Abstract
Obesity is currently considered a global epidemic, with rising prevalence worldwide and rather pessimistic projections. Based on its close interconnection with various co-morbidities, such as diabetes mellitus and cardiovascular disease, obesity is associated with significant increases in morbidity and mortality, while it also poses a substantial economic burden for national healthcare systems. Apparently, the majority of individuals classified as obese do not achieve adequate weight loss with the adoption of a healthy lifestyle intervention, including dietary modification and physical activity. Fortunately, during the last decade, a significant progress in pharmacotherapy of obesity has been observed, with the introduction of agents that have gained approval from regulatory authorities, namely semaglutide, liraglutide and tirzepatide, due to their impressive results in body weight reduction, alongside their beneficial, pleiotropic effects. The aim of the present review article is to discuss on evidence retrieved from real-world studies regarding the efficacy of those agents in obesity treatment, with emphasis on cost-effectiveness data, towards an effort to tackle efficiently the progression of obesity epidemic.
Introduction
Obesity has evolved over the last decades as an epidemic, with continuous increase in its prevalence; according to evidence generated by the NCD Risk Factor Collaboration, the global age-standardized prevalence of obesity has increased from 8.8% in 1990 to 18.5% in 2022 among women and from 4.8% in 1990 to 14% in 2022 among men, resulting in 504 million women and 374 million living with obesity worldwide.Citation1 Based on previously published data, body mass index (BMI) greater than 35 kilograms(kg)/m2, classified as grade 2 or 3 obesity, was associated with a significant increase in the risk for all-cause death by 29%, whereas, a non-significant association between overweight or grade 1 obesity and all-cause mortality was shown.Citation2 However, newer data supported that even at lower BMI levels, there is a significant increase in the risk for all-cause death, which increases approximately log-linearly with BMI, for BMI levels higher than 25 kg/m2.Citation3 Of note, the association between obesity and all-cause mortality was consistent in all continents, with the disease burden being greater for younger versus older obese individuals.Citation3
More recent data from the UK primary care data from the Clinical Practice Research Datalink demonstrated a J-shaped association between BMI and all-cause mortality, whereas, a similar association was documented between BMI and more specific causes of death, including cardiovascular disease (CVD), cancer or respiratory disease.Citation4 This results in a significant reduction in life expectancy, which is 4.2 years shorter for men and 3.5 years shorter for women that are obese and older than 40 years.Citation4 Besides the strong association between obesity and all-cause mortality, it also significantly correlates with increased risk for all-cause hospitalization, compared to normal weight individuals, regardless of gender or age, as shown in several observational studies over the last years.Citation5–8
Apart from the significant rise in morbidity among the affected individuals, obesity is also accompanied by a substantially increased healthcare cost, leading to a significant economic burden for national healthcare systems.Citation8,Citation9 According to data from the United States (U.S.), obesity accounts for more than 170 billion dollars of annual expenditures,Citation10 whereas the corresponding annual expenditure in Germany amounts approximately 30 billion euros.Citation11 Thus, it appears that, even in the most developed countries worldwide, obesity is associated with an enormous economic burden.
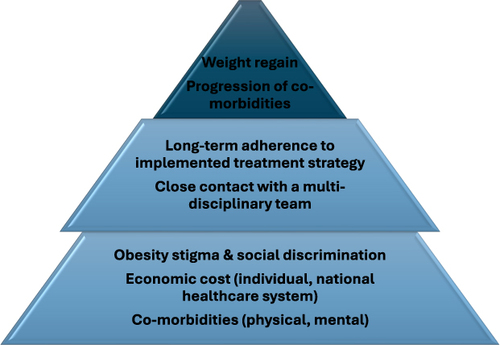
Obesity is strongly and directly related to sedentary behavior and physical inactivity, especially in Western countries; according to recent evidence from a large meta-analysis, combined prevalence of sedentary behavior physical inactivity among individuals with obesity was 31% and 43%, respectively.Citation12 Therefore, multilevel lifestyle intervention is always considered as the first step in the therapeutic management of obesity, including energy restriction, regular physical activity and frequent evaluation by healthcare professionals.Citation13 Behavioral treatment strategies are of utmost importance for those individuals, since they have been demonstrated to significantly increase their adherence to lifestyle intervention programmes.Citation14 Unfortunately, lifestyle interventions are associated with long-term weight regain, especially if they are stopped or interrupted,Citation15,Citation16 with this weight regain being correlated with a more modest improvement in several cardiometabolic risk factors (eg blood pressure, glycemia, lipid profile parameters) after a multilevel lifestyle intervention, compared to those individuals that did not experience any weight regain.Citation17 Based on the fact that individuals with obesity are at significantly higher risk for the development of type 2 diabetes (T2DM) and CVD, mainly coronary artery disease (CAD),Citation18 it appears that this weight regain and subsequent amelioration of improvement in cardio-metabolic parameters of interest can have deleterious effect on morbidity and mortality among those individuals. Therefore, additional treatment strategies, besides lifestyle intervention, are required, for the achievement of greater weight loss and the maintenance of that ().
Outstanding progress in the therapeutic, pharmacological, management of obesity has been observed over the last decade, with the development of novel drug classes, mainly glucagon-like peptide-1 (GLP-1) receptor agonists and dual GLP-1/glucose-dependent insulinotropic polypeptide (GIP) receptor agonists, which produce significant weight loss and substantial improvement in a number of various cardio-metabolic outcomes, according to large randomized controlled trials (RCTs) in the field.
