Abstract
Objective
Cardiac microvascular obstruction (CMVO) remains a severe complication in non-ST elevation myocardial infarction (NSTEMI) patients with reperfusion therapy. We aimed at developing and validating the nomogram to predict the possibility of CMVO after primary percutaneous coronary intervention (PCI) by integrating clinical and laboratory-based information.
Methods
A total of 325 patients undergoing primary PCI for NSTEMI were recruited and divided into the training cohort (n=226) and the validating cohort (n = 99). The development of the nomogram was based on independent predictors of CMVO, and these variables were selected by multivariable logistic regression analysis.
Results
Independent predictors contained in nomogram were identified by multivariable logistic regression analysis, and these independent predictors included neutrophils (OR 1.166, 95% CI 1.044–1.303, P<0.01), hemoglobin (OR 1.037, 95% CI 1.013–1.062, P<0.01), triglyceride (OR 1.343, 95% CI 1.059; 1.704, P=0.015), Killip grade (OR 2.190, 95% CI 1.065–4.503, P=0.033), high thrombus load (OR 3.146, 95% CI 1.424–6.952, P<0.01), no-reflow (OR 3.142, 95% CI 1.419–6.955, P<0.01) and ischemic postconditioning (OR 0.445, 95% CI 0.209–0.944, P=0.035). The nomogram accurately predicted the presentation of CMVO in both the training set and validating set (AUC, 0.835 and 0.881, respectively). The results predicted by nomogram were confirmed to be highly consistent with the results of DE-CMR, both the training and validating cohorts, by Calibration plot and Hosmer-Lemeshow test. Decision curve analysis (DCA) also suggested that the nomogram was applicable in the clinic.
Conclusion
The nomogram showed good performance in predicting CMVO, and it could help clinicians optimize the clinical treatments to improve the prognosis of NSTEMI patients.
Introduction
Non-ST segment elevation myocardial infarction (NSTEMI) is a severe type of acute coronary syndrome (ACS), and its incidence is growing.Citation1 In a myocardial infarction registry, 63.1% were NSTEMI, and 4.2% of NSTEMI patients died in the hospital.Citation2 Therefore, more attention should be paid to the treatment and prognosis of NOSTEMI patients. Currently, reperfusion therapy, as the main treatment for NSTEMI patients, is mainly aimed to prompt intervention for the culprit artery. However, in some circumstances, the culprit’s vessel is opened, yet main adverse cardiovascular events (MACE), such as death, rehospitalization, or heart failure, would still happen to patients due to coronary microvascular obstruction (CMVO).Citation3 The occurrence of CMVO is a result of multiple factors. Mechanisms involved in pathogenesis of CMVO include blocking of the vascular lumen due to formation of leukocyte–platelet aggregates and erythrocyte aggregates, myocardial cell edema induced by coronary microvasculature compression after reperfusion injury, which will be aggravated by the lack of blood flow in functional vessels, and the distal microvascular embolization elicited by atherothrombotic debris during percutaneous coronary intervention (PCI).Citation4 In addition, oxidative stress and intracardial hemorrhage after acute myocardial infarction (AMI), coronary spasm and dissection, endothelial dysfunction, inflammation in microvasculature, and individual susceptibility can also be implicated in CMVO.Citation5,Citation6
Currently, preventive treatment strategies for CMVO include ischemic postconditioning, drug treatment, and interventional procedures, and in order to translate clinically into effective treatment for AMI patients, especially, the elderly, with comorbidities, and with receiving several medications, we need to focus not only on the reduction in infarct size, but also on attenuation of CMVO size.Citation7–Citation9 In addition, studies showed that active and effective treatment early would make CMVO improved.Citation10 Therefore, early detection of CMVO is particularly vital for the treatment and clinical prognosis of patients. Currently, diagnosis of CMVO includes invasive and non-invasive detection methods. In invasive detection methods, coronary flow reserve (CFR) is considered the gold standard for evaluating CMD, but it is difficult to be widely promoted in clinical practice due to trauma, time consumption, expensiveness, and many potential complications. Thrombolysis in myocardial infarction (TIMI), TIMI myocardial perfusion grade (TMPG), and myocardial blush grading (MBG) are the primary detection methods for CMVO during PCI. However, the results of angiography may not reflect the perfusion status of capillaries.Citation11 Studies have confirmed that 29–38% of patients with TIMI 3 still have CMVO.Citation12,Citation13 Vicente et al found that MBG underestimated CMVO after optimal revascularization in AMI.Citation14 In addition, Wu et al demonstrated that a noninvasive method for MRI-determined microvascular obstruction can predict prognosis within 2 years.Citation15 Judd et al conducted a study in the dog model and confirmed that the coronary microvascular hypoperfusion area distinguished by delay-enhanced cardiovascular magnetic resonance (DE-CMR) corresponds to the coronary microvascular occlusion area of thioflavin-negative regions.Citation16 Therefore, compared with invasive detection methods, DE-CMR has been regarded as the gold standard for noninvasive detecting CMVO. However, DE-CMR may have many shortcomings that make it less clinically used, such as high cost, time consumption, poor tolerance, Most current studies focus on looking for independent risk factors of arising CMVO after PCI, but the occurrence of CMVO is the result of multiple risk factors interacting, and not the result of a certain risk factor.Citation3 Accordingly, one single indicator may not be valid enough to evaluate CMVO after PCI. According to the above statement, a simple scoring system needs to be established to assess quickly and easily whether NSTEMI patients have CMVO after PCI.
