Abstract
Hereditary factors are increasingly attracting the interest of behavioral scientists and practitioners. Our aim in the present article is to introduce some state-of-the-art topics in behavioral genetics, as well as selected findings in the field, in order to illustrate how genetic makeup can modulate the impact of environmental factors. We focus on the most-studied polymorphism to date for antisocial responses to adversity: the monoamine oxidase A gene. Advances, caveats, and promises of current research are reviewed. We also discuss implications for the use of genetic information in applied settings.
Introduction
Our aim in the present paper is to provide an update of studies on the monoamine oxidase A (MAOA) gene as a moderator of adversity-induced antisocial behavior (ASB). After a brief introduction of some key behavioral and molecular genetic concepts, we move on to review selected evidence on the MAOA–adversity–ASB triad; unlike previous reviews that focused only on correlational approaches, we also include experimental work that has shed light on the specific mechanisms underlying the aforementioned relationships. Moreover, we also discuss current and future issues in the applied use of genotyping.
Genes moderate behavior
Can genes moderate our daily behavioral responses? If so, how do genes moderate them? The moderation of behavior by genes, namely genetic sensitivity to environmental stimuli, is known as gene–environment (G × E) interaction. More specifically, G × E interactions are said to occur when the effects of the environment on individuals vary by their genotype, or conversely, when environments modulate genetic effects.Citation1 Behavioral G × E interaction studies thus operate according to the notion that individuals may have distinct genetic sensitivity to environmental conditions in the determination of a given outcome. Under this apparently simple reasoning, colliding views have been proposed regarding how G × E interactions take place.Citation2 Does genetic makeup confer modified sensitivity to environmental stressors, or does it provide differential responsiveness to both favoring and detrimental environments? The former perspective corresponds to the diathesis–stress model, wherein genes are thought to determine individuals’ vulnerability to environmental risk factors. The latter view matches the differential susceptibility model, which assumes that constitutional features shape general individual plasticity to environmental influences.Citation2,Citation3
While most G × E studies assume implicitly or explicitly the diathesis–stress model,Citation2,Citation3 some research has provided disagreeing evidence. For instance, van Ijzendoorn et alCitation4 found support for the differential susceptibility model on the serotonin transporter gene’s promoter region polymorphism (5HTTLPR) with a meta-analytic approach. Caucasian participants carrying the short allele – the risk variant – of the aforementioned polymorphism were more susceptible than individuals with the long allele to both positive environments (r=0.21 versus r=0.11) and negative environments (r=0.22 versus r=0.06) for different behavioral, psychiatric, and developmental measures. These results support the assertion that certain assumedly risk-related genetic variants may help determining the degree to which individuals can benefit from favorable environmental conditions.Citation5 In fact, carriers of risk alleles often have the lowest outcome scores in absence of environmental stressors and the highest scores when those stressors are present.Citation2 As commented later on in this paper, the differential susceptibility hypothesis deserves further investigation.
Despite the appeal of the approaches briefly outlined above, it must be noted that they conceive of individuals as passive recipients of environmental influences, which are considered as external and independent from the person. In this sense, a line of research led by Robert Plomin caused an upheaval in our understanding of behavioral genetics when he treated the environment as a dependent variable, only to find that environmental measures could be partially determined by genetic variation.Citation6
This led to the notion of gene–environment correlations (rG × E), which emerge when genetic and environmental factors covary synergistically.Citation7 Active rG × E occurs if individuals’ genes drive the environments they select (niche-picking). Passive rG × E is said to happen when individuals receive genetic and environmental characteristics that reinforce mutually. Evocative rG × E takes place when individuals elicit responses in the environment in a way that matches their own inherited qualities. School achievement constitutes an excellent example to illustrate how rG × E can be relevant. Youngsters with greater intellectual ability are more likely to attend class (active rG × E), where they may be stimulated by an academic environment (passive rG × E) in which they evoke intellect-enhancing behaviors from their teachers (eg, pedagogical attention) and peers (eg, amusing discussions or debates). Given the ubiquitous influence of rG × E, it has become customary to control for rG × E in interactional approaches,Citation8–Citation10 although this is not always accomplished properly.
Aiming to reunite the existing perspectives on G × E relationships, a new framework emerged under the name of gene–environment transactions,Citation8 which paralleled the development of the person–environment transactions models in personality and social psychology.Citation11 The neutrality of the term “transactions” is on purpose, to include both interactional and correlational approaches to G × E relationships. This seems to be the next direction in behavioral genetics to fully account for the boundaries, direction, and magnitude of gene-to-environment and environment-to-gene effects. In this section, we have reviewed how genes and environment relate to each other, either interacting or correlating in a number of ways.
Specific genes involved in behavioral responses
In the previous section, we reviewed theoretical and empirical relationships between genes, environment, and behavior. But, how does this translate into specific findings? Some polymorphisms have been associated to psychological outcomes, ranging from basic processes (eg, working memory capacity,Citation12 emotion perceptionCitation13) to complex social behaviors (eg, altruismCitation14); as time goes by, the number of such polymorphisms increases. In this sense, some genes have received more attention than others, depending on the existing preliminary evidence (eg, animal models) and theory on the role of certain genes (eg, putative implication in response to pathogen) when selecting candidate polymorphisms.Citation1
The most extensively studied G × E relationships to date are 5HTTLPR and stressful life events in depression,Citation15 and the MAOA gene and childhood maltreatment in antisocial behavior.Citation16 Genes related to dopaminergic activity, such as the catechol-O-methyl transferase (COMT) gene,Citation17 the dopamine transporter gene,Citation18 or polymorphisms encoding for distinct dopamine receptors (eg, DRD4),Citation19 have also been widely examined. Whereas infantile conduct disordersCitation19 and addictionsCitation20 are prominent outcomes in such studies, perhaps the most renowned G × E association involving dopamine systems is the effect of adolescent cannabis consumption in psychosis, varying upon COMT Val158/108Met genotype.Citation21
One cautionary note must be stated here. Most of the research presented so far is based on association studiesCitation1 involving a single gene in conjunction with environmental measures; therefore, findings thus far have to be confirmed either via meta-analyses or complementary approaches that include causal exploration and more-complex models, such as gene–gene interactions, neuroimaging genetics, or experimental G × E research.