Parallel to the documentation of the impressive cardio-reno-metabolic benefits of GLP-1 and GLP-1/GIP receptor agonists in various patients’ populations, a significant progress in the understanding of underlying mechanisms of action has also been demonstrated. GLP-1 and GIP receptors are widely expressed in multiple organs beyond the pancreas, therefore receptors’ agonism mediates the various, pleiotropic effects, including reduction of appetite and food intake, along with increase in satiety, through effects on central nervous system, slowing of gastric emptying and reduction of gastrointestinal motility, promotion of insulin synthesis and insulin secretion, enhancement of beta-cell proliferation and reduction of beta-cell apoptosis in the pancreas, suppression of hepatic gluconeogenesis, induction of lipolysis in white adipose tissue and enhancement of glucose uptake in white adipose tissue and skeletal muscle.Citation19–23 GLP-1 receptor agonism has also been shown to possess significant anti-inflammatory effects in multiple organs and cell types, whereas, GIP receptor agonism suppresses macrophage-dependent inflammation and regulates inflammation within brown adipose tissue.Citation22,Citation23 Of note, whereas GLP-1 receptor agonism has been shown to exert cardio-renal beneficial effects via increase in glucose uptake, reduction of fatty acid metabolism, enhancement of vasodilation, promotion of diuresis and natriuresis, current insights into the cardiovascular and renal biology of GIP receptor agonism is limited.Citation22
The aim of the present review article is to discuss on major findings from real-world studies in the field, with emphasis on data concerning cost-effectiveness of the currently officially approved for use by the regulatory authorities GLP-1 and GLP-1/GIP receptor agonists, in an effort to assess the applicability of such pharmacological interventions in the real-world setting.
Semaglutide
Semaglutide has emerged over the last decade as a potent antidiabetic drug, administered either subcutaneously or orally, which induces a significant reduction in body weight in adults with concomitant T2DM, also exerting a significant number of cardio-renal benefits.Citation24–28 Based on evidence generated by the STEP (Semaglutide Treatment Effect in People with obesity) clinical trials programme, semaglutide 2.4 mg, administered subcutaneously once-weekly, has been established as a highly efficacious treatment option against obesity, gaining approval for use by the US Food and Drug Administration (FDA) in June 2021. According to data from the STEP clinical trials programme, administration of semaglutide 2.4 mg, compared to placebo, in overweight or obese individuals without baseline T2DM results in a significant reduction in body weight by 11.8%, equal to a mean reduction of 12.2 kg in absolute body weight, along with a significant decrease in BMI by 4.5 kg/m2 and in waist circumference (WC) by 9.4 cm.Citation29 In addition, semaglutide was associated with significantly higher odds for achievement of weight loss ≥5%, ≥10%, ≥15% and ≥20%, compared to baseline, along with significant reductions in fasting plasma glucose, systolic and diastolic blood pressure, and a significant improvement in fasting lipid profile parameters.Citation29 These results are in line with another meta-analysis, suggesting that semaglutide 2.4 mg is highly effective for weight management, when conjugated with appropriate lifestyle intervention, regardless of the history of concomitant T2DM, and of note, more effective than liraglutide.Citation30
The high efficacy of semaglutide observed in relevant RCTs in terms of body weight reduction has also been demonstrated in real-world studies in the field, enrolling obese individuals with or without concomitant diabetes mellitus. Semaglutide administration has resulted in a substantial weight loss, similar to that observed in RCTs, in individuals with obesity but without diabetes mellitus,Citation31,Citation32 in individuals with concomitant T2DM,Citation33–36 or even among individuals with type 1 diabetes mellitus (T1DM).Citation37 Real-world evidence suggests that, besides the significant improvement in body weight, individuals assigned to semaglutide, regardless of concomitant diabetes mellitus, experienced a significant improvement in other cardio-metabolic parameters of interest, indicative of a substantial cardiovascular risk reduction, although there are no observational studies addressing the cardiovascular efficacy and safety of semaglutide in obese individuals without diabetes mellitus,Citation32,Citation36 similar to the recently published cardiovascular outcome SELECT trial, which demonstrated a significant 20% reduction in the risk for the primary composite cardiovascular endpoint with semaglutide 2.4 mg in obese individuals with pre-existing CVD.Citation38
Cost-Effectiveness of Semaglutide versus Lifestyle Intervention
In terms of cost-effectiveness, a base-case analysis from the United Kingdom (UK) utilizing data from the STEP 1 trial demonstrated that semaglutide 2.4 mg was associated with greater economic costs and health benefits, with an incremental cost-effectiveness ratio (ICER) of £14,827 per quality-adjusted life-year (QALY) gained.Citation39 Probabilistic sensitivity analysis documented that semaglutide was cost-effective in 90% of all cases at a willingness-to-pay (WTP) threshold of £20,000 per QALY, suggesting that, in the UK, semaglutide, compared to lifestyle intervention alone, is a cost-effective treatment option for individuals with obesity and weight-related comorbidities.Citation39 Another recently published study from the US demonstrated that, among obese individuals requiring pharmacological treatment, semaglutide was the most costly treatment over a lifetime horizon, with an ICER of $24,274 per QALY gained.Citation40 Unfortunately, according to a probabilistic sensitivity analysis performed over a lifetime horizon, neither semaglutide nor the rest GLP-1 receptor agonists were shown to be cost-effective, across a wide range of WTP values up to $400,000 per QALY.Citation40
Cost-Effectiveness of Semaglutide versus Other GLP-1 Receptor Agonists
A very interesting analysis from the US has also been recently published, showing that utilization of semaglutide 2.4 mg for the treatment of obesity has a total annual cost of $130,040, resulting in a total of 13.492 QALYs.Citation41 When researchers defined a WTP threshold of $150,000/QALY, they observed that the estimated probability of semaglutide 2.4 mg to be co-effective was 82% compared to diet and exercise, 98% compared with liraglutide 3.0 mg and 100% compared to no treatment, over a 30-year horizon.Citation41
