Abstract
In vitro, in vivo animal, and human clinical data show a broad field of application for mesenchymal stem cells (MSCs). There is overwhelming evidence of the usefulness of MSCs in regenerative medicine, tissue engineering, and immune therapy. At present, there are a significant number of clinical trials exploring the use of MSCs for the treatment of various diseases, including myocardial infarction and stroke, in which oxygen suppression causes widespread cell death, and others with clear involvement of the immune system, such as graft-versus-host disease, Crohn’s disease, and diabetes. With no less impact, MSCs have been used as cell therapy to treat defects in bone and cartilage and to help in wound healing, or in combination with biomaterials in tissue engineering development. Among the MSCs, allogeneic MSCs have been associated with a regenerative capacity due to their unique immune modulatory properties. Their immunosuppressive capability without evidence of immunosuppressive toxicity at a global level define their application in the treatment of diseases with a pathogenesis involving uncontrolled activity of the immune system. Until now, the limitation in the number of totally characterized autologous MSCs available represents a major obstacle to their use for adult stem cell therapy. The use of premanufactured allogeneic MSCs from controlled donors under optimal conditions and their application in highly standardized clinical trials would lead to a better understanding of their real applications and reduce the time to clinical translation.
Introduction
Mesenchymal stem cells (MSCs) are multipotent adult cells that were first isolated and characterized from bone marrow. They were further identified by their ability to attach to the plastic of tissue culture dishes.Citation1 In the bone marrow, the multipotent stromal mesenchymal cells that have been isolated are part of the marrow microenvironment, together with endothelial and reticular cells, adipocytes, osteoblasts, and macrophages.Citation2,Citation3 In this context, MSCs are involved in many key events related to hematopoiesis, immune cell generation and activation, immunomodulation, and immune tolerance.Citation4 These processes are mediated by physical and chemical signals to which MSCs are responsive through phenotypic changes and growth factor production and secretion.Citation5 Furthermore, they are distributed in an undifferentiated state in their primary location throughout the bone marrow.Citation6 Most of the basic scientific and preclinical studies have been done using MSCs isolated from bone marrow. However, many different sources can be used, and fat could be especially relevant. Stem cells have been isolated from bone marrow aspirates, fat, striated, smooth, and cardiac muscle, the spleen, placenta, and umbilical cord blood, and may perhaps be isolated from other sources in which they are resident components or part of the reticular tissue associated with the vasculature.Citation7–Citation17 Human MSCs do not express the hematopoietic markers CD11a/lymphocyte function-associated antigen 1, CD14, CD31, CD34, or CD45, or the costimulatory molecules CD80, CD86, and CD40. However, they do express CD44, CD49, CD54/CD102, CD71, CD73, CD90, CD105, CD166, and CD271, among other cytokines and receptors that define their behavior under different conditionsCitation18–Citation20 (). Given optimal stimuli, MSCs can differentiate into phenotypes of many different types of mesenchymal cells including, but not limited to, the connective tissue of different organs, stroma, fat, muscle, bone, cartilage, and tendon, along with endothelial and neural lineages.Citation15 It has been observed that the beneficial effects of MSCs are not restricted to a unique tissue source. Human MSCs derived from adipose tissue have been shown to have an effect similar to those of the bone marrow in a murine model of graft-versus-host-disease (GVHD).Citation21 MSCs can be isolated, expanded in culture, and characterized in vitro.Citation6,Citation22 Due to these characteristics, MSCs have been used quickly in clinical trials and treatments. Autologous human MSCs were first infused in cancer patients, after a long culture period, without evidence of adverse effects.Citation23
Table 1 Surface markers for isolation and characterization of bone marrow mesenchymal stem cells
The goal of cell-based therapies is to use a strategy that includes a combination of activities that are aimed overall to replace, repair, or enhance the function of a cell type, tissue, organ, or system, using intact, amplified, or modified autologous or allogeneic cells. From the philosophical point of view, adult MSCs have a clear advantage over embryonic or fetal stem cells, in terms of basic biological aspects related to immunotolerance, differentiation, and transformation. MSCs are immunologically competent cells.Citation24 They are fully capable of undertaking an immunological response, and modulate some of the most important mechanisms in this complex response, and so have been used widely in a variety of alternatives for cell therapy.
Allogeneic or syngeneic MSCs
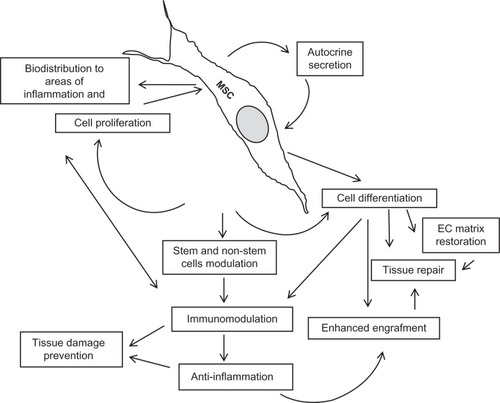
Clinical use of allogeneic MSCs for preventing or treating acute GVHD dates back to the 1970s.Citation25 While more limited application of allogeneic MSCs has been undertaken in tissue engineering and reparative medicine. In this therapeutic alternative, in which syngeneic cells are routinely used, the use of allogeneic MSCs is mostly restricted to regeneration of bone and restoration of the myocardium. Bone marrow MSCs are mobilized in response to tissue injury, such as hypoxia, ischemia, or necrosis, along with a variety of mediators associated with tissue damage.Citation26–Citation29 These cells migrate and settle in the damaged tissues and interact with resident cells and the stroma, secreting very important bioactive molecules that modify the redox potential, modulate apoptosis, induce cell proliferation, and recruit other cells, both stem and nonstem, which continue the reparative process and regulate the local immune response.Citation30–Citation32 This complex chain of events results mostly in incomplete tissue regeneration, but is the basis of resolution of tissue damage. In any case, there are two major mechanisms that support the rationale for use of MSCs, ie, replacement of damaged cells and local delivery of bioactive molecules.Citation33 Replacement of cells is the goal of cell therapy for regenerative medicine, and the release of biological signals, as mediators and receptors, is the basis of many cellular processes, including immunomodulation induced by stem cells (). These two mechanisms are clearly attributed to the effects reported after infusion of allogeneic MSCs. Meanwhile, additional immune effects are not clearly described in clinical trials for regenerative purposes using syngeneic cells. It is very likely and expected that both mechanisms are involved in the ideal reparative process.
