Abstract
Background
Despite use of a lower mycophenolate dose in Thai kidney transplant patients, acceptable graft and patient outcomes can be achieved. We therefore examined the pharmacokinetics of mycophenolic acid (MPA) by area under the curve (AUC) and investigated genetic contribution in mycophenolate metabolism in this population.
Methods
Kidney transplant recipients with stable graft function who were receiving mycophenolate mofetil 1,000 mg/d in combination with either cyclosporine or tacrolimus, and prednisolone were studied. The MPA concentration was measured by fluorescence polarization immunoassay (FPIA), at predose and 1, 1.5, 2, 4, 6, 8, 10, and 12 hours after dosing. Genetic polymorphisms in UGT1A8, UGT1A9, and UGT2B7 were examined by denaturing high-performance liquid chromatography (DHPLC)-based single-base extension (SBE) analysis.
Results
A total 138 patients were included in study. The mean AUC was 39.49 mg-h/L (28.39–89.58 mg-h/L), which was in the therapeutic range. The correlation between the predose MPA concentration and AUC was poor. The mean AUC in the tacrolimus group was higher than that in the cyclosporine group. Polymorphisms in UGT2B7 showed significant association with AUC.
Conclusion
Most of our patients with reduced mycophenolate dose had the AUC within the therapeutic range. Genetic polymorphisms in UGT2B7 may play a role in MPA metabolism in Thai kidney transplant patients.
Keywords:
Introduction
Mycophenolate is a highly effective immunosuppressive drug that has been used to prevent rejection in organ transplant recipients. The active metabolite, mycophenolic acid (MPA), acts by inhibiting inosine monophosphate dehydrogenase enzyme (IMPDH), which mediates the synthesis of purine and therefore controls lymphocyte proliferation. Mycophenolate was formulated as prodrugs, called mycophenolate mofetil (MMF), and as a salt form, mycophenolate sodium (MPS). The current recommended doses in adults is 1,000 mg twice daily for MMF and 720 mg twice daily for MPS.Citation1 Adjustment for body weight is not necessary. The area under the curve (AUC) of MPA as MMF and MPS at these doses was found to be equivalent.Citation2 Previous study has shown that an appropriate AUC at 0–12 hours after drug administration, between 30–60 mg-h/L, was associated with significant decrease in acute graft rejection in kidney transplant patients.Citation3
MPA is primarily metabolized by the liver, through glucuronidation. Several uridine glucuronosyltransferases (UGTs) are involved in the metabolism process. UGT1A8 and UGT1A9 are mainly responsible for mycophenolate 7-o-glucuronide (MPAG) production, while UGT1A8 and UGT2B7 are responsible for producing acyl glucuronide (AcMPAG). Genetic polymorphisms exist on the UGT family of genes and may contribute to differences in MPA metabolism and drug level in organ transplant patients. Reports showed that several polymorphisms on the UGTs were associated with side effects in these patients receiving MPA.Citation4,Citation5
From our experience, use of the recommended dose of MPA in Thai kidney transplant recipients was associated with high prevalence of adverse effects, such as nausea/vomiting, severe diarrhea, neutropenia, and opportunistic infection (Pithukpakorn, unpublished data, 2010). Study in Asian patients showed similar problems, and AUC studies with the recommended dose of MPA were found to be much higher than those from Western countries.Citation6,Citation7 Current practice at the Siriraj Organ Transplantation Center suggests that our kidney transplant patients receive only half of recommended doses of mycophenolate (MMF 1,000 mg/day or MPS 720 mg/day), in combination with either cyclosporine or tacrolimus, and prednisolone. The incidence of acute rejection is 10%–15% during the first 6 months and graft survival at 1 year and 5 years was 95.2% and 88.7% for living related transplantation and 74.5% and 57.8% for cadaveric transplantation, respectively. These data were comparable with other international centers.Citation8
There was a previous study on pharmacokinetics of MPA in Thai kidney transplant patients, but that study involved small number of subjects, and no study had been done on pharmacogenetic factors.Citation9 Therefore, this study was conducted to investigate the pharmacokinetics of MPA as well as the pharmacogenetic effect of UGTs on MPA level in Thai kidney transplant patients.
