Abstract
Osteoporotic fracture carries an enormous public health burden in terms of mortality and morbidity. Current approaches to identify individuals at high risk for fracture are based on assessment of bone mineral density and presence of other osteoporosis risk factors. Bone mineral density and susceptibility to osteoporotic fractures are highly heritable, and over 60 loci have been robustly associated with one or both traits through genome-wide association studies carried out over the past 7 years. In this review, we discuss opportunities and challenges for incorporating these genetic discoveries into strategies to prevent osteoporotic fracture and translating new insights obtained from these discoveries into development of new therapeutic targets.
Introduction
Osteoporosis is a major public health problem that is associated with significant mortality and morbidity. Although osteoporosis is often regarded as a disorder of women, and indeed women are disproportionately affected, the disorder also carries an enormous health burden in men. An estimated 20% of women and 4% of men in the US were diagnosed with osteoporosis in 2005–2006, with rates increasing to 48% in women and 12% in men over the age of 85 years.Citation1 It has further been estimated that up to one half of all women and one quarter to one third of all men over the age of 50 years will experience a hip fracture during their lifetime.Citation2 The economic impact is enormous, with an estimated health care cost of $17 billion in direct costs in the US alone.Citation3
Osteoporosis is a systemic skeletal disorder characterized by microarchitectural deterioration in bone tissue that results in reduced bone strength, bone fragility, and increased susceptibility to fracture.Citation4 The most common fracture sites are the hip and spine, although any bone can be affected. Identifying individuals with low bone strength and high risk for fracture is important so that strategies to slow bone loss, including pharmacotherapy, can be deployed. Although the molecular basis underlying these defects and the triggers that initiate them are not completely understood, a strong familial contribution to bone mineral density (BMD) is well established, with genes estimated to contribute 60%–80% of the variability in BMD.Citation5,Citation6 It is hoped that current efforts aimed at identifying the specific genes involved will provide further insight into the molecular basis underlying osteoporosis and will eventually lead to new therapeutic targets and prevention strategies.
The goal of this review is to summarize the current knowledge of factors contributing to increased fracture risk in osteoporosis, with an emphasis on genetic factors, and to discuss the potential impact of new genetic discoveries on identifying individuals at risk for osteoporotic fracture and preventing future fractures.
Development and sequelae of osteoporosis
Bone is a dynamic tissue that is continually being formed and resorbed during one’s lifetime. Bone mass at any time of life is the integral of the amount of bone accrued during growth and consolidation (peak bone mass) and the loss of bone that occurs with aging. The importance of achieving an optimal level of bone mass in early adulthood has highlighted the role of early nutritional and lifestyle factors in bone health, and led some to characterize osteoporosis as a pediatric disease,Citation7 or at least an adult disease with its seeds in childhood.
There is substantial variability both in the acquisition of peak bone mass, attained during early adulthood (by women in the early 20s and by men in the mid 20s), and in the rate of bone loss that begins during the perimenopausal years. In both men and women, peak bone mass is generally maintained in the 30s and early 40s, with a slow loss of bone mass that accelerates in women during the menopause. Both peak mass and rate of bone loss contribute to fracture risk; that is, the fracture risk will be high in individuals who achieved a low peak bone mass even if the subsequent rate of bone loss is small, as well as in individuals who achieve a relatively high peak bone mass but who experience a relatively high rate of bone loss in their later years. In a given year, about 10% of the skeleton is broken down and reformed by bone remodeling.Citation8
Bone remodeling occurs at numerous focal areas throughout the skeleton. The net amount of bone formation and resorption can be crudely assessed by bone turnover markers that are routinely measured in many laboratories. Bone formation markers, which are protein products of the osteoblast, include bone-specific alkaline phosphatase, procollagen-1 amino peptide, and osteocalcin, while resorption markers include C-telopeptide and N-telopeptide, which are breakdown products of collagen (the main protein in bone).
Osteoporosis is associated with increased morbidity and mortality. In the first 6 months following hip fracture, there is an excess mortality of at least 18%.Citation9 A meta-analysis showed that mortality 1 year after hip fracture was more than twice as high in men as in women.Citation10 Although men are younger than women at the time of hip fracture, they fare worse than women following hip fracture due to greater comorbidity and higher postoperative infection rates (reviewed by SterlingCitation11). Increased mortality also follows vertebral compression fracture.Citation12 In addition to increased mortality, osteoporotic fracture is frequently followed by chronic pain, functional limitation, and psychologic difficulties, that include fear of falling and social isolation.Citation13 Overall quality of life is reduced in both womenCitation14 and menCitation15 following osteoporotic fracture. Medical treatment of osteoporosis reduces fracture risk and enhances quality of life,Citation16 and some medications have been associated with improved overall survival.Citation17
Diagnosis, risk estimation, and treatment
Bone strength is determined by two factors, ie, BMD and bone quality.Citation18 BMD is determined by dual energy X-ray absorptiometry (DXA) and is usually performed at the lumbar spine and hip. Bone quality can be assessed by three-dimensional imaging modalities, such as peripheral computed tomography and high resolution magnetic resonance imaging,Citation19 although these are currently used primarily in research settings and are not available clinically. The assessment of fracture risk using only BMD has limitations.