In a former study from the US, conducted in 2019, prior to FDA approval of semaglutide for the treatment of obesity, it was demonstrated that semaglutide 1.0 mg with an ICER of $135,467/QALY was the most cost-effective GLP-1 receptor agonist, compared to liraglutide, exenatide and dulaglutide, for the treatment of obesity, with a proposed WTP of $195,000/QALY and a rate of cost-effectiveness equal to 75.3%.Citation42 According to another relevant analysis from the US, also conducted in 2019, it was shown that semaglutide 1.0 mg was highly efficacious in terms of gained QALYs; however, it was not cost-effective, with an ICER of $661,326/QALY in year 3 and of $520,262/QALY in year 5 after treatment initiation.Citation43 Because of its high cost, after adjustment for a WTP threshold of $100,000/QALY, semaglutide was not found to be cost-effective for the treatment of obesity.Citation43
The main side effects associated with the use of semaglutide, when utilized for the treatment of obesity, are nausea (44%), diarrhea (30%), vomiting (24%), constipation (24%), abdominal pain (20%), headache (14%) and fatigue (10%).Citation44
Therefore, current evidence appears to be conflicting from available studies regarding the cost-effectiveness of semaglutide for the treatment of obesity (), based on the different dosing regimens and the different pricing of semaglutide before and after FDA approval for the treatment of obesity. However, it appears to be a trend towards a substantial reduction in its cost, with a subsequent, significant reduction in ICER, which might facilitate its broader use of appropriate dosages for the treatment of obesity, as an adjunct to lifestyle intervention.
Table 1 Cost-Effectiveness of Semaglutide for the Treatment of Obesity
Liraglutide
Liraglutide is another potent GLP-1 receptor agonist, primarily designed for the treatment of patients with T2DM, which has also been approved for chronic weight management by the US FDA since December 2014, whereas it has also been approved for use in obesity by the European Medicines Agency (EMA) in March 2015. Similar to semaglutide, liraglutide has been shown to produce significant improvement in glycemic control and weight loss in patients with T2DM, while it also provides a significant number of cardio-renal benefits, according to the results of hallmark, dedicated trials.Citation24,Citation25,Citation27
According to the hallmark SCALE Obesity and Prediabetes trial, published in 2015, treatment with liraglutide 3.0 mg once daily, injected subcutaneously, in a total of 3731 individuals with overweight or obesity without T2DM at baseline, was shown to result in a significant reduction in body weight by 5.6 kg versus placebo, equal to 5.4% of body weight compared to baseline, along with a significant reduction in BMI by 2.0 kg/m2 and in WC by 4.2 cm.Citation45 Of note, a significantly greater proportion of individuals assigned to liraglutide versus placebo achieved weight loss greater than 5% and 10%, compared to baseline, whereas, a significant improvement in cardio-metabolic parameters of interest was also documented.Citation45 Importantly, in a post-hoc analysis of the SCALE Obesity and Prediabetes trial it was demonstrated that liraglutide 3.0 mg for the management of obesity is not associated with excess cardiovascular risk; however, it does not exert any cardiovascular benefit as well.Citation46 Several meta-analyses of RCTs in the field have confirmed the significant weight-lowering effects of liraglutide among individuals with obesity, which are, however, numerically lower compared to that observed with semaglutide.Citation47–49
There is a significant number of real-world studies addressing the safety and efficacy of liraglutide 3.0 mg once daily as a treatment option for individuals with obesity, with or without concomitant T2DM, most of which provide results similar to that reported in the SCALE Obesity and Prediabetes trial, in various clinical settings.Citation5,Citation50 Interesting findings have been reported by more recently published real-world studies in the field. Concerning the always important topic of ethnic disparities in the treatment of obesity, a recent study from the UK demonstrated that, among individuals with overweight or obesity treated with liraglutide 3.0 mg, those of Black African or Caribbean ethnicity experienced significantly less body weight reduction and had higher attrition rates.Citation51 According to another real-world study from the UK, liraglutide 3.0 mg is a highly efficacious and safe treatment option for body weight management among obese individuals being on the waiting list for bariatric surgery, with substantially achieved weight loss and high percentages of prediabetes remission.Citation52 Another study demonstrated that treatment with liraglutide was associated with the greatest persistence rates compared to other treatment options for obesity, including orlistat and lorcaserin.Citation53 As far as weight regain is concerned, a real-world study showed that net fat mass loss was associated with weight regain, but the magnitude of weight regain was significantly lower with liraglutide versus lifestyle intervention alone among individuals with obesity.Citation54 Finally, liraglutide 3.0 mg appears to be a safe, viable and efficacious treatment option for subjects who experience either insufficient weight loss or weight regain after bariatric surgery, potentially extending the therapeutic potential of this drug class even for patients who have undergone bariatric surgery that failed.Citation55
Cost-Effectiveness of Liraglutide versus Other GLP-1 Receptor Agonists
Regarding cost-effectiveness, according to a previously mentioned analysis from the US, liraglutide treatment resulted in similar QALYs with semaglutide (29.229 with liraglutide vs 29.233 with semaglutide); however, with substantially higher ICER, equal to $39,665, compared to that of semaglutide, which was estimated to be $24,274.Citation40 Of note, for a WTP threshold up to $100,000, liraglutide appeared to have the same, very low cost-effectiveness, similar to semaglutide, which remained very low for greater WTP thresholds, unlike semaglutide.Citation40 Similar results were obtained from other relevant analyses,Citation42,Citation43 which demonstrated that liraglutide is substantially less cost-effective versus semaglutide for the therapeutic management of obesity.