Materials and methods
The research protocol was approved by Siriraj Institutional Review Board. The study was conducted according to the Declaration of Helsinki principles. Participants were 138 patients who underwent kidney transplantation at least 3 months prior and had regular follow up at Siriraj Organ Transplantation Center between February 1, 2009 and July 31, 2009. All patients received MMF or MPS as one of the immunosuppressive drugs, and the dosage had to be stable and not be adjusted during 3 months prior to study enrollment. Any subjects with clinical evidence of infection or pregnancy were excluded. All patients gave informed consent after a full detail of study protocol had been explained and discussed.
All patients visited the clinic in the morning and stayed for 12 hours, during the study. Blood samples from the patients were collected at time 0 (predose) and at 1, 1.5, 2, 4, 6, 8, 10, and 12 hours after oral MMF or MPS was given. Genomic DNA from each patient was extracted from the leftover cellular portion from blood samples and used for pharmacogenetic study. MPA measurement from collected serum was performed at the certified clinical laboratory, by fluorescence polarization immunoassay (FPIA), with a Cobas Integra® 400 system (F Hoffman-La Roche Ltd, Basel, Switzerland). The system yielded excellent correlation with liquid chromatography with tandem mass spectrometry (LC-MS/MS) technique.Citation10
For pharmacogenetic testing, DNA regions of interest were amplified from genomic DNA collected as described above, with multiplex polymerase chain reaction (PCR) to create six distinct fragments. Investigation for six well-established polymorphisms of UGT1A8, 1A9, and 2B7 was performed by multiplex PCR and denaturing high-performance liquid chromatography (DHPLC)-based single-base extension (SBE) analysis. The SBE products were analyzed on a fully denaturing mode of the WAVE™ DHPLC system equipped with an OLIGOSep™ cartridge (catalog number NUC-99-3550; Transgenomic, Omaha, NE, USA).
Analyses of clinical and pharmacokinetic data were done by SPSS for Windows, version 16.0. Spearman’s correlation was used for correlation analysis between pharmacokinetic parameters and clinical data. Pharmacogenetic data were tested for deviation from Hardy–Weinberg equilibrium. Based on the previous data that AUC between 30–60 mg-h/L was associated with reduced rejection rate, individuals with AUC at 30 mg-h/L or higher were defined as the case group and those with AUC below 30 mg-h/L were defined as the control group. The degree of linkage disequilibrium between loci was measured using Lewontin’s D′ and r2, using the JLIN software program.Citation11 Test for association was done with analysis of allele frequency difference, heterozygous states, homozygous states, and allele positivity, using Pearson’s goodness-to-fit chi-square test. The Bonferroni correction (P<0.05 divided by the number of single nucleotide polymorphisms analyzed) was used to account for multiple testing.
Results
Clinical and AUC data
Of 138 patients, 76 (55.1%) patients underwent living-related kidney transplantation, while 62 (44.9%) patients underwent deceased-donor kidney transplantation. Mean serum creatinine was 1.35±0.41 mg/dL. All patients received half of recommended dose of mycophenolate, by either 1,000 mg of MMF or 720 mg of MPS daily (76.8% and 23.2%, respectively), as our standard protocol. Further demographic data are demonstrated in .
