Abstract
Silent mating type information regulation 2 homolog 1 (SIRT1) is implicated in the control of skeletal muscle mitochondrial content and function through deacetylation of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and participation in the SIRT1/PGC-1α axis. The SIRT1/PGC-1α axis control of skeletal muscle mitochondrial biogenesis is an important therapeutic target for obesity and obesity-related metabolic dysfunction, as skeletal muscle mitochondrial dysfunction is implicated in the pathogenesis of multiple metabolic diseases. This review will establish the importance of the SIRT1/PGC-1α axis in the control of skeletal muscle mitochondrial biogenesis, and explore possible pharmacological and physiological interventions designed to activate SIRT1 and the SIRT1/PGC-1α axis in order to prevent and/or treat obesity and obesity-related metabolic disease. The current evidence supports a role for therapeutic activation of SIRT1 and the SIRT1/PGC-1α axis by both pharmaceuticals and exercise in the treatment and prevention of metabolic disease. Future research should be directed toward the feasibility of pharmaceutical activation of SIRT1 in humans and refining exercise prescriptions for optimal SIRT1 activation.
Introduction
Decreases in mitochondrial content and function accompanying the development of overweight and obesity represent an underlying mechanism of several metabolic disorders including insulin resistance, dyslipidemia, type II diabetes, hypertension, and cardiovascular disease.Citation1,Citation2 In 2005, an estimated 23.2% of the worldwide adult population was overweight and 9.8% was obese.Citation3 Current projections predict that 58.7% of the global adult population will be overweight or obese by 2030,Citation4 an estimate that potentially represents a massive economic burden for healthcare systems worldwide. In the United States, these rates would increase obesity-related costs to 16% (USD860 billion) of total healthcare expenditure.Citation4 Type II diabetes alone is already costing USD376 billion each year globally, a figure that is projected to grow 30% in the next 20 years.Citation5,Citation6 Given the economic and health costs associated with overweight and obesity, and their associated diseases, there is an urgent need for effective preventative and therapeutic interventions targeting weight gain and metabolic disease.
Skeletal muscle accounts for approximately 40% of body massCitation7 and plays a critical role in the maintenance of metabolic health. Healthy skeletal muscle has a highly oxidative phenotype, readily oxidizes lipids, is sensitive to insulin, and efficiently stores glucose.Citation8,Citation9 Consistent with the importance of mitochondria in healthy muscle, impairments in skeletal muscle function that contribute to obesity-related disease (eg, decreased fatty acid oxidation, impaired insulin sensitivity) are causally linked to decreases in mitochondrial content and function.Citation10–Citation15 In fact, decreases in mitochondrial content are apparent in early-stage overweight and obesity, and appear to contribute to both weight gain per se and to the metabolic dysfunction that underlies the development of metabolic disease.Citation10,Citation16
Genetic control of skeletal muscle mitochondrial content is regulated via a complex network of signaling pathways, transcriptional factors, and transcription cofactors. Peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) is a transcription factor coactivator that has been implicated as a key regulator of mitochondrial gene expression and mitochondrial biogenesis ().Citation17 The central role of PGC-1α in the regulation of mitochondrial content has positioned this protein as an important target in therapies aimed at preventing and/or treating disease.Citation18,Citation19 Silent mating type information regulation 2 homolog 1 (SIRT1) interacts with PGC-1α in skeletal muscle increasing PGC-1α’s transcriptional activity through deacetylation.Citation20 Given its apparent ability to activate PGC-1α and increase mitochondrial content in skeletal muscle,Citation20 SIRT1 has itself been implicated as a key player in metabolic health.Citation21,Citation22 It should be noted that SIRT1 is not solely responsible for the control of PGC-1α acetylation status, and evidence of normal mitochondrial biogenesis in mice lacking SIRT1 deacetylase activity suggests an inherent redundancy in the PGC-1α deacetylation pathway.Citation23 Despite this redundancy, the bulk of evidence suggests an important role of SIRT1 in skeletal muscle adaptations; thus, there is considerable interest in interventions designed to target SIRT1 and the SIRT1/PGC-1α axis as a means of increasing mitochondrial content, skeletal muscle function, and metabolic health.
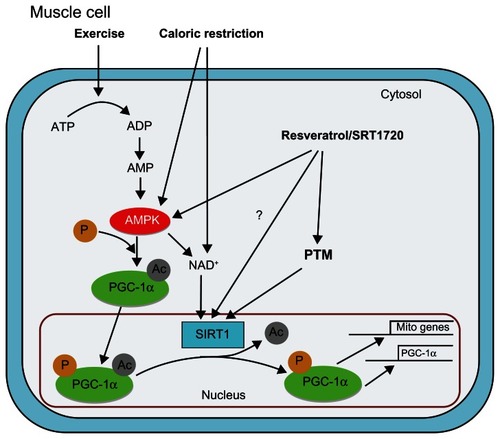
Figure 1 Model of pathways leading to activation of the silent mating type information regulation 2 homolog 1 (SIRT1)/peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) axis in skeletal muscle: SIRT1 deacetylase activity is directly influenced through posttranslational modification and indirectly through exercise, caloric restriction, and pharmaceutical activation.
Abbreviations: Ac, acetyl; ADP, adenosine diphosphate; AMP, adenosine monophosphate; AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; NAD+, nicotinamide adenine dinucleotide; P, phosphate; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PTM, posttranslational modification; SIRT1, silent mating type information regulation 2 homolog 1.
Within this context, this review will first examine the role of skeletal muscle mitochondria in the development of obesity and metabolic disorders and the importance of the SIRT1/PGC-1α axis in the determination of skeletal muscle mitochondrial content. Once the importance of the SIRT1/PGC-1α axis to metabolic health has been established, potential interventions – pharmacological and otherwise – designed to activate SIRT1 in an attempt to prevent and/or treat obesity and obesity-related metabolic disorder will be examined.