An insightful cost-effectiveness analysis with direct comparison between semaglutide and liraglutide, utilized data from the SCALE and the STEP 1 trials, was recently published, demonstrating a higher annual drug cost for semaglutide ($17,543) versus liraglutide ($16,373), corresponding to a weekly cost $336,44 for semaglutide versus $314,01 for liraglutide; however, according to the more than double weight loss achieved with semaglutide compared to liraglutide, the cost needed to treat per 1% of body weight reduction with liraglutide is estimated at $3256 versus $1845 with semaglutide.Citation56 Of course, the main limitation of this analysis is that it did not account for the future treatment costs for the maintenance of the achieved result; however, semaglutide, even at the start of treatment, appears to be more cost-effective than liraglutide.
It should be highlighted that all relevant evidence on the comparative effectiveness of liraglutide versus other GLP-1 receptor agonists for the management of obesity stems from analyses performed in the US population, whereas no relevant analyses have been performed elsewhere so far, despite the fact that several, comparative, cost-effectiveness analyses for T2DM have been performed.Citation57–61
The main side effects reported by consumers with the use of liraglutide for the treatment of obesity include nausea (39%), diarrhea (21%), constipation (19%), vomiting (16%), injection site reaction (14%), headache (14%) and dyspepsia (10%).Citation44
Overall, despite the fact that liraglutide was historically the first GLP-1 receptor agonist to be the FDA approved for the treatment of obesity in adults, it seems that semaglutide is a more potent drug for body weight reduction, with similar safety and efficacy profile, but with significantly better cost-effectiveness (). It has to be admitted that there is no evidence, to date, regarding the impact of recently observed shortages in semaglutide supplies on the long-term cost-effectiveness of this novel drug.
Table 2 Cost-Effectiveness of Liraglutide for the Treatment of Obesity
Tirzepatide
Tirzepatide is a novel, dual, GLP-1/GIP receptor agonist, recently incorporated into the treatment algorithm of T2DM,Citation62 based on outstanding data regarding its efficacy from the SURPASS Clinical Trials Programme.Citation63 SURMOUNT-1 was the first trial to assess the safety and efficacy of tirzepatide in adults with obesity without baseline T2DM; a total of 2539 adults with obesity were randomized either to tirzepatide, administered once-weekly subcutaneously or placebo and were followed-up for 72 weeks.Citation64 At the end of the trial, the percentage change in body weight compared to the baseline was 15% with tirzepatide 5 mg, 19.5% with tirzepatide 10 mg and 20.9% with tirzepatide 15 mg, corresponding to a mean body weight reduction, compared to placebo, of 11.9, 16.4 and 17.8 kg, respectively.Citation64 Of note, at the end of the trial, more than two-third of those participants assigned to the maximum dose of 15 mg experienced a body weight reduction greater than 15% compared to baseline, more than 50% of them experienced a weight reduction greater than 20% compared to baseline, whereas, one-third of those participants lost more than 25% of their baseline body weight at the end of the trial.Citation64 Therefore, tirzepatide was finally approved for the therapeutic management of obesity by the F.D.A. on November 2023, promising to revolutionize the treatment of obesity.
Evidence retrieved from systematic reviews and meta-analyses of relevant RCTs in the field have suggested that tirzepatide provides significantly greater body weight reduction compared to GLP-1 receptor agonists in overweight or obese individuals, with a similar safety profile.Citation65 To date, there is a scarcity of real-world studies evaluating the safety and efficacy of tirzepatide for the treatment of obesity. A recently published retrospective cohort study addressed the comparative efficacy of tirzepatide and semaglutide in patients with weight regain after bariatric surgery.Citation66 After 6 months of treatment, patients in both treatment arms experienced a significant body weight reduction compared to baseline; however, patients assigned to tirzepatide had a significantly greater weight loss compared to those administered semaglutide (15.5% versus 10.3%, respectively), with no reporting of serious adverse events.Citation66
In another, recently published retrospective cohort study from the US assessing the comparative efficacy of tirzepatide and semaglutide for the therapeutic management of individuals being overweight or obese, it was demonstrated that those receiving tirzepatide were significantly more likely to achieve weight loss greater than 5%, 10% and 15%, compared to those receiving semaglutide, with a significantly greater on-treatment difference on body weight, reaching up to 6.9% at 12 months of treatment (difference, −6.9%; 95% CI, −7.9% to −5.8%).Citation67 Of note, incidence rates of gastrointestinal adverse events were similar between the treatment groups.Citation67 In addition, consistent treatment effects of tirzepatide versus semaglutide were observed regardless of concomitant T2DM.Citation67