Hence, some genes can interact with other genes at different loci in a process called epistasis, or gene–gene interaction.Citation8 The extent to which epistatic phenomena are relevant is still unclear, but their underlying logic is straightforward: because most complex traits have polygenic influences, might one gene buffer or enhance the effect of another? Epistatic interactions have been described in moodCitation22 and behaviorCitation23 disorders, heralding a thrilling area of inquiry.
Once associations have been established between genes, environment, and behavior, a more-complete understanding of these relationships can be achieved by measuring genes’ functional and biochemical correlates at a lower level of abstraction than the behavioral phenotype of interest.Citation24 The rationale behind this strategy is that outcomes intermediate to behavior should be influenced by a lesser number of genes with more-distinguishable effects.Citation24 For example, the COMT gene should have stronger effects on speed of performance than on general intelligence scores; in turn, concentration of the COMT enzyme in the frontal cortex should depend more on the COMT gene than on speed of performance.Citation25
Functional neuroimaging and other in vivo techniques can lead to the characterization of these intermediate phenotypes, which Gottesman and Gould labeled “endophenotypes.”Citation26 They restricted the concept to heritable, state-independent, outcome-related, and co-segregated features.Citation26 In the case mentioned above, one could test frontal levels of COMT as an endophenotype for intelligence.Citation25 Although the concept of endophenotype is not universally accepted, confirming previously hypothesized – or unexpected – biochemical, neuropsychological, or cognitive pathways for gene action gives G × E relationship findings a sounder ground.Citation9,Citation27
In addition, we can experimentally study the moderation of behavioral responses by the genotype in order to confirm the causal role of genes or the potential roles of expected moderators and to consequently bring these phenomena into the lab. In this sense, van Ijzendoorn and Bakermans-KranenburgCitation28 review different alternatives to study G × E interactions experimentally and they provide some specific examples, from the serotonin transporter gene (5-HTT) to the DRD4 gene. Although they do not mention any study involving the MAOA gene, they do provide some examples on substance abuse, which is closely related to ASB.
In the following, we will attempt to provide an update of studies on the MAOA gene as a moderator of adversity-induced ASB. Unlike previous reviews that focused only on correlational approaches, we also include experimental work that has shed light on the specific causal pathways through which genetic effects are exerted and discuss applied genotyping in behavioral settings.
The MAOA gene and its role in antisocial behavior
The MAOA gene was one of the first genes linked to antisocial behavior. Back in 1993, Brunner et alCitation29 detected a rare mutation of the MAOA gene in three generations of a Dutch family. This mutation hinders the synthesis of MAOA enzyme, which is responsible for degrading neurotransmitters such as serotonin or dopamine. All males carrying this structural anomaly had borderline intelligence and consistently displayed severe antisocial conducts such as arson and rape attempts, whereas females remained unaffected. (Brunner’s finding must not be confused with another recently identified mutation in the MAOA gene associated with a similar phenotypeCitation30). Located at the short arm of the X chromosome (position 11.23–11.4), the MAOA gene has ever since been central in genetic studies of antisocial behavior.
Given that the mutation described by Brunner et alCitation29 was highly uncommon, researchers inquired whether normal variations of the MAOA gene could be relevant for ASB. Sabol et alCitation31 found a polymorphic area in which a nucleotide sequence can be repeated 2, 3, 3.5, 4, or 5 times – although the authors did not report the 2 -repeat variant in their original paper. Differences in sequence repeats are called “ variable number of tandem repeats” (VNTR) and are generally associated to differential rates of transcription. In the case of the MAOA gene, the 3.5-repeat and 4-repeat alleles had a markedly higher transcriptional efficiency (ie, led to an increased production of the MAOA enzyme) than did the 3-repeat and 5-repeat alleles. Hence, individuals are usually classified as possessing high-efficiency (3.5-repeat or 4-repeat) or low-efficiency (2-repeat, 3-repeat, or 5-repeat) alleles for this gene. It must be noted that the MAOA gene is located at the X chromosome, and hence males are homozygous (ie, have only one copy of the allele). Females have two copies, and it is unclear how the expression of the MAOA gene is affected by the natural random inactivation of one of the X chromosomes.
Caspi et alCitation16 provided the first evidence that common variants of the MAOA gene could modulate individuals’ antisocial tendencies in interaction with childhood maltreatment in a longitudinal cohort comprehensively – the Dunedin study. Specifically, severely maltreated participants carrying the low-activity allele of the MAOA gene displayed the highest scores in disposition toward violence and antisocial personality disorder scores, and demonstrated the greatest proportions of adolescent conduct disorder and convictions due to violent behavior.
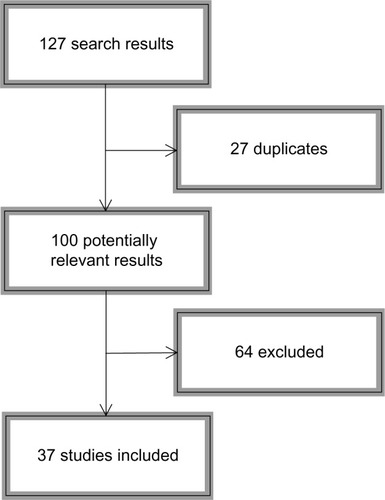
Since Caspi et alCitation16 published their milestone article, many studies have been conducted attempting to replicate and expand their findings to other domains of ASB. Given that Taylor and Kim-CohenCitation32 provided the last meta-analysis on the topic, we sought to review papers published in 2007 through 2013 tapping the MAOA gene–adversity–ASB triad. We conducted searches on PubMed and Web of Science, selecting only empirical (not theoretical) articles conducted on human subjects using at least one environmental measure of adversity. References were hand-searched for possible nonretrieved articles. depicts the search sequence; shows the search syntax used for each source.
Table 1 Search syntax to locate articles conducted on human subjects with at least one environmental measure of adversity
The studies reviewed are presented in and . A majority of the studies included in this review present statistically significant evidence for the interaction between the MAOA gene and environmental adversity measures (31 of 37). Overall, the pattern of results suggests that carriers of the low-activity variant of the MAOA gene are more likely to incur ASB when reared in adverse environments.Citation5,Citation13,Citation23,Citation33–Citation43 This pattern holds across disparate outcomes such as delinquency, aggressive dispositions, or attention deficit hyperactivity disorder. Among the several environmental stressors that are labeled as adversity, such as neglect, sexual abuse, or family dysfunction, childhood maltreatment shows the strongest effects in predicting ASB. There is thus remarkable variability across studies regarding measurement, sampling, and analytical procedures. Even if this complicates comparison between studies, it also increases confidence in the robustness of the MAOA-by-adversity interaction for ASB, as it has been found in a considerable range of samples assessed in a number of ways.