Table 1 Demographic data of 138 patients who underwent pharmacokinetic study
For patients receiving MMF, MPA concentration rose rapidly after oral administration, and peak level was reached at 1 hour. The concentration decreased gradually after 2 hours and became stable after 6 hours. The concentration curve from each patient showed very similar pattern without delayed peak level. In contrast, patients receiving MPS showed a sustained level of MPA concentration after oral administration, with no peak level throughout the monitoring period. The mean AUC was 39.49±13.28 (28.39–89.58) mg-h/L. We also compared AUC between patients who were on different calcineurin inhibitors. The mean AUC in the tacrolimus group was higher than that in the cyclosporine group, but there was no statistical difference (41.27±15.82 vs 35.69±13.67 mg-h/L) (P=0.1). The patients who were on MMF had similar AUC to the patients with equivalent dose of MPS. The correlation between the predose MPA concentration and AUC was poor (r2=0.255).
Pharmacogenetic data
Genotype data on selected polymorphisms showed that for UGT1A8, S43L (rs372427845), H53N (rs45504099), A231T (rs72551325), T240A (rs72551326), and C277Y (rs17863762); and for UGT1A9, -2152C/T (rs17868320) and -275 T/A (rs6714486) were all homozygous, in both patients with AUC above 30 mg-h/L (case group) and below 30 mg-h/L (control group). We found that for UGT2B7, -824G/A (rs7438135) and H268Y (rs7439366); and for UGT1A8, A173G (rs1042597) showed heterozygosity on both loci. These alleles were in Hardy–Weinberg equilibrium, for both case and control groups. Tests for association showed that for UGT2B7, -824G/A and H268Y both had statistically significant allele frequency differences between the case and control groups. In addition, -824G/A and H268Y were highly linked (D′= 1) (r2=0.966). For UGT2B7 at position -824, the common odds ratio of having G allele in case group was 0.604 when compared with A allele (P=0.03674). The presence of G allele (G/G or G/A) had odds ratio of 0.461 compared with the absence of G allele (A/A). Heterozygous G/A at this position also had significant odds of 0.451 when compared with homozygous A/A. Meanwhile, for UGT2B7 at position 268, the common odds ratio of having a C (Y) allele in the case group was 0.557 compared with the T (H) allele (P=0.01858). The presence of C allele (C/C: YY or C/T: HY) had odds ratio of 0.433 compared with the absence of C allele (T/T: HH). Heterozygous C/T (HY) at this position also had significant odds of 0.435 compared with homozygous C/C (HH). After Bonferroni correction was used, UGT2B7 polymorphism remained statistically significant. Among the 138 patients who participated in the study, we found that patients who carried G allele at position -824 also had a C (Y) allele at position 268, and patients who carried C allele at position -824 also had a T (H) allele at position 268 of UGT2B7. In contrast, we did not see significant association on UGT1A8 at position A173G and AUC (). Since two alleles of UGT2B7 were linked, we chose to examine the correlation between AUC in percentile and the UGT2B7 H268Y allele. We found that patients who were HY heterozygous had lower average AUC (38.5 mg-h/L) than did patients who were HH homozygous (44.18 mg-h/L). The AUC percentile comparison found the highest frequency of HY heterozygote was in the fourth decile (30–40 mg-h/L), while the highest frequency of the HH homozygote was in the fifth decile (40–50 mg-h/L) ().
Figure 1 (A) Comparison of AUC decile distribution between patients who carried TC alleles (dark bars) and patients who carried TT alleles (light bars) in UGT2B7 at position 268. (B) Comparison of allele frequency between the group with AUC above 30 mg-h/L (left pie chart) and the group with AUC below 30 mg-h/L (right pie chart).