Figure 1 Key mechanisms involved in beneficial effects of cell therapy using allogeneic mesenchymal stem cells.
Controversial data concerning the effects of MSCs on regulation of tumor growth have been reported for animal and in vitro models.Citation34–Citation37 However, no tumors have been found in human recipients of MSCs thus far, and remarkably, even aneuploidy MSCs have not given rise to tumors.Citation36 It remains controversial as to whether MSCs stimulate growth of other tumors, but there is no clinical validation of this as yet.Citation37–Citation39 The immunosuppression described in association with allogeneic MSC should be carefully evaluated in cancer patients.
Autologous MSCs have a clear advantage in that they constitute a closed therapeutic system. Their clinical use has been promoted based on avoidance of undesirable immune reactions and lack of contamination by unknown pathogens. In fact, the enormous amount of data generated for MSCs in regenerative medicine is based mainly on the use of autologous cells.
However, autologous MSCs have some obvious limitations. Their procurement requires surgical, albeit minimal, intervention via bone marrow aspiration in sometimes highly compromised patients. Many patients who could benefit from MSC infusion are elderly, in poor nutritional condition, and/or suffering from disorders associated with critical oxidative stress. With advancing age, significant changes in the function and composition of mature blood cells are observed. It has been reported that age-related changes also occur in the human hematopoietic stem cell system.Citation38 Similar results have been found in the MSC population obtained from bone marrow in animals.Citation39,Citation40 These issues might also affect the conclusions and validity of many clinical trials, the results of which are difficult to compare because of the characteristics of the patients included. Another limitation to the use of autologous cells is pre-existence of a proliferative or degenerative disease of the bone marrow, which will not only limit the amount of available cells, but may contribute to the original disease. Cultured MSCs derived from multiple myeloma patients have a distinctive array comparative genomic hybridization profile from that observed in their normal counterparts.Citation41 This may explain why MSCs from myeloma patients show an altered functional pattern with potential involvement in worsening of disease.Citation42,Citation43 A similar phenomenon can be seen in autoimmune diseases.Citation44 Further, the limited number of available autologous MSCs represents a major obstacle to their application. For a long time, it was assumed that MSCs were a stromal cell population with a high capacity for in vitro expansion. In reality, this assumption included three critical issues that needed to be addressed, ie, use of a heterogeneous cell population, the culture period, the cell duplications required to achieve the desired number of cells, and the use of xenogeneic supplements and/or potent cytokines. Culture conditions could have important effects on the phenotype of MSCs, affecting their efficiency, potency, and safety via immunogenicity, sensitizing to animal antigens, allergic reactions, or chromosomal stability.Citation45–Citation47 These effects can be avoided using defined media or autologous serum. Both procedures can prevent the formation of xenoantibodies and some of the undesirable reactions associated with administration of these cells.Citation48,Citation49 Unfortunately, defined media requires addition of cytokines, the long-term effects of which, on MSCs in culture is not well understood, and autologous serum collection has obvious limitations.
In contrast with the aforementioned difficulties associated with autologous MSCs, allogeneic MSCs have evident advantages, ie, immediate availability, no limitation of amount, the ability to do a donor selection based on different parameters (including age), the small time frame needed to perform complex quality control, and product stability. In addition, but with special importance, allogeneic MSCs are natural immune-privileged cells, as demonstrated by their persistence in maternal blood.Citation50 However, it is necessary to have a careful balance between the advantages of using an “off-the-shelf” product and the characteristics of each particular case, the preferences of the patient, the necessity for a Good Manufacturing Practice facility, and extensive preclinical evaluation prior to their application.
Allogeneic MSCs in human therapy
As previously mentioned, allogeneic MSCs are immune-privileged cells. Perhaps for this reason, allogeneic MSCs have been more widely used in the area of immunotherapy than in regenerative medicine. Encouraged by the early application and promising results using autologous bone marrow cells in clinical trials, the most studied model of tissue regeneration using allogeneic MSCs has been myocardial infarction.Citation51 Allogeneic MSCs have also been used in this model for regenerative purposes. Intramyocardial injection of allogeneic swine MSCs three days after myocardial infarction stimulated cardiac regeneration, decreasing infarct size. The animals showed remarkable improvement in ejection fraction and near normalization, without any associated immune events.Citation52 No arrhythmogenic events were observed, even when multiple doses were used.Citation53 Allogeneic MSCs are able to migrate and persist in the infarcted area, and act in a dose-dependent manner when they are administered intravenously.Citation54 In an acute myocardial infarct model using a combined single-photon emission/computed tomography scanner for imaging of the labeled cells, both focal and diffuse uptake of allogeneic MSCs in the infarcted myocardium was visible in the first 24 hours after intravenous injection and persisted until seven days after injection.Citation55 In another swine model, allogeneic MSCs showed the capacity to survive and engraft when injected into the affected myocardium 12 weeks following induced infarction. In female swine that received catheter-based transendocardial injection of male allogeneic MSCs, Y chromosome-positive cells expressed GATA-4, Nkx2.5, and alpha-sarcomeric actin. Some of the cells showing a vascular smooth muscle and endothelial phenotype appeared to contribute to local angiogenesis.Citation56 Induction of angiogenesis in the long-term post infarction area has been confirmed in fully immunocompetent pigs, but limited to viable myocardium adjacent to the infarct.Citation57,Citation58 Clearly, homing of allogeneic MSCs is associated with cooperative morphological and functional change in the restoration of damaged tissue.