Table 2 Recent studies replicating the relationship between MAOA-uVNTR, adversity, and at least one kind of antisocial outcome in humans
Table 3 Recent studies failing to confirm the relationship between MAOA-uVNTR, adversity, and at least one kind of antisocial outcome in humans
Extrafamilial social environment appears to be a powerful moderator of the MAOA–adversity interaction. Material deprivation,Citation42 neighborhood disadvantage,Citation5,Citation44,Citation45 and adoption of the “street code”Citation43 all seem to add up to the effect of maltreatment, with low-activity allele carriers being at slightly greater risk for ASB in such rearing environments. Indeed, maltreatment is more likely to occur in an unfavorable broad social environment, which in turn offers fewer resources to overcome its pernicious consequences.Citation45
Consistent with the rationale above, G × E effects for convictions are not as strong as for milder indices of ASB.Citation5,Citation38,Citation42 This suggests that the types of ASB that lead to imprisonment may be more strongly influenced by macrosocial variables than solely by individual or familial features. However, failures to confirm this G × E interaction have also been reported.Citation46,Citation47 Perhaps the most outstanding among such findings are the results obtained by Haberstick et al,Citation48 who placed formidable effort into replicating the original study by Caspi et alCitation16 and did not find any interactive effect in a large sample.
Other studies have found G × E interactions with discordant results, associating the high-activity variant with greater ASB scores in the presence of early adversity;Citation49–Citation53 the reasons for these results are unclear. Even more intriguing, this trend is usual in female samples or subsamples.Citation13,Citation39,Citation54–Citation57
The latter results may be reflective of a gene–by–sex interaction, such that the low activity (MAOA-L) variant would be the predominant risk or plasticity variant in males, whereas the high activity (MAOA-H) variant would act as such in females. Unfortunately, the answer will remain subject to speculation until we have a clearer picture of the MAOA gene’s action in females. One of the main sources of general discrepancy across studies is the aforementioned methodological variability; the use of longitudinal versus cross-sectional approaches or the selection of instruments may influence results dramatically, let alone the ubiquitous measurement and sampling error.Citation58 Furthermore, other artifacts such as population stratification (ie, differences in allelic frequencies between subpopulations) might have also biased some results in multiethnic samples.Citation47,Citation48 Such bias could be supported by reports from Lea and Chambers,Citation59 who stirred controversy claiming that the increased percentage of 3-repeat alleles in a Maori population could represent a positive selection for behavioral aggression. However, their claims are not related to modern violence and are hardly generalizable to other populations, but they could explain a certain degree of population stratification. In fact, different allelic frequencies and variants could certainly alter the association between a gene and a complex genotype, which is the case of the oxytocin receptor geneCitation60 and the serotonin transporter gene.Citation61
Among noninteractive effects of the MAOA gene, the 2 repeat (2R) allele has been linked to enhanced delinquency, as well as to strikingly lower levels of transcriptional activity, in comparison to all other variants.Citation62,Citation63 Nonetheless, monogenic effects tend to yield relatively low effect sizes, and should not be considered separately from the environment in applied work.
ASB, as well as aggression, shows an exaggeratedly sex-specific prevalence. In fact, being a male dramatically increases the risk of displaying ASB, and one of the reasons that has been pointed out as an important determinant of these differences is the homozygosity of males for the MAOA gene.Citation64 Females’ heterozygosis may help to compensate the detrimental effects of the MAOA-L allele through developmental deactivation of the X chromosome that carries the low-activity allele. There is some evidence suggesting that the MAOA-H allele puts females in greater risk for overt ASB.Citation51 In any case, females have been shown to predominantly manifest ASB in subtler ways than males (eg, verbal rather than physical aggression).Citation65 Relational, nonphysical aggression is a field of research that should yield relevant results in the future. Interestingly, the few studies that have examined the role of MAOA in psychopathy – characterized by instrumental aggressive tendencies – have yielded small or nonexistent effects, thus reinforcing the implication of this gene in reactive, rather than proactive, aggression.Citation47,Citation66
So far we have presented correlational research in which outcomes are often aggregated or self-reported measures.Citation1 As in the case of other genes, as mentioned previously, some authors have brought this question into the lab and have shown that the MAOA gene also moderates specific, pinpointed behaviors. In this sense, McDermott et alCitation67 showed that after provocation, reactive aggression was higher among carriers of the low-activity allele of the MAOA gene than among carriers of the high-activity allele, a recently replicated finding.Citation68 Social exclusion has been usually put forward as a major source of provocation that leads to personal distress and eventually to aggression.Citation69 In this sense, Gallardo-Pujol et alCitation70 experimentally replicated the finding from Caspi et alCitation16 using an ad hoc procedure in which they combined behavioral genetics and social psychology. More specifically, they found that under conditions of social exclusion, MAOA-L carriers were twice as likely to engage in aggressive behaviors than were MAOA-H carriers. Another important contribution is that they provided guidelines for experimental research on G × E interactions. Interestingly, they suggest that experimental G × E research should follow these steps:
1) confirm that there is evidence of a given G × E interaction from epidemiological genetic research; 2) search for analogs of environmental risk and target behavior; 3) search for independent effects of these analogs upon the dependent variable (behavioral task); 4) check the plausibility of the effect of the environmental analog on the biological systems involved in the task; 5) check the evidence of an association for the candidate gene with similar laboratory tasks; 6) control for any possible confounding variables (by blocking, covariates, etc); 7) test for G × E interaction; and finally 8) perform independent replication and meta-analysis.Citation70
Once we have correlational and experimental evidence on the interaction of environmental adversity and the MAOA gene, what is the next step? What do we know about the causal mechanisms from the gene to behavior and its interaction with environmental stimuli? Some clues may lie in the neural correlates of the MAOA gene, as well as in brain areas responsive to adversity. If the cerebral circuitry associated to genetic variation couples with that observed in response to adversity, a clearer picture of the MAOA–adversity relationship may emerge. As commented next, this approach has yielded interesting results.