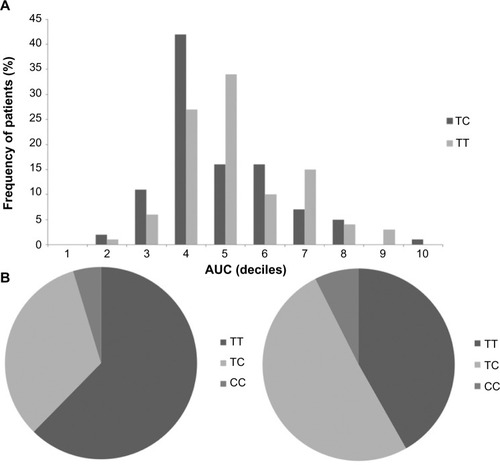
Table 2 Test for association between polymorphisms and AUC
Discussion
Our study was the first report of pharmacokinetic and pharmacogenetic studies in Thai kidney transplant patients who received reduced dose of mycophenolate. We demonstrated that the majority of patients had adequate AUC level despite that all patients received smaller dose of mycophenolate than the Caucasian population. These results were similar to other reports of higher AUC among Asian kidney transplant individuals who were on mycophenolate, compared with Western patients.Citation6,Citation7 These findings also support our clinical observation that many Thai kidney transplant patients experienced high incidence of mycophenolate-related adverse reaction with full dose and that most of the Thai transplanted patients at Siriraj Organ Transplant Center were able to tolerate reduced mycophenolate dosage with acceptable graft survival outcome and acute rejection rate. A recent study in Japanese renal transplant patients also found that the majority of patients who were using a MMF plus cyclosporine-based regimen were unable to tolerate the original dosage of 2 g/day due to adverse effects and subsequently reduced to a mean dosage of 1.24 g/day.Citation12
There were previous studies on the relationship between MPA pharmacokinetics and graft rejection, and on adverse effects in various organ transplant cohorts. Some results showed that high MPA level (peak or trough) or high AUC were associated with increased risk of side effects, while other results did not.Citation13–Citation15 A systematic review published in 2008 showed that MPA AUC and total dose had good correlation with risk of acute graft rejection and that free MPA level may better predict toxicity.Citation16 The study by Yau et al brought an interesting concept that current recommended fixed dose should not be ideal for all patients.Citation17 The authors concluded that the body weight-adjusted MMF dosage tended to be lower than that reported from the Western population. Yau et al further concluded that the 12 mg/kg twice daily dose of MMF (24 mg/kg/day) should provide adequate AUC for Asian patients. However, whether there are ethnic differences in mycophenolate disposition in the Asian population needs further investigation. Studies to determine whether genetic differences in the drug-metabolizing enzymes for MPA could be a contributing factor were suggested. Our finding confirmed the conclusion of Yau et al that Asian patients required lower MMF dosage than do Caucasian patients. The range of body weight-adjusted MMF dosages in our study was 13–19.6 mg/kg/day. Our study showed that polymorphisms in UGT2B7 – a drug metabolizing enzyme gene – were associated with MPA AUC. As expected, we did not find the difference in AUC between the suggested doses of MMF and MPS, thus supported the bioequivalence of these two preparations. It is of interest to observe that patients treated with cyclosporine tend to have lower AUC than those with tacrolimus. It has been known that enterohepatic recirculation of MPA involves excretion of MPAG into the intestinal lumen through the biliary system. Multidrug resistance-associated protein 2 (MRP2) at the apical surface of hepatocytes plays an active role in MPAG excretion. Inactive MPAG is then deconjugated by intestinal bacteria to become active MPA and is absorbed into circulation. The enterohepatic recirculation was estimated to contribute about 40% of the total AUC. Cyclosporine is an inhibitor of MRP2 at the apical surface of hepatocytes and may cause an AUC reduction in those patients who are on cyclosporine. The results of our study indicated that most Thai kidney transplant patients achieve an adequate AUC value even though lower dose of mycophenolate was administered. Similar findings from several populations suggested that a genetic factor may play role in difference in MPA metabolism.Citation18–Citation20 We were interested in UGT1A8, 1A9, and 2B7 polymorphisms, due to their involvement in the MPA metabolic pathway. Our results showed that polymorphisms at position -824 and 268 of UGT2B7 were associated with the AUC