largely focuses on anti-VEGF drugs.
The VEGF gene is located on chromosome 6p21.3 and is highly polymorphic as demonstrated in previous studies.Citation71–Citation73 Of particular interest is the VEGF gene C-634G polymorphism rs2010963 in the 5-untranslated region. Studies including Japanese and Slovenian populations suggest strong association of this polymorphism with development of macular edema.Citation70,Citation74,Citation75 Recent study in an Egyptian cohort of 392 patients demonstrated that the CC genotype of C-634G polymorphism resulted in a significant risk for developing macular edema independently of the grade of DR.Citation76 The −634G to C substitution enhances VEGF expression.Citation77
Many SNPs in the VEGF gene have been identified to be associated with the development of DR. Studies that have tested the role of VEGF gene polymorphisms in patients with DR conclude that patients with the CC genotype have a higher level of VEGF in serum comparing the CG and the GG genotypes. The CC genotype is associated with more favorable response to anti-VEGF therapy, 76% compared to the CG (23%) and GG genotype (0.0%). The GG phenotype has been found to be an independent predictor of neovascularization and development of proliferative DR.Citation78
A recent study found an increased association of DR with the CA genotype of the -2578 polymorphism.Citation79 This polymorphism is also located in the promoter region. A meta-analysis including a number of case–control studies suggested an association between the −2578C/A polymorphism and DR in various ethnic groups except the Caucasian population.Citation80 Another meta-analysis studying the association of the VEGF gene polymorphisms with DR found that retinopathy is associated with the VEGF gene -460T/C polymorphism but not the −2578C/A polymorphism.Citation81 SNPs in the splicing region have also shown to be associated with DR. These include the SRp55 2994 polymorphism that controls alternative splicing of VEGF pre-RNA exon. Such properties may affect the balance between pro- and antiangiogenic VEGF isoforms.Citation82
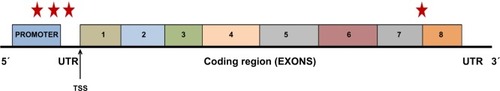
The VEGF-A gene structure and its associated major polymorphisms associated with DR are summarized in . There is, indeed, a need for additional studies in confirming the role of these polymorphisms in patients with DR and DME. The aim of the studies, which should evaluate genetic polymorphisms and mutations involving a variety of loci on the human genome, is to be able to comprehensively identify all the potential pharmacogenomics markers for poor prognosis in DR. While pharmacogenomic markers have been identified to a great extent in ocular diseases such as age-related macular degeneration and glaucoma, information on DR is not very definitive. summarizes a list of potential pharmacogenomic markers that have been identified, but we still need further evidences before they can be clinically applicable.
Figure 2 Structure and functional anatomy of the vascular endothelial growth factor-A (VEGF-A) gene.
Genetic basis of response to treatment
With the knowledge that there are a number of genetic polymorphisms associated with the development of DR, it may be plausible that these polymorphisms could be responsible, at least partly, for variable response to anti-VEGF agents. Higher levels of VEGF associated with certain genetic compositions and polymorphisms in the VEGF gene itself may indicate that, in order to achieve clinical benefit, either improvisation in the dosing regimen or combination therapy (of different targets) may be essential.
There is very little information available regarding the efficacy of various anti-VEGF agents used to treat DME based on the genetic profile of patients. On the other hand, there have been studies that evaluated such questions for AMD.Citation83–Citation85 The large multicenter CATT study evaluated the response of anti-VEGF treatment in 835 patients based on VEGF gene polymorphisms.Citation86 The study could not conclusively identify any pharmacogenetic associations between gene SNPs and response to different anti-VEGF therapies. Current literature does not provide adequate information of pharmacogenetic associations in patients with DR.
On the other hand, it has been identified that there may be signaling pathways and abnormal gene expressions in patients with DR that are nonresponsive to therapy. In a study by Dabir et al,Citation87 a difference in the gene expression was found between responders and nonresponders to treatment. More than 60 genes were upregulated and approximately 50 downregulated in patients with DR who were nonresponsive to therapy. Identifying the specific signaling pathway involved in DR at different stages of retinopathy and in response to therapy may provide insights that can help to solve the puzzle between responders and nonresponders.
