Abstract
Background
Fever is a common problem in adults visiting the emergency department. Extensive studies have been done in children comparing the efficacy of various antipyretics. However, studies on the efficacy of antipyretic drugs in adults are very scarce. To the best of our knowledge, no controlled trial has been carried out comparing the antipyretic efficacy of paracetamol (oral and intravenous) and intramuscular diclofenac in adults.
Methods
In this parallel-group, open-label trial, participants aged 14–75 years presenting with fever who had a temperature of more than 38.5°C were enrolled and treated. Participants were randomly allocated to receive treatment with 1,000 mg oral paracetamol (n = 145), 1,000 mg intravenous paracetamol (n = 139), or 75 mg intramuscular diclofenac (n = 150). The primary outcome was degree of reduction in mean oral temperature at 90 minutes. The efficacy of diclofenac versus oral and intravenous paracetamol was assessed by superiority comparison. Analysis was done using intention to treat principles.
Results
After 90 minutes, all three groups showed a significant reduction in mean temperature, with intramuscular diclofenac showing the greatest reduction (−1.44 ± 0.43, 95% confidence interval [CI] −1.4 to −2.5) and oral paracetamol the least (−1.08 ± 0.51, 95% CI −0.99 to −2.2). After 120 minutes, there was a significant difference observed in the mean change from baseline temperature between the three treatment groups (P < 0.0001). Significant changes in temperature were observed in favor of intramuscular diclofenac over oral and intravenous paracetamol at each time point from 60 minutes through 120 minutes inclusive.
Conclusion
Both intramuscular diclofenac and intravenous paracetamol showed superior antipyretic activity than oral paracetamol. However, in view of its ease of administration, intramuscular diclofenac can be used as a first-choice antipyretic in febrile adults in the emergency department.
Introduction
Fever accounts for a substantial proportion of adult emergency consultations or visits. It is one of the leading patient complaints aside from abdominal pain and chest pain in all emergency department visits.Citation1 Although there may be physiologic benefits of fever, it also associated with arthralgia, myalgia, nausea, and vomiting. Treatment with antipyretics improves these accompanying symptomsCitation2 and reduces patient discomfort.Citation3 Both pharmacologic and nonpharmacologic methods like tepid spongingCitation4 have been used to reduce body temperature in febrile patients. Extensive studies have been done in children comparing the efficacy of various antipyretics. These have included paracetamol, ibuprofen, nimesulide, ketoprofen, propacetamol, and dipyrone.Citation5–Citation19
Studies on the efficacy of antipyretic drugs in adults are very scarce. Most of the available studies on acetaminophen were carried out in endotoxin-induced febrile modelsCitation20–Citation22 and others in intensive care patients.Citation23 Few studies have been done on oral diclofenac using varying dosesCitation24 or comparing it with ibuprofenCitation25 or acetylsalicylic acid.Citation26 Intravenous ketorolac has also been studied as an antipyretic in adults.Citation27 To the best of our knowledge, no controlled trial has been carried out comparing the antipyretic efficacy of paracetamol (both oral and intravenous) and intramuscular diclofenac in adults. Therefore, we decided to compare the antipyretic efficacy of oral and intravenous paracetamol with that of intramuscular diclofenac in febrile adults in the emergency department.
Materials and methods
Study design, participants, and randomization
A randomized controlled clinical trial was conducted in the emergency department at Alkhor Hospital, Hamad Medical Corporation, in the state of Qatar from June 2008 to December 2011. Adults aged 14–75 years were included if they had an oral temperature of more than 38.5°C. A complete clinical assessment including past and present medical history was performed. The main exclusion criteria were a history of allergy to any of the trial medications, antipyretics within the previous 8 hours, renal, hepatic or hematologic disorders, bronchial asthma, peptic ulcer disease, and frequent vomiting. Pregnant or lactating women were also excluded. The study was approved by the local ethics committee at the Medical Research Center, Hamad Medical Corporation (number 9070/09) and was done in accordance with the principles of the Declaration of Helsinki and guidelines for Good Clinical Practice. All patients provided their written informed consent prior to enrollment. The trial is registered with ClinicalTrials.gov (NCT01891435).
The randomization sequence list was created using a computerized random number generator. Participants were assigned to one of the three treatment groups using an equal allocation ratio of 1:1:1. The allocation sequence was concealed from the investigators enrolling the patients in sequentially numbered and sealed envelopes. The corresponding envelopes were opened only after the participants completed all baseline assessments and it was time to allocate the intervention. Randomization codes were kept secure until all data entry was complete.