Other, recently published real-world studies from different ethnic groups, enrolling overweight or obese individuals with T2DM, have confirmed results obtained from relevant RCTs in the field, showing that tirzepatide is a highly efficacious treatment option for glycemic control and body weight loss, regardless of prior treatment with other GLP-1 receptor agonists.Citation68,Citation69 It is of utmost importance to highlight that, despite the small sample size and the limitations of specific ethnicity, Suzuki et al also demonstrated in their cohort that tirzepatide can ameliorate eating disorders among individuals with T2DM and concomitant obesity, although the observed change did not correlate with improvement in metabolic parameters of interest.Citation69,Citation70
Similarly, there is scarcity of evidence regarding the cost-effectiveness of tirzepatide as a therapeutic option for obesity. In their analysis, Gómez Lumbreras et al demonstrated that, if projected in a lifetime horizon, total treatment cost with tirzepatide would be $234,084, however, resulting in the highest QALYs, compared to other pharmacological interventions.Citation40 According to their probabilistic sensitivity analysis, researchers demonstrated a relatively low cost-effectiveness of tirzepatide, reaching up to 40–45% for WTP thresholds greater than $400,000 per QALY.Citation40
Of importance, there is a limited number of cost-effectiveness analyses assessing the comparative effectiveness of tirzepatide versus other drug classes; however, those analyses were all performed in T2DM populations with emphasis on glycemic control. As far as cost-effectiveness of tirzepatide for body weight reduction is concerned, Azuri et al demonstrated that the cost needed to treat per 1% of body weight reduction with tirzepatide was $985 (95% CI: $908-$1075) compared with $1845 (95% CI: $1707-$1989) with semaglutide.Citation71 Similarly, Zhang and McAdam Marx showed in their cost-effectiveness analysis, utilizing data from the T2DM dedicated SURPASS-2 trial that tirzepatide 10 mg once-weekly has an ICER of $237 per 1 kg weight loss, compared to semaglutide 1.0 mg once-weekly, suggesting that tirzepatide is a more cost-effective option for weight loss induction in individuals with T2DM, compared to semaglutide.Citation72
It has to be emphasized that the main adverse events associated with the administration of tirzepatide in overweight or obese subjects include nausea (28%), diarrhea (23%), vomiting (13%), constipation (11%), abdominal pain (10%) and dyspepsia (10%).Citation44
Therefore, despite the fact that tirzepatide is the most promising drug for the therapeutic management of obesity, there is no relevant real-world evidence to reinforce results demonstrated in RCTs, whereas there is also no solid evidence concerning its cost-effectiveness.
Other Anti-Obesity Agents
Several other agents are on the way for the therapeutic management of obesity in the near future. Oral semaglutide, at a single daily dose of 50 mg, has been recently shown in the OASIS 1 trial to produce a significant body weight reduction equal to 15.1% from baseline after 68 weeks of treatment in adults with obesity without T2DM.Citation73 Another oral, nonpeptide GLP-1 receptor agonist, named orforglipron, has been recently demonstrated in a small RCT to result in a significant body weight change up to 14.7% after 36 weeks of treatment in adults with obesity without concomitant T2DM.Citation74 Other drugs, such as cagrilintide, a once-weekly, subcutaneously administered, amylin analogue,Citation75 or survodutide, a dual glucagon and GLP-1 receptor agonist, also administered once-weekly subcutaneously,Citation76 have recently been tested in Phase 2 RCTs for the treatment of adults with obesity without T2DM, demonstrating significant effect on body weight reduction with a favorable safety profile.
Retatrutide is a triple agonist of the GIP, GLP-1 and glucagon receptors that has been tested in phase 2 trial enrolling adults with overweight or obesity, producing impressive weight loss, reaching up to 24.2% with the maximum dose after 48 weeks of treatment, while its use is typically associated with mild-to-moderate severity gastrointestinal adverse events.Citation77 Another agent under development for the treatment of obesity is bimagrumab (BYM338), a fully human monoclonal antibody that binds to the activin type II receptor (ActRII); data from a small, phase 2 trial enrolling individuals with T2DM and obesity demonstrated that bimagrumab resulted in a modest weight loss reduction, up to 6.5%, after 48 weeks of treatment, leading to significant reduction in total body fat mass, up to 20.5%, with an additional mild increase in total body lean mass.Citation78 Other agents with a shared mechanism of action include apitegromab, taldefgrobep, and the mitochondrial uncoupling agent HU6.Citation79 All those agents are under development, in early-phase trials (phase 2 or earlier), with no definitive conclusions regarding safety and efficacy so far.Citation79
However, there is no real-world evidence to date concerning the efficacy of those agents, whereas there are no cost-effectiveness analyses, as well. Therefore, the future in the pharmacological treatment of obesity appears to be bright, although there is an absolute need for adequate evidence from the real-world setting, in different patients’ populations worldwide.