Skeletal muscle mitochondria: mechanisms of metabolic disease
Although not all individuals who are overweight or obese develop metabolic disorders, in general, there appears to be a progression from overweight, to obese, to metabolically diseased. Consistent with this idea, being overweight as a young adult is highly predictive of obesity later in life,Citation24 and adult obesity is strongly associated with increased risk for many diseases, including insulin resistance, type II diabetes, and cardiovascular disease.Citation1,Citation2,Citation11,Citation25 Changes in mitochondrial content and function are implicated in the development of several metabolic diseases. While the importance of mitochondrial dysfunction is controversial,Citation26 there is significant evidence that mitochondrial content is altered in metabolic dysfunction. For example, the decline in skeletal muscle mitochondria associated with altered metabolic health is eloquently demonstrated by observations that mitochondrial oxidative capacity is lower in obese than in lean adults, and further decreased in type II diabetics (ie, lean > obese > type II diabetes).Citation10 Further, a positive correlation has been observed between mitochondrial oxidative capacity and insulin sensitivity in lean and obese young adultsCitation27,Citation28 and in sedentary and exercise-trained older adults.Citation29 While it is not clear whether mitochondrial dysfunction (defined as either decreases in individual mitochondrial oxidative capacity and/or decreased mitochondrial content) precedes initial weight gain, there is substantial evidence that mitochondrial function is impaired in both obesityCitation11,Citation28,Citation30,Citation31 and type II diabetes.Citation14,Citation32 At present, there is still debate regarding the exact mechanism(s) linking mitochondrial dysfunction to metabolic disease; however, the current literature suggests that either impaired fatty acid oxidation and/or increased oxidative stress resulting from altered mitochondrial function are central in the etiology of metabolic disease.
Fatty acid oxidative capacity is correlated with insulin sensitivity in obese individualsCitation30 and impairments in lipid oxidation, accompanied by a concurrent upregulation of fatty acid transport,Citation30 appear to underlie the accumulations of intramuscular fat associated with obesity and metabolic disease.Citation11 Impaired lipid oxidation in obese skeletal muscle appears to be related to dysfunctional mitochondria lacking appropriate oxidative enzyme activity, decreased mitochondrial content within skeletal muscle, or a combination of both.Citation10–Citation12,Citation16,Citation26,Citation33 As individuals progress from obesity to metabolic disease, there is a decrease in the content or quality of mitochondria, which results in an accumulation of intramuscular fat that is intimately related to decreases in metabolic health.
The accumulation of intramuscular fat is implicated in the development of a number of comorbidities, particularly insulin insensitivity and type II diabetes.Citation8,Citation34–Citation37 Intramuscular lipid accumulation and the associated increases in lipid species, particularly diacylglycerolCitation38 and ceramides,Citation35,Citation39 have been linked to the disruption of the insulin receptor cascade.Citation26 Decreased insulin action (insulin insensitivity and the eventual development of insulin resistance and type II diabetes) is independently associated with the progression of hypertension and hyperlipidemia, and is known to be atherogenic.Citation34,Citation40
In addition to a decreased capacity to oxidize fat, impaired mitochondrial function also contributes to chronic increases in oxidative stress via increased lipid peroxidation. Decreased fatty acid usage results in slowed intramuscular lipid turnover and a resulting maladaptive peroxidation of intramuscular lipids and reactive lipid species accumulation.Citation41,Citation42 Lowered lipid turnover rates, coupled with dysfunctional mitochondria prone to reactive oxygen species production, facilitate the peroxidation of lipids and the production of lipotoxic lipid species in the intramuscular lipid pool.Citation43 These lipotoxic lipid species further interfere with mitochondrial function and intracellular signaling through the disruption of protein and DNA structure, potentiating lipid peroxidation in a damaging cycle that contributes to the development of metabolic disease (see Schrauwen and Hesselink for a detailed review).Citation43
The importance of skeletal muscle mitochondria for overall metabolic health is illustrated through the association of mitochondrial dysfunction and metabolic disease. The accumulation of damaging reactive lipid species within the muscle is hypothesized to result from impaired mitochondrial function, and the ensuing oxidative stress is implicated in the pathophysiology of a host of metabolic diseases. Specifically, oxidative stress is suggested to contribute to the development of obesity and several other diseases associated with metabolic syndrome, including coronary artery disease, hypertension, and type II diabetes. For more detailed information on the role of oxidative stress in obesity and metabolic disease, the reader is referred to Furukawa et al and Roberts and Sindhu.Citation44,Citation45 The activation of PGC-1α has been implicated in the control of antioxidant expression and the prevention of mitochondrial oxidative damage in mice,Citation46 reinforcing the importance of targeting the SIRT1/PGC-1α axis to improve metabolic health. Improving skeletal muscle mitochondrial content may reverse this lipid accumulation and potentially ameliorate the associated metabolic dysfunction. Thus, skeletal muscle mitochondrial content, through its role in the determination of intramuscular fat content, is intimately related to systemic metabolic health.
Genetic control of skeletal muscle mitochondrial content: the SIRT1/PGC-1α axis
Plasticity is a defining characteristic of skeletal muscle; thus, there is great potential for the remodeling of metabolic function towards a healthy phenotype in all populations. For example, exercise training can significantly increase mitochondrial oxidative activity and fat oxidation, decrease intramuscular lipid accumulation and oxidative stress, and improve insulin sensitivity – all parameters of healthy skeletal muscle.Citation36,Citation47–Citation49 These adaptations are largely due to increases in mitochondrial content and function resulting from the upregulation of a genetic program of mitochondrial protein controlled through the SIRT1/PGC-1α axis.