Sample size
The primary outcome measure was the mean oral temperature decrease from baseline at 90 minutes. It was assumed that the mean oral temperature decrease for the three treatments was 1.4°C (intramuscular diclofenac), 1.22°C (intravenous paracetamol), and 1.1°C (oral paracetamol), respectively, with a constant standard deviation of 0.5°C for the three treatments. The sample size needed to achieve the desired power of 80% (β = 0.20) at the 5% (α = 0.05) level of significance was 130 patients per treatment group.
Procedures
Baseline oral temperature was recorded at enrollment. Patients were randomly assigned to receive oral paracetamol 1,000 mg, intravenous paracetamol 1,000 mg, or intramuscular diclofenac sodium 75 mg supplied from the hospital pharmacy free of cost to the patients. Intravenous paracetamol was given as an infusion over 15 minutes. Oral temperature was recorded at 30, 60, 90, and 120 minutes after drug administration. All temperature recordings were done using a standard thermometer. The primary outcome was the degree of reduction in mean oral temperature at 90 minutes and the secondary outcome was the degree of reduction in mean oral temperature at 30, 60, and 120 minutes. Patients were also monitored for any adverse effects pertaining to the trial medications.
Statistical analysis
Categoric and continuous values were expressed as the frequency (percentage) and mean ± standard deviation. Descriptive statistics were used to summarize all demographic and other clinical characteristics of the patients. Baseline participant characteristics in the three groups were compared using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for categoric variables. For the primary outcome variable, ie, reduction in mean oral temperature at 90 minutes was compared using one-way ANOVA. The results are presented with the associated 95% confidence interval. Where an overall group difference was found to be statistically significant, pairwise comparisons were made using the appropriate post hoc test. Reduction in mean oral temperature at 30, 60, and 120 minutes was analyzed using repeated-measures ANOVA. Means of quantitative variables between two independent groups were analyzed using the unpaired t-test. Associations between two or more qualitative or categoric variables were assessed using the chi-square test. For small cell frequencies, the chi-square test was used with a continuity correction factor. Pictorial presentations of the key results were made using appropriate statistical graphs. A two-sided P-value < 0.05 was considered to be statistically significant. All statistical analyses were done using Statistical Package for the Social Sciences version 19 software (SPSS Inc., Chicago, IL, USA).
Results
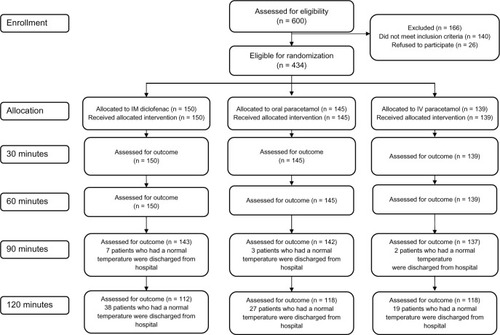
Six hundred patients were screened for study eligibility (). Of these, 166 patients could not participate for various reasons (140 did not fulfill the inclusion criteria or met an exclusion criterion and 26 did not give their consent to participate in the trial). Therefore, a total of 434 adults were enrolled, randomized, and received the study medications, ie, oral paracetamol (n = 145), intravenous paracetamol (n = 139), and intramuscular diclofenac (n = 150). Baseline demographic and clinical characteristics were similar across the three treatment groups (P > 0.05, ). The majority of the study subjects enrolled were males. This can be attributed to the location of the hospital, which caters mainly for the migrant labor community of the surrounding industrial area. The mean patient age was 36. 1 ± 15.81 years. Mean oral temperature at baseline was similar across the three treatment groups. shows the enrollment of participants through each stage of the trial.
Table 1 Baseline demographic and clinical characteristics of enrolled patients
After 90 minutes, all three groups showed a significant reduction in mean temperature, with intramuscular diclofenac showing the greatest (−1.44 ± 0.43, 95% confidence interval [CI] −1.4 to −2.5) and oral paracetamol the least (−1.08 ± 0.51, 95% CI −0.99 to −2.2). After 120 minutes, there was a significant difference observed in the mean change from baseline temperatures between the three treatment groups (P < 0.0001). Significant changes in temperature were observed in favor of intramuscular diclofenac over oral and intravenous paracetamol at each time point from 60 minutes through 120 minutes inclusive (). Repeated-measures ANOVA revealed a significant reduction in the change from mean baseline temperature within each treatment group, particularly at 60, 90, and 120 minutes ( and ). Patients who received intravenous fluids showed a significantly higher reduction in mean temperature from baseline, particularly at 30 minutes (−0.45 ± 0.38 versus −0.33 ± 0.34; P = 0.002) and 120 minute (−1.7 ± 0.59 versus −1.5 ± 0.51; P = 0.030) Reduction in mean temperature at the various time points was not significantly different among patients receiving antibiotics.