The safety of intravenous injection of allogeneic MSCs has been reported in a randomized, double-blind, placebo-controlled, dose-escalation study using Prochymal® (Osiris Therapeutics Inc, Columbia, MD) after acute myocardial infarction. Prochymal is a premanufactured, universal donor formulation of human MSCs from different donors screened and tested according to US Food and Drug Administration requirements and processed under Good Manufacturing Practice guidelines in a scaled adaptation of the method described by Pittenger et al.Citation6 The ex vivo cultured MSC manufacturing process requires a total of five cell passages according to Food and Drug Administration Good Manufacturing Practice.Citation59 Global symptom score and ejection fraction was significantly improved in treated patients. After six months, a cardiac magnetic resonance imaging substudy showed that MSC treatment, but not placebo, increased left ventricular ejection fraction and led to reverse remodeling. There was also improvement in pulmonary function tests and a reduction in ventricular arrhythmias.Citation60
The beneficial effects of allogeneic MSCs in cardiac repair should be evaluated further, considering the particular immune capacities of these cells. Inflammation is a critical factor in evolution of myocardial ischemia, and the immunomodulation exerted by allogeneic MSCs could be a major factor in the generation of a favorable microenvironment for muscle protection and repair.Citation61 In addition, allogeneic MSCs could cooperate with local cells and provide the antiapoptotic activity necessary to limit the damage and sequelae of ischemic events. This activity is exerted by MSCs in the bone marrow, at the hematopoietic stem cell niche, where MSCs control the proliferation and differentiation needed to avoid the apoptotic events associated with quiescent cells.Citation18,Citation62
The capacity of allogeneic bone morphogenetic protein-2 (BMP-2)-engineered allogeneic MSCs to facilitate bone healing was studied in rats with a femoral segmental defect. The results showed that BMP-2-engineered allogeneic MSCs repaired bone defects to the same degree as in rats treated with BMP-2-engineered autologous MSCs. It was also demonstrated that allogeneic gene-transferred MSCs are directly involved in bone repair, in addition to acting as gene deliverers. The positive clinical benefits of allogeneic MSCs were dependent on their immunosuppressive and regenerative properties.Citation63 Nevertheless, another study using allogeneic MSCs loaded on hydroxyapatite-tricalcium phosphate implants enhanced, the repair of a critical-sized segmental defect in dog femurs without the use of immunosuppressive therapy. In this case, no adverse immune response was detected.Citation64 Furthermore, the absence of immunogenicity of allogeneic MSCs in orthopedics is an advantage for the clinical application of preconstructed tissue-engineered bone.Citation65 This lack of induction of an immune response should be considered a unique advantage in the use of genetically modified MSCs as carriers of therapeutic agents. The use of nonautologous genetically modified cells is beyond the scope of this review.
Allogeneic MSCs as immunomodulators
The first reported immunomodulatory activity of allogeneic MSCs was inhibition of T cell proliferation in vitro and in vivo.Citation66,Citation67 As with their reparative activity in tissues, allogeneic MSCs act through intercellular interaction and release a myriad soluble bioactive factors. Allogeneic MSCs have an inhibitory effect on the activity of antigen-presenting cells which impacts on T cell function.Citation68 The immunosuppressive activity of allogeneic MSCs is exerted via inhibitory mediators, such as prostaglandin E2, transforming growth factor beta-1, hepatocyte growth factor, and the human leukocyte antigen G isoform.Citation67–Citation70 Allogeneic MSCs induce upregulation of the indoleamine 2,3 intracellular pathway, dioxygenase expression, and inducible nitric oxide synthetase and heme oxygenase-1 that contribute to immune suppression.Citation71–Citation73 MSCs inhibit CD4+ and CD8+ T cells, the cytotoxic function of resting natural killer cells, and generation of innate and adaptive immune regulatory cell populations. MSCs upmodulate secretion of interleukin-10 by dendritic cells, that acts on T cells and downregulates production of interferon-gamma and interleukin-2. It has also been suggested that MSCs suppress the B cell proliferative response with regard to generation of antibodies.Citation69
Clinical application of allogeneic MSCs
In addition to the use of allogeneic MSCs in regenerative medicine, there has been a large amount of data generated in preclinical models of disease treated with allogeneic MSCs, mostly taking advantage of their capacity to modulate the local immune reaction. The first report of allogeneic MSCs acting as useful immunosuppressive agents demonstrated their capacity to prolong skin graft survival.Citation66 There is also diverse literature supporting the efficiency of allogeneic MSC infusion to enhance hematopoietic stem cell engraftment and prevention of GVHD.Citation74 Patient survival is poor when GVHD is unresponsive to steroid therapy. The successful use of allogeneic MSCs for the treatment of GVHD has been reported in isolated cases and multicenter nonrandomized trials.Citation75–Citation77 Prochymal in combination with steroids has been used in patients with Grade II–IV GVHD. Study endpoints included safety of Prochymal administration, induction of response, and overall response of GVHD by day 28, as well as long-term safety. Ninety-four percent of patients had an initial response to Prochymal (77% complete response and 16% had a partial response). No infusional toxicity or ectopic tissue formation was reported. There was no difference with respect to safety or efficacy between low and high Prochymal doses.Citation78 Further, a beneficial effect of Prochymal infusion was observed in pediatric patients, and more than 50% of treated patients responded after only one dose of cells.Citation59 The response rate varies in different studies, and without a clear explanation for these different responses. As previously mentioned, the methodology to expand the cells appears to be a critical step in the generation of allogeneic MSCs as a therapeutic tool. It is clear that allogeneic MSCs are potential candidates for the treatment of other conditions in which immune disorders, such as autoimmunity, are part of the pathogenesis. For example, Prochymal cells are been used in clinical trials for Crohn’s disease and osteoarthritis.Citation79