PGC-1α drives the transformation of skeletal muscle towards an oxidative phenotype via interaction with, and activation of, a plethora of transcription factors involved in the induction of mitochondrial genes encoded within both nuclear and mitochondrial DNA.Citation17,Citation18,Citation50–Citation52 While the regulation of the transcriptional activity of PGC-1α is complex,Citation53,Citation54 induction of PGC-1α-mediated transcription is accomplished through the acute activation of PGC-1αCitation55,Citation56 and chronic upregulation of PGC-1α protein content via an autoregulatory loop.Citation57 Importantly, the acute activation of PGC-1α, accomplished via posttranslational modification, appears to be an essential first step in the induction of PGC-1α-mediated transcription of mitochondrial genes.Citation21 PGC-1α is posttranslationally modified by phosphorylation via p38 mitogen-activated protein kinase and adenosine monophosphate-activated protein kinase (AMPK).Citation58–Citation60 In addition, PGC-1α acetylation status is also implicated in PGC-1α transcriptional activity and mitochondrial biogenesis.Citation20,Citation61 For example, acetylation levels of PGC-1α are inversely correlated with oxidative capacity in murine skeletal muscle,Citation62 while genetic mutation of acetylation sites on PGC-1α (mimicking the deacetylated state) markedly increase PGC-1α transcriptional activity and mitochondrial biogenesis in skeletal muscle cells.Citation62
Through the deacetylation of acetylated-lysine residues, the sirtuins (a family of evolutionarily conserved deacetylase) modify protein activity and have been implicated in a variety of cellular processes, including the stress response and cellular energy control in mammals.Citation22,Citation63,Citation64 While seven sirtuins have been identified in humans, several of which are implicated in metabolic control, including the mitochondrial sirtuins SIRT3, SIRT4, and SIRT5,Citation65 the current review will focus on SIRT1 in skeletal muscle and metabolic disease. SIRT1 directly interacts with PGC-1α in mouse hepatocytes,Citation61 and is responsible for deacetylation of PGC-1α in 293T cellsCitation61 and PC12 neuronal cells.Citation66 In addition, SIRT1 interacts with PGC-1α in C2C12 skeletal muscle cells,Citation20 and SIRT1-mediated deacetylation of PGC-1α appears to play an important, but perhaps nonessential,Citation23 role in the induction of PGC-1α-mediated transcription.Citation62 In support of this evidence from cells, SIRT1 is implicated in caloric restriction, exercise, and aminoimidazole carboxamide ribonucleotide-induced upregulation of mitochondrial content and function in skeletal muscle of mice.Citation20,Citation62,Citation67 Further, in transgenic animal models, overexpression of SIRT1 consistently improves insulin sensitivityCitation68,Citation69 and exerts a protective effect against metabolic disorders commonly associated with high-fat feeding.Citation70 This evidence implicates the SIRT1/PGC-1α axis in skeletal muscle in the upregulation of genes implicated in mitochondrial content,Citation71,Citation72 as well as fatty acid oxidation and energy expenditure,Citation20 and illustrates the importance of this axis for any intervention designed to reverse skeletal muscle dysfunction. As such, targeting the SIRT1/PGC-1α axis may be a practical treatment model for obesity and metabolic disorder.
Physiological control of SIRT1 activity
In vivo, SIRT1 activity is regulated by substrate availability, posttranslational modification, and the formation of both inhibiting and activating complexes.Citation73 Activation of SIRT1 through one, or several, of these mechanisms is expected to upregulate mitochondrial gene expression via the SIRT1/PGC-1α axis, resulting in improved mitochondrial and metabolic function.
Substrate availability is implicated in SIRT1 activation, primarily through changes in cellular redox potential. Nicotinamide adenine dinucleotide (NAD+) is a substrate in SIRT1-mediated deacetylation, which suggests changes in NAD+ can affect SIRT1 activity.Citation61,Citation74 Consistent with this hypothesis, SIRT1 activity (measured as a decrease in PGC-1α acetylation status) and NAD+ are increased concomitantly during nutrient deprivation in hepatocytesCitation61 and C2C12 skeletal muscle cells,Citation20,Citation61 and following nutrient deprivationCitation67 and exerciseCitation62,Citation67 in intact mouse muscle. Recent reports suggest that AMPK, an intracellular energy sensor,Citation75 mediates increases in NAD+ during periods of nutrient restriction, and thus plays a key role in regulating SIRT1 activity and transcriptional programs controlled through the SIRT1/PGC-1α axis.Citation62,Citation67 Additionally, nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in the NAD+ salvage pathway,Citation76,Citation77 is implicated in the control of intramuscular NAD+ and thus SIRT1 activity. NAMPT is upregulated following interventions known to activate SIRT1 in skeletal muscle – specifically, exercise training in humansCitation78 and acute exercise in mice.Citation67 These results comprise the main evidence supporting the hypothesis that SIRT1 activity is increased in response to altered energy status through increased substrate availability via AMPK, NAMPT, or both. Interestingly, in both cell and animal models,Citation49,Citation79–Citation81 SIRT1 activity does not always correspond to changes in NAD+. These results suggest that SIRT1 activity is under more complex control than can be explained through alterations in substrate availability alone.
Several recent lines of evidence have implicated posttranslational modifications of SIRT1 and the formation of inhibitoryCitation82,Citation83 and activating complexes in the control of SIRT1 deacetylase activity.Citation84,Citation85 A number of phosphorylation sites have been identified on SIRT1,Citation86,Citation87 and phosphorylation of these sites consistently increases SIRT1 deacetylase activity.Citation86–Citation88 In addition to phosphorylation, SIRT1 activity is influenced by sumoylation,Citation89 methylation,Citation90 and nitrosylation.Citation91 Sumoylation by the small ubiquitin-like modifier family of enzymes increases SIRT1 catalytic activity,Citation89 while it is unclear whether methylation directly affects SIRT1 deacetylase activity.Citation90 Transnitrosylation of SIRT1 has also been demonstrated, resulting in increased acetylation of SIRT1 targets, suggesting an inhibitory effect on SIRT1 deacetylase activity with nitrosylation.Citation91 In addition to posttranslational modifications, SIRT1 deacetylase activity can be modified through the formation of regulatory complexes. Specifically, SIRT1 deacetylase activity is inhibited when forming a complex with deleted in breast cancer-1Citation85 and activated when forming a complex with active regulator of SIRT1.Citation84 These types of SIRT1 activity regulations (both posttranslational modifications and regulatory complex formation) have not been thoroughly explored in skeletal muscle, making it an important direction for future research.