Nomogram, as a graphical tool, can be used to quickly calculate the morbidity of a certain disease in an individual patient, as well as evaluate the prognosis.Citation17 To determine quickly and conveniently CMVO probability after primary PCI and stratify high-risk patients, this retrospective study based on the analysis of independent risk factors of laboratory examinations and clinical indicators aims to establish a nomogram that can quickly calculate the probability of CMVO individually.
Materials and Methods
Study Design and Participants
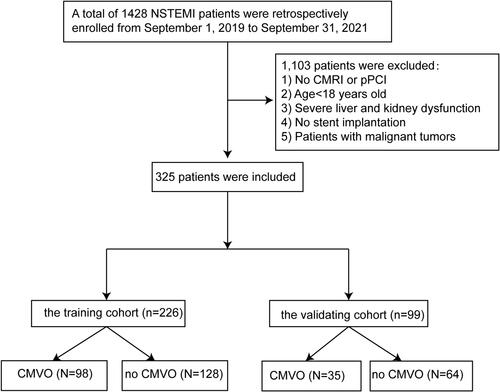
The consecutive patients were retrospectively reviewed for the data, which was recorded in the medical record management system of the Affiliated Hospital of Xuzhou Medical University and the Second Affiliated Hospital of Xuzhou Medical University from September 1, 2019, to September 31, 2021, and 1428 patients with NSTEMI were included in the study. According to the inclusion and exclusion criteria, 325 NSTEMI patients were eventually divided into the training cohort (70%, n=226) and validation cohort (30%, n=99), which were used for the development and verification of nomogram. showed the flow chart of the study. In additional, Supplementary Material shows detailed design program for the study.
Figure 1 Flow chart.
Inclusion criteria were as follows: 1) The diagnosis of NSTEMI met the diagnostic criteria of the relevant guidelines;Citation1 2) The patients were performed primary PCI within 24 hours of the onset of symptoms.
Meanwhile, exclusion criteria contain any one of the following: 1) age <18 years old; 2) Patients had not undergone DE-CMR; 3) Patients with any one of the following diseases: cardiogenic shock, immunological or rheumatic diseases, severe liver, and kidney dysfunction (require liver and kidney replacement therapy), malignant tumors, bleeding disorders, and contraindication for the anticoagulant or antiplatelet agents; 4) Killip IV grade; 5) Patients with severely missing clinical data.
Clinical Treatment Process
All patients were sent to digital subtraction angiography (DSA) room, and then underwent primary PCI treatment. Patients received 300 mg aspirin, 600 mg clopidogrel, or 180 mg ticagrelor as pre-treatment before primary coronary angiography and primary PCI. During the angiography, 3000U unfractionated heparin and 200ug of nitroglycerin were injected through the sheath and 100 IU/kg unfractionated heparin was injected through the sheath during primary PCI. Intraoperative medication including nitroprusside, nitroglycerin, tirofiban, and other drugs that improve the symptoms of coronary ischemia, and adjuvant therapy including thrombus aspiration and cardiac pacing was determined according to the patient’s condition and the operators. Patients who were not suitable for PCI should be treated with percutaneous transluminal coronary angioplasty (PTCA). They should be treated with regular programs after the operation, such as Dual antiplatelet [aspirin (100mg QD) combined with clopidogrel (75mg QD) or aspirin (100mg QD) combined with ticagrelor (90mg BID) and statins.