In postmenopausal women and men over the age of 50 years, the diagnosis of osteoporosis can be made either by the occurrence of a fragility fracture (following a fall from standing height, after ruling out malignancy) or by a DXA T-score of ≤−2.5 at the lumbar spine, total hip, or femoral neck (). This T-score threshold corresponds to a BMD that is 2.5 standard deviation (SD) units or more lower than the BMD for an “average” young adult female. The World Health Organization has published guidelines further classifying a T-score ≥−1.0 as normal and a T-score of −1.0 to −2.5 in the absence of fracture as “low bone mass” (or osteopenia).Citation20 The definition of osteoporosis in premenopausal women and men younger than 50 years is controversial, but most agree that the patient must have experienced a fragility fracture and have a BMD ≤2 SD that of the average BMD for individuals of the same age and gender (ie, a Z-score ≤−2.0).Citation21
Table 1 Diagnosis of osteoporosis
Pharmacologic treatment to prevent fracture is currently targeted to those at high risk of fracture. According to guidelines established by the US National Osteoporosis Foundation, postmenopausal women and men older than 50 years and with osteoporosis (fracture or T-score ≤−2.5 at the spine or femoral neck) should be treated.Citation22 Current pharmacotherapies to prevent osteoporotic fracture are designed to slow bone loss. A number of different drugs are available, most of which act as antiresorptive agents on osteoclasts to decrease bone loss, although there is currently one that acts by stimulating bone formation (teriparatide). There is a wide variation among patients in response to drugs, both in terms of efficacy and frequency/type of adverse reactions.Citation23,Citation24
Because of the high prevalence of osteoporosis and the proven benefit of pharmacologic treatment in reducing fractures,Citation25 there is a strong incentive to identify individuals at risk for future fracture. DXA is the most widely used tool for this purpose, although there are concerns that DXA utilization rates have dropped since 2007, coincident with a lowering of Medicare reimbursement rates for non-facility-based DXAs.Citation26–Citation28 Nevertheless, in a large meta-analysis involving approximately 90,000 years of follow-up and over 2,000 fractures, it was estimated that each SD decrease in BMD from the age-adjusted mean was associated with a 2.3-fold increase in incidence of vertebral fractures and a 2.6-fold increase in hip fractures. The predictive ability was roughly similar to (or, for hip or spine measurements, better than) that of a one SD increase in blood pressure for stroke and better than a one SD increase in serum cholesterol concentration for cardiovascular disease.Citation29 In a later meta-analysis, Johnell et al reported that each SD decrease in BMD corresponded approximately to a 2.9-fold increase in hip fracture risk.Citation30
While these results illustrate the utility of DXA as a screening tool, DXA does not have a high sensitivity at the individual level for fracture prediction.Citation31,Citation32 Indeed, while the lifetime risk of fracture in women and men over age 50 years is estimated to be 50% and 30%, respectively,Citation2 more than 50% of women and 70% of men who experienced a fracture did not meet DXA criteria for osteoporosis.Citation33 Tools that assess bone quality (eg, high resolution peripheral computed tomography) improve fracture risk prediction over DXA but are not available clinically.Citation34 To improve fracture prediction, the FRAX® tool (http://www.shef.ac.uk/FRAX/) was developed in 2008 by the World Health Organization as an online calculator that integrates information from BMD and clinical risk factors to calculate the 10-year probability of hip fracture and the 10-year probability of any major osteoporotic fracture (clinical spine, forearm, hip, or shoulder fracture). The clinical risk factors included in FRAX fracture prediction include age, gender, height, weight, prior fracture, parental history of hip fracture, current glucocorticoid use, current smoking, excess alcohol intake, rheumatoid arthritis, and secondary causes of osteoporosis. Separate prediction equations have been developed for different geographic regions and ethnic groups. The National Osteoporosis Foundation guidelines recommend pharmacologic treatment of those with low bone mass who have a 10-year risk of fracture of at least 20% for any fracture or 3% for hip fracture as calculated by FRAX.Citation22 Using these treatment guidelines, approximately 30% of women and 19% of men over age 50 years in the US meet the criteria for pharmacologic treatment.Citation35
Risk factors for osteoporotic fracture
Because of the wide availability of DXA, the high precision with which it can be measured, and the correlation of BMD with fracture risk, much of the research into the determinants of osteoporotic fracture has focused on identifying the risk factors associated with variability in BMD. We provide below and in a brief summary of risk factors for BMD and fracture; more extensive reviews on this subject have been published elsewhere.Citation36–Citation38
Table 2 Non-genetic risk factors for osteoporosis
BMD is lower and hip fracture rates are higher in women compared with men and in populations of Caucasian origin compared with non-Caucasian populations. Higher body weight is also consistently associated with higher BMD and lower hip fracture risk, a correlation that may be attributable to multiple factors, including higher mechanical loading on skeletal bone that may promote mineralization and bone strength, skeletoprotective effects of adipokine secretion from fat tissue, and cushioning that may protect bone from the trauma of falls. Most non-vertebral fractures are associated with falls, and this dependence on falls complicates the ability to predict fractures accurately.