The physiological pathways controlling SIRT1 activity, outlined above, can be activated by both exercise and caloric restriction. The exact mechanism by which caloric restriction impacts SIRT1 activity remains unclear, although it is hypothesized that caloric restriction initiates a disturbance to metabolic homeostasis, activating AMPK and subsequently increasing cellular NAD+, resulting in an increased activation of SIRT1.Citation20,Citation21,Citation61,Citation62,Citation67 Caloric restriction may also increase SIRT1 activity through activation of NAMPT, increasing flux through the NAD+ salvage pathway.Citation92 Despite the uncertainty surrounding the mechanism of caloric restriction-mediated SIRT1 activation, it is apparent that caloric restriction increases SIRT1 deacetylation activity and can play a role in the activation of the SIRT1/PGC-1α axis, increasing fatty acid oxidation in skeletal muscle,Citation62 and providing protective metabolic effects against an energy imbalance.Citation93 For a more detailed review on the role of SIRT1 in caloric restriction, the reader is referred to Canto and Auwerx.Citation21,Citation93
Pharmacological activation of SIRT1
In addition to physiological activation, SIRT1 can also be pharmacologically activated through interactions with a number of natural and synthetic molecules. Resveratrol, a naturally occurring polyphenol found in grape skins, has been implicated in the activation of SIRT1.Citation72,Citation94–Citation96 While resveratrol administration can increase SIRT1 activity by as much as eight-fold,Citation96 the exact mechanism of activation remains elusive. Although it was initially believed that resveratrol interacts with and activates SIRT1 directly, more recent investigations have demonstrated that this interaction may be an artifact of the fluorophore detection method,Citation97,Citation98 and in vivo resveratrol-mediated activation of SIRT1 appears to result from activation of upstream targets.Citation72,Citation99,Citation100 The synthetic production of SIRT1 activators has also been successful; in particular, SRT1720 increases SIRT1 deacetylase activity more potently than resveratrol in both cell lines and within skeletal muscle of mice.Citation94,Citation96,Citation101 However, as with resveratrol, whether SRT1720 activates SIRT1 directly or indirectly remains unclear.Citation98,Citation101 Despite the uncertainty surrounding the mechanism of these molecules and their interaction with SIRT1, their ability to increase SIRT1 deacetylase activity is well established. The potential for activation of the SIRT1/PGC-1α axis through pharmacological means makes SIRT1 an intriguing target for future therapeutic intervention.
Targeting SIRT1 activation as therapeutic intervention
The remainder of this review will explore the potential benefits and limitations of targeting SIRT1, through pharmacological and physiological interventions, for the purpose of activating the SIRT1/PGC-1α axis to stimulate mitochondrial biogenesis and improve skeletal muscle function and metabolic health.
Health benefits associated with pharmaceutical activation of SIRT1
The pharmacological activation of SIRT1 exerts its therapeutic effects on skeletal muscle through the SIRT1/PGC-1α axis. By increasing mitochondrial content and function through PGC-1α-mediated transcription, improved skeletal muscle function may prevent or reverse obesity and metabolic disease. In skeletal muscle, resveratrol activates SIRT1 resulting in deacetylation of PGC-1α and the induction of a genetic profile associated with improved mitochondrial function and fatty acid metabolism.Citation72 As mentioned previously, while SIRT1 is required for resveratrol-mediated deacetylation of PGC-1α in mouse embryonic fibroblasts,Citation72 there is some debate regarding resveratrol’s direct interaction with SIRT1,Citation98,Citation102 with some evidence suggesting that resveratrol’s effects may occur via an AMPK-mediated mechanism.Citation72,Citation99,Citation100 Regardless, AMPK and SIRT1 are believed to function in concert in skeletal muscle,Citation67 and this uncertainty only questions whether resveratrol acts directly on SIRT1, not whether resveratrol activates the SIRT1/PGC-1α axis.
Consistent with the above, activation of the SIRT1/PGC-1α axis following resveratrol treatment in mice increases mitochondrial and fatty acid gene expression and increases mitochondrial content in skeletal muscle.Citation72 Accompanying these improvements in skeletal muscle function are improved insulin sensitivity in mouse modelsCitation72,Citation99,Citation103 and a resistance to the deleterious effects of aging and a high-fat diet on metabolic health.Citation72,Citation103,Citation104 At present, there are relatively few studies examining the metabolic impact of resveratrol in humans. However, a recent study in obese males indicates that 30 days of dietary resveratrol supplementation increased skeletal muscle SIRT1, PGC-1α protein content, and intrinsic mitochondrial function and improved several markers of cardiovascular and metabolic health.Citation105 Two other studies have also reported beneficial effects of dietary resveratrol in humans, including improved glucose tolerance in older adultsCitation106 and a reduction of the oxidative and inflammatory response to a high-fat meal.Citation107 While these studies provide promising initial findings indicating that targeting SIRT1 via resveratrol supplementation may prove therapeutic for obesity and obesity-related disease, randomized controlled studies are still needed to confirm these beneficial effects in a larger population. In addition, there are little data regarding whether a prolonged intake of resveratrol has any negative implications either within skeletal muscle, within other organs (liver for example), or on overall metabolic health in humans.