Collection of Candidate Variables
Relevant demographic variables included age, gender, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), smoking history, drinking status, pre-infarction angina, and Killip grade. Previous history contained hypertension, diabetes, cerebral infarction, and history of stent implantation. Laboratory examination was consist of white blood cell (WBC) count, neutrophil (N) count, lymphocyte (L) count, red blood cell (RBC) count, hemoglobin (Hb), platelet (PLT) count, high-sensitivity C-reactive protein (hs-CRP), albumin, creatinine, estimated glomerular filtration rate (eGFR), serum uric acid (SUA), glucose, glycated hemoglobin, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A, apolipoprotein B, lipoprotein a, small dense low-density lipoprotein cholesterol (sdLDL-C), lactate dehydrogenase, creatine kinase isoenzyme, high-sensitivity troponin T (hsTnT), cardiac troponin I (cTnI), N-terminal pro-brain natriuretic peptide (NT-proBNP), fibrinogen (FIB), and D-dimer. Image data during PCI included symptom onset-to-balloon time (SBT), door-to-balloon (D2B) time, history of stent implantation, multiple vascular disease, left anterior descending (LAD), left circumflex branch (LCX), right coronary artery (RCA), calcified lesions, proximal lesions, bifurcation lesion, stent length, stent diameter, number of stent, high thrombus load, no-reflow, ischemic postconditioning, temporary pacemaker, and the use drug of tirofiban, atropine, dopamine or hydroxylamine and nitroprusside.
Assessment of CMVO and Definitions
DE-CMR (3.0T) was performed to assess CMVO within 5–7 days after successful reperfusion. In DE-CMR, the infarct area shows as the high signal area in the enhanced area, and CMVO appears as a low signal area in the center of the high signal. Related definitions of important candidate variables included the following: History of CAD is defined as either one or more major epicardial coronary arteries that have more than 50% lumen stenosis on imaging. The multivessel disease is defined as more than 50% stenosis of either one or more main epicardial vessels. Bifurcation lesions are defined as coronary artery stenosis occurring nearing and/or involving the origin of important branch openings.Citation18 If one of the following characteristics is met, it is indicated as a high thrombotic load: 1) The vessel diameter of the infarct-related arteries (IRA) ≥4.0 mm; 2) Thrombus length tripled the inner diameter of the reference vessel or more; 3) There is no tapering lumen proximal to the occlusion site for truncation-like occlusion; 4) Thrombus accumulation proximal to the occlusion site; 5) Floating thrombus presented near the proximal occlusion site; 6) Persistent contrast retention near the distal end of occlusion.Citation19 “No reflow” was defined as TIMI flow grade <3 with the reopening of occluded coronary artery evidenced in angiography.Citation20 After reperfusion by stents implantation, ischemic postconditioning was defined as inflation and deflation frequently (approximately four times) of the balloon within 1 minute of reperfusion.Citation21
Statistical Analysis
Continuous variables were indicated as mean ± standard deviation, If the test of normality and the homogeneity test of variance is performed, the t-test is appropriate. Otherwise, the Mann–Whitney U-test is appropriate. Categorical variables were presented as percentages in the tables and compared by using the X2 test or Fisher exact test. The candidate variables were first selected by univariate logistic regression analysis in the train set, and the candidate variables from P-value < 0.05 in the univariate logistic regression analysis were analyzed in the multivariable logistic regression analysis. Finally, variables with a P-value < 0.05 in the multivariable logistic regression analysis were included in the nomogram. Nomogram evaluation consisted of discrimination capacity, calibration, and clinical effectiveness. The concordance index (C-index) was measured to quantify the discrimination capacity of the nomogram, and C-index is equal to the area under the receiver operating characteristics curve (AUC-ROC) in logistic regression analysis. A calibration plot was drawn to evaluate the prediction accuracy of the nomogram, and the Hosmer-Lemeshow test was calculated to assess the consistency of the predicted with actual probability. Decision curve analysis (DCA) was performed to evaluate Clinical effectiveness. Stata (Version 15.0, https://www.stata.com/) and R Studio (Version 4.1.1, https://www.Rproject.org) were utilized to analyze data. All statistical tests with a P-value < 0.05 were significant.