MSCs have been shown to pass through the blood–brain barrier and migrate throughout the forebrain and cerebellum without disrupting the host brain architecture.Citation80 Administration of allogeneic MSCs to mice with pre-established experimental autoimmune encephalomyelitis led to a significant decrease in the disease score over time comparable with that achieved with syngeneic MSCs. It was correlated with a blunting of immune cell infiltration in the spinal cord and reduced circulating levels of interferon-gamma and interleukin-17.Citation81 Human MSCs administered to mice with proteolipid protein-induced experimental autoimmune encephalomyelitis resulted in a reduction in disease severity, and this correlated well with an increase in axonal density and cells expressing nerve growth factor in association with immune modulatory events.Citation82,Citation83 MSCs appear to act in the central nervous system according to two general mechanisms, ie, replacement of cells and intense paracrine activity. In a focal ischemia animal model of middle cerebral artery occlusion in the rat, xenotransplantation of human MSCs induced functional improvement, reduced infarct volume, and conferred neuroprotection, possibly by providing insulin-like growth factor-1 and inducing vascular endothelial growth factor, epithelial growth factor, and basic fibroblast growth factor in the host brain.Citation84 Other authors report upregulation of interleukin-10 and downregulation of tumor necrosis factor-alpha, and an even earlier decrease of infarct volume.Citation85 In a similar experiment, significant increases in brain-derived neurotrophic factor and nerve growth factor were detected, while the number of apoptotic cells was significantly reduced in the ischemic area. Exposure of neurons to brain-derived neurotrophic factor increased activation of Akt pathways and protected neurons from trophic factor withdrawal. Treated animals showed proliferation of lymphocytes without induction of cytotoxic T lymphocytes.Citation86,Citation87 Beneficial effects in the treatment of neurological disorders are augmented by the high in vitro culture passages of MSCs, again stressing the relevance of culture technique and environment.Citation88
Multiple sclerosis is a major neurological and autoimmune problem in medicine, in which anti-inflammatory treatments have been used in the repair of damaged tissue without major success. It has been assumed that the central nervous system lesions are irreversible. Cell-based therapies have the potential to provide an alternative approach, according to data obtained from animal models of inflammatory nervous disease. The results from an animal model of experimental autoimmune encephalomyelitis give the rationale for use of MSCs in multiple sclerosis. MSCs apparently have an immunoregulatory or immunosuppressive action, but also stimulate the repair of neural structures. Under experimental conditions, MSCs induce immune tolerance, production of neurotrophins, and inhibit production of myelin-specific antibodies.Citation83,Citation89,Citation90 This capacity of MSCs has interested neurologists for more reasons than just the above-mentioned transdifferentiation that can occur in them under certain circumstances.Citation91,Citation92 Both autologous and allogeneic MSCs have been administered to a limited number of patients with multiple sclerosis. Preliminary data suggest the absence of major complications or toxicity with autologous expanded MSCs in patients with multiple sclerosis and amyotrophic lateral sclerosis.Citation93,Citation94
Based on a similar rationale, MSCs have been tested in different models of lung disease. Human allogeneic MSCs have been shown to restore alveolar epithelial fluid transport and the lung fluid balance from acute lung injury in an ex vivo perfused human lung preparation injured by Escherichia coli endotoxin. The treatment reduced extravascular lung water, improved lung endothelial barrier permeability, and restored alveolar fluid clearance. Of note, the authors refer to similar results using culture media conditioned by MSCs, and identify keratinocyte growth factor as essential for the beneficial effects.Citation95 Attenuation of obliterative bronchiolitis associated with trachea transplantation was observed in mice treated with MSCs. This effect was associated with a significant increase in secretion of interleukin-10 and a decrease in the expression of transforming growth factor-beta.Citation96 It has been reported that systemic injection of allogeneic MSCs protected the airway from allergen-induced pathology by reduction of IgE. This effect was associated with an increase in interleukin-10 and a decrease in interleukin-4 in bronchial fluid, and appears to be mediated by induction of regulatory T cells and secretion of immunosuppressive molecules, such as hepatocyte growth factor, which negatively regulates allergic airway inflammation and hyper-responsiveness.Citation97 The above data show the potential therapeutic use of allogeneic MSCs in many respiratory diseases, including chronic asthma.Citation98
The regenerative and immunomodulatory properties of allogeneic MSCs make them natural candidates for the treatment of diabetes.Citation99 Ongoing clinical trials are evaluating the effects of bone marrow-derived (in most cases autologous) MSCs for engraftment and survival of transplanted islets as well as possibly halting the complications of type 1 and type 2 diabetes.Citation100
Islets are destroyed in type 1 diabetes by an autoimmune process against beta cells, whereas in diabetes type 2, the islets have a functional alteration that results in inadequate glycemic control. Current clinical therapy using insulin and oral antidiabetic agents does not achieve complete metabolic control or avoid the complications associated with the disease. In this scenario, a genuinely substitutive therapy which restores functional pancreatic tissue appears to be the best alternative for patients with diabetes.