The use of the synthetic SIRT1 activator SRT1720 has been proposed to selectively activate SIRT1 with greater potency, efficacy, and selectivity than resveratrol.Citation94,Citation96,Citation101 The chronic administration of SRT1720 in mice decreases acetylation of PGC-1α and a number of other SIRT1 targetsCitation94 and increases oxidative capacity, lipid oxidation, insulin sensitivity, and provides a protective effect against diet-induced obesity.Citation94,Citation95,Citation101 In addition to protective metabolic effects, mice on high-fat diets given SRT1720 ran twice the distance in endurance trials and improved muscle function in a variety of functional tests when compared to controls fed the same diet.Citation94 These skeletal muscle adaptations make SRT1720 an appealing molecule for therapeutic intervention. Similar to resveratrol, controversy exists regarding whether SRT1720 activates SIRT1 through direct interaction, or acts indirectly via activation of AMPK through alterations in cellular energy status.Citation98 Thus the metabolic adaptations attributed directly to SRT1720–SIRT1 interaction versus an AMPK-mediated SIRT1 activation are difficult to distinguish.Citation94 Phase I and II clinical trials examining the safety and metabolic benefits of a drug closely related to SRT1720 (ie, SRT2104) are currently underway and represent an important look into the ability of SRT1720/2104 to increase mitochondrial content in skeletal muscle and improve metabolic function in obesity and metabolic disease.
At present, interventions with both resveratrol and SRT1720 in mice and in preliminary human studies (resveratrol only) appear to activate the SIRT1/PGC-1α axis, improve mitochondrial content in skeletal muscle, and improve metabolic health. These findings support the suggestion that these pharmaceuticals are promising potential agents for the prevention and treatment of obesity and its related diseases, and warrant further investigation.
Health benefits of the SIRT1/PGC-1α axis activation during exercise
Exercise, as a component of lifestyle intervention, is an alternative to pharmacological interventions aimed at improving skeletal muscle mitochondrial function and metabolic health.Citation108 An emerging line of evidence suggests that exercise targets SIRT1, and the resulting activation of the SIRT1/PGC-1α axis underlies many of the beneficial effects associated with exercise.Citation109,Citation110
An acute bout of exercise in rat and mouse muscle increases SIRT1 activityCitation109 and deacetylation of PGC-1α immediately, and for several hours following exercise.Citation62,Citation67 Exercise training is also implicated in the activation of SIRT1 in animal models, with both increases in SIRT1 activityCitation49,Citation80,Citation81,Citation111 and deacetylation of PGC-1αCitation112 observed following chronic contractile activity and treadmill running. Activation of SIRT1 was also observed in human skeletal muscle after 2 weeks and 6 weeks of exercise training.Citation71,Citation109 While skeletal muscle SIRT1 protein content is also elevated following acute exercise and exercise training in both ratsCitation113,Citation114 and humans,Citation115,Citation116 the implications of elevated SIRT1 content in muscle remain unclear.Citation117 It should also be noted that although PGC-1α deacetylation was normal in mice expressing SIRT1 lacking deacetylase activity,Citation23 the majority of observations in intact animal and human skeletal muscle support the involvement of SIRT1 in mediating PGC-1α acetylation status. Also consistent with the contention that SIRT1 and the SIRT1/PGC-1α axis play a critical role in the control of skeletal muscle mitochondrial content are numerous animal studies reporting that increased SIRT1 activity is accompanied by increases in PGC-1α transcriptional activity.Citation80,Citation81,Citation113,Citation114,Citation118
The activation of the SIRT/PGC-1α axis by exercise is accompanied by increases in markers of oxidative capacity and mitochondrial content in animalsCitation71,Citation80,Citation81 and humans.Citation71,Citation116 Further, markers of improved fatty acid oxidation and insulin sensitivity are also found in animals and humans accompanying activation of the SIRT1/PGC-1α axis by exercise.Citation71,Citation114,Citation116 In addition to mitochondrial adaptations, activation of the SIRT1/PGC-1α axis is linked to the attenuation of age-related decline in skeletal muscle health in exercised rodentsCitation49,Citation111,Citation113 via decreased oxidative stress and DNA damage, factors also implicated in the pathogenesis of many metabolic disorders associated with obesity.Citation44 Consistent with these observations, exercise training improves oxidative capacity and fatty acid oxidation in skeletal muscle from obese adults,Citation13,Citation119,Citation120 improves insulin sensitivity in obesity and type II diabetes,Citation121,Citation122 and decreases both risk factors for, and symptoms of, metabolic disease.Citation123,Citation124 In summary, exercise appears to activate the SIRT1/PGC-1α axis and improve skeletal muscle mitochondrial function and metabolic health. These results highlight the preventative and therapeutic potential of exercise for obesity and obesity-related disease.
As an alternative treatment in obesity and metabolic disease, exercise has several inherent advantages over pharmaceutical intervention. First, the improved metabolic function associated with exercise comes at minimal financial cost, while a pharmaceutical intervention carries a substantial financial commitment from both the individual and healthcare provider.Citation4,Citation6 Second, in addition to improved skeletal muscle mitochondrial function and metabolic/cardiovascular health, regular exercise is associated with a myriad of beneficial effects ranging from the prevention and treatment of mental disordersCitation125 and cancerCitation126 to alleviating symptoms and improving quality of life in many chronic diseases.Citation127 Third, exercise is implicated in a systemic improvement of health with little to no risk of adverse side effects.Citation108,Citation128 Pharmaceuticals are often associated with undesirable side effects, and are inherently designed to be specific, eliminating the possibility of a systemic health improvement. Finally, there is evidence that exercise, as part of a lifestyle intervention, induces superior improvements compared to pharmaceutical intervention in subjects with metabolic disease.Citation121 In light of these arguments, it makes both health and financial sense that exercise becomes a first-line tool in both the prevention and treatment of obesity and obesity-related disease.
Conclusion and future direction
The current review has focused on the contribution of SIRT1 to skeletal muscle function and metabolic health. Future studies should not only continue to investigate SIRT1 function, but should also focus on other members of the sirtuin family. The contribution of SIRT1 to overall metabolic health occurs, in part, through SIRT1’s influence on PGC-1α and skeletal muscle mitochondrial function. As skeletal muscle mitochondrial dysregulation is implicated in obesity and obesity-related metabolic disease, SIRT1 has become an attractive target for therapeutic intervention. The activation of SIRT1, and consequently the SIRT1/PGC-1α axis, results in upregulation of mitochondrial genes and improved skeletal muscle mitochondrial content and function. SIRT1 activity can be modified by pharmaceuticals and exercise, providing an array of options to pursue for implementing a therapeutic intervention. A clear need for further investigation of the feasibility of pharmaceutical intervention in humans is evident as, for the most part, human trials are in their infancy or are nonexistent. With exercise, exploration of the optimal dose and intensity will expand the possibility of tailored prescriptions targeting SIRT1 activity.