Reproductive and hormonal factors play an important role in regulating bone metabolism, with bone loss accelerating in women at the onset of menopause. Bone sites most affected by estrogen deficiency are those with high trabecular content, such as the spine and ultradistal forearm.Citation36 Estradiol is the most important hormone for bone health in both genders, but other hormones are important, including growth hormone, testosterone, and insulin. Nutritional factors, including calcium, vitamin D, and protein intake, play an important role in both the acquisition of peak bone mass and its maintenance in later life. However, a more precise understanding of the role of nutritional factors on BMD and bone health has been hampered by methodologic limitations in assessing dietary intakes and the time lag between dietary intake and its effects on bone health.
Other important risk factors for osteoporosis and reduced BMD include physical inactivity (which may increase fracture risk in part by reducing muscle mass) cigarette smoking, alcohol use, low sun exposure (for production of vitamin D), and use of some medications, including glucocorticoids and anticonvulsants. The impact of some of these osteoporosis risk factors differs slightly according to age and across sites, perhaps due in part to differing compositions of cortical versus trabecular bone.Citation39 Recent studies have also indicated that hip geometry contributes to fracture risk.Citation40,Citation41
Recent studies of genetic factors associated with osteoporosis
There is a strong genetic contribution to susceptibility to osteoporosis, with genes estimated to account for about 25% of the variance in liability to osteoporotic fractures,Citation42 25%–54% for fractures of the wrist,Citation43,Citation44 and up to 48% for fractures of the hip.Citation42 Despite these moderate to high heritabilities, the fracture phenotype is a very challenging one for use in genetic studies because fracture risk is influenced by a number of diverse physiologic factors, including BMD and age-related declines in bone microarchitecture and quality, muscle strength, balance, cognition, cardiovascular function, and vitamin D status. Since each of these factors is itself under at least partial genetic control, variants that influence fracture susceptibility entirely through any of these other factors should be more easily detectable in an analysis of the factor itself, rather than fracture.
Because of its wide use and acceptability as a screening tool, numerous genetic studies have been carried out on BMD, providing highly consistent estimates that genes account for 60%–80% of the total variability in BMD.Citation5 That low BMD is a significant mechanism through which fracture risk can be transmitted through families was demonstrated by Seeman et al over 20 years ago, who showed that BMD in the lumbar spine and femoral neck was lower in the daughters of women with osteoporotic fractures than in the daughters of non-osteoporotic women.Citation45 Significant heritabilities of BMD in young adulthood and premenopausal adults have been reported,Citation46–Citation48 implying a genetic contribution to the acquisition of peak bone mass. It is also presumed that genes contribute significantly to variability in aging-related bone loss, and in fact a few small studies have reported moderate heritabilities of bone loss.Citation46,Citation49–Citation52 However, precise estimates of the genetic contribution to bone loss are lacking because of the difficulty in assessing bone change reliably, ie, specific trajectories of bone change are non-linear and highly age-dependent, making it critical that multiple measurements over time be obtained in a standardized fashion.Citation53 These difficulties notwithstanding, it would be immensely valuable to identify bone change-related genes because many may fall in pathways that are more amenable to therapeutic modification than pathways specific for bone acquisition.
Early efforts to identify specific genes related to variation in BMD and fracture risk focused on identifying biologically motivated candidate genes and testing specific genotyped variants for association with BMD (or fracture). While many positive association results were published, with few exceptions (eg, the estrogen receptor 1 [ER1 gene] and low-density lipoprotein receptor-related proteins 4 and 5 [LRP4, LRP5] genes), most initially reported associations turned out to be difficult to replicate. With advances in genomic technology, numerous genome-wide association studies (GWAS) of BMD and related traits have been published over the last 6–7 years. It is important to appreciate that the GWAS approach is designed to test the hypothesis of association with genetic variants that are common in the population, ie, typically variants with minor allele frequencies of 5% or greater. Although the variants (typically single nucleotide polymorphisms [SNPs]) included on GWAS arrays are generally neutral (ie, do not have functional effects), they may be highly correlated with numerous other surrounding SNPs, including some that may influence gene expression or function. The largest of the BMD GWAS studies published to date was from the Genetic Factors of Osteoporosis Consortium and included a discovery sample of over 32,000 subjects from 17 different populations and an independent replication sample of over 50,000 subjects.Citation54 In this study, 56 loci significantly associated with BMD, of which 14 were found to associate also with fracture. Several additional BMD-associated loci have since been identified, some from non-Caucasian populations, bringing the total number of BMD-associated loci to 62.Citation55 The loci that have been robustly associated with BMD through GWAS studies to date are listed in . The effect sizes of these loci are uniformly small, each accounting for far less than 1% of the total variation in BMD; even collectively, all identified loci account for less than 6% of the total variation in femoral neck BMD.Citation54 Future GWAS studies including even more subjects will undoubtedly be published, through which even more BMD-associated loci will be identified. However, these newly associated loci will almost certainly have even smaller effect sizes than those already discovered.