Eisenberger et alCitation71 found that carriers of the low-activity MAOA allele displayed greater activity in the anterior cingulate cortex as a response to experimentally induced social exclusion, as compared to MAOA-H and MAOA-H/L participants. This evidence suggested that carriers of the MAOA-L variant would experience greater distress when confronted with adverse conditions.
Alia-Klein et alCitation72 confirmed the functional relevance of the MAOA enzyme by showing moderate negative correlations of brain MAOA activity with trait aggression. However, no differences in enzymatic activity were found regarding genotype, in line with other research failing to find such relationship.Citation73 These puzzling findings suggest that MAOA genotype does not determine basal enzymatic action, in apparent contradiction with differences detected in structural and functional measures.Citation74 Speculations converge in pointing out that MAOA genotype may be especially crucial during early stages of development,Citation72,Citation73 such that the effects of genotype would only be relevant upon the action of a developmental or environmental disruption of this enzyme’s activity. In line with this formulation, Huizinga et alCitation75 found no G × E interaction when victimization was limited to adolescence.
Another study reported that MAOA-L males displayed structural reductions in emotional processing areas (ie, bilateral amygdala and cingulate cortex) but increased functional activity in these same areas when evaluating angry versus fearful faces.Citation74
Drawing upon this work, Buckholtz and Meyer-LindenbergCitation76 posited that the low-activity allele of the MAOA gene conferred a more labile sociocognitive processing system, characterized by an increased tendency to respond hostilely to aggression cues – even if ambiguous. Biochemically speaking, these differences may translate into an excess of amygdaline reactivity that demands greater frontal regulatory action in MAOA-L subjects; supporting this view, functional connectivity between ventromedial prefrontal cortex and amygdala has been associated with high levels of harm avoidance and angry hostility,Citation77 as well as low reward dependence, in MAOA-L (but not MAOA-H) males.Citation76 By default, this pattern would only be manifest in the shape of temperamental variations within the normal range. Nevertheless, early disruptions in the serotonin and epinephrine circuits, such as those caused by maltreatment or other forms of environmental adversity, would render MAOA-L participants more susceptible to reacting aggressively in social interactions. The serotonin deficiency hypothesis, which states that low serotonin levels would be associated with greater aggression, has been demonstrated to be an oversimplification,Citation78 but it appears clear nonetheless that the MAOA gene influences reactive aggression mainly through serotonergic imbalance. Note that the brain mechanisms mentioned above apply to structurally intact brains; however, a lesion study with war veterans has shown that an intact prefrontal cortex may be a prerequisite to detect genetic effects.Citation57 See .
Taken together, these studies shed light about the causal mechanisms by means of which environmental adversity is moderated by the genotype, therefore providing intervention targets if they are needed.
Personalized genomics in the management of antisocial behavior
In this section, we will focus on interventions for ASB, since it has been the most important outcome associated to the MAOA gene, and how they could be improved by taking into account existing knowledge. Personalized genomics in this particular case could be fitted into the broader framework of neuroprediction. Neuroprediction is the use of biological data to predict future behavior. This term recently gained prominence in the realm of risk management after the publication of a longitudinal study in which the use of neuroimaging data successfully predicted rearrest.Citation79,Citation80 As we will see by the end of this section, taking genomics into account can significantly improve risk management. The incremental validity of including genomics in applied behavioral management remains to be tested, although this practice has been routinely incorporated into cancer protocols.Citation81 Genetic effects are often small, and therefore it is very unlikely that a single gene would yield a large increment in validity. Nonetheless, the more we know about multiple genes in relation to ASB and their interactions with other risk factors, the more prepared we will be to properly manage them.
Most interventions to prevent or treat ASB are based on single psychosocial risk factors.Citation82 For instance, training in peer-group skills or mentoring programs are aimed at reducing the influence of deviant peers, a well-established risk factor. However, coming together with delinquent partners may well reflect active rG × E, as youngsters might seek out such environments partially due to their own inherited tendencies. Hence, it is no surprise that success rates obtained by programs of this kind are inconsistent.Citation82 Further quantitative genetics studies are required to draw clearer delineation between true criminogenic factors and those confounded with genetic influence. An accurate targeting of the factors to be modified could benefit not only society as a whole (eg, avoiding inefficient preventive policies), but also individuals (eg, by helping to adapt treatments for specific patients).Citation83 Noteworthy, there is a growing interest in targeting specific risk factors as the most efficient way to cure a disorder, or as some authors suggest, “cause should inform cure”.Citation84,Citation85
Following this line of reasoning, Collins,Citation86 among others, argued that diagnoses based on etiology rather than symptomatology should be more reliable and should allow for better-tailored environmental and behavioral treatments. This constitutes the core idea of what he called “personalized medicine”. Hence, the identification of specific genetic variants probabilistically associated with certain outcomes directly taps into Collins’ prophecy by allowing the assessment of constitutional risk and protective factors. In fact, this is so-called “therapygenetics”,Citation87 similar to pharmacogenetics.
The only study where the MAOA gene has been tested with therapeutic outcomes found that carriers of the high-activity allele had a worse response than low-activity carriers to cognitive–behavioral treatment for panic disorder with agoraphobia.Citation88 More specifically, participants with the high-activity variant displayed less improvement than their counterparts in self-reported anxiety, avoidance (only in females who completed the whole study), and clinician-rated global severity. Furthermore, participants’ responses in a behavioral avoidance test (BAT) revealed that subjects bearing the high activity allele had a faster heart rate in all BAT phases, benefitted less from repeated exposure, and reported more anxiety in anticipation and throughout the BAT than did low-activity allele carriers. This latter group also showed a neural activity pattern in a fear-conditioning paradigm suggestive of a better discrimination between anxiogenic stimuli. The implications of this study, pending replication, are manifold: carriers of the high-activity MAOA variant may benefit more from treatments focused on autonomic arousal (eg, relaxation, beta-adrenergic blockers) and might have an increased tendency to generalize learned fear responses, whereas individuals with the low-activity variants may have better response to usual cognitive-behavioral therapies, and thus might require less intensive pharmacological interventions. Similar studies with ASB as the main outcome would bring findings on the MAOA–adversity–ASB relationship into clinical practice, allowing the retest of epidemiological, correlational, and experimental evidence in a still-unexplored ambit.