Responders to treatment showed dramatic decrease in the extracellular matrix receptor gene and notable decrease in the transforming growth factor beta (TGF-β), while no changes were observed in these genes in the nonresponder group. Nonresponders, on the other hand, demonstrated increased expression of cellular adhesion molecules, WNT signaling pathway (which is implicated in diabetic complications such as vascular leakage and retinal neovascularization), several transcription factors, as well as stress-related genes.
In addition to signaling pathways, patients with DR are known to have altered and mitigated genetic expression of antioxidant enzymes.Citation88 There are a variety of microRNAs (miRNAs) that are implicated in a variety of pathophysiological processes responsible for changes of DR. miRNAs control posttranscriptional gene expression and are important potential mediator and biomarker of diabetic complications and response to conventional therapy.Citation89,Citation90
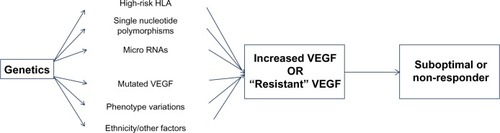
Thus, the benefit in the response to antiangiogenic therapy may be limited due to redundancy of the VEGF target, thereby making the VEGF “resistant” to a particular therapeutic agent.Citation91 The summary of the pharmacogenomic basis for nonresponse or suboptimal response in certain cohorts of patients is elucidated in .
Figure 3 Summary of the most important pathways that may be responsible for variability of response from patient to patient in diabetic retinopathy (DR).
Personalized medicine in diabetic retinopathy
With advances in prospective, genetic, diagnostic, and therapeutic strategies for managing patients with DR, personalized treatment of patients with DR may soon be coming to the clinics. It may be possible to intervene early in patients identified to be at high genetic or phenotypic risk of progression, or in those who have a high risk of nonresponse to available therapies.Citation92
Due to the enormous amount of financial constraints and health care cost concerns, research is now focusing on individualizing health care strategies based on extensive data available from studies.Citation93 Through recognition of genomic biomarkers to identify patients at risk who may be incomplete responders or may not respond at all, innovative and early treatment options may be tailored for better outcomes.Citation94 The ever-increasing burden of diabetes and its microvascular complications makes it very relevant to introduce the concept of personalized health care for this disease. Prevention of difficult-to-treat entities such as macular edema, iris and retinal neovascularization, and high-risk proliferative DR may become possible by early characterization of genomic features in every individual.
Personalized medicine in DR must also focus on individualization of glycemic target control, HbA1c levels, blood pressure, and lipid-lowering strategies. There has been only modest progress in the pharmacogenomics of glucose-lowering medications.Citation90 Largely, the treatment of diabetes thus far remains empirical.Citation95
Because of the wide variation in the clinical presentation, progression, and complications of DR, certainly a tailored therapeutic approach may be more effective. Personalized medicine can be also helpful in DR prediction and prevention. With advances in the identification of potential genetic associations of high-risk genes such as the 1704T allele (RAGE gene)Citation96 and MTHFR 677C/T polymorphisms,Citation97–Citation99 data from proteomics and genomics may soon become relevant in clinical practice.Citation100
Clinical developments in treatment strategies based on pharmacogenomics
The availability of information on various genetic influences on the incidence and severity of DR has enabled newer treatment options targeting molecular pathways to be on the horizon. Studies have been initiated in order to target alternate pathways, such as the insulin-like growth factor pathway and tyrosine kinase (TIE-2) pathway, which may be overactive in certain sets of patients based on their genetic composition.Citation101,Citation102
RNA interference (RNAi) technology has been used to potentially identify newer therapeutic targets for DR.Citation103 RNAi allows silencing of practically any gene that may be overactive and responsible for phenotype manifestations resulting in disease. Such properties provide a promising strategy to treat diseases such as DR or DME.
Gene therapy for DR is still in its infancy. Animal studies have been initiated to deliver targeted molecules using adeno-associated virus to improve manifestations of DR with success.Citation104,Citation105 There have been no definitive data to support full clinical usage.