Table 3 Loci associated with bone mineral density at the hip or spine at genome-wide levels of significance
One goal of GWAS is to obtain insights into disease pathogenesis by determining whether the associated loci map to any novel molecular pathways. Many of the 62 associated loci do indeed map to genes that fall within pathways related to bone biology, such as the Wnt/β-catenin signaling pathway, the RANK-RANKL-osteoprotegerin (OPG) pathway, mesenchymal stem cell differentiation, and endochondral ossification, although the involvement of these pathways in osteoporosis pathophysiology had generally been known before the advent of GWAS. The Wnt/β-catenin signaling pathway, which is mediated by interactions of Wnt proteins with their receptors, causes an accumulation of β-catenin in the cytoplasm that then translocates into the nucleus where it participates in gene transcription. The importance of this pathway in bone health was first recognized with the discovery of a mutation in LRP5 as the cause of osteoporosis pseudoglioma syndrome,Citation56 and it is now appreciated that this pathway is crucially important for a variety of processes, including bone cell differentiation, proliferation, and apoptosis. The RANK-RANKL-OPG pathway is an important regulator of bone resorption that involves receptor activator of nuclear factor-κB (RANK), its ligand (RANKL), and OPG, a so-called decoy receptor of RANKL. RANK is expressed by osteoclasts and their precursors, RANKL is expressed on osteoblast surfaces, and OPG is produced by osteoblasts. It is the binding of RANKL to its receptor, RANK, that controls the differentiation, proliferation, and survival of osteoclasts. illustrates how the Wnt and RANK/RANKL/OPG pathways interact with each other to regulate the balance between bone formation and resorption.
Figure 1 RANK/RANKL/OPG pathway in bone remodeling. The balance between bone formation and resorption is largely regulated by the Wnt pathway (bone formation), the RANK (pink symbols)/RANKL (blue symbols) pathway (osteoclast activation), and sclerostin (negative regulation of bone formation). Osteoblasts express the cell surface receptors RANKL and Wnt and also secrete a soluble decoy receptor, OPG (green symbols). Wnt protein binds coreceptors Fizzle-Fz and LRP5/6, leading to stabilization of β-catenin and its translocation to the nucleus to regulate target genes, resulting in increased bone formation. In the absence of OPG, RANKL on the osteoblast surface is available to bind RANK present on osteoclast precursors. Binding of RANK/RANKL leads to osteoclast maturation and resorption of bone. Sclerostin, secreted by osteocytes, inhibits Wnt from binding LRP5.
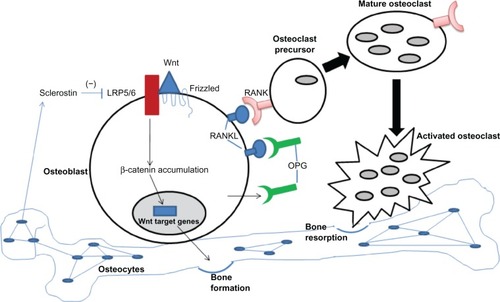
The process of mesenchymal stem cell differentiation is highly relevant to bone turnover because mesenchymal stem cells are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, and adipocytes. The endochondral ossification pathway involves processes active during fetal development of the mammalian skeleton. A fuller description of the Wnt, RANK-RANKL-OPG, and endochondral ossification pathways is beyond the scope of this review, but excellent reviews are available on these subjects.Citation57
Finding genes for fracture risk is likely to be more difficult than for BMD due to the much smaller sample sizes generally available for studies of fracture and the complexity of the fracture phenotype. As previously noted, it is difficult enough to identify genes/SNPs associated with intermediate traits. For example, although over 60 SNPs have now been associated with BMD, for which the heritability is very high, the effect sizes of all are very small, and enormous sample sizes were required to identify these. Of the 16 SNPs that have been associated with fracture to date (see ), most were tested because of their initial association with BMD,Citation54,Citation55 and all have odds ratios for fracture of 1.11 or lower for the risk allele with the exception of one, ie, rs13182402 in ALDH7A1 (odds ratio 2.25). This SNP was identified in a GWAS of fractures in a Chinese population.Citation58ALDH7A1 is a gene in the aldehyde dehydrogenase 7 family (member A1) that degrades and detoxifies acetaldehyde, which inhibits osteoblast proliferation and results in decreased bone formation.Citation59 For further reading, the reader is referred to several of the numerous excellent reviews that have been published recently on the genetics of BMD and hip fracture.Citation60–Citation63
Can genetic discoveries help to reduce the burden of osteoporotic fracture?
The last 10 years has been an exciting time for osteoporosis genetics insofar as GWAS studies carried out during this time have led to the discovery of over 60 new loci robustly associated with variation in BMD, including some subsequently found to be associated with fracture. So how might these findings, or future discoveries coming down as even larger sample sizes become available, be translatable? Will knowledge gleaned from these discoveries improve our ability to predict individuals at risk for future fracture, allowing initiation of early treatment? Will the discovered loci provide novel insights about bone biology and suggest new therapeutic targets? What new genetics/genomics approaches will be applied and what are the prospects for their translatability?