Results
Participants Characteristics
From September 1, 2019, to September 31, 2021, 325 participants were enrolled in the testing dataset. 133 (41%) of 325 participants were diagnosed with CMVO by DE-CMR. Among all patients, 226 patients were placed in the train dataset and 99 in the validation dataset in chronological order (). The training set was divided into 98 patients with CMVO and 128 patients without CMVO, and the validating dataset was divided into 35 patients with CMVO and 64 patients without CMVO. The patients with CMVO after primary PCI had a higher level of Killip grade, higher using tirofiban, a higher proportion of No-reflow, heavier thrombus load, a higher baseline level of N, NLR, Hb, TG, LDH, CKMB, hsTnT, cTnI (all P<0.05). However, lower levels ofWBC, a lower proportion of women, a lower proportion of ischemic postconditioning were found in CMVO (all P<0.05).
Table 1 Patient Characteristics
Predictors of CMVO After Primary PCI
The predictors with P-value <0.05 in the univariate analysis results were sex (OR 0.397, 95% CI 0.177–0.891, P=0.025), N (OR 1.231, 95% CI 1.124–1.348, P<0.001), Hb (OR 1.048, 95% CI 1.028–1.067, P<0.001), TG (OR 1.387, 95% CI 1.154–1.667, P=0.001), LDH (OR 1.0006, 95% CI 1.0001–1.0012, P=0.015), CKMB (OR 1.005, 95% CI 1.002–1.008, P=0.003), hsTnT (OR 1.0002, 95% CI 1.0001–1.0003, P<0.001), cTnI (OR 1.022, 95% CI 1.005–1.038, P=0.01), SBT (OR 1.108, 95% CI 1.003–1.224, P=0.043), Killip grade (OR 2.098, 95% CI 1.222–3.602, P=0.007), high thrombus load (OR 3.42, 95% CI 1.827–6.400, P<0.001), no-reflow (OR 2.891, 95% CI 1.575–5.306, P=0.001) and ischemic postconditioning (OR 0.37, 95% CI 0.207–0.663, P=0.001) (). Finally, the following 7 variables selected by multivariate regression analysis were considered as independent influencing factors for CMVO in NSTEMI patients after primary PCI: N (OR 1.166, 95% CI 1.044–1.303, P<0.01), Hb (OR 1.037, 95% CI 1.013–1.062, P<0.01), TG (OR 1.343, 95% CI 1.059; 1.704, P=0.015), Killip grade (OR 2.190, 95% CI 1.065–4.503, P=0.033), high thrombus load (OR 3.146, 95% CI 1.424–6.952, P<0.01), no-reflow (OR 3.142, 95% CI 1.419–6.955, P<0.01) and ischemic postconditioning (OR 0.445, 95% CI 0.209–0.944, P=0.035) ().
Table 2 Univariate Logistic Regression Analysis of the Data from the Training Cohort
Table 3 Multivariate Logistic Regression Analysis of the Data from the Training Cohort
Development and Validation of Nomogram to the Predictive Probability of CMVO After Primary PCI
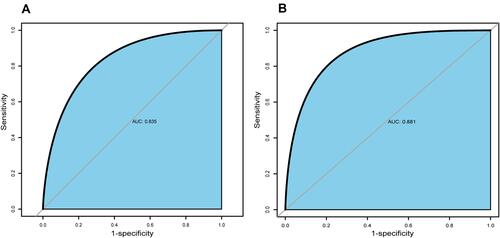
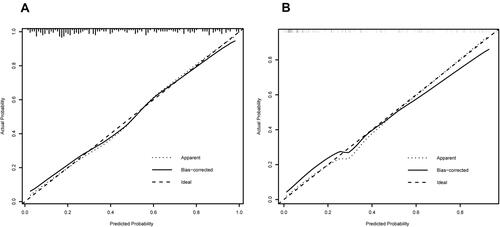
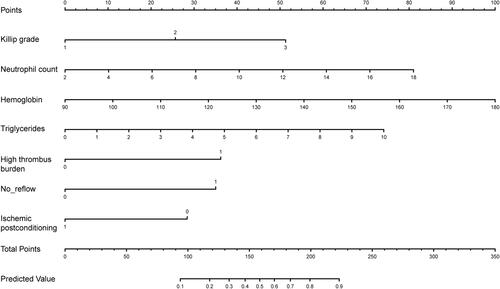
These 7 independent influencing factors selected by multivariate regression analysis were incorporated to generate a nomogram, and the nomogram was showed in . The AUC for the nomogram of the training cohort and the validation cohort was 0.835 (95% CI: 0.780–0.882) and 0.881 (95% CI: 0.803–0.939), respectively, which suggested a nomogram with good discriminative capacity, and ROC curves were showed in and . The calibration of the nomogram was assessed by using the calibration curve and Hosmer-Lemeshow test. The calibration plot showed good agreement between the nomogram and the validating sets ( and ), and the Hosmer-Lemeshow test also suggested high consistency for predicted and actual probability in both the nomogram (P = 0.684) and validation (P = 0.727) cohort.