Disclosure
The authors report no conflicts of interest in this work.
References
- LauDCDouketisJDMorrisonKMHramiakIMSharmaAMUrE2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and childrenCMAJ20071768S1S1317420481
- KopelmanPGObesity as a medical problemNature2000404677863564310766250
- KellyTYangWChenCSReynoldsKHeJGlobal burden of obesity in 2005 and projections to 2030Int J Obes (Lond)20083291431143718607383
- WangYBeydounMALiangLCaballeroBKumanyikaSKWill all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemicObesity (Silver Spring)200816102323233018719634
- ZhangPZhangXBrownJGlobal healthcare expenditure on diabetes for 2010 and 2030Diabetes Res Clin Pract201087329330120171754
- ShawJESicreeRAZimmetPZGlobal estimates of the prevalence of diabetes for 2010 and 2030Diabetes Res Clin Pract201087141419896746
- KameiYMiuraSSuzukiMSkeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic controlJ Biol Chem200427939411144112315272020
- MuoioDMNewgardCBObesity-related derangements in metabolic regulationAnnu Rev Biochem20067536740116756496
- DysonPAThe therapeutics of lifestyle management on obesityDiabetes Obes Metab2010121194194620880340
- KelleyDEHeJMenshikovaEVRitovVBDysfunction of mitochondria in human skeletal muscle in type 2 diabetesDiabetes200251102944295012351431
- KimJYHicknerRCCortrightRLDohmGLHoumardJALipid oxidation is reduced in obese human skeletal muscleAm J Physiol Endocrinol Metab20002795E1039E104411052958
- RitovVBMenshikovaEVHeJFerrellREGoodpasterBHKelleyDEDeficiency of subsarcolemmal mitochondria in obesity and type 2 diabetesDiabetes200554181415616005
- MenshikovaEVRitovVBFerrellREAzumaKGoodpasterBHKelleyDECharacteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesityJ Appl Physiol20071031212717332268
- MoothaVKLindgrenCMErikssonKFPGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetesNat Genet200334326727312808457
- PattiMEButteAJCrunkhornSCoordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1Proc Natl Acad Sci U S A2003100148466847112832613
- HollowayGPBonenASprietLLRegulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individualsAm J Clin Nutr2009891455S462S19056573
- LinJWuHTarrPTTranscriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibresNature2002418689979780112181572
- WuZPuigserverPAnderssonUMechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1Cell199998111512410412986
- HandschinCSpiegelmanBMPeroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolismEndocr Rev200627772873517018837
- Gerhart-HinesZRodgersJTBareOMetabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1 alphaEMBO J20072671913192317347648
- CantoCAuwerxJPGC-1 alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditureCurr Opin Lipidol20092029810519276888
- GuarenteLSirtuins as potential targets for metabolic syndromeNature2006444712186887417167475
- PhilpAChenALanDSirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) deacetylation following endurance exerciseJ Biol Chem201128635305613057021757760
- HermanKMCraigCLGauvinLKatzmarzykPTTracking of obesity and physical activity from childhood to adulthood: the Physical Activity Longitudinal StudyInt J Pediatr Obes20094428128819922043
- BoykoEJde CourtenMZimmetPZChitsonPTuomilehtoJAlbertiKGFeatures of the metabolic syndrome predict higher risk of diabetes and impaired glucose tolerance: a prospective study in MauritiusDiabetes Care20002391242124810977013
- HollowayGPMitochondrial function and dysfunction in exercise and insulin resistanceAppl Physiol Nutr Metab200934344044619448712
- HickeyMSWeidnerMDGaviganKEZhengDTyndallGLHoumardJAThe insulin action-fiber type relationship in humans is muscle group-specificAm J Physiol19952691 Pt 1E150E1547631770
- SimoneauJAColbergSRThaeteFLKelleyDESkeletal-muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese womenFASEB J1995922732787781930
- HoumardJAEganPCNeuferPDElevated skeletal muscle glucose transporter levels in exercise-trained middle-aged menAm J Physiol19912614 Pt 1E437E4431928336
- SimoneauJAVeerkampJHTurcotteLPKelleyDEMarkers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight lossFASEB J199913142051206010544188
- KelleyDEGoodpasterBWingRRSimoneauJASkeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight lossAm J Physiol19992776 Pt 1E1130E114110600804
- AsmannYWStumpCSShortKRSkeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemiaDiabetes200655123309331917130474
- ThyfaultJPKrausRMHicknerRCHowellAWWolfeRRDohmGLImpaired plasma fatty acid oxidation in extremely obese womenAm J Physiol Endocrinol Metab20042876E1076E108115280153
- PetersenKFShulmanGIPathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitusAm J Cardiol2002905A11G18G
- HollandWLKnottsTAChavezJAWangLHoehnKLSummersSALipid mediators of insulin resistanceNutr Rev2007656 Pt 2S39S4617605313
- GoodpasterBHTheriaultRWatkinsSCKelleyDEIntramuscular lipid content is increased in obesity and decreased by weight lossMetabolism200049446747210778870
- PanDALilliojaSKriketosADSkeletal muscle triglyceride levels are inversely related to insulin actionDiabetes19974669839889166669
- TimmersSSchrauwenPde VogelJMuscular diacylglycerol metabolism and insulin resistancePhysiol Behav200894224225118207474
- SummersSACeramides in insulin resistance and lipotoxicityProg Lipid Res2006451427216445986
- DefronzoRAFerranniniEInsulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular diseaseDiabetes Care19911431731942044434
- GoodpasterBHHeJWatkinsSKelleyDESkeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletesJ Clin Endocrinol Metab200186125755576111739435
- Samocha-BonetDHeilbronnLKLichtenbergDCampbellLVDoes skeletal muscle oxidative stress initiate insulin resistance in genetically predisposed individuals?Trends Endocrinol Metab2010212838819854062
- SchrauwenPHesselinkMKOxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetesDiabetes20045361412141715161742