Prediction
There is good evidence that treating individuals who have already experienced a low trauma fracture, or who are osteoporotic by virtue of low BMD, with medications to either reduce bone resorption or increase bone formation can reduce the incidence of subsequent fractures.Citation64 Unfortunately, about one-half of hip fractures occur in women without a a pre-existing fracture and who have a BMD that is not in the osteoporotic range,Citation65 and prediction algorithms based on BMD and clinical information fractures (eg, DXA and FRAX) are imperfect for predicting individuals destined to experience a future fracture. An important question therefore is whether addition of genetic variants can improve prediction accuracy for fracture beyond that which can be determined by clinical risk factors alone.
Based on prospective studies, the discriminatory value of prognostic models based on clinical risk factors is moderate to good, with areas under the receiver operating characteristic curve ranging from 0.7 to 0.8.Citation66,Citation67 Tran et al evaluated the improvement of these models for fracture prediction that would be achieved by adding into the model a variant in the collagen I alpha 1 (COL1A1) gene (rs1800012) that has been consistently associated with fracture risk (reviewed by Tran et al).Citation68 This variant, which marks an Sp1 binding site in the first intron of COLIA1, has a minor allele frequency in Caucasians of approximately 19%, and individuals homozygous for this variant have a 2–4-fold increased risk of fracture. Adding this variant to the hip fracture prediction model increased the area under the receiver operating curve only modestly from 0.86 to 0.88.Citation68 There was likewise only a very modest improvement in reclassification from normal to high risk. In the large Genetic Factors of Osteoporosis Consortium meta-analysis, a summary genotype score based on genotypes for all 62 BMD-associated loci had modest ability for prediction of osteoporosis (area under the receiver operating characteristic curve 0.59), but addition of this score to a model that included age and weight increased predictive discrimination only negligibly (from area under the receiver operating characteristic curve of 0.75 to 0.76).Citation54 It should not be surprising that the marginal increase in discriminatory value achieved by adding genotypes at BMD-associated loci is minute given their small effect sizes. Moreover, one might expect variants associated with BMD to add very little predictive value to models that already include BMD as a clinical variable.
Although single variants have very small effect sizes and do not add to prediction, there is a theoretical benefit to considering multiple risk variants in aggregate in genetic profiling. One problem is that many of the known variants associated with fracture risk exert their effects through BMD, and thus their additional predictive value would be expected to be very marginal. The number of SNPs associated with fracture independently of BMD is very small. In simulation studies, Nguyen and Eisman estimated that 50 SNPs with odds ratios of 1.3–1.5 would be needed to provide a significant increase in reclassification.Citation69 However, given the complexity of the fracture phenotype, it seems unlikely that many associated SNPs will have effects this large. In fact, even in the absence of BMD screening, it seems unlikely at this point that SNPs will play a meaningful role in fracture prediction.
Novel pathways
A second area where genetic discoveries could potentially be translatable for osteoporosis lies in identifying new pathways that could serve as therapeutic targets. Despite the growing numbers of SNPs associated with BMD, few have led to major new insights about bone biology to date, largely because the genes marked by these SNPs have either fallen into pathways already known to be related to bone biology or the associated SNPs are either intergenic or appear to be correlated with genes having functions which are not clearly known. To make more sense of GWAS findings, more comprehensive analyses may be required that integrate additional information, such as tissue expression of implicated genes and functional annotation of identified SNPs and genes. In the large Genetic Factors of Osteoporosis Consortium meta-analysis, many of the identified SNPs mapped to genes in the RANK-RANKL-OPG, mesenchymal stem cell differentiation, and Wnt signaling pathways (see Estrada et alCitation54 and ). Integrating expression data to GWAS findings can prioritize the most biologically relevant candidate genes, as in the GWAS findings carried out by the Framingham StudyCitation70 and Genetic Factors of Osteoporosis Consortium.Citation54 In an analysis of 180 SNPs associated with height in prior GWAS meta-analyses, Lui et al used rat, mouse, and human databases to identify genes expressed in growth plates to help localize the causal gene within many of the associated loci.Citation71
One important application of new discoveries will be in the development of medications that more precisely target key mechanistic pathways, such as the RANK-ligand pathway, cathepsin K inhibition, and Wnt signaling manipulation. Identifying the key players in these pathways, especially the ones most amenable to targeting, represents an active area of ongoing research.Citation72 For example, the antiresorptive agent, denosumab, is an antibody that inhibits RANKL and has been on the market for 3 years, and an sclerostin (SOST) antibody has recently completed 5 years of testing and is likely to be approved soon. Although these particular targets were identified before the advent of GWAS, it is possible that genomic approaches will lead to new discoveries in these or other pathways that will prove druggable.