Figure 2 Nomogram for predicting the possibility of CMVO after primary PCI in NSTEMI patients.
Use of the Nomogram
The possibility of CMVO after NSTEMI is predicted by combining N, Hb, TG, Killip grade, high thrombus load, No-reflow, and ischemic postconditioning. The score of each variable is calculated by drawing a vertical line between each variable axis and the top line of the nomogram. Then, we can add the scores of each variable and find the corresponding score on the total scoreline. Finally, we can draw a vertical line from the total scoreline to the bottom predicted probability scale to obtain the individual probability of CMVO in NSTEMI patients.
Clinical Effectiveness
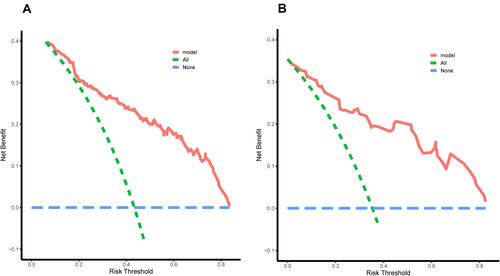
The clinical validity of the nomogram was evaluated by the decision curve analysis (DCA). As shown decision curve, the blue dotted line represents the intervention-none and the net benefit with zero, the green dotted line shows intervention-all-patients. The upper part of the green dotted line represents positive income, meanwhile, the lower part represents negative income. Redline represents a threshold of the model. The threshold of the nomogram and validation sets exceed widely threshold range and all range shows from approximately 5% to 85%, which suggests that the nomogram was clinically useful ( and ).
Figure 5 The decision curve analysis (DCA) for the nomogram in the training cohort (A) and the validating cohort (B). The blue dotted line represents the intervention-none and the net benefit with zero, the green dotted line shows intervention-all-patients. The upper part of the green dotted line represents positive income, meanwhile, the lower part represents negative income. The red line represents a threshold of the model.
Discussion
At present, in clinical practice, primary PCI is the main treatment method for NSTEMI. However, even after primary PCI, some NSTEMI patients still have main adverse cardiovascular events, which seriously affect people’s quality of life. However, the current evaluation of CMVO still lacks a unified prediction and scoring system. Therefore, the focus of the present study is to develop a nomogram and identify the possibility of CMVO after PCI for NSTEMI patients. Based on multivariate logistic regression analysis, we identified the following 7 independent influencing factors for CMVO: N, Hb, TG, Killip grade, high thrombus load, no-reflow and ischemic postconditioning. The present study demonstrates that a simple diagnostic score composed of the above independent risk factors is a valid clinical tool for calculating the possibility of CMVO after primary PCI. The excellent correspondence of results in both the nomogram and verification cohort demonstrated that the score is useful enough to provide accurate risk stratification of NSTEMI patients even among people in different periods.