Future of pharmacogenomics and personalized medicine in diabetic retinopathy
The era of pharmacogenomics will undoubtedly allow tailoring treatment based on the individual’s genetic profile. Such a critical step will help to select the best therapy for the patient that will aid achieving the best visual outcome at the least possible cost. Future research strategies focusing on the identification of more phenotypic variations in DR based on clinical characteristics of the disease are certainly very relevant. In addition, consolidation of knowledge on genomic biomarkers of DR associated with suboptimal treatment response is necessary. It is imperative for clinical trials focusing on DR to analyze the possible reasons behind incomplete response or no response.
There have been advances in pharmacogenomics in other ocular diseases such as AMD and glaucoma. Therefore, further knowledge on the polymorphisms of the VEGF gene in DR and development of newer molecules to target the “resistant” allele may help improve visual outcomes in a larger number of patients. The level of evidence from various studies and practice guidelines will continue to evolve as more research is conducted in the field of pharmacogenomics.
Disclosure
Quan Dong Nguyen and Diana V. Do serve on the Scientific Advisory Boards for Genentech, Inc. and Regeneron, Inc. The authors report no other conflicts of interest in this work.
References
- International Diabetes FederationIDF Diabetes Atlas5th ed2011 Available from: http://www.idf.org/diabetesatlas
- BourneRRStevensGAWhiteRAVision Loss Expert GroupCauses of vision loss worldwide, 1990–2010: a systematic analysisLancet Glob Health201316e339e34925104599
- AhmadiehHShoeibiNEntezariMMonshizadehRIntravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trialOphthalmology2009116101943194819699531
- ChenELoomanMLaouriMBurden of illness of diabetic macular edema: literature reviewCurr Med Res Opin20102671587159720429823
- NguyenQDBrownDMMarcusDMRISE and RIDE Research GroupRanibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDEOphthalmology2012119478980122330964
- AbouammohMARanibizumab injection for diabetic macular edema: meta-analysis of systemic safety and systematic reviewCan J Ophthalmol201348431732323931473
- BandelloFCasalinoGLoewensteinAGoldsteinMPelayesDBattaglia ParodiMPharmacological approach to diabetic macular edemaOphthalmic Res2014512889524356667
- BoyerDSHopkinsJJSorofJEhrlichJSAnti-vascular endothelial growth factor therapy for diabetic macular edemaTher Adv Endocrinol Metab20134615116924324855
- PatelliFRadicePGiacomottiEDiabetic macular edemaDev Ophthalmol20145416417325196766
- YangJWangXFuhGComparison of binding characteristics and in vitro activities of three inhibitors of vascular endothelial growth factor AMol Pharm201411103421343025162961
- StewartMWRosenfeldPJPredicted biological activity of intravitreal VEGF TrapBr J Ophthalmol200892566766818356264
- RajendramRFraser-BellSKainesAA 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3Arch Ophthalmol2012130897297922491395
- DoDVSchmidt-ErfurthUGonzalezVHThe DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edemaOphthalmology201111891819182621546089
- MartinDFMaguireMGYingGSGrunwaldJEFineSLJaffeGJRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- KorobelnikJFDoDVSchmidt-ErfurthUIntravitreal aflibercept for diabetic macular edemaOphthalmology2014
- NguyenQDShahSMKhwajaAAREAD-2 Study GroupTwo-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) studyOphthalmology2010117112146215120855114
- MassinPBandelloFGarwegJGSafety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II studyDiabetes Care201033112399240520980427
- MitchellPBandelloFSchmidt-ErfurthURESTORE Study GroupThe RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edemaOphthalmology2011118461562521459215