Variations in the 5-HTT gene and in two DRD4 polymorphisms were tested in an early intervention program devised to improve mother–child attachment in maltreated and nonmaltreated 2 year olds.Citation89 Whereas no genetic effects were found in maltreated children, there was a greater proportion of risk gene carriers among insecurely attached, nonmaltreated controls. This result is suggestive that the possible genetic influences in the development of mother– child relationships in normative populations may be diminished in presence of maltreatment. Considering the strong associations between maternal attachment and all kinds of psychopathology, it would be of great value to test the role of the MAOA gene in attachment processes, and especially in the response to preventive interventions.Citation90
Overall, mapping patients’ genotypes seems to be especially useful in the prediction of stimulus-contingent outcomes, but it may not translate well to the prediction of general dispositions. Genetic effects for broad personality dimensions were found to be inconsistent in an exhaustive meta-analysis, with effect sizes of specific polymorphisms on personality being predominantly small to moderate.Citation91 However, as already commented, the MAOA gene may predispose toward reactive aggression after provocation; it is therefore interesting to consider MAOA genotype as a predictive tool in forensic or penitentiary settings, where hostile interpersonal interactions are common and estimating the risk of recidivism is crucial. We further argue that inquiring about the past maltreatment history of inmates or indicted individuals, complemented with their genetic makeup, can give important clues when it comes to assessing the likelihood of future ASB arising from social interactions.
It is almost compulsory to cite, in this regard, the work by Bernet et al,Citation92 who depicted a series of cases in which genetic information was presented as evidence in murder trials. The authors first reviewed precedent cases, in two of which they raised the possibility that conviction reductions could have been influenced by genotypic evidence. Subsequently, the authors related their own experience in the use of genotype in murder trials, where they exposed the extenuatory influence of either the MAOA or the 5-HTT risk allele in combination with past history of harsh discipline or maltreatment. It is noteworthy, however, that the authors selectively presented the genetic markers that most favored the defendants, obviating putative gene–gene interactive effects – only one case had the risk allele in both measured genes. Furthermore, they alleged a general predisposition toward violence conferred by genes instead of relating it to specific instances of the situation in which murder occurred. Nonetheless, genotypic evidence has also been presented in an Italian murder trial, in which the defense alleged genetic risk for aggressive reactions to provocation, without considering the defendant’s upbringing; in this case, the indicted saw his conviction reduced.Citation93
At this point we want to draw attention to the fact that utilizing specific genes in trials is a relatively novel practice, but the allegation of “genetic predisposition” – usually on the basis of familial aggregation – is more established; Pioro et al have recently found this term in 468 different legal decisions when reviewing Canadian judicial databases.Citation94 Farahany and ColemanCitation95 encouraged caution regarding the use of genetic factors as evidence in court, considering that behavioral genetic evidence was still poorly suited to apprehend individual cases, and that the prevailing concepts on liability – such as legal free will – drained a great deal of importance from genetic factors in law; see BaumCitation96 for further discussion. Although we agree on the need for wariness until more-thorough studies come out, the recently observed effects of the MAOA gene in experimental situations,Citation67,Citation70 obtained with very small samples and in controlled conditions, permit greater confidence in G × E interaction results at an individual level of analysis. Therefore, we suggest that the use of genotype in trials should be limited to cases of impulsive (rather than premeditated) crimes, with clearly demonstrable risk factors (eg, childhood severe adversity), and offering explicit and specific relationships between genetic makeup and the circumstances in which the offense occurred – such as provocation – that might have served as a trigger.
Finally, although internalizing psychopathology is not our present focus, we will introduce a brief note on the topic due to its relevance in forensic practice. In this ambit, it is worth mentioning a recent meta-analysis that has discarded the implication of the MAOA gene on suicidal behavior.Citation97 While the high activity variant of the MAOA gene seems to protect males from ASB, it may heighten liability to anxiety and depression in females.Citation52 This has led some researchers to label the MAOA as the “warrior–worrier gene”,Citation88 but more evidence is needed to test the validity of this somewhat simplistic tag. It appears wise, in future research, to explore how the MAOA gene may relate differently to the internalizing and externalizing spectra, or even with the recently derived general psychopathology factor, or p.Citation98
Conclusions and therapy implications
Future G × E studies should place special effort in sampling not only adverse environments, but also beneficial ones. The fact that presumed risk alleles have been related to greater positive responses to protective factorsCitation5,Citation99 questions several results presented here, as only adverse environmental factors are considered in the majority of G × E studies. Although great effort is expended to make samples representative of the different socioeconomic strata, a more-comprehensive assessment of identifiable positive factors would allow testing of whether some alleles are indeed simple risk variants, or if they map into greater behavioral variability in the presence of both detrimental and reinforcing environments.
Even if we only focus on the pathological side, multidimensional measures of environmental features can help uncover differential response patterns; Kinnaly et al,Citation100 for instance, used a self-report of parental care and a separate questionnaire of stressful life events (eg, death of a close relative, physical or sexual abuse), and, as explained in , found different effects for each environmental variable. Indeed, studies that go beyond the usual two-level or three-level scale for adversity often find subtle effects that cannot be captured in dichotomic measures.Citation49,Citation56,Citation101 These are just a few examples of how the impact of environmental variables can be diverse. This is of crucial importance for those theories of crime that focus only on environmental aspects, as disentangling the relative importance of each level of environmental factors can lead to more successful environmental interventions.
As we have seen so far, the MAOA gene, in conjunction with environmental adversity, seems to play a central role in the genesis of ASB and related outcomes. Even though there is still some controversy, epidemiological, clinical, and experimental research converges in providing evidence for it.