For AMI, what was once considered to be a disease with a high risk for mortality have been treated effectively with low mortality because of primary PCI, during the past 2 decades. However, despite the general success of primary PCI, a large proportion of patients do not achieve myocardial reperfusion owing to CMVO after primary PCI.Citation22 Several studies have clearly shown that CMVO is interrelated to various adverse prognoses, such as ventricular remodeling, heart failure, and death.Citation3 Therefore, the diagnosis of CMVO is particularly important for clinicians. Xiao et al established a model for predicting CMVO after primary PCI in STEMI patients,Citation23 but their results may have certain controversy. Firstly, they defined TMPG 3 grade as the gold standard for CMVO, however, the current gold standard for diagnosing CMVO is DE-CMR in our study. Early studies had shown that even if coronary angiography showed complete recovery of epicardial blood flow, a sizeable proportion of patients still had CMVO.Citation11–Citation14 And secondly, their model has not been tested for clinical effectiveness, whether the model can be effectively applied in a clinic is still unknown. Meanwhile, in the present study, not only do we regard DE-CMR as the gold standard for diagnosing CMVO, but also our model has good clinical effectiveness by conducting clinical effectiveness tests.
Previous studies have shown that the emergence of CMVO was caused by multiple interactive mechanisms, which makes the diagnosis of CMVO unable to be predicted by a single factor. Among the various CMVO mechanisms, the following four mechanisms are widely accepted: distant embolization, ischemia-related injury, reperfusion-related injury, and individual susceptibility.Citation24–Citation26 In this study, multiple risk factors were identified to develop a nomogram that can predict the possibility of CMVO after primary PCI. For the constituent elements of nomogram in the present study, neutrophils result in capillary blocking in the coronary microcirculation and can mediate endothelial function injury leading to the “no-reflow” phenomenon.Citation27,Citation28 As one of the major risk factors in cardiovascular disease, the mechanism of the contribution of dyslipidemia in atherosclerosis has been widely accepted. Hypertriglyceridaemia exerts detrimental effects on nitric oxide bioavailability resulting in endothelial dysfunction and stimulating the accumulation of inflammatory factors to the endothelial surface decreasing stability of coronary artery plaques.Citation29,Citation30 For AMI patients, the Killip grade indicates their quality of heart function, and the high Killip grade represents poor heart function and larger infarct size.Citation31 Jang and De Luca et al found that high Killip grade was associated with a high coronary microcirculation resistance index and myocardial reperfusion injury.Citation32,Citation33
In addition, the study also found high thrombus load, no-reflow were independent risk factors and ischemic postconditioning was an independent protective factor for CMVO after primary PCI. The high-load thrombus may break up to form small or tiny thrombus to block the distal capillaries either spontaneously or after mechanical dilation of the culprit lesion, which can cause the “no-reflow” or slow-reflow of the culprit’s vessel during primary PCI,Citation34 which may aggravate ischemia-reperfusion injury. In clinical practice, we often regard “no-reflow” phenomenon as CMVO, yet CMVO may be more complicated than the “no-reflow”.Citation35 Most “no-reflow” phenomena are reversible by applying vasodilators into the coronary arteries,Citation26 In addition, Kim and Vicente et al found that the possibility of CMVO diagnosed by coronary angiography is often lower than the actual incidence of CMVO.Citation11–Citation14 Meanwhile, Engstrøm et al found that ischemic postconditioning had no obvious positive effect on death and hospitalization,Citation36 but the study from Zhao et al found that ischemic postconditioning was cardio-protective effect on the heart by attenuating myocardial edema after reperfusion injury.Citation37 The cardioprotection by ischemic preconditioning could be achieved through several complex signal transduction pathways. Although several risk factors and comorbidities, including advanced age, diabetes, hypertension, and Hyperemia, may attenuate or abrogate the cardioprotection effects of ischemic postconditioning, this cardioprotection effect could be not affected by coronary microembolization.Citation7,Citation38 The protective effect of ischemic postconditioning is consistent with the results of this study.
Currently, some studies have proved that age, smoking, and other factors are independent risk factors for CMVO. With the increase of age, aging-related vascular endothelial dysfunction can induce coronary artery disease.Citation39,Citation40 In addition, endothelial dysfunction has been confirmed in the coronary arteries of long-term smokers, in whom coronary flow reserve was reduced by 21% by comparing with the value in nonsmoking.Citation41 However, the present study did not get similar results with the above. We analyzed the following reasons for the above results: 1) In this study, only 30% of patients were elderly, which reduced the proportion of risk factors for advanced age. 2) In this study, about 97% of smoking patients were young and middle-aged, which may increase the influence of age as a risk factor on the results. 3) The data collection bias has a certain impact on the experimental results because of the retrospective study. Although the nomogram does not include the above influencing factors, it can still predict the occurrence of CMVO accurately.