Conclusion
A significant progress in the therapeutic management of obesity has been made over the last decade, with the development and approval for use of three highly efficacious drugs, namely semaglutide, liraglutide and tirzepatide. Real-world studies are confirmatory of the evidence retrieved from relevant RCTs in the field, demonstrating that semaglutide is more effective than liraglutide and tirzepatide might be more effective than the other two agents. Cost-effectiveness analyses are rather limited and restricted in the US and the UK, highlighting the superiority of semaglutide versus liraglutide, whereas no definitive conclusion regarding the cost-effectiveness of tirzepatide can be drawn, based on the limited relevant data. Thus, current knowledge can be utilized to inform and update relevant treatment algorithms of obesity, although there is an absolute need for additional cost-effectiveness analyses from different healthcare systems across the world, since obesity is a global epidemic with rapid increase in its prevalence even in the developing world.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024;403(10431):1027–1050. doi:10.1016/S0140-6736(23)02750-2
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi:10.1001/jama.2012.113905
- Di Angelantonio E, Bhupathiraju S, Wormser D, et al.; Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi:10.1016/S0140-6736(16)30175-1
- Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–953. doi:10.1016/S2213-8587(18)30288-2
- Morgen CS, Haase CL, Oral TK, Schnecke V, Varbo A, Borlaug BA. Obesity, cardiorenal comorbidities, and risk of hospitalization in patients with heart failure with preserved ejection fraction. Mayo Clin Proc. 2023;98(10):1458–1468. doi:10.1016/j.mayocp.2023.07.008
- Ramezankhani A, Azizi F, Hasheminia M, Hadaegh F. The impact of general and central obesity for all-cause hospitalization among Iranian adults: a 20 year follow-up-results from the TLGS cohort. BMC Public Health. 2023;23(1):903. doi:10.1186/s12889-023-15851-0
- Han E, Truesdale KP, Taber DR, Cai J, Juhaeri J, Stevens J. Impact of overweight and obesity on hospitalization: race and gender differences. Int J Obes Lond. 2009;33(2):249–256. doi:10.1038/ijo.2008.193
- Migliore E, Pagano E, Mirabelli D, et al. Hospitalization rates and cost in severe or complicated obesity: an Italian cohort study. BMC Public Health. 2013;13:544. doi:10.1186/1471-2458-13-544
- Biener A, Cawley J, Meyerhoefer C. The high and rising costs of obesity to the US health care system. J Gen Intern Med. 2017;32(Suppl 1):6–8. doi:10.1007/s11606-016-3968-8
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Effertz T, Engel S, Verheyen F, Linder R. The costs and consequences of obesity in Germany: a new approach from a prevalence and life-cycle perspective. Eur J Health Econ. 2016;17(9):1141–1158. doi:10.1007/s10198-015-0751-4
- Silveira EA, Mendonça CR, Delpino FM, et al. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: a systematic review and meta-analysis. Clin Nutr ESPEN. 2022;50:63–73. doi:10.1016/j.clnesp.2022.06.001
- Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447–1463. doi:10.1016/j.jand.2015.02.031
- Burgess E, Hassmén P, Welvaert M, Pumpa KL. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: a systematic review and meta-analysis. Clin Obes. 2017;7(2):105–114. doi:10.1111/cob.12180
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi:10.1056/NEJMoa1014296
- Machado AM, Guimarães NS, Bocardi VB, et al. Understanding weight regain after a nutritional weight loss intervention: systematic review and meta-analysis. Clin Nutr ESPEN. 2022;49:138–153. doi:10.1016/j.clnesp.2022.03.020
- Hartmann-Boyce J, Theodoulou A, Oke JL, et al. Long-term effect of weight regain following behavioral weight management programs on cardiometabolic disease incidence and risk: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2023;16(4):e009348. doi:10.1161/CIRCOUTCOMES.122.009348
- Riaz H, Khan MS, Siddiqi TJ, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of Mendelian randomization studies. JAMA Network Open. 2018;1(7):e183788. doi:10.1001/jamanetworkopen.2018.3788
- Drucker DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57:101351. doi:10.1016/j.molmet.2021.101351
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi:10.1016/j.cmet.2013.04.008
- Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20(7):463–474. doi:10.1038/s41569-023-00849-3
- Hammoud R, Drucker DJ. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol. 2023;19(4):201–216. doi:10.1038/s41574-022-00783-3
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756. doi:10.1016/j.cmet.2018.03.001
- Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173(4):278–286. doi:10.7326/M20-0864
- Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021;23(9):2116–2124. doi:10.1111/dom.14451
- Patoulias D, Popovic DS, Stoian AP, Janez A, Sahebkar A, Rizzo M. Effect of semaglutide versus other glucagon-like peptide-1 receptor agonists on cardio-metabolic risk factors in patients with type 2 diabetes: a systematic review and meta-analysis of head-to-head, Phase 3, randomized controlled trials. J Diabetes Complications. 2023;37(8):108529. doi:10.1016/j.jdiacomp.2023.108529
- Yao H, Zhang A, Li D, et al. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. doi:10.1136/bmj-2023-076410
- Shi Q, Nong K, Vandvik PO, et al. Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2023;381:e074068. doi:10.1136/bmj-2022-074068
- Qin W, Yang J, Deng C, Ruan Q, Duan K. Efficacy and safety of semaglutide 2.4 mg for weight loss in overweight or obese adults without diabetes: an updated systematic review and meta-analysis including the 2-year STEP 5 trial. Diabetes Obes Metab. 2024;26(3):911–923. doi:10.1111/dom.15386
- Smith I, Hardy E, Mitchell S, Batson S. Semaglutide 2.4 Mg for the management of overweight and obesity: systematic literature review and meta-analysis. Diabetes Metab Syndr Obes. 2022;15:3961–3987. doi:10.2147/DMSO.S392952
- Alabduljabbar K, Alsaqaaby M, Neff KJ, Crotty M, le Roux CW. Weight loss response in patients with obesity treated with injectable semaglutide in a real-world setting. Endocrine. 2024;83(2):392–398. doi:10.1007/s12020-023-03534-0
- Ruseva A, Michalak W, Zhao Z, Fabricatore A, Hartaigh BÓ, Umashanker D. Semaglutide 2.4 mg clinical outcomes in patients with obesity or overweight in a real-world setting: a 6-month retrospective study in the United States (SCOPE). Obes Sci Pract. 2024;10(1):e737. doi:10.1002/osp4.737
- Marzullo P, Daffara T, Mele C, et al. Real-world evaluation of weekly subcutaneous treatment with semaglutide in a cohort of Italian diabetic patients. J Endocrinol Invest. 2022;45(8):1587–1598. doi:10.1007/s40618-022-01799-2
- Thewjitcharoen Y, Yenseung N, Butadej S, et al. Real-world use of once-weekly semaglutide in Thai patients with type 2 diabetes mellitus in a private hospital setting. J ASEAN Fed EndocrSoc. 2023;38(1):21–28.