- FurukawaSFujitaTShimabukuroMIncreased oxidative stress in obesity and its impact on metabolic syndromeJ Clin Invest2004114121752176115599400
- RobertsCKSindhuKKOxidative stress and metabolic syndromeLife Sci20098421–2270571219281826
- WenzTRossiSGRotundoRLSpiegelmanBMMoraesCTIncreased muscle PGC-1 alpha expression protects from sarcopenia and metabolic disease during agingProc Natl Acad Sci U S A200910648204052041019918075
- BruceCRThrushABMertzVAEndurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid contentAm J Physiol Endocrinol Metab20062911E99E10716464906
- GibalaMJLittleJPvan EssenMShort-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performanceJ Physiol2006575Pt 390191116825308
- FerraraNRinaldiBCorbiGExercise training promotes SIRT1 activity in aged ratsRejuvenation Res200811113915018069916
- PuigserverPSpiegelmanBMPeroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulatorEndocr Rev2003241789012588810
- ScarpullaRCNuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivatorAnn N Y Acad Sci2008114732133419076454
- LiraVABentonCRYanZBonenAPGC-1 alpha regulation by exercise training and its influences on muscle function and insulin sensitivityAm J Physiol Endocrinol Metab20102992E145E16120371735
- HolloszyJORegulation by exercise of skeletal muscle content of mitochondria and GLUT4J Physiol Pharmacol200859Suppl 751819258654
- GibalaMMolecular responses to high-intensity interval exerciseAppl Physiol Nutr Metab200934342843219448710
- WrightDCHanDGarcia-RovesPMGeigerPCJonesTEHolloszyJOExercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1 alpha expressionJ Biol Chem2007282119419917099248
- PerryCGLallyJHollowayGPHeigenhauserGJBonenASprietLLRepeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscleJ Physiol2010588Pt 234795481020921196
- HandschinCRheeJLinJDTarrPTSpiegelmanBMAn autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1 alpha expression in muscleProc Natl Acad Sci U S A2003100127111711612764228
- PuigserverPRheeJLinJCytokine stimulation of energy expenditure through p38 MAP kinase activation of PPAR gamma coactivator-1Mol Cell20018597198211741533
- JaegerSHandschinCSt-PierreJSpiegelmanBMAMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alphaProc Natl Acad Sci U S A200710429120171202217609368
- WrightDCGeigerPCHanDHJonesTEHolloszyJOCalcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1 alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activationJ Biol Chem200728226187931879917488713
- RodgersJTLerinCHaasWGygiSPSpiegelmanBMPuigserverPNutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1Nature2005434702911311815744310
- CantoCGerhart-HinesZFeigeJNAMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activityNature200945872411056106019262508
- Dali-YoucefNLagougeMFroelichSKoehlCSchoonjansKAuwerxJSirtuins: the “magnificent seven,” function, metabolism and longevityAnn Med200739533534517701476
- YangTSauveAANAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicityAAPS J200684E632E64317233528
- FlickFLuscherBRegulation of sirtuin function by posttranslational modificationsFront Pharmacol201232922403547
- NemotoSFergussonMMFinkelTSIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1 alphaJ Biol Chem200528016164561646015716268
- CantoCJiangLQDeshmukhASInterdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscleCell Metab201011321321920197054
- BanksASKonNKnightCSirT1 gain of function increases energy efficiency and prevents diabetes in miceCell Metab20088433334118840364
- BordoneLCohenDRobinsonASIRT1 transgenic mice show phenotypes resembling calorie restrictionAging Cell20076675976717877786
- PflugerPTHerranzDVelasco-MiguelSSerranoMTschopMHSirt1 protects against high-fat diet-induced metabolic damageProc Natl Acad Sci U S A2008105289793979818599449
- GurdBJYoshidaYMcFarlanJTNuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscleAm J Physiol Regul Integr Comp Physiol20113011R67R7521543634
- LagougeMArgmannCGerhart-HinesZResveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alphaCell200612761109112217112576
- ZschoernigBMahlknechtUSIRTUIN 1: regulating the regulatorBiochem Biophys Res Commun2008376225125518774777
- SauveAAWolbergerCSchrammVLBoekeJDThe biochemistry of sirtuinsAnnu Rev Biochem20067543546516756498
- HardieDGAMP-activated/SNF1 protein kinases: conserved guardians of cellular energyNat Rev Mol Cell Biol200781077478517712357
- RevolloJRGrimmAAImaiSThe NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cellsJ Biol Chem200427949507545076315381699
- YangHYangTBaurJANutrient-sensitive mitochondrial NAD+ levels dictate cell survivalCell200713061095110717889652
- CostfordSRBajpeyiSPasaricaMSkeletal muscle NAMPT is induced by exercise in humansAm J Physiol Endocrinol Metab20102981E117E12619887595
- AndersonRMLatorre-EstevesMNevesARYeast life-span extension by calorie restriction is independent of NAD fluctuationScience200330256532124212614605207
- ChabiBAdhihettyPJO’LearyMFMenziesKJHoodDARelationship between Sirt1 expression and mitochondrial proteins during conditions of chronic muscle use and disuseJ Appl Physiol200910761730173519797682
- GurdBJYoshidaYLallyJHollowayGPBonenAThe deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesisJ Physiol2009587Pt 81817182819237425
- KimJChenJLouZDBC1 is a negative regulator of SIRT1Nature2008451717858358618235501
- EscandeCChiniCCNinVDeleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in miceJ Clin Invest2010120254555820071779
- KimEJKhoJHKangMRUmSJActive regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activityMol Cell200728227729017964266
- NinVEscandeCChiniCCRole of deleted in breast cancer 1 (DBC1) in SIRT1 activation induced by protein kinase A and AMP activated protein kinaseJ Biol Chem532012 [Epub ahead of print.]