- CampochiaroPABrownDMPearsonAFAME Study GroupSustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edemaOphthalmology2012119102125213222727177
- BoyerDSYoonYHBelfortRJrOzurdex MEAD Study GroupThree-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edemaOphthalmology2014121101904191424907062
- LoboCLBernardesRCFigueiraJPde AbreuJRCunha-VazJGThree-year follow-up study of blood-retinal barrier and retinal thickness alterations in patients with type 2 diabetes mellitus and mild nonproliferative diabetic retinopathyArch Ophthalmol2004122221121714769598
- NunesSRibeiroLLoboCCunha-VazJThree different phenotypes of mild nonproliferative diabetic retinopathy with different risks for development of clinically significant macular edemaInvest Ophthalmol Vis Sci20135474595460423745006
- Cunha-VazJRibeiroLLoboCPhenotypes and biomarkers of diabetic retinopathyProg Retin Eye Res2014419011124680929
- JešićMSajićSJešićMMicroalbuminuria in relation to metabolic control and blood pressure in adolescents with type 1 diabetesArchives of medical science2011761037104122328888
- ArarNHFreedmanBIAdlerSGFamily Investigation of Nephsropathy and Diabetes Research GroupHeritability of the severity of diabetic retinopathy: the FIND-Eye studyInvest Ophthalmol Vis Sci20084993839384518765632
- ChenJLLuvianoDMChenJCYuFSarrafDComparison of diabetic retinopathy phenotype between Latinos and BlacksJ Diabetes Complications200923637137518599323
- YohannanJBittencourtMSepahYJAssociation of retinal sensitivity to integrity of photoreceptor inner/outer segment junction in patients with diabetic macular edemaOphthalmology201312061254126123499060
- Cunha-VazJPhenotypes and biomarkers of diabetic retinopathy. Personalized medicine for diabetic retinopathy: the Weisenfeld awardInvest Ophthalmol Vis Sci20145585412541925169433
- BaiYMaJXGuoJMuller cell-derived VEGF is a significant contributor to retinal neovascularizationJ Pathol2009219444645419768732
- HietalaKForsblomCSummanenPGroopPHHeritability of proliferative diabetic retinopathyDiabetes20085782176218018443200
- Clustering of long-term complications in families with diabetes in the diabetes control and complications trialThe Diabetes Control and Complications Trial Research GroupDiabetes19974611182918399356033
- Simo-ServatOHernandezCSimoRGenetics in diabetic retinopathy: current concepts and new insightsCurr Genomics201314528929924403848
- GrassiMATikhomirovARamalingamSReplication analysis for severe diabetic retinopathyInvest Ophthalmol Vis Sci20125342377238122427569
- HuangYCLinJMLinHJGenome-wide association study of diabetic retinopathy in a Taiwanese populationOphthalmology2011118464264821310492
- FuYPHallmanDMGonzalezVHIdentification of diabetic retinopathy genes through a genome-wide association study among Mexican-Americans from Starr County, TexasJ Ophthalmol20102010ii:861291
- ImperatoreGHansonRLPettittDJKobesSBennettPHKnowlerWCSib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes GroupDiabetes19984758218309588456
- LookerHCNelsonRGChewEGenome-wide linkage analyses to identify Loci for diabetic retinopathyDiabetes20075641160116617395753
- RandLIKrolewskiASAielloLMWarramJHBakerRSMakiTMultiple factors in the prediction of risk of proliferative diabetic retinopathyN Engl J Med198531323143314383864010
- CruickshanksKJVadheimCMMossSEGenetic marker associations with proliferative retinopathy in persons diagnosed with diabetes before 30 yr of ageDiabetes19924178798851612203
- AbharySHewittAWBurdonKPCraigJEA systematic meta-analysis of genetic association studies for diabetic retinopathyDiabetes20095892137214719587357
- ChiuCMossCFThe role of the external ear in vertical sound localization in the free flying bat, Eptesicus fuscusJ Acoust Soc Am200712142227223517471736
- HudsonBISticklandMHFutersTSGrantPJEffects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathyDiabetes20015061505151111375354