All in all, the take-home messages about incorporating G × E research in applied settings are three. First, with respect to ASB management, incorporating genetic data into current risk assessment procedures, such as HCR-20 or Offender Group Reconviction Scale,Citation102 could improve risk management protocols, especially in forensic settings.Citation103,Citation104 Second, taking genetic data into account could be useful in order to detect individuals at a greater risk of victimization,Citation89 or at least, the most vulnerable ones. And last but not least, the use of genetic data could be useful to compare the performance of specific psychological treatments versus specific pharmacological treatments, as Lester and Eley suggested.Citation105
Future research in this area should seek to fill the gaps in four main points. First, research testing the incremental validity of genomics testing in risk management settings must be conducted, especially using longitudinal designs. NeuropredictionCitation79 has proven successful in spite of criticisms concerning the reliability of neuroimaging data. Genetic data is often considered more reliable, though their effects are smaller. Also, modeling their interactions with environmental risk factors should lead to better risk management. Second, research on factors predisposing to victimization has been considered taboo, as some nonacademic organizations consider this as blaming the victims. However, this type of research would help focus our interventions on them. In turn, this would lead to a rationalization in the allocation of resources. Actually, personality allows identification of those individuals that lack most resources to cope with severe forms of maltreatment, such as childhood sexual abuse,Citation11 and we should not forget that personality traits are highly heritable.Citation106 Third, little is known about why different genotypes confer differential sensitivities to psychological treatments, although some hypotheses have been suggested. These hypotheses are yet to be tested. Finally, as some other researchers have pointed out, understanding the MAOA –adversity–ASB triad can lead to unraveling the causal mechanisms and therefore allow more-successful interventions.
In conclusion, the moderating role of the MAOA gene on the development of antisocial behavior is not only useful for basic research and advancing in the ethiological knowledge of ASB, but also for managing risk and interventions in applied clinical and forensic settings.
Disclosure
The authors report no conflicts of interest in this work.
References
- CaspiAMoffittTEGene-environment interactions in psychiatry: joining forces with neuroscienceNat Rev Neurosci20067758359016791147
- BelskyJJonassaintCPluessMStantonMBrummettBWilliamsRVulnerability genes or plasticity genes?Mol Psychiatry200914874675419455150
- BelskyJPluessMBeyond diathesis stress: differential susceptibility to environmental influencesPsychol Bull2009135688590819883141
- van IjzendoornMHBelskyJBakermans-KranenburgMJSerotonin transporter genotype 5 HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studiesTransl Psychiatry20122e14722872162
- BeaverKMNedelecJLWildeMLippoffCJacksonDExamining the association between MAOA genotype and incarceration, anger and hostility: The moderating influences of risk and protective factorsJ Res Pers2011453279284
- ScarrSMccartneyKHow people make their own environments: a theory of genotype greater than environment effectsChild Dev19835424244356683622
- O’ConnorTGDeater-DeckardKFulkerDRutterMPlominRGenotype-environment correlations in late childhood and early adolescence: antisocial behavioral problems and coercive parentingDev Psychol19983459709819779743
- JohnsonWPenkeLSpinathFMHeritability in the era of molecular genetics: some thoughts for understanding genetic influences on behavioural traitsEur J Pers2011254254266
- RutterMBiological implications of gene-environment interactionJ Abnorm Child Psychol200836796997518642072
- PlominRBergemanCSThe nature of nurture: genetic influence on “environmental” measuresBehav Brain Sci1991143373386
- Gallardo-PujolDPeredaNPerson-environment transactions: personality traits moderate and mediate the effects of child sexual victimization on psychopathologyPersonal Ment Health20137210211324343936
- ZiermansTDumontheilIRoggemanCWorking memory brain activity and capacity link MAOA polymorphism to aggressive behavior during developmentTransl Psychiatry20122e8522832821
- WakschlagLSKistnerEOPineDSInteraction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behaviorMol Psychiatry201015992893719255579
- KnafoAIsraelSEbsteinRPHeritability of children’s prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 geneDev Psychopathol2011231536721262039
- CaspiASugdenKMoffittTEInfluence of life stress on depression: moderation by a polymorphism in the 5 -HTT geneScience2003301563138638912869766
- CaspiAMcClayJMoffittTERole of genotype in the cycle of violence in maltreated childrenScience2002297558285185412161658
- QianQJLiuJWangYFYangLGuanLLFaraoneSVAttention Deficit Hyperactivity Disorder comorbid oppositional defiant disorder and its predominately inattentive type: evidence for an association with COMT but not MAOA in a Chinese sampleBehav Brain Funct20095819228412
- LangleyKTuricDRiceFTesting for gene x environment interaction effects in attention deficit hyperactivity disorder and associated antisocial behaviorAm J Med Genet B Neuropsychiatr Genet2008147B1495317579368
- LiDShamPCOwenMJHeLMeta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD)Hum Mol Genet200615142276228416774975
- GorwoodPLe StratYRamozNDubertretCMoalicJMSimonneauMGenetics of dopamine receptors and drug addictionHum Genet2012131680382222350797
- CaspiAMoffittTECannonMModeration of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interactionBiol Psychiatry200557101117112715866551
- Priess-GrobenHAHydeJS5-HTTLPR X stress in adolescent depression: moderation by MAOA and genderJ Abnorm Child Psychol201341228129422836288
- ReifARöslerMFreitagCMNature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environmentNeuropsychopharmacology200732112375238317342170
- MartinezDBroftALaruelleMImaging neurochemical endophenotypes: promises and pitfallsPharmacogenomics20012322323711535111
- BarnettJHScorielsLMunafòMRMeta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphismBiol Psychiatry200864213714418339359
- GottesmanIIGouldTDThe endophenotype concept in psychiatry: etymology and strategic intentionsAm J Psychiatry2003160463664512668349
- MoffittTECaspiARutterMStrategy for investigating interactions between measured genes and measured environmentsArch Gen Psychiatry200562547348115867100