- Di Loreto C, Minarelli V, Nasini G, Norgiolini R, Del Sindaco P. Effectiveness in real world of once weekly semaglutide in people with type 2 diabetes: glucagon-like peptide receptor agonist naïve or switchers from other glucagon-like peptide receptor agonists: results from a retrospective observational study in Umbria. Diabetes Ther. 2022;13(3):551–567. doi:10.1007/s13300-022-01218-y
- Ghusn W, Fansa S, Anazco D, et al. Weight loss and cardiovascular disease risk outcomes of semaglutide: a one-year multicentered study. Int J Obes Lond. 2024;48:662–667. doi:10.1038/s41366-023-01456-5
- Garg SK, Kaur G, Haider Z, Rodriquez E, Beatson C, Snell-Bergeon J. Efficacy of semaglutide in overweight and obese patients with type 1 diabetes. Diabetes Technol Ther. 2024;26(3):184–189. doi:10.1089/dia.2023.0490
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–2232. doi:10.1056/NEJMoa2307563
- Sandhu H, Xu W, Olivieri AV, Lübker C, Smith I, Antavalis V. Once-weekly subcutaneous semaglutide 2.4 mg injection is cost-effective for weight management in the United Kingdom. Adv Ther. 2023;40(3):1282–1291. doi:10.1007/s12325-022-02423-8
- Gómez Lumbreras A, Tan MS, Villa-Zapata L, Ilham S, Earl JC, Malone DC. Cost-effectiveness analysis of five anti-obesity medications from a US payer’s perspective. Nutr Metab Cardiovasc Dis. 2023;33(6):1268–1276. doi:10.1016/j.numecd.2023.03.012
- Kim N, Wang J, Burudpakdee C, et al. Cost-effectiveness analysis of semaglutide 2.4 mg for the treatment of adult patients with overweight and obesity in the United States. J Manag Care Spec Pharm. 2022;28(7):740–752. doi:10.18553/jmcp.2022.28.7.740
- Hu Y, Zheng SL, Ye XL, et al. Cost-effectiveness analysis of 4 GLP-1RAs in the treatment of obesity in a US setting. Ann Transl Med. 2022;10(3):152. doi:10.21037/atm-22-200
- Lee M, Lauren BN, Zhan T, et al. The cost-effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obes Sci Pract. 2019;6(2):162–170. doi:10.1002/osp4.390
- Gudzune KA, Kushner RF. Medications for Obesity: a Review. JAMA. 2024. doi:10.1001/jama.2024.10816
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi:10.1056/NEJMoa1411892
- Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20(3):734–739. doi:10.1111/dom.13125
- Ansari HUH, Qazi SU, Sajid F, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists on body weight and cardiometabolic parameters in individuals with obesity and without diabetes: a systematic review and meta-analysis. Endocr Pract. 2024;30(2):160–171. doi:10.1016/j.eprac.2023.11.007
- Lin Q, Xue Y, Zou H, Ruan Z, Ung COL, Hu H. Efficacy and safety of liraglutide for obesity and people who are overweight: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol. 2022;15(12):1461–1469. doi:10.1080/17512433.2022.2130760
- Iqbal J, Wu HX, Hu N, et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes Rev. 2022;23(6):e13435. doi:10.1111/obr.13435
- Ahmad NN, Robinson S, Kennedy-Martin T, Poon JL, Kan H. Clinical outcomes associated with anti-obesity medications in real-world practice: a systematic literature review. Obes Rev. 2021;22(11):e13326. doi:10.1111/obr.13326
- Dobbie LJ, Coelho C, Mgaieth F, et al. Liraglutide 3.0 mg in the treatment of adults with obesity and prediabetes using real-world UK data: a clinical evaluation of a multi-ethnic population. Clin Obes. 2024;14. doi:10.1111/cob.12649
- Wilmington R, Ardavani A, Simenacz A, Green C, Idris I. Liraglutide 3.0 mg (Saxenda©) for weight loss and remission of pre-diabetes. real-world clinical evaluation of effectiveness among patients awaiting bariatric surgery. Obes Surg. 2024;34(1):286–289. doi:10.1007/s11695-023-06895-7
- Leventhal-Perek S, Shani M, Schonmann Y. Effectiveness and persistence of anti-obesity medications (liraglutide 3 mg, lorcaserin, and orlistat) in a real-world primary care setting. Fam Pract. 2023;40(5–6):629–637. doi:10.1093/fampra/cmac141
- Grannell A, Al-Najim W, le Roux C. Long-term weight outcomes in patients treated with liraglutide 3.0 mg in real-world clinical practice. Clin Obes. 2024;14(1):e12622. doi:10.1111/cob.12622
- Vinciguerra F, Di Stefano C, Baratta R, et al. Efficacy of high-dose liraglutide 3.0 mg in patients with poor response to bariatric surgery: real-world experience and updated meta-analysis. Obes Surg. 2024;34(2):303–309. doi:10.1007/s11695-023-07053-9
- Azuri J, Hammerman A, Aboalhasan E, Sluckis B, Arbel R. Liraglutide versus semaglutide for weight reduction-a cost needed to treat analysis. Obesity. 2023;31(6):1510–1513. doi:10.1002/oby.23752