- TongZYangZPatelSGenetics of Diabetes and Diabetic Complication Study GroupPromoter polymorphism of the erythropoi-etin gene in severe diabetic eye and kidney complicationsProc Natl Acad Sci U S A2008105196998700318458324
- ChoSEHongKSShinGJChungWSThe methylenetetrahydrofolate reductase C677T gene mutation is associated with hyperhomocysteinemia, cardiovascular disease and plasma B-type natriuretic peptide levels in KoreaClinical Chemistry and Laboratory Medicine20064491070107516958597
- NakaiKItohCNakaiKHabanoWGurwitzDCorrelation between C677T MTHFR gene polymorphism, plasma homocysteine levels and the incidence of CADAm J Cardiovasc Drugs20011535336114728017
- MalaguarneraGGaglianoCGiordanoMHomocysteine serum levels in diabetic patients with non proliferative, proliferative and without retinopathyBiomed Res Int2014201419149724877066
- AydemirOTürkçüoğluPGülerMPlasma and vitreous homocysteine concentrations in patients with proliferative diabetic retinopathyRetina200828574174318463519
- GoldsteinMLeibovitchIYeffimovIGavendoSSelaBALoewensteinAHyperhomocysteinemia in patients with diabetes mellitus with and without diabetic retinopathyEye200418546046515131674
- LiangSPanMHuNAssociation of angiotensin-converting enzyme gene 2350 G/A polymorphism with diabetic retinopathy in Chinese Han populationMol Biol Rep201340146346823065222
- ManeaSARobciucAGujaCHeltianuCIdentification of gene variants in NOS3, ET-1 and RAS that confer risk and protection against microangiopathy in type 2 diabetic obese subjectsBiochem Biophys Res Commun2011407348649021406182
- dos SantosKGCananiLHGrossJLTschiedelBSoutoKERoisenbergIThe -106CC genotype of the aldose reductase gene is associated with an increased risk of proliferative diabetic retinopathy in Caucasian-Brazilians with type 2 diabetesMol Genet Metab200688328028416545977
- WangJYangMMLiYBLiuGDTengYLiuXMAssociation of CFH and CFB gene polymorphisms with retinopathy in type 2 diabetic patientsMediators Inflamm2013201374843523864767
- ChenMLinWRLuCHChimerin 2 genetic polymorphisms are associated with non-proliferative diabetic retinopathy in Taiwanese type 2 diabetic patientsJ Diabetes Complications201428446046324854763
- ArndtCLeclercqINazeyrollasPAssociation of endothelial lipase Thr111Ile polymorphism with proliferative retinopathy in type 2 diabetes patientsDiabetes and Metabolism2014
- TavernaMJSolaAGuyot-ArgentonCeNOS4 polymorphism of the endothelial nitric oxide synthase predicts risk for severe diabetic retinopathyDiabet Med200219324024511918626
- AbharySBurdonKPCassonRJGogginMPetrovskyNPCraigJEAssociation between erythropoietin gene polymorphisms and diabetic retinopathyArch Ophthalmol2010128110210620065225
- GongJYDengDTSunYHAssociation of platelet glycoprotein receptor alpha2beta1 integrin and glycoprotein IIIa gene polymorphisms with diabetic retinopathy: evidence from 3007 subjectsCurr Eye Res2014301824979111
- KatakamiNMatsuhisaMKanetoHMonocyte chemoattractant protein-1 (MCP-1) gene polymorphism as a potential risk factor for diabetic retinopathy in Japanese patients with type 2 diabetesDiabetes Res Clin Pract2010891e9e1220488574
- YigitSKarakusNInanirAAssociation of MTHFR gene C677T mutation with diabetic peripheral neuropathy and diabetic retinopathyMol Vis2013191626163023901246
- Mankoc RamusSKumseTGlobocnik PetrovicMPetrovicDCilensekISNP rs2073618 of the osteoprotegerin gene is associated with diabetic retinopathy in Slovenian patients with type 2 diabetesBiomed Res Int2013201336407324228244
- NgZXKuppusamyURIqbalTChuaKHReceptor for advanced glycation end-product (RAGE) gene polymorphism 2245G/A is associated with pro-inflammatory, oxidative-glycation markers and sRAGE in diabetic retinopathyGene2013521222723323545311
- ZhangHMChenLLWangLAssociation of 1704G/T and G82S polymorphisms in the receptor for advanced glycation end products gene with diabetic retinopathy in Chinese populationJ Endocrinol Invest200932325826219542745