- Van IjzendoornMHBakermans-KranenburgMJDifferential susceptibility experiments: going beyond correlational evidence: comment on beyond mental health, differential susceptibility articlesDev Psychol201248376977422545852
- BrunnerHGNelenMBreakefieldXORopersHHvan OostBAAbnormal behavior associated with a point mutation in the structural gene for monoamine oxidase AScience199326251335785808211186
- PitonAPoquetHRedinC20 ans après: a second mutation in MAOA identified by targeted high-throughput sequencing in a family with altered behavior and cognitionEur J Hum Genet201422677678324169519
- SabolSZHuSHamerDA functional polymorphism in the monoamine oxidase A gene promoterHum Genet199810332732799799080
- TaylorAKim-CohenJMeta-analysis of gene-environment interactions in developmental psychopathologyDev Psychopathol20071941029103717931432
- NilssonKWSjöbergRLWargeliusHLLeppertJLindströmLOrelandLThe monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumptionAddiction2007102338939817298646
- DucciFEnochMAHodgkinsonCInteraction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult womenMol Psychiatry200813333434717592478
- SjöbergRLDucciFBarrCSA non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behaviorNeuropsychopharmacology200833242543017429405
- EnochMASteerCDNewmanTKGibsonNGoldmanDEarly life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in childrenGenes Brain Behav201091657419804559
- AslundCNordquistNComascoELeppertJOrelandLNilssonKWMaltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescentsBehav Genet201141226227220734127
- FergussonDMBodenJMHorwoodLJMillerALKennedyMAMAOA, abuse exposure and antisocial behaviour: 30-year longitudinal studyBr J Psychiatry2011198645746321628708
- NilssonKWComascoEÅslundCNordquistNLeppertJOrelandLMAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumptionAddict Biol201116234735520731636
- RetiIMXuJZYanofskiJMonoamine oxidase A regulates antisocial personality in whites with no history of physical abuseCompr Psychiatry201152218819421295226
- CicchettiDRogoschFAThibodeauELThe effects of child maltreatment on early signs of antisocial behavior: genetic moderation by tryptophan hydroxylase, serotonin transporter, and monoamine oxidase A genesDev Psychopathol201224390792822781862
- FergussonDMBodenJMHorwoodLJMillerAKennedyMAModerating role of the MAOA genotype in antisocial behaviourBr J Psychiatry2012200211612322297589
- SimonsRLLeiMKStewartEASocial adversity, genetic variation, street code, and aggression: a geneticlly informed model of violent behaviorYouth Violence Juv Justice201210132423785260
- HillJBreenGQuinnJTibuFSharpHPicklesAEvidence for interplay between genes and maternal stress in utero: monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5 weeksGenes Brain Behav201312438839623480342
- HartDMarmorsteinNRNeighborhoods and genes and everything in between: understanding adolescent aggression in social and biological contextsDev Psychopathol200921396197319583892
- VerhoevenFEBooijLKruijtAWCeritHAntypaNDoesWThe effects of MAOA genotype, childhood trauma, and sex on trait and state-dependent aggressionBrain Behav20122680681323170243
- SadehNJavdaniSVeronaEAnalysis of monoaminergic genes, childhood abuse, and dimensions of psychopathyJ Abnorm Psychol2013122116717922985017
- HaberstickBCLessemJMHewittJKMAOA genotype, childhood maltreatment, and their interaction in the etiology of adult antisocial behaviorsBiol Psychiatry2014751253023726513
- PrichardZMackinnonAJormAFEastealSNo evidence for interaction between MAOA and childhood adversity for antisocial behaviorAm J Med Genet B Neuropsychiatr Genet2008147B222823218023041
- Va n der VegtEJOostraBAArias-VásquezAvan der EndeJVerhulstFCTiemeierHHigh activity of monoamine oxidase A is associated with externalizing behaviour in maltreated and nonmaltreated adopteesPsychiatr Genet200919420921119398936
- LeeSSDeviant peer affiliation and antisocial behavior: interaction with monoamine oxidase A (MAOA) genotypeJ Abnorm Child Psychol201139332133221152968
- NikulinaVWidomCSBrzustowiczLMChild abuse and neglect, MAOA, and mental health outcomes: a prospective examinationBiol Psychiatry201271435035722030358
- VanyukovMMMaherBSDevlinBThe MAOA promoter polymorphism, disruptive behavior disorders, and early onset substance use disorder: gene-environment interactionPsychiatr Genet200717632333218075472
- SjöbergRLNilssonKWWargeliusHLLeppertJLindströmLOrelandLAdolescent girls and criminal activity: role of MAOA-LPR genotype and psychosocial factorsAm J Med Genet B Neuropsychiatr Genet2007144B215916417034017
- KinnallyELHuangYYHaverlyRParental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult womenPsychiatr Genet200919312613319357553
- Prom-WormleyECEavesLJFoleyDLMonoamine oxidase A and childhood adversity as risk factors for conduct disorder in femalesPsychol Med200939457959018752729
- PardiniMKruegerFHodgkinsonCPrefrontal cortex lesions and MAO-A modulate aggression in penetrating traumatic brain injuryNeurolog y2011761210381045
- MonroeSMReidMWGene-environment interactions in depression research: genetic polymorphisms and life-stress polyproceduresPsychol Sci2008191094795619000200
- LeaRChambersGMonoamine oxidase, addiction, and the “warrior” gene hypothesisN Z Med J20071201250U244117339897
- KimHSShermanDKMojaverianTGene–culture interaction: oxytocin receptor polymorphism (OXTR) and emotion regulationSoc Psychol Personal Sci201126665672
- ChiaoJYBlizinskyKDCulture-gene coevolution of individualism-collectivism and the serotonin transporter geneProc Biol Sci2010277168152953719864286
- GuoGOuXMRoettgerMShihJCThe VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activityEur J Hum Genet200816562663418212819
- BeaverKMWrightJPBoutwellBBBarnesJCDelisiMVaughnMGExploring the association between the 2-repeat allele of the MAOA gene promoter polymorphism and psychopathic personality traits, arrests, incarceration, and lifetime antisocial behaviorPers Individ Dif2013542164168
- EmeRMale life-course-persistent antisocial behavior: the most important pediatric mental health problemArch Pediatr Adolesc Med2010164548648720439801
- LiJJLeeSLatent class analysis of antisocial behavior: interaction of serotonin transporter genotype and maltreatmentJ Abnorm Child Psychol201038678980120405199
- TikkanenRAuvinen-LintunenLDucciFPsychopathy, PCL-R, and MAOA genotype as predictors of violent reconvictionsPsychiatry Res2011185338238620850185
- McDermottRTingleyDCowdenJFrazzettoGJohnsonDDMonoamine oxidase A gene (MAOA) predicts behavioral aggression following provocationProc Natl Acad Sci U S A200910672118212319168625
- KuepperYGrantPWielpuetzCHennigJMAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocationBehav Brain Res2013247737823499704