New approaches/future directions
As for many other complex diseases, the future of genomics research in osteoporosis will almost certainly include a thorough investigation of the role of rare variants discovered by deep sequencing of osteoporosis candidate genes as well as those discovered through a variety of other sources, including exome sequencing and the 1000 Genomes Project. An association study targeted to rare variants will be potentially useful for two reasons. First, because GWAS SNP arrays do not generally tag variants with minor allele frequencies less than 5% well, direct genotyping of these SNPs is the only way to assess the low frequency spectrum of allelic variation. Second, rare variants located within the exons of genes may include some that disrupt gene function, thus potentially having large effects on phenotype expression. Even if these mutations are rare, their identification can shed important insights into the role of that particular gene (and perhaps gene pathway) in bone biology.
Other approaches for identifying genetic variants associated with BMD and/or fracture risk are in their infancy. Epigenetic factors that do not modify the DNA sequence itself, but rather gene expression, may play important roles. The most well known epigenetic mechanisms are DNA methylation and histone modifications, which act at the level of gene transcription, and miRNAs (microRNAs), which act at the post-transcriptional level. Epigenetic marks may drive differentiation programs for cell fate, including osteoblastic lineage differentiation, and/or may be important regulators of bone remodeling during osteoclastogenesis.Citation73 In fact, recent evidence suggests that methylation-dependent mechanisms may influence the transcription of RANKL and OPG expression.Citation74 One speculation is that epigenetic marks may be modified by nutritional factors, intrauterine growth influences, and/or other environmental factors, although at the present time it is not clear what these modifying factors are and what are the key epigenetic marks most relevant to osteoporosis. Resolving these questions has been difficult for a number of reasons, including obtaining accurate measurement of the relevant exposures, identifying the relevant epigenetic marks to measure, and the cell and/or tissue specificity of epigenetic marks. These challenges notwithstanding, elucidation of epigenetic control of bone regulation may have translational implications; for example, some demethylating agents are already used to treat neoplastic disorders.Citation75,Citation76
There has been very limited research to date into the genetic determinants of response to treatment with antiresorptive agents used to slow bone loss in osteoporosis. With increased knowledge of the molecules and pathways that drive the balance between bone formation and bone resorption, one can imagine that drug treatments could be refined and optimized according to the patient’s individual genetic profile to allow the most beneficial treatment for each patient. The degree to which this can be realized is hard to say, because it may depend not only on how individual polymorphisms influence response to treatment, but perhaps also on how combinations of polymorphisms in aggregate influence response.
Conclusion
Over 60 variants identified over the past 7 years have been reliably associated with variation in BMD, some of which are also associated with fracture risk. Many of these variants map to genes in known pathways that are related to bone biology, although some map to genes with a relation to bone biology that is not readily apparent. A key point is that the effect sizes of variants are very small, because they collectively account for less than 6% of the variation in BMD. Although predicting individuals at risk for future fracture would be of enormous value so that treatment can be initiated early, the current panel of variants is not useful for prediction of future fracture, given that adding them to prediction models that already include age, gender, and BMD offers only a negligible improvement in predictive value. One hope is that future genetic discoveries made through even larger meta-analyses or new discoveries based on analyses of rare variants will identify new pathways relevant to the pathophysiology of bone metabolism or lead to a more granular characterization of known pathways. Such knowledge could inspire the development of more targeted therapeutics to prevent age-related bone loss.
Acknowledgment
Partial funding for this project was provided by the Mid-Atlantic Nutrition and Obesity Research Center (P30 DK072488).
Disclosure
The authors report no conflicts of interest in this work.
References
- United States Bone and Joint InitiativeThe burden of musculoskeletal diseases in the United States2011 Available from: http://www.boneandjointburden.org/Accessed September 4, 2013
- NguyenNDAhlborgHGCenterJREismanJANguyenTVResidual lifetime risk of fractures in women and menJ Bone Miner Res20072278178817352657
- BurgeRDawson-HughesBSolomonDHWongJBKingATostesonAIncidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025J Bone Miner Res20072246547517144789