Figure 2 Mean temperatures at different time points for the three treatment groups.
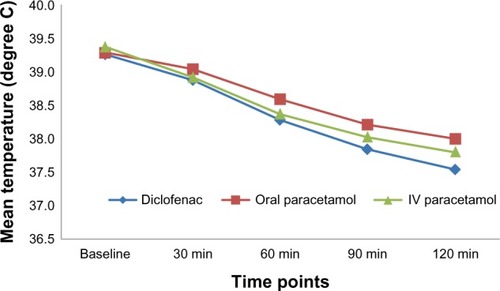
Table 2 Comparison of mean temperature at different time points between the three treatment groups
Table 3 Between-treatment comparisons of mean change from baseline in temperatures at different time points
Discussion
Fever occurs as a response to a variety of infectious and noninfectious inflammatory conditions. It is due to abnormally high hypothalamic thermostasis caused by the actions of interleukin or pyrogenic cytokines on the hypothalamic thermoregulatory center. Although treating the underlying cause should be the primary objective, reducing the fever is also important because this decreases constitutional symptoms and the discomfort to the patient.
We believe that this is the first study to compare directly the antipyretic efficacy of intramuscular diclofenac and paracetamol (both oral and intravenous), which are the two drugs commonly used in adults. Our results are in partial agreement with the findings of a previous study done by Peacock et alCitation20 comparing intravenous and oral paracetamol, which showed a statistically significant difference in temperature in the first 2 hours favoring intravenous over oral acetaminophen. Similarly, another study done by Kett et alCitation21 showed a significant reduction in temperature at 30 minutes from baseline by intravenous acetaminophen 1,000 mg in comparison with placebo. However, both these studies were done using endotoxin-induced febrile models. Another study done in an endotoxin-induced model showed oral acetaminophen to have a greater effect than aspirin.Citation22
Gehanno et al compared the antipyretic and analgesic effects of oral paracetamol with that of various doses of oral diclofenac. They found that diclofenac at doses of 12. 5 mg and 25 mg had a greater analgesic and antipyretic effect than paracetamol.Citation24
There has been no study done on the antipyretic effect of intramuscular diclofenac. However, a study comparing three intravenous antipyretics, ie, diclofenac 75 mg, metamizole 2,500 mg or 1,000 mg, and propacetamol 2,000 mg or 1,000 mg in hematology/oncology patients found metamizole to be the most effective, while propacetamol 1,000 mg had the least antipyretic efficacy. However, major drawbacks of this study are that it was nonrandomized and done in cancer patients.Citation2 The limitation of our study is that the majority of the subjects enrolled were males. This can be attributed to the location of the hospital which mainly caters for the migrant labor community in the surrounding industrial area.
Conclusion
In this study, intramuscular diclofenac and intravenous paracetamol were more effective in achieving a significant reduction in temperature at each time point from 60 minutes through 120 minutes inclusive. Of the two agents, intramuscular diclofenac showed the greatest effect. Beyond 90 minutes, all three drugs showed significant antipyretic activity, with oral paracetamol having the least effect and intramuscular diclofenac having the greatest effect. Hence we conclude that, in the emergency department, intramuscular diclofenac can be used as an antipyretic of choice to reduce temperature within a short time. However, if rapid reduction is not required, patients can be treated with oral paracetamol.