- WilliamsKDOstracism: The kiss of social deathSoc Personal Psychol Compass200711236247
- Gallardo-PujolDAndrés-PueyoAMaydeu-OlivaresAMAOA genotype, social exclusion and aggression: an experimental test of a gene-environment interactionGenes Brain Behav201312114014523067570
- EisenbergerNIWayBMTaylorSEWelchWTLiebermanMDUnderstanding genetic risk for aggression: clues from the brain’s response to social exclusionBiol Psychiatry20076191100110817137563
- Alia-KleinNGoldsteinRZKriplaniABrain monoamine oxidase A activity predicts trait aggressionJ Neurosci200828195099510418463263
- FowlerJSAlia-KleinNKriplaniAEvidence that brain MAO A activity does not correspond to MAO A genotype in healthy male subjectsBiol Psychiatry200762435535817141746
- Meyer-LindenbergABuckholtzJWKolachanaBNeural mechanisms of genetic risk for impulsivity and violence in humansProc Natl Acad Sci U S A2006103166269627416569698
- HuizingaDHaberstickBCSmolenAChildhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotypeBiol Psychiatry200660767768317008143
- BuckholtzJWMeyer-LindenbergAMAOA and the neurogenetic architecture of human aggressionTrends Neurosci200831312012918258310
- BuckholtzJWCallicottJHKolachanaBGenetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personalityMol Psychiatry200813331332417519928
- DukeAABègueLBellREisenlohr-MoulTRevisiting the serotonin-aggression relation in humans: a meta-analysisPsychol Bull201313951148117223379963
- AharoniEVincentGMHarenskiCLNeuroprediction of future rearrestProc Natl Acad Sci U S A2013110156223622823536303
- NadelhofferTBibasSGraftonSNeuroprediction, violence, and the law: setting the stageNeuroethics201051679925083168
- WeitzelJNBlazerKRMacdonaldDJCulverJOOffitKGenetics, genomics, and cancer risk assessment: state of the art and future directions in the era of personalized medicineCA Cancer J Clin Epub8192011
- MoffittTEThe new look of behavioral genetics in developmental psychopathology: gene-environment interplay in antisocial behaviorsPsychol Bull2005131453355416060801
- RutterMPlominRPathways from science findings to health benefitsPsychol Med200939452954218700990
- UherRThe implications of gene-environment interactions in depression: will cause inform cure?Mol Psychiatry200813121070107818679406
- UherRGenes, environment, and individual differences in responding to treatment for depressionHarv Rev Psychiatry201119310912421631158
- CollinsFHas the revolution arrived?Nature2010464728967467520360716
- LesterKJEleyTCTherapygenetics: Using genetic markers to predict response to psychological treatment for mood and anxiety disordersBiol Mood Anxiety Disord201331423388219
- ReifARichterJStraubeBMAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapyMol Psychiatry201419112212823319006
- CicchettiDRogoschFAGene × Environment interaction and resilience: effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genesDev Psychopathol201224241142722559122
- CicchettiDRogoschFATothSLThe effects of child maltreatment and polymorphisms of the serotonin transporter and dopamine D4 receptor genes on infant attachment and intervention efficacyDev Psychopathol201123235737223786683
- MunafòMRClarkTGMooreLRPayneEWaltonRFlintJGenetic polymorphisms and personality in healthy adults: a systematic review and meta-analysisMol Psychiatry20038547148412808427
- BernetWVnencak-JonesCLFarahanyNMontgomerySABad nature, bad nurture, and testimony regarding MAOA and SLC6A4 genotyping at murder trialsJ Forensic Sci20075261362137117944904
- FeresinELighter Sentence for Murderer with “Bad Genes.”London, UKNature Publishing Group2014 Available from: http://www.nature.com/news/2009/091030/full/news.2009.1050.htmlAccessed May 28, 2014
- PioroMMykitiukRFinklerLNiskerJUnderstanding the use of “genetic predisposition” in canadian legal decisionsMcGill J Law Heal201371165
- FarahanyNAColemanJEJrGenetics and responsibility: to know the criminal from the crimeLaw Contemp Probl200669115164
- BaumMLThe monoamine oxidase A (MAOA) genetic predisposition to impulsive violence: is it relevant to criminal trials?Neuroethics201162287306
- HungCFLungFWHungTHMonoamine oxidase A gene polymorphism and suicide: an association study and meta-analysisJ Affect Disord2012136364364922041522
- CaspiAHoutsRMBelskyDWThe p Factor: one general psychopathology factor in the structure of psychiatric disorders?Clin Psychol Sci201322119137
- van IjzendoornMHBelskyJBakermans-KranenburgMJSerotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studiesTransl Psychiatry20122e14722872162
- KinnallyELHuangYHaverlyRParental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult womenPsychiatr Genet200919312613319357553
- WederNYangBZDouglas-PalumberiHMAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of traumaBiol Psychiatry200965541742418996506
- GrayNSSnowdenRJMacCullochSPhillipsHTaylorJMacCullochMJRelative efficacy of criminological, clinical, and personality measures of future risk of offending in mentally disordered offenders: a comparative study of HCR-20, PCL:SV, and OGRSJ Consult Clin Psychol200472352353015279535
- HudsonJLLesterKJLewisCMPredicting outcomes following cognitive behaviour therapy in child anxiety disorders: the influence of genetic, demographic and clinical informationJ Child Psychol Psychiatry201354101086109423772677
- DoehrmannOGhoshSSPolliFEPredicting treatment response in social anxiety disorder from functional magnetic resonance imagingJAMA Psychiatry2013701879722945462
- LesterKJEleyTCTherapygenetics: Using genetic markers to predict response to psychological treatment for mood and anxiety disordersBiol Mood Anxiety Disord201331423388219
- EbsteinRPThe molecular genetic architecture of human personality: beyond self-report questionnairesMol Psychiatry200611542744516534505
- NilssonKWWargeliusH-LSjöbergRLLeppertJOrelandLThe MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviourDrug Alcohol Depend2008931–2516218029114
- BeachSRBrodyGHGunterTDPackerHWernettPPhilibertRAChild maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorderJ Fam Psychol2010241122020175604
- DerringerJKruegerRFIronsDEIaconoWGHarsh discipline, childhood sexual assault, and MAOA genotype: an investigation of main and interactive effects on diverse clinical externalizing outcomesBehav Genet201040563964820364435
- EdwardsACDodgeKALatendresseSJMAOA-uVNTR and early physical discipline interact to influence delinquent behaviorJ Child Psychol Psychiatry201051667968719951362
- McGrathLMMustanskiBMetzgerAA latent modeling approach to genotype-phenotype relationships: maternal problem behavior clusters, prenatal smoking, and MAOA genotypeArch Womens Ment Health201215426928222610759
- WilliamsLMGattJMKuanSAA polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial indexNeuropsychopharmacology20093471797180919194374