- SobrinLGreenTSimXFamily Investigation of Nephropathy and Diabetes-Eye Research Group, Wellcome Trust Case Control Consortium 2Candidate gene association study for diabetic retinopathy in persons with type 2 diabetes: the Candidate gene Association Resource (CARe)Invest Ophthalmol Vis Sci201152107593760221873659
- LuoJZhaoLChenAYTCF7L2 variation and proliferative diabetic retinopathyDiabetes20136272613261723434931
- BrondaniLAde SouzaBMDuarteGCThe UCP1 -3826A/G polymorphism is associated with diabetic retinopathy and increased UCP1 and MnSOD2 gene expression in human retinaInvest Ophthalmol Vis Sci201253127449745723033381
- CrispimDFagundesNJdos SantosKGPolymorphisms of the UCP2 gene are associated with proliferative diabetic retinopathy in patients with diabetes mellitusClin Endocrinol2010725612619
- HanLZhangLXingWThe associations between VEGF gene polymorphisms and diabetic retinopathy susceptibility: a meta-analysis of 11 case-control studiesJ Diabetes Res2014201480580124868559
- IshidaSUsuiTYamashiroKVEGF164 is proinflammatory in the diabetic retinaInvest Ophthalmol Vis Sci20034452155216212714656
- FunatsuHYamashitaHSakataKVitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edemaOphthalmology2005112580681615878060
- PetrovicMGKorosecPKosnikMLocal and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathyMol Vis2008141382138718682813
- VincentiVCassanoCRocchiMPersicoGAssignment of the vascular endothelial growth factor gene to human chromosome 6p21.3Circulation1996938149314958608615
- BroganIJKhanNIsaacKHutchinsonJAPravicaVHutchinsonIVNovel polymorphisms in the promoter and 5’ UTR regions of the human vascular endothelial growth factor geneHum Immunol199960121245124910626738
- StevensASodenJBrenchleyPERalphSRayDWHaplotype analysis of the polymorphic human vascular endothelial growth factor gene promoterCancer Res200363481281612591731
- WatsonCJWebbNJBottomleyMJBrenchleyPEIdentification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein productionCytokine20001281232123510930302
- AwataTKuriharaSTakataNFunctional VEGF C-634G polymorphism is associated with development of diabetic macular edema and correlated with macular retinal thickness in type 2 diabetesBiochem Biophys Res Commun2005333367968515963467
- El-ShazlySFEl-BradeyMHTameeshMKVascular endothelial growth factor gene polymorphism prevalence in patients with diabetic macular oedema and its correlation with anti-vascular endothelial growth factor treatment outcomesClin Experiment Ophthalmol201442436937823927080
- HuezIBornesSBressonDCreancierLPratsHNew vascular endothelial growth factor isoform generated by internal ribosome entry site-driven CUG translation initiationMol Endocrinol200115122197221011731620
- FeghhiMNikzamirAEsteghamatiAMahmoudiTYekaninejadMSRelationship of vascular endothelial growth factor (VEGF) +405 G/C polymorphism and proliferative retinopathy in patients with type 2 diabetesTransl Res20111582859121757152
- BledaSDe HaroJVarelaCEsparzaLFerrueloAAcinFVascular endothelial growth factor polymorphisms are involved in the late vascular complications in Type II diabetic patientsDiab Vasc Dis Res201291687422064697
- WangHChengJWZhuLSMeta-analysis of association between the -2578C/A polymorphism of the vascular endothelial growth factor and retinopathy in type 2 diabetes in Asians and CaucasiansOphthalmic Res20145211824751925
- GongJYSunYHAssociation of VEGF gene polymorphisms with diabetic retinopathy: a meta-analysisPLoS One2013812e8406924376787
- CarterJGCherryJWilliamsKTurnerSBatesDOChurchillAJSplicing factor polymorphisms, the control of VEGF isoforms and association with angiogenic eye diseaseCurr Eye Res201136432833521309690