Limitations
Several limitations should be pointed out. Firstly, the present study is a single-center retrospective design. The number of patients is small, and the selected population is relatively limited, which may lead to experimental bias. Further studies, especially multi-center registration, large-scale prospective research should be needed to verify the transportability and generalizability of our nomogram. Secondly, some indicators of echocardiography were not included in the nomogram. Finally, the nomogram cannot be applied to those patients with Killip IV grade, as these patients were excluded in this study.
Conclusions
The nomogram developed by using 7 factors, including N, Hb, TG, Killip grade, high thrombotic load, no-reflow, and ischemic post-treatment, could be used to calculate the possibility of CMVO in NSTEMI patients after primary PCI, and it could help clinicians contribute to the risk stratification, judge the prognosis and make a decision for treatment in NSTEMI patients.
Data Sharing Statement
The datasets are available by contacting the corresponding author ([email protected]).
Ethics Statement
This study was conducted by the Declaration of Helsinki and was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. The review committee waived the requirement for written informed consent because of the retrospective nature of the study. Prior to analysis, confidential patient information was deleted from the entire data set prior to analysis.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
- Balzi D, Di Bari M, Barchielli A, et al. Should we improve the management of NSTEMI? Results from the population-based “acute myocardial infarction in Florence 2” (AMI-Florence 2) registry. Intern Emerg Med. 2013;8(8):725–733. doi:10.1007/s11739-012-0817-6
- Crea F. Coronary microvascular obstruction–a puzzle with many pieces. N Engl J Med. 2015;372(15):1464–1465. doi:10.1056/NEJMe1501882
- Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. 2019;114(6):45. doi:10.1007/s00395-019-0756-8
- Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118(10):1643–1658. doi:10.1161/CIRCRESAHA.116.308640
- Kleinbongard P, Heusch G. A fresh look at coronary microembolization. Nat Rev Cardiol. 2021;18:1–16. doi:10.1038/s41569-020-00473-5
- Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17(12):773–789. doi:10.1038/s41569-020-0403-y
- Niccoli G, Montone RA, Ibanez B, et al. Optimized treatment of ST-elevation myocardial infarction. Circ Res. 2019;125(2):245–258. doi:10.1161/CIRCRESAHA.119.315344
- Heusch G. Treatment of myocardial ischemia/reperfusion injury by ischemic and pharmacological postconditioning. Compr Physiol. 2015;5(3):1123–1145.
- Kofler T, Hess S, Moccetti F, et al. Efficacy of ranolazine for treatment of coronary microvascular dysfunction-A systematic review and meta-analysis of randomized trials. CJC Open. 2021;3(1):101–108. doi:10.1016/j.cjco.2020.09.005
- Kim JS, Ko YG, Yoon SJ, et al. Correlation of serial cardiac magnetic resonance imaging parameters with early resolution of ST-segment elevation after primary percutaneous coronary intervention. Circ j. 2008;72(10):1621–1626. doi:10.1253/circj.CJ-08-0232
- Jesel L, Morel O, Ohlmann P, et al. Role of pre-infarction angina and inflammatory status in the extent of microvascular obstruction detected by MRI in myocardial infarction patients treated by PCI. Int J Cardiol. 2007;121(2):139–147. doi:10.1016/j.ijcard.2006.10.022
- Rogers WJ Jr., Kramer CM, Geskin G, et al. Early contrast-enhanced MRI predicts late functional recovery after reperfused myocardial infarction. Circulation. 1999;99(6):744–750. doi:10.1161/01.CIR.99.6.744
- Vicente J, Mewton N, Croisille P, et al. Comparison of the angiographic myocardial blush grade with delayed-enhanced cardiac magnetic resonance for the assessment of microvascular obstruction in acute myocardial infarctions. Catheter Cardiovasc Interv. 2009;74(7):1000–1007. doi:10.1002/ccd.22157
- Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97(8):765–772. doi:10.1161/01.CIR.97.8.765
- Judd RM, Lugo-Olivieri CH, Arai M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92(7):1902–1910. doi:10.1161/01.CIR.92.7.1902
- Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793. doi:10.1016/j.jtcvs.2017.12.107
- Louvard Y, Thomas M, Dzavik V, et al. Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter Cardiovasc Interv. 2008;71(2):175–183. doi:10.1002/ccd.21314
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54(4):281–292. doi:10.1016/j.jacc.2009.03.054
- Ndrepepa G, Tiroch K, Keta D, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. 2010;3(1):27–33. doi:10.1161/CIRCINTERVENTIONS.109.896225
- Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112(14):2143–2148. doi:10.1161/CIRCULATIONAHA.105.558122
- Piek JJ. Beyond epicardial reperfusion. N Engl J Med. 2007;356(18):1880–1882. doi:10.1056/NEJMe078024
- Xiao Y, Fu X, Wang Y, Wu Y, Wang W, Zhang Q. Development and validation of risk nomogram model predicting coronary microvascular obstruction in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous catheterization. Med Sci Monitor. 2019;25:5864–5877. doi:10.12659/MSM.915960
- Scarabelli T, Stephanou A, Rayment N, et al. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation. 2001;104(3):253–256. doi:10.1161/01.CIR.104.3.253
- Stakos DA, Kambas K, Konstantinidis T, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405–1414. doi:10.1093/eurheartj/ehv007
- Lee CH, Tse HF. Microvascular obstruction after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2010;75(3):369–377. doi:10.1002/ccd.22234
- Mehta JL, Nichols WW, Mehta P. Neutrophils as potential participants in acute myocardial ischemia: relevance to reperfusion. J Am Coll Cardiol. 1988;11(6):1309–1316. doi:10.1016/0735-1097(88)90297-5
- Meegan JE, Yang X, Coleman DC, Jannaway M, Yuan SY. Neutrophil-mediated vascular barrier injury: role of neutrophil extracellular traps. Microcirculation. 2017;24(3):e12352. doi:10.1111/micc.12352
- Dart AM, Chin-Dusting JP. Lipids and the endothelium. Cardiovasc Res. 1999;43(2):308–322. doi:10.1016/S0008-6363(99)00150-9
- Yang S, Yuan HQ, Hao YM, et al. Macrophage polarization in atherosclerosis. Clin Chim Acta. 2020;501:142–146. doi:10.1016/j.cca.2019.10.034
- Canali E, Masci P, Bogaert J, et al. Impact of gender differences on myocardial salvage and post-ischaemic left ventricular remodelling after primary coronary angioplasty: new insights from cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2012;13(11):948–953. doi:10.1093/ehjci/jes087
- Jang JH, Lee MJ, Ko KY, et al. Mechanical and pharmacological revascularization strategies for prevention of microvascular dysfunction in ST-segment elevation myocardial infarction: analysis from index of microcirculatory resistance registry data. J Interv Cardiol. 2020;2020:5036396. doi:10.1155/2020/5036396
- De Luca G, Gibson CM, Huber K, et al. Association between advanced Killip class at presentation and impaired myocardial perfusion among patients with ST-segment elevation myocardial infarction treated with primary angioplasty and adjunctive glycoprotein IIb-IIIa inhibitors. Am Heart J. 2009;158(3):416–421. doi:10.1016/j.ahj.2009.06.029
- Li P, Ruan JW, Liu M, Li SY, Wang ZD, Xie WC. Thrombus aspiration catheter improve the myocardial reperfusion of STEMI patients with high thrombus load during the emergency PCI operation. J Cardiothorac Surg. 2019;14(1):172. doi:10.1186/s13019-019-0974-z
- Kloner RA. The importance of no-reflow/microvascular obstruction in the STEMI patient. Eur Heart J. 2017;38(47):3511–3513. doi:10.1093/eurheartj/ehx288
- Engstrøm T, Kelbæk H, Helqvist S, et al. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA cardiol. 2017;2(5):490–497. doi:10.1001/jamacardio.2017.0022
- Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi:10.1152/ajpheart.01064.2002
- Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38(11):774–784. doi:10.1093/eurheartj/ehw224
- Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis. 2019;1865(7):1802–1809. doi:10.1016/j.bbadis.2018.08.008
- Herrmann J, Lerman A. The endothelium: dysfunction and beyond. J Nuclear Cardiol. 2001;8(2):197–206. doi:10.1067/mnc.2001.114148
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. doi:10.1056/NEJMra061889