- Gerhart-HinesZDominyJEJrBlattlerSMThe cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+Mol Cell201144685186322195961
- SasakiTMaierBKoclegaKDPhosphorylation regulates SIRT1 functionPLoS One2008312e402019107194
- NasrinNKaushikVKFortierEJNK1 phosphorylates SIRT1 and promotes its enzymatic activityPLoS One2009412e841420027304
- YangYFuWChenJSIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stressNat Cell Biol20079111253126217934453
- LiuXWangDZhaoYMethyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1)Proc Natl Acad Sci U S A201110851925193021245319
- KornbergMDSenNHaraMRGAPDH mediates nitrosylation of nuclear proteinsNat Cell Biol201012111094110020972425
- AndersonRMBittermanKJWoodJGMedvedikOSinclairDANicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiaeNature2003423693618118512736687
- CantoCAuwerxJCaloric restriction, SIRT1 and longevityTrends Endocrinol Metab200920732533119713122
- FeigeJNLagougeMCantoCSpecific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidationCell Metab20088534735819046567
- SmithJJKenneyRDGagneDJSmall molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivoBMC Syst Biol200933119284563
- HowitzKTBittermanKJCohenHYSmall molecule activators of sirtuins extend Saccharomyces cerevisiae lifespanNature2003425695419119612939617
- BorraMTSmithBCDenuJMMechanism of human SIRT1 activation by resveratrolJ Biol Chem200528017171871719515749705
- PacholecMBleasdaleJEChrunykBSRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1J Biol Chem2010285118340835120061378
- ChenSLiJZhangZEffects of resveratrol on the amelioration of insulin resistance in KKAy miceCan J Physiol Pharmacol201290223724222309033
- ParkSJAhmadFPhilpAResveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterasesCell2012148342143322304913
- MilneJCLambertPDSchenkSSmall molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetesNature2007450717071271618046409
- DaiHKustigianLCarneyDSIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activatorJ Biol Chem201028543326953270320702418
- BaurJAPearsonKJPriceNLResveratrol improves health and survival of mice on a high-calorie dietNature2006444711733734217086191
- PearsonKJBaurJALewisKNResveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life spanCell Metab20088215716818599363
- TimmersSKoningsEBiletLCalorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humansCell Metab201114561262222055504
- CrandallJPOramVTrandafirescuGPilot study of resveratrol in older adults with impaired glucose toleranceJ Gerontol A Biol Sci Med Sci142012 [Epub ahead of print.]
- GhanimHSiaCLKorzeniewskiKA resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate mealJ Clin Endocrinol Metab20119651409141421289251
- HaskellWLLeeIMPateRRPhysical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart AssociationMed Sci Sports Exerc20073981423143417762377
- GurdBJDeacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesisAppl Physiol Nutr Metab201136558959721888529
- BentonCRWrightDCBonenAPGC-1 alpha-mediated regulation of gene expression and metabolism: implications for nutrition and exercise prescriptionsAppl Physiol Nutr Metab200833584386218923559
- KoltaiESzaboZAtalayMExercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged ratsMech Ageing Dev20101311212819913571
- LiLPanRLiRMitochondrial biogenesis and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity: intact adipocytokine signaling is requiredDiabetes201160115716720929977
- LjubicicVJosephAMAdhihettyPJMolecular basis for an attenuated mitochondrial adaptive plasticity in aged skeletal muscleAging (Albany, NY)20091981883020157569
- SuwaMNakanoHRadakZKumagaiSEndurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1 alpha protein expressions in rat skeletal muscleMetabolism200857798699818555842
- GuerraBGuadalupe-GrauAFuentesTSIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: influence of glucose ingestionEur J Appl Physiol2010109473174320217115
- LittleJPSafdarAWilkinGPTarnopolskyMAGibalaMJA practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanismsJ Physiol2010588Pt 61011102220100740
- GurdBJLittleJPPerryCGDoes SIRT1 determine exercise-induced skeletal muscle mitochondrial biogenesis: differences between in vitro and in vivo experiments?J Appl Physiol2012112592692822096123
- DumkeCLDavisJMMurphyEASuccessive bouts of cycling stimulates genes associated with mitochondrial biogenesisEur J Appl Physiol2009107441942719657668
- MenshikovaEVRitovVBToledoFGFerrellREGoodpasterBHKelleyDEEffects of weight loss and physical activity on skeletal muscle mitochondrial function in obesityAm J Physiol Endocrinol Metab20052884E818E82515585590
- GoodpasterBHKatsiarasAKelleyDEEnhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesityDiabetes20035292191219712941756
- KnowlerWCBarrett-ConnorEFowlerSEReduction in the incidence of type 2 diabetes with lifestyle intervention or metforminN Engl J Med2002346639340311832527
- LaaksonenDELindstromJLakkaTAPhysical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention studyDiabetes200554115816515616024
- HawleyJAGibalaMJExercise intensity and insulin sensitivity: how low can you go?Diabetologia20095291709171319557385
- TjonnaAELeeSJRognmoOAerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot studyCirculation2008118434635418606913
- WolffEGaudlitzKvon LindenbergerBLPlagJHeinzAStrohleAExercise and physical activity in mental disordersEur Arch Psychiatry Clin Neurosci2011261Suppl 2S186S19121935629
- PekmeziDWDemark-WahnefriedWUpdated evidence in support of diet and exercise interventions in cancer survivorsActa Oncol201150216717821091401
- PedersenBKSaltinBEvidence for prescribing exercise as therapy in chronic diseaseScand J Med Sci Sports200616Suppl 136316451303
- WarburtonDENicolCWBredinSSHealth benefits of physical activity: the evidenceCMAJ2006174680180916534088