- VelosoCEde AlmeidaLNRecchiaFMPelayesDNehemyMBVEGF gene polymorphism and response to intravitreal ranibizumab in neovascular age-related macular degenerationOphthalmic Res20145111824157918
- LoteryAJGibsonJCreeAJAlternative Treatments to Inhibit VEGF in Patients with Age-Related Choroidal Neovascularisation (IVAN) Study GroupPharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age-related macular degeneration in the IVAN StudyOphthalmology2013120122637264324070809
- AbediFWickremasingheSRichardsonAJVariants in the VEGFA gene and treatment outcome after anti-VEGF treatment for neovascular age-related macular degenerationOphthalmology2013120111512123149126
- HagstromSAYingGSPauerGJComparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research GroupVEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy: comparison of age-related macular degeneration treatments trials (CATT)JAMA Ophthalmol2014132552152724652518
- DabirSSDasDNallathambiJMangaleshSYadavNKSchoutenJSDifferential systemic gene expression profile in patients with diabetic macular edema: responders versus nonresponders to standard treatmentIndian J Ophthalmol2014621667324492504
- El-BabMFZakiNSMojaddidiMAAl-BarryMEl-BeshbishyHADiabetic retinopathy is associated with oxidative stress and mitigation of gene expression of antioxidant enzymesInt J Gen Med2013679980624092995
- MastropasquaRTotoLCipolloneFSantovitoDCarpinetoPMastropasquaLRole of microRNAs in the modulation of diabetic retinopathyProg Retin Eye Res201443C9210725128741
- KatoMCastroNENatarajanRMicroRNAs: potential mediators and biomarkers of diabetic complicationsFree Radic Biol Med201364859423770198
- JainRKDudaDGWillettCGBiomarkers of response and resistance to antiangiogenic therapyNat Rev Clin Oncol20096632733819483739
- SnydermanRWilliamsRSProspective medicine: the next health care transformationAcad Med200378111079108414604864
- ChawlaNVDavisDABringing big data to personalized healthcare: a patient-centered frameworkJ Gen Intern Med201328Suppl 3S660S66523797912
- ChenJJLinWJChenHCPharmacogenomic biomarkers for personalized medicinePharmacogenomics201314896998023746190
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Diabetes Care20123561364137922517736
- NiuWQiYWuZLiuYZhuDJinWA meta-analysis of receptor for advanced glycation end products gene: four well-evaluated polymorphisms with diabetes mellitusMol Cell Endocrinol2012358191722402134
- NiuWQiYAn updated meta-analysis of methylenetetrahydrofolate reductase gene 677C/T polymorphism with diabetic nephropathy and diabetic retinopathyDiabetes Res Clin Pract201295111011822056717
- MaedaMYamamotoIFukudaMMTHFR gene polymorphism is susceptible to diabetic retinopathy but not to diabetic nephropathy in Japanese type 2 diabetic patientsJ Diabetes Complications200822211912518280442
- MaedaMYamamotoIFukudaMMTHFR gene polymorphism as a risk factor for diabetic retinopathy in type 2 diabetic patients without serum creatinine elevationDiabetes Care200326254754812547903
- GlauberHSRisheNKarnieliEIntroduction to personalized medicine in diabetes mellitusRambam Maimonides Med J201451e000224498509
- Aerpio TherapeuticsThe TIME-2 Study: A Phase 2 Study of AKB-9778, A Novel Tie-2 Activator, in Patients with Diabetic Macular Edema2014 Available from: https://clinicaltrials.gov/ct2/show/NCT02050828
- River Vision Development CorporationA Phase 1, Open-Label Study of Teprotumumab in Patients with Diabetic Macular Edema (DME)2014
- Guzman-AranguezALomaPPintorJSmall-interfering RNAs (siRNAs) as a promising tool for ocular therapyBr J Pharmacol2013170473074723937539
- VaccaODarcheMSchafferDVAAV-mediated gene delivery in Dp71-null mouse model with compromised barriersGlia201462346847624382652
- AdhiMCashmanSMKumar-SinghRAdeno-associated virus mediated delivery of a non-membrane targeted human soluble CD59 attenuates some aspects of diabetic retinopathy in micePLoS One2013810e7966124167638