Bone marrow transplantation has been shown to contribute to the prevention of islet destruction,Citation101 and clinical trials have demonstrated the ability of allogeneic islet transplants to impact positively on glycemic control in patients with type 1 diabetes. However, the need for lifelong immunosuppression currently limits the indication of islet transplantation.Citation100,Citation101 Another potential application of MSCs could be enhancement of allogeneic islet cell engraftment and survival. This property has already been demonstrated in a nonhuman primate model.Citation102 Additional infusions of donor-specific or third party MSCs resulted in reversal of rejection episodes in animals. In sublethally irradiated mice with type 1 diabetes induced by streptozotocin, serum blood glucose and insulin returned to normal levels in parallel with efficient tissue regeneration after a single injection of bone marrow cells and MSCs. The cell therapy was only effective when both types of cells were combined. This was the result of a reparative and not regenerative process, since no donorderived cells were found in the pancreas of treated animals. Beta cell-specific T lymphocytes disappeared in the pancreas as a result of MSC injection, demonstrating a dual effect of cell repair and immunomodulation.Citation103
In addition to the abovementioned immunomodulatory effect, there is evidence that MSCs are able to participate in the islet regenerative process on the basis of their capacity to generate insulin-producing cells.Citation104,Citation105 These insulin-producing cells express multiple genes related to the development or function of pancreatic beta cells, including high expression of pancreatic and duodenal homeobox 1, insulin, and glucagon, and could release insulin in a glucose-dependent manner that led to amelioration of diabetes in streptozotocin-treated nude mice.Citation104,Citation105 It is interesting that in vivo hyperglycemia appears to be an important factor in bone marrow-derived MSC differentiation into insulin-producing cells capable of normalizing hyperglycemia in a diabetic animal model.Citation106–Citation108
The well studied effects of MSCs on angiogenesis and myogenesis also have importance in the treatment of the cardiovascular complications of diabetes. MSCs induce myogenesis and angiogenesis by releasing angiogenic, mitogenic, and antiapoptotic factors, including vascular endothelial growth factor, insulin-like growth factor-1, adrenomedullin, and hepatocyte growth factor.Citation109 Transplanted MSCs were shown to differentiate into cardiomyocytes and improve myogenesis and angiogenesis, with improvement in cardiac disorders.Citation109 This effect has also been attributed to the release of MSC-derived paracrine factors capable of cardioprotection. Autologous MSCs have been successfully used in the treatment of severe diabetic limb ischemia.Citation110 Bradycardia, decreased left ventricular pressure, decreased contractility index, and increased arterial pressure occur in diabetic animals because of cardiac sympathetic nerve impairment.Citation111 It is accepted that insulin can improve cardiac function by its inotropic effect of reducing blood glucose levels to prevent further myocardial remodeling.Citation112 Treatment of diabetic rats with allogeneic MSCs results in a significant increase in heart rate, left ventricular pressure, and contractility index, as well as a notable reduction of systolic blood pressure. These results appear to be associated with lowering of serum glucose and increased serum insulin levels, with homing of implanted cells detected in the pancreas and heart.Citation113 In mice with streptozotocin-induced type 1 diabetes, injection of MSCs reduced albuminuria, and the glomeruli were histologically normal. In the corresponding control group, untreated diabetic mice showed glomerular hyalinosis and mesangial expansion, in association with pancreatic islet degeneration.Citation114 Data from studies using NOD/SCID mice transplanted with human MSCs and C57Bl/6 mice transplanted with murine MSCs indicate that injected MSCs engraft in damaged kidneys, differentiate into renal cells, and regulate the immune response, resulting in efficient treatment of diabetic nephropathy because MSCs are able to reconstitute the necrotic segments of diabetic kidneys.Citation114–Citation116 Due to their intense paracrine activity, with release of angiogenic and neurotrophic factors, as well as their already mentioned potential capacity to convert in bone marrow mononuclear cells, MSCs have been studied for their capacity to improve diabetic neuropathy and associated wound healing impairment.Citation117,Citation118 A list of clinical trials using autologous and allogeneic MSCs for a wide range of conditions is showed in .
Table 2 Clinical evaluation of autologous and allogeneic MSCsTable Footnote*
Conclusion
In vitro, in vivo animal, and human clinical data show a wide range of potential applications for MSCs. There is overwhelming evidence of their usefulness in regenerative medicine, tissue engineering, and immune therapy. Although adult MSC transformation can be observed in vitro and in animals, no malignant transformation of implanted cells has been reported in patients. Infusion of allogeneic MSCs appears to be free of major hazardous events and does not raise any ethical issues, such as those related to use of human embryonic stem cells. MSCs migrate to sites of tissue injury in response to local signals, with critical phenotypic changes and intense paracrine activity that contributes to the reparative process. Among the MSCs, allogeneic MSCs have unique immunomodulatory properties. Their immunosuppressive capabilities without evidence of immunosuppressive toxicity at a global level define their application in the treatment of diseases in which the pathogenesis involves uncontrolled activity of the immune system. Infusion of allogeneic MSCs or their coinfusion with autologous MSCs has shown promising results in GVHD, diabetes, lung injury, Crohn’s disease, and multiple sclerosis. It is important to mention the data related to the regenerative capacity of allogeneic MSCs in cardiac and neural disease, as well as in diabetes-related loss of functional mass in the kidney. In addition, the capacity of allogeneic MSCs to facilitate engraftment of other cells and their potential association with smart scaffolds used to seed other stem cells with regenerative purposes should be explored further.