- NIH Consensus Development PanelOsteoporosis prevention, diagnosis, and therapyJAMA200128578579511176917
- PeacockMTurnerCHEconsMJForoudTGenetics of osteoporosisEndocr Rev20022330332612050122
- NgMYShamPCPatersonADChanVKungAWEffect of environmental factors and gender on the heritability of bone mineral density and bone sizeAnn Hum Genet20067042843816759177
- ChesnutCH3rdIs osteoporosis a pediatric disease? Peak bone mass attainment in the adolescent femalePublic Health Rep1989Suppl 10450542517701
- WattsNBClinical utility of biochemical markers of bone remodelingClin Chem1999451359136810430819
- TostesonANGottliebDJRadleyDCFisherESMeltonLJ3rdExcess mortality following hip fracture: the role of underlying health statusOsteoporos Int2007181463147217726622
- HaentjensPMagazinerJColon-EmericCSMeta-analysis: excess mortality after hip fracture among older women and menAnn Intern Med201015238039020231569
- SterlingRSGender and race/ethnicity differences in hip fracture incidence, morbidity, mortality, and functionClin Orthop Relat Res20114691913191821161737
- PongchaiyakulCNguyenNDJonesGCenterJREismanJANguyenTVAsymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: a long-term prospective studyJ Bone Miner Res2005201349135516007332
- HallbergIRosenqvistAMKartousLLöfmanOWahlströmOTossGHealth-related quality of life after osteoporotic fracturesOsteoporos Int20041583484115045468
- AdachiJDAdamiSGehlbachSGLOW InvestigatorsImpact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in womenMayo Clin Proc20108580681320634496
- SolimeoSLSilvermanSLCalderonADNguyenAGoldDTMeasuring health-related quality of life (HRQOL) in osteoporotic males using the male OPAQOsteoporos Int20122384185221528362
- BlackDMDelmasPDEastellRHORIZON Pivotal Fracture TrialOnce-yearly zoledronic acid for treatment of postmenopausal osteoporosisN Engl J Med20073561809182217476007
- LylesKWColon-EmericCSMagazinerJSHORIZON Recurrent Fracture TrialZoledronic acid and clinical fractures and mortality after hip fractureN Engl J Med20073571799180917878149
- BonoCMEinhornTAOverview of osteoporosis: pathophysiology and determinants of bone strengthEur Spine J200312Suppl 2S90S9613680312
- BouxseinMLSeemanEQuantifying the material and structural determinants of bone strengthBest Pract Res Clin Rheumatol20092374175319945686
- World Health OrganizationAssessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study GroupWorld Health Organ Tech Rep Ser199484311297941614
- Martinez-MorilloMGradosDHolgadoSPremenopausal osteoporosis: how to treat?Reumatol Clin20128939722089064
- National Osteoporosis FoundationClinician’s Guide to Prevention and Treatment of OsteoporosisWashington, DCNational Osteoporosis Foundation2008
- MariniFBrandiMLPharmacogenetics of osteoporosis: future perspectivesCalcif Tissue Int20098433734719271099
- MariniFBrandiMLPharmacogenetics of osteoporosis: what is the evidence?Curr Osteoporos Rep20121022122722760517
- CompstonJClinical and therapeutic aspects of osteoporosisEur J Radiol20097138839119660883
- KingABFiorentinoDMMedicare payment cuts for osteoporosis testing reduced use despite tests’ benefit in reducing fracturesHealth Aff (Millwood)2011302362237022147865
- O’MalleyCDJohnstonSSLenhartGCherkowskiGPalmerLMorganSLTrends in dual-energy x-ray absorptiometry in the United States, 2000–2009J Clin Densitom20111410010721787516
- TannerSBDual-energy x-ray absorptiometry in clinical practice: new guidelines and concernsCurr Opin Rheumatol20112338538821637082
- MarshallDJohnellOWedelHMeta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fracturesBMJ1996312125412598634613
- JohnellOKanisJAOdenAPredictive value of BMD for hip and other fracturesJ Bone Miner Res2005201185119415940371
- JonesGNguyenTSambrookPKellyPJEismanJAProgressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo osteoporosis epidemiology studyBMJ19943096916957950520
- SmithJAVentoJASpencerRPTendlerBEAortic calcification contributing to bone densitometry measurementJ Clin Densitom1999218118310499978
- NguyenNDEismanJACenterJRNguyenTVRisk factors for fracture in nonosteoporotic men and womenJ Clin Endocrinol Metab20079295596217164302
- NishiyamaKKMacdonaldHMHanleyDABoydSKWomen with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCTOsteoporos Int2013241733174023179565
- Dawson-HughesBLookerACTostesonANJohanssonHKanisJAMeltonLJ3rdThe potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008Osteoporos Int20122381182021717247
- SamelsonEJHannanMTEpidemiology of osteoporosisCurr Rheumatol Rep20068768316515770
- KielDPRosenCJDempsterDAge-Related Bone Loss Chapter 20John Wiley & Sons Inc2009
- LaneNEEpidemiology, etiology, and diagnosis of osteoporosisAm J Obstet Gynecol2006194S3S1116448873
- MitchellBDKammererCMSchneiderJLPerezRBauerRLGenetic and environmental determinants of bone mineral density in Mexican Americans: results from the San Antonio Family Osteoporosis StudyBone20033383984614623060
- FaulknerKGWackerWKBardenHSFemur strength index predicts hip fracture independent of bone density and hip axis lengthOsteoporos Int20061759359916447009
- PulkkinenPJämsäTLochmüllerEMKuhnVNieminenMTEcksteinFExperimental hip fracture load can be predicted from plain radiography by combined analysis of trabecular bone structure and bone geometryOsteoporos Int20081954755817891327