- Evans M, Berry S, Malkin SJP, Hunt B, Sharma A. Evaluating the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus liraglutide 1.8 mg: a health economic analysis in the UK. Diabetes Ther. 2023;14(6):1005–1021. doi:10.1007/s13300-023-01408-2
- Wilke T, Mueller S, Fuchs A, Kaltoft MS, Kipper S, Cel M. Diabetes-related effectiveness and cost of liraglutide or insulin in German patients with type 2 diabetes: a 5-year retrospective claims analysis. Diabetes Ther. 2020;11(10):2357–2370. doi:10.1007/s13300-020-00903-0
- Johansen P, Chubb B, Hunt B, Malkin SJP, Sandberg A, Capehorn M. Evaluating the long-term cost-effectiveness of once-weekly semaglutide versus once-daily liraglutide for the treatment of type 2 diabetes in the UK. Adv Ther. 2020;37(5):2427–2441. doi:10.1007/s12325-020-01337-7
- Bain SC, Hansen BB, Malkin SJP, et al. Oral semaglutide versus empagliflozin, sitagliptin and liraglutide in the UK: long-term cost-effectiveness analyses based on the PIONEER clinical trial programme. Diabetes Ther. 2020;11(1):259–277. doi:10.1007/s13300-019-00736-6
- Gæde P, Johansen P, Tikkanen CK, Pollock RF, Hunt B, Malkin SJP. Management of patients with type 2 diabetes with once-weekly semaglutide versus dulaglutide, exenatide ER, liraglutide and lixisenatide: a cost-effectiveness analysis in the Danish setting. Diabetes Ther. 2019;10(4):1297–1317.
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925–1966. doi:10.1007/s00125-022-05787-2
- Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65(8):1251–1261. doi:10.1007/s00125-022-05715-4
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi:10.1056/NEJMoa2206038
- Pan XH, Tan B, Chin YH, et al. Efficacy and safety of tirzepatide, GLP-1 receptor agonists, and other weight loss drugs in overweight and obesity: a network meta-analysis. Obesity. 2024;32(5):840–856. doi:10.1002/oby.24002
- Jamal M, Alhashemi M, Dsouza C, et al. Semaglutide and tirzepatide for the management of weight recurrence after sleeve gastrectomy: a retrospective cohort study. Obes Surg. 2024;34(4):1324–1332. doi:10.1007/s11695-024-07137-0
- Rodriguez PJ, Goodwin Cartwright BM, Gratzl S, et al. Semaglutide vs tirzepatide for weight loss in adults with overweight or obesity. JAMA Intern Med. 2024. doi:10.1001/jamainternmed.2024.2525
- Buckley A, Suliman S, Allum M, et al. Real world use of tirzepatide in the treatment of type 2 diabetes in an Arab population. Diabetes Obes Metab. 2024;26(8):3381–3391. doi:10.1111/dom.15680
- Suzuki T, Sato T, Tanaka M, et al. Tirzepatide ameliorates eating behaviors regardless of prior exposure to glucagon-like peptide receptor agonists in Japanese patients with type 2 diabetes mellitus. J Diabetes Complications. 2024;38(7):108779. doi:10.1016/j.jdiacomp.2024.108779
- Patoulias D, Karakasis P, El-Tanani M, Rizzo M. Can tirzepatide untie the Gordian knot of eating disorders among individuals with type 2 diabetes and obesity? J Diabetes Complications. 2024;38:108812. doi:10.1016/j.jdiacomp.2024.108812
- Azuri J, Hammerman A, Aboalhasan E, Sluckis B, Arbel R. Tirzepatide versus semaglutide for weight loss in patients with type 2 diabetes mellitus: a value for money analysis. Diabetes Obes Metab. 2023;25(4):961–964. doi:10.1111/dom.14940
- Zhang X, McAdam Marx C. Short-term cost-effectiveness analysis of tirzepatide for the treatment of type 2 diabetes in the United States. J Manag Care Spec Pharm. 2023;29(3):276–284. doi:10.18553/jmcp.2023.29.3.276
- Knop FK, Aroda VR, Do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402(10403):705–719. doi:10.1016/S0140-6736(23)01185-6
- Wharton S, Blevins T, Connery L, et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N Engl J Med. 2023;389(10):877–888. doi:10.1056/NEJMoa2302392
- Lau DCW, Erichsen L, Francisco AM, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398(10317):2160–2172. doi:10.1016/S0140-6736(21)01751-7
- le Roux CW, Steen O, Lucas KJ, Startseva E, Unseld A, Hennige AM. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: a randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024;12(3):162–173. doi:10.1016/S2213-8587(23)00356-X
- Jastreboff AM, Kaplan LM, Frías JP, et al. Triple–hormone-receptor agonist retatrutide for obesity — a phase 2 trial. N Engl J Med. 2023;389(6):514–526. doi:10.1056/NEJMoa2301972
- Heymsfield SB, Coleman LA, Miller R, et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Network Open. 2021;4(1):e2033457. doi:10.1001/jamanetworkopen.2020.33457
- Henderson K, Sloan CE, Bessesen DH, Arterburn D. Effectiveness and safety of drugs for obesity. BMJ. 2024;384:e072686. doi:10.1136/bmj-2022-072686