The limited availability of autologous MSCs has been a major obstacle to their application. This has been resolved by extended culture methods. However, this generalized procedure is not free of potential risks. The variability in culture methods conspires to make the establishment of standard treatment procedures and generation of definitive data for clinical application more difficult. Minimal changes in the culture procedure will produce heterogeneous cell populations. The longer the culture period, the greater the risk of cytogenetic changes being observed in vitro. The use of supplements to reduce culture time may have undesirable and unpredictable effects on the immunogenicity and biological activity of the cells implanted. The use of premanufactured allogeneic MSCs from controlled donors under optimal conditions and their application in highly standardized clinical trials would lead to a better understanding of their clinical applications and reduce the time to clinical translation.
Disclosure
The authors report no conflicts of interest in this work.
References
- FriedensteinAJChailakhyanRKLatsinikNVPanasyukAFKeiliss-BorokIVStromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivoTransplantation19741743313404150881
- Davis-SproulJMHarrisMPDavidsonNEKobrinBJJaffeeEMEmensLACost-effective manufacture of an allogeneic GM-CSF-secreting breast tumor vaccine in an academic cGMP facilityCytotherapy200571465616040383
- BordignonCCarlo-StellaCColomboMPCell therapy: achievements and perspectivesHaematologica199984121110114910586214
- MorrisonSJShahNMAndersonDJRegulatory mechanisms in stem cell biologyCell19978832872989039255
- AugelloADe BariCThe regulation of differentiation in mesenchymal stem cellsHum Gene Ther201021101226123820804388
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- WilliamsJTSoutherlandSSSouzaJCalcuttAFCartledgeRGCells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypesAm Surg199965122269915526
- HansonSEKimJJohnsonBHCharacterization of mesenchymal stem cells from human vocal fold fibroblastsLaryngoscope2010120354655120131365
- JazedjeTPerinPMCzeresniaCEHuman fallopian tube: a new source of multipotent adult mesenchymal stem cells discarded in surgical proceduresJ Transl Med200974619538712
- HuangTFChenYTYangTHIsolation and characterization of mesenchymal stromal cells from human anterior cruciate ligamentCytotherapy200810880681419023768
- D’AndreaFDe FrancescoFFerraroGALarge-scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue bankingTissue Eng Part C Methods200814323324218781836
- SarugaserRLickorishDBakshDHosseiniMMDaviesJEHuman umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitorsStem Cells200523222022915671145
- HoogduijnMJCropMJPeetersAMHuman heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacitiesStem Cells Dev200716459760417784833
- LinTMChangHWWangKHIsolation and identification of mesenchymal stem cells from human lipoma tissueBiochem Biophys Res Commun2007361488388917679141
- MiaoZJinJChenLIsolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cellsCell Biol Int200630968168716870478
- DickerALe BlancKAstromGFunctional studies of mesenchymal stem cells derived from adult human adipose tissueExp Cell Res2005308228329015925364
- LeeOKKuoTKChenWMLeeKDHsiehSLChenTHIsolation of multipotent mesenchymal stem cells from umbilical cord bloodBlood200410351669167514576065
- UccelliAMorettaLPistoiaVMesenchymal stem cells in health and diseaseNat Rev Immunol20088972673619172693
- ChamberlainGFoxJAshtonBMiddletonJConcise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homingStem Cells200725112739274917656645
- DominiciMLe BlancKMuellerIMinimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statementCytotherapy20068431531716923606
- YanezRLamanaMLGarcia-CastroJColmeneroIRamirezMBuerenJAAdipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host diseaseStem Cells200624112582259116873762
- BruderSPJaiswalNHaynesworthSEGrowth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservationJ Cell Biochem19976422782949027588
- LazarusHMHaynesworthSEGersonSLRosenthalNSCaplanAIEx vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic useBone Marrow Transplant19951645575648528172
- MedawarPBThe homograft reactionProc R Soc Lond B Biol Sci195814993514516613614379
- DickeKAvan der WaaijDvan BekkumDWThe use of stem-cell grafts in combined immune deficienciesBirth Defects Orig Artic Ser1975111391396238682
- GaspardoneADe FabritiisPScaffaRStem cell mobilization after coronary artery bypass graftingItal Heart J Suppl200451232815253141
- CoralliniFSecchieroPBeltramiAPTNF-alpha modulates the migratory response of mesenchymal stem cells to TRAILCell Mol Life Sci20106781307131420063037
- DasRJahrHvan OschGJFarrellEThe role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approachesTissue Eng Part B Rev201016215916819698058
- Mobius-WinklerSHilbergTMenzelKTime-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individualsJ Appl Physiol200910761943195019797690
- PayneTROshimaHOkadaMA relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic heartsJ Am Coll Cardiol200750171677168417950150
- LiHZuoSHeZParacrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survivalAm J Physiol Heart Circ Physiol2010299617721781
- GnecchiMZhangZNiADzauVJParacrine mechanisms in adult stem cell signaling and therapyCirc Res2008103111204121919028920
- GenoveseJASpadaccioCRivelloHGToyodaYPatelANElectrostimulated bone marrow human mesenchymal stem cells produce follistatinCytotherapy200911444845619530028
- LiXLingWPennisiAHuman Placenta-Derived Adherent Cells Prevent Bone Loss, Stimulate Bone Formation, and Suppress Growth of Multiple Myeloma in BoneStem Cells201129226327321732484
- LiLTianHYueWZhuFLiSLiWHuman mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vitroJ Cell Physiol201122671860186721442622