of MPA. Because both alleles were linked, the data did not allow us to conclude the real contribution from each allele. Study of UGT2B7 glucuronidation in human hepatocytes showed that T/T (HH) polymorphism had modestly lower activity than C/C (YY) polymorphism. Though H268Y polymorphism was sufficient to affect MPA kinetics,Citation20 there was evidence that promoter polymorphism may also have influence on MPA metabolism, in addition to H268Y allele.Citation21 Lower enzyme activity gives rise to slower conversion from MPA to MPAG. This result may explain the lower AUC in patients who carried the HY allele, when compared with HH allele. No association was demonstrated in the YY allele group, due to inadequate sample size. However, the present study had several limitations. First, the purpose of this study was mainly to demonstrate that majority of Thai kidney transplanted patients who received half of the recommended fixed-dose regimen without rejection could achieve an AUC target above 30 mg-h/L. Though this result supported the fact that Thai patients, similar to other Asian cohorts, require a smaller mycophenolate dose than Caucasian patients, we could not conclude that the low dose regimen is suitable for long-term immunosuppression since the outcomes on graft survival, development of donor-specific antibody, and other clinical parameters require ongoing follow-up study of this cohort. Second, we did not include all genetic polymorphisms that could affect the pharmacokinetics and pharmacodynamics of MPA. Furthermore, no significant association in selected UGT1A8 and 1A9 polymorphisms was observed due to uninformative alleles (for UGT1A8, these were S43L, H53N, A231T, T240A, and C277Y; and for UGT1A9, these were -2152C/T and -275 T/A). The lack of informative alleles did not allow us to observe additive effects of several UGTs on MPA metabolism. We also did not observe an association in polymorphism at position 173 of UGT1A8. One explanation is that in vitro study revealed no significant effect of UGT1A8 activity for the codon 173 variation because MPA metabolism involves many UGTs as well as other cellular transporters, such as ABCC2 (MRP2), ABCG2, and SLCO proteins.Citation22 As expected, we detected moderate contribution in UGT2B7 polymorphisms. Other studies on the effect of UGT polymorphisms and MPA pharmacokinetics have previously been done and revealed similar results; however these were mainly performed in healthy individuals using a single dose of MMF.Citation20,Citation23 Our study in transplanted recipients should better represent real clinical scenarios in which patients have regular and concurrent use of multiple immunosuppressive drugs.
In conclusion, the majority of Thai patients could maintain adequate AUC despite a lower mycophenolate dosage than recommendation. We also demonstrated the significant association between UGT2B7 polymorphisms and AUC level, suggesting the role of genetic polymorphisms in MPA metabolism and AUC, in our kidney transplant patients. However, further investigation of other polymorphisms in UGTs and other transporter genes would be crucial to elucidate a better picture of pharmacogenetic roles of MPA metabolism.
Author contributions
Manop Pithukpakorn participated in research design, data analysis, and writing of the manuscript. Tiwat Tiwawanwong, Yupaporn Lalerd, and Nalinee Premasathian performed data collection and data analysis. Anunchai Assawamakin performed data analysis. Adis Tasanarong participated in research design. Wanna Thongnoppakhun participated in research design and data analysis. Attapong Vongwiwatana participated in research design, data collection, data analysis, and writing of the manuscript. All authors participated in manuscript revision, ensured data integrity and approved the final version of the manuscript.
Acknowledgments
We wish to thank all the patients for their cooperation and participation in this study.
This work was supported by funding from Siriraj Research Development Grant, Siriraj Kidney Transplant Grant, Siriraj Core Research Facility, and the Thai Transplantation Society.
Disclosure
The authors report no conflicts of interest in this work.