- MichaëlssonKMelhusHFermHAhlbomAPedersenNLGenetic liability to fractures in the elderlyArch Intern Med20051651825183016157825
- DengHWChenWMReckerSGenetic determination of Colles’ fracture and differential bone mass in women with and without Colles’ fractureJ Bone Miner Res2000151243125210893672
- AndrewTAntioniadesLScurrahKJMacgregorAJSpectorTDRisk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMDJ Bone Miner Res200520677415619671
- SeemanEHopperJLBachLAReduced bone mass in daughters of women with osteoporosisN Engl J Med19893205545582915666
- HuiSLKollerDLForoudTMEconsMJJohnstonCCPeacockMHeritability of changes in bone size and bone mass with age in premenopausal white sistersJ Bone Miner Res2006211121112516813533
- ShafferJRKammererCMDressenASBruderJMBauerRLMitchellBDRate of bone loss is greater in young Mexican American men than women: the San Antonio Family Osteoporosis studyBone201047495420347056
- BrownLBStreetenEAShapiroJRGenetic and environmental influences on bone mineral density in pre- and post-menopausal womenOsteoporos Int2005161849185615997421
- KellyPJNguyenTHopperJPocockNSambrookPEismanJChanges in axial bone density with age: a twin studyJ Bone Miner Res1993811178427043
- ChristianJCYuPLSlemendaCWJohnstonCCJrHeritability of bone mass: a longitudinal study in aging male twinsAm J Hum Genet1989444294332916585
- ShafferJRKammererCMBruderJFive-year change in bone mineral density is heritable in Mexican Americans: The San Antonio Family Osteoporosis StudyJ Bone Miner Res200520Suppl 1S67
- MakoveyJNguyenTVNaganathanVWarkJDSambrookPNGenetic effects on bone loss in peri- and postmenopausal women: a longitudinal twin studyJ Bone Miner Res2007221773178017620052
- MitchellBDYerges-ArmstrongLMThe genetics of bone loss: challenges and prospectsJ Clin Endocrinol Metab2011961258126821346070
- EstradaKStyrkarsdottirUEvangelouEGenome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fractureNat Genet20124449150122504420
- RichardsJBZhengHFSpectorTDGenetics of osteoporosis from genome-wide association studies: advances and challengesNat Rev Genet20121357658822805710
- GongYSleeRBFukaiNOsteoporosis-Pseudoglioma Syndrome Collaborative GroupLDL receptor-related protein 5 (LRP5) affects bone accrual and eye developmentCell200110751352311719191
- ClarkeBNormal bone anatomy and physiologyClin J Am Soc Nephrol20083Suppl 3S131S13918988698
- GuoYTanLJLeiSFGenome-wide association study identifies ALDH7a1 as a novel susceptibility gene for osteoporosisPLoS Genet20106e100080620072603
- GiulianiNGirasoleGVescoviPPPasseriGPedrazzoniMEthanol and acetaldehyde inhibit the formation of early osteoblast progenitors in murine and human bone marrow culturesAlcohol Clin Exp Res19992338138510069572
- RalstonSHUitterlindenAGGenetics of osteoporosisEndocr Rev20103162966220431112
- LiWFHouSXYuBLiMMFérecCChenJMGenetics of osteoporosis: accelerating pace in gene identification and validationHum Genet201012724928520101412
- FarberCRLusisAJFuture of osteoporosis genetics: enhancing genome-wide association studiesJ Bone Miner Res2009241937194219888896
- DuncanELBrownMAGenetic determinants of bone density and fracture risk – state of the art and future directionsJ Clin Endocrinol Metab2010952576258720375209
- HopkinsRBGoereeRPullenayegumEThe relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal womenBMC Musculoskelet Disord20111220921943363
- WainwrightSAMarshallLMEnsrudKEStudy of Osteoporotic Fractures Research GroupHip fracture in women without osteoporosisJ Clin Endocrinol Metab2005902787279315728213
- NguyenNDFrostSACenterJREismanJANguyenTVDevelopment of a nomogram for individualizing hip fracture risk in men and womenOsteoporos Int2007181109111717370100
- NguyenNDFrostSACenterJREismanJANguyenTVDevelopment of prognostic nomograms for individualizing 5-year and 10-year fracture risksOsteoporos Int2008191431144418324342
- TranBNNguyenNDCenterJREismanJANguyenTVEnhancement of absolute fracture risk prognosis with genetic marker: the collagen 1 alpha 1 geneCalcif Tissue Int20098537938819789904
- NguyenTVEismanJAGenetics and the individualized prediction of fractureCurr Osteoporos Rep20121023624422851044
- HsuYHZillikensMCWilsonSGAn integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility loci for osteoporosis-related traitsPLoS Genet20106e100097720548944
- LuiJCNilssonOChanYSynthesizing genome-wide association studies and expression microarray reveals novel genes that act in the human growth plate to modulate heightHum Mol Genet2012215193520122914739
- BoneHFuture directions in osteoporosis therapeuticsEndocrinol Metab Clin North Am20124165566122877435
- Delgado-CalleJGarmillaPRianchoJADo epigenetic marks govern bone mass and homeostasis?Curr Genomics20121325226323115526
- Delgado-CalleJSañudoCFernándezAFGarcía-RenedoRFragaMFRianchoJARole of DNA methylation in the regulation of the RANKL-OPG system in human boneEpigenetics20127839122207352
- La ThangueNBHistone deacetylase inhibitors and cancer therapyJ Chemother200416Suppl 4646715688613
- SantosFPKantarjianHGarcia-ManeroGIssaJPRavandiFDecitabine in the treatment of myelodysplastic syndromesExpert Rev Anticancer Ther20101092220014881