- RhodesLVMuirSEElliottSAdult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independenceBreast Cancer Res Treat2010121229330019597705
- ZhuYSunZHanQHuman mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1Leukemia200923592593319148141
- KurandaKVargaftigJde la RocherePAge-related changes in human hematopoietic stem/progenitor cellsAging Cell201110354254621418508
- DresslerMRButlerDLBoivinGPEffects of age on the repair ability of mesenchymal stem cells in rabbit tendonJ Orthop Res200523228729315734238
- KatsaraOMahairaLGIliopoulouEGEffects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cellsStem Cells Dev20112091549156121204633
- GarayoaMGarciaJLSantamariaCMesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donorsLeukemia20092381515152719357701
- LiBFuJChenPZhuangWImpairment in immunomodulatory function of mesenchymal stem cells from multiple myeloma patientsArch Med Res201041862363321199732
- WangXZhangZYaoCAngiogenic activity of mesenchymal stem cells in multiple myelomaCancer Invest2011291374121166497
- NieYLauCLieAChanGMokMDefective phenotype of mesenchymal stem cells in patients with systemic lupus erythematosusLupus201019785085920511276
- HaynesworthSEBaberMACaplanAICytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alphaJ Cell Physiol199616635855928600162
- UeyamaHHoribeTHinotsuSChromosomal variability of human mesenchymal stem cells cultured under hypoxic conditionsJ Cell Mol Med2011 [Epub ahead of print.]
- SundinMRingdenOSundbergBNavaSGotherstromCLe BlancKNo alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipientsHaematologica20079291208121517666368
- SpeesJLGregoryCASinghHInternalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapyMol Ther20049574775615120336
- ChachquesJCHerrerosJTraininiJAutologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplastyInt J Cardiol200495Suppl 1S29S3315336842
- O’DonoghueKChanJde la FuenteJMicrochimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancyLancet2004364942917918215246731
- WollertKCMeyerGPLotzJIntracoronary autologous bonemarrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trialLancet2004364942914114815246726
- AmadoLCSaliarisAPSchuleriKHCardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarctionProc Natl Acad Sci U S A200510232114741147916061805
- PohKKSperryEYoungRGFreymanTBarringhausKGThompsonCARepeated direct endomyocardial transplantation of allogeneic mesenchymal stem cells: safety of a high dose, “off-the-shelf”, cellular cardiomyoplasty strategyInt J Cardiol2007117336036416889857
- WolfDReinhardASeckingerAKatusHAKuechererHHansenADose-dependent effects of intravenous allogeneic mesenchymal stem cells in the infarcted porcine heartStem Cells Dev200918232132918435573
- KraitchmanDLTatsumiMGilsonWDDynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarctionCirculation2005112101451146116129797
- QuevedoHCHatzistergosKEOskoueiBNAllogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacityProc Natl Acad Sci U S A200910633140221402719666564
- PonceletAJHielALVercruysseJHermansDZechFGianelloPIntracardiac allogeneic mesenchymal stem cell transplantation elicits neo-angiogenesis in a fully immunocompetent ischaemic swine modelEur J Cardiothorac Surg201038678178720434353
- PatelANSpadaccioCKuzmanMImproved cell survival in infarcted myocardium using a novel combination transmyocardial laser and cell delivery systemCell Transplant200716989990518293888
- PrasadVKLucasKGKleinerGIEfficacy and safety of ex vivo cultured adult human mesenchymal stem cells (prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use studyBiol Blood Marrow Transplant201117453454120457269
- HareJMTraverseJHHenryTDA randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarctionJ Am Coll Cardiol200954242277228619958962
- FrantzSBauersachsJErtlGPost-infarct remodelling: contribution of wound healing and inflammationCardiovasc Res200981347448118977766
- JonesSHorwoodNCopeADazziFThe antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cellsJ Immunol200717952824283117709496
- TsuchidaHHashimotoJCrawfordEManskePLouJEngineered allogeneic mesenchymal stem cells repair femoral segmental defect in ratsJ Orthop Res2003211445312507579
- ArinzehTLPeterSJArchambaultMPAllogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defectJ Bone Joint Surg Am200385-A101927193514563800
- GuoSQXuJZZouQMJiangDMImmunological study of allogeneic mesenchymal stem cells during bone formationJ Int Med Res20093761750175920146873
- BartholomewASturgeonCSiatskasMMesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivoExp Hematol2002301424811823036
- Di NicolaMCarlo-StellaCMagniMHuman bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuliBlood200299103838384311986244
- AggarwalSPittengerMFHuman mesenchymal stem cells modulate allogeneic immune cell responsesBlood200510541815182215494428
- TolarJLe BlancKKeatingABlazarBRConcise review: hitting the right spot with mesenchymal stromal cellsStem Cells20102881446145520597105
- SelmaniZNajiAZidiIHuman leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cellsStem Cells200826121222217932417
- MeiselRZibertALaryeaMGobelUDaubenerWDillooDHuman bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradationBlood2004103124619462115001472
- RenGZhangLZhaoXMesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxideCell Stem Cell20082214115018371435
- ChabannesDHillMMerieauEA role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cellsBlood2007110103691369417684157
- MaitraBSzekelyEGjiniKHuman mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activationBone Marrow Transplant200433659760414716336
- Le BlancKRasmussonISundbergBTreatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cellsLancet200436394191439144115121408
- RingdenOUzunelMRasmussonIMesenchymal stem cells for treatment of therapy-resistant graft-versus-host diseaseTransplantation200681101390139716732175
- Le BlancKFrassoniFBallLMesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II studyLancet200837196241579158618468541
- KebriaeiPIsolaLBahceciEAdult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host diseaseBiol Blood Marrow Transplant200915780481119539211
- NewmanREYooDLeRouxMADanilkovitch-MiagkovaATreatment of inflammatory diseases with mesenchymal stem cellsInflamm Allergy Drug Targets20098211012319530993
- KopenGCProckopDJPhinneyDGMarrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brainsProc Natl Acad Sci U S A19999619107111071610485891
- RafeiMBirmanEFornerKGalipeauJAllogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitisMol Ther200917101799180319602999
- ZhangJLiYLuMBone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis miceJ Neurosci Res200684358759516773650
- GerdoniEGalloBCasazzaSMesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitisAnn Neurol200761321922717387730
- WakabayashiKNagaiASheikhAMTransplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia modelJ Neurosci Res20108851017102519885863
- LiuNChenRDuHWangJZhangYWenJExpression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cellsCell Mol Immunol20096320721319567204
- LiYChenJChenXGHuman marrow stromal cell therapy for stroke in rat: neurotrophins and functional recoveryNeurology200259451452312196642
- WilkinsAKempKGintyMHaresKMallamEScoldingNHuman bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitroStem Cell Res200931637019411199
- LiWYChoiYJLeePHMesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturingCell Transplant20081791045105919177841
- ZappiaECasazzaSPedemonteEMesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergyBlood200510651755176115905186
- ZhangJLiYChenJHuman bone marrow stromal cell treatment improves neurological functional recovery in EAE miceExp Neurol20051951162615904921
- KassisIGrigoriadisNGowda-KurkalliBNeuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitisArch Neurol200865675376118541795
- FreedmanMSBar-OrAAtkinsHLThe therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study GroupMult Scler201016450351020086020
- YamoutBHouraniRSaltiHBone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot studyJ Neuroimmunol20102271–218518920728948
- KarussisDKarageorgiouCVaknin-DembinskyASafety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosisArch Neurol201067101187119420937945
- LeeJWFangXGuptaNSerikovVMatthayMAAllogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lungProc Natl Acad Sci U S A200910638163571636219721001
- GroveDAXuJJoodiRAttenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantationJ Heart Lung Transplant201130334135021093298
- KavanaghHMahonBPAllogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cellsAllergy201166452353121091718
- BonfieldTLKolozeMLennonDPZuchowskiBYangSECaplanAIHuman mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma modelAm J Physiol Lung Cell Mol Physiol20102996L760L77020817776
- AbdiRFiorinaPAdraCNAtkinsonMSayeghMHImmunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetesDiabetes20085771759176718586907
- FotinoCRicordiCLauriolaVAlejandroRPileggiABone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetesRev Diabet Stud20107214415721060973
- InverardiLRicordiCTolerance and pancreatic islet transplantationPhilos Trans R Soc Lond B Biol Sci2001356140975976511375078
- BermanDMWillmanMAHanDMesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primatesDiabetes201059102558256820622174
- UrbanVSKissJKovacsJMesenchymal stem cells cooperate with bone marrow cells in therapy of diabetesStem Cells200826124425317932424
- XieQPHuangHXuBHuman bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitroDifferentiation200977548349119505629
- SunYChenLHouXGDifferentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitroChin Med J (Engl)2007120977177617531117
- TangDQCaoLZBurkhardtBRIn vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrowDiabetes20045371721173215220196
- ChenLBJiangXBYangLDifferentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cellsWorld J Gastroenterol200410203016302015378785
- VolarevicVArsenijevicNLukicMLStojkovicMConcise review: Mesenchymal stem cell treatment of the complications of diabetes mellitusStem Cells201129151021280154
- ZhangNLiJLuoRJiangJWangJABone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathyExp Clin Endocrinol Diabetes2008116210411118286426
- ComerotaAJLinkADouvilleJBurchardtERUpper extremity ischemia treated with tissue repair cells from adult bone marrowJ Vasc Surg201052372372920576396
- FazanRJrBallejoGSalgadoMCMoraesMFSalgadoHCHeart rate variability and baroreceptor function in chronic diabetic ratsHypertension1997303 Pt 26326359322994
- StroedterDSchmidtTBretzelRGFederlinKGlucose metabolism and left ventricular dysfunction are normalized by insulin and islet transplantation in mild diabetes in the ratActa Diabetol19953242352438750762
- Abdel AzizMTEl-AsmarMFHaidaraMEffect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic ratsMed Sci Monit20081411BR249BR25518971868
- EzquerFEEzquerMEParrauDBCarpioDYanezAJCongetPASystemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic miceBiol Blood Marrow Transplant200814663164018489988
- LeeRHSeoMJRegerRLMultipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid miceProc Natl Acad Sci U S A200610346174381744317088535
- HerreraMBBussolatiBBrunoSFonsatoVRomanazziGMCamussiGMesenchymal stem cells contribute to the renal repair of acute tubular epithelial injuryInt J Mol Med20041461035104115547670
- KimHParkJSChoiYJBone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathyStem Cells20092771686169619544451
- KwonDSGaoXLiuYBTreatment with bone marrow-derived stromal cells accelerates wound healing in diabetic ratsInt Wound J20085345346318593394