References
- SollingerHWMycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. US Renal Transplant Mycophenolate Mofetil Study GroupTransplantation19956032252327645033
- ArnsWBreuerSChoudhurySEnteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophe-nolate mofetilClin Transplant200519219920615740555
- StaatzCETettSEClinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipientsClin Pharmacokinet2007461135817201457
- YangJWLeePHHutchinsonIVPravicaVShahTMinDIGenetic polymorphisms of MRP2 and UGT2B7 and gastrointestinal symptoms in renal transplant recipients taking mycophenolic acidTher Drug Monit200931554254819730281
- BetônicoGNAbbud-FilhoMGoloni-BertolloEMInfluence of UDP-glucuronosyltransferase polymorphisms on mycophenolate mofetil-induced side effects in kidney transplant patientsTransplant Proc200840370871018454993
- SuhailSMVathsalaALouHXWooKTSafety and efficacy of mycophenolate mofetil for prophylaxis in Asian renal transplant recipientsTransplant Proc20003271757175811119922
- ZhouPJXuDYuZCWangXHShaoKZhaoJPPharmacokinetics of mycophenolic acid and estimation of exposure using multiple linear regression equations in Chinese renal allograft recipientsClin Pharmacokinet200746538940117465638
- KrämerBKDel CastilloDMargreiterREuropean Tacrolimus versus Ciclosporin Microemulsion Renal Transplantation Study GroupEfficacy and safety of tacrolimus compared with ciclosporin A in renal transplantation: three-year observational resultsNephrol Dial Transplant20082372386239218258740
- JirasirithamSSumethkulVMavichakVNa-BangchangKThe pharmacokinetics of mycophenolate mofetil in Thai kidney transplant recipientsTransplant Proc20043672076207815518751
- van GelderTDomkeIEngelmayerJClinical utility of a new enzymatic assay for determination of mycophenolic acid in comparison with an optimized LC-MS/MS methodTher Drug Monit200931221822319214147
- CarterKWMcCaskiePAPalmerLJJLIN: a java based linkage disequilibrium plotterBMC Bioinformatics200676016466584
- TakahashiKUchidaKYoshimuraNEfficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month resultsTransplant Res2013211423866828
- KuypersDRClaesKEvenepoelPLong-term changes in mycophenolic acid exposure in combination with tacrolimus and corticosteroids are dose dependent and not reflected by trough plasma concentration: a prospective study in 100 de novo renal allograft recipientsJ Clin Pharmacol200343886688012953344
- FigurskiMJPawińskiTGoldbergLRPharmacokinetic monitoring of mycophenolic acid in heart transplant patients: correlation the side-effects and rejections with pharmacokinetic parametersAnn Transplant2012171687822466911
- MouradMMalaiseJChaib EddourDCorrelation of myco-phenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetilClin Chem2001471889411148182
- KnightSRMorrisPJDoes the evidence support the use of myco-phenolate mofetil therapeutic drug monitoring in clinical practice? A systematic reviewTransplantation200885121675168518580456
- YauWPVathsalaALouHXChanEIs a standard fixed dose of mycophenolate mofetil ideal for all patients?Nephrol Dial Transplant200722123638364517640939
- BernardOTojcicJJournaultKPerusseLGuillemetteCInfluence of nonsynonymous polymorphisms of UGT1A8 and UGT2B7 metabolizing enzymes on the formation of phenolic and acyl glucuronides of mycophenolic acidDrug Metab Dispos20063491539154516790554
- KagayaHInoueKMiuraMInfluence of UGT1A8 and UGT2B7 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipientsEur J Clin Pharmacol200763327928817211619
- LévesqueEDelageRBenoit-BiancamanoMOThe impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteersClin Pharmacol Ther200781339240017339869
- DuguayYBáárCSkorpenFGuillemetteCA novel functional polymorphism in the uridine diphosphate-glucuronosyltransferase 2B7 promoter with significant impact on promoter activityClin Pharmacol Ther200475322323315001974
- MiuraMSatohSInoueKInfluence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacoki-netics in Japanese renal transplant recipientsEur J Clin Pharmacol200763121161116917906856
- GuoDPangLFHanYPolymorphisms of UGT1A9 and UGT2B7 influence the pharmacokinetics of mycophenolic acid after a single oral dose in healthy Chinese volunteersEur J Clin Pharmacol201369484384923052409