Acknowledgments
We thank the doctors and nursing staff of the emergency department, Alkhor Hospital, for their support and cooperation during this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- National Hospital Ambulatory Medical Care Survey 2010: Emergency Department Summary Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdfAccessed August 8, 2013
- OborilováAMayerJPospísilZKorístekZSymptomatic intravenous antipyretic therapy: efficacy of metamizol, diclofenac, and propacetamolJ Pain Symptom Manage20022460861512551812
- KramerMSNaimarkLERoberts-BrauerRMcDougallALeducDGRisks and benefits of paracetamol antipyresis in young children with fever of presumed viral originLancet19913375915941671951
- ThomasSVijaykumarCNaikRAntonisamyBComparative effectiveness of tepid sponging and antipyretic drug versus only antipyretic drug in the management of fever among children: a randomized controlled trialIndian Pediatr20094613313619242030
- JeanMTSylvieTPhilippeDAAntipyretic efficacy of an initial 30 mg/kg loading dose of acetaminophen versus a 15 mg/kg maintenance dosePediatrics2001108e7311581481
- KarbasiSAModares-MosadeghMGolestanMComparison of antipyretic effectiveness of equal doses of rectal and oral acetaminophen in childrenJ Pediatr (Rio J)20108622823320436978
- PaulIMSturgisSAYangCWattsHBerlinCMJrEfficacy of standard doses of ibuprofen alone, alternating, and combined with acetaminophen for the treatment of febrile childrenClin Ther2010322433244021353111
- MaryamSRahebGSemiraMSetarehAParvizBA comparison of the antipyretic effect antipyretic acetaminophen and ibuprofen in febrile children hospitalized at Amir-al-Momenin Hospital in SemnanIran J Pharmacol Ther200762213215
- NabulsiMMTamimHMahfoudZAlternating ibuprofen and acetaminophen in the treatment of febrile children: a pilot study [ISRCTN30487061]BMC Med20064416515705
- Van EschAVan Steensel-MollHASteyerbergEWOffringaMHabbemaJDDerksen-LubsenGAntipyretic efficacy of ibuprofen and acetaminophen in children with febrile seizuresArch Pediatr Adolesc Med19951496326377767417
- HayADRedmondNMCostelloeCParacetamol and ibuprofen for the treatment of fever in children: the PITCH randomized controlled trialHealth Technol Assess200913iiiivixx116319454182
- McIntyreJHullDComparing efficacy and tolerability of ibuprofen and paracetamol in feverArch Dis Child1996741641678660083
- VinhHParryCMHanhVTDouble blind comparison of ibuprofen and paracetamol for adjunctive treatment of uncomplicated typhoid feverPediatr Infect Dis J20042322623015014297
- LalAGomberSTalukdarBAntipyretic effects of nimesulide, paracetamol and ibuprofen-paracetamolIndian J Pediatr20006786587011262983
- KapoorSKSharmaJBatraBPaulEAnandKSharmaDComparison of antipyretic effect of nimesulide and paracetamol in children attending a secondary level hospitalIndian Pediatr20023947347712037280
- KokkiHKokkiMKetoprofen versus paracetamol or ibuprofen in the management of fever: results of two randomized, double-blind, double-dummy, parallel-group, repeated-dose, multicentre, phase III studies in childrenClin Drug Investig201030375386
- WalsonPDJonesJChesneyRRodarteAAntipyretic efficacy and tolerability of a single intravenous dose of the acetaminophen prodrug propacetamol in children: a randomized, double blind placebo controlled trialClin Ther20062876276916861098
- PradoJDazaRChumbesOLoayzaIHuichoLAntipyretic efficacy and tolerability of oral ibuprofen, oral dipyrone and intramuscular dipyrone in children: a randomized controlled trialSao Paulo Med J2006124136140
- CelebiSHacimustafaogluMArisoyESAntipyretic effect of ketoprofenIndian J Pediatr20097628729119129989
- PeacockWFBreitmeyerJBPanCSmithWBRoyalMAA randomized study of the efficacy and safety of intravenous acetaminophen compared to oral acetaminophen for the treatment of feverAcad Emerg Med20111836036621496138
- KettDHBretmeyerJBAngRRoyalMAA randomized study of the efficacy and safety of intravenous acetaminophen vs intravenous placebo for the treatment of feverClin Pharmacol Ther201190323921544074
- PernerstorferTSchmidRBieglmayerCEichlerHGKapiotisSJilmaBAcetaminophen has greater antipyretic efficacy than aspirin in endotoxemia: a randomized, double blind placebo controlled trialClin Pharmacol Ther199966515710430109
- MullinsMEEmpeyMJaramilloDA prospective randomized study to evaluate the antipyretic effect of the combination of acetaminophen and ibuprofen in neurological ICU patientsNeurocrit Care20111537537821503807
- GehannoPDreiserRLIonescuEGoldMLiuJMLowest effective single dose diclofenac for antipyretic and analgesic effects in acute febrile sore throatClin Drug Investig200323263271
- GrebeWIonescuEGoldMSLiuJMFrankWOA multicentre, randomized, double-blind, double-dummy, placebo and active controlled, parallel-group comparison of diclofenac-K and ibuprofen for the treatment of adults with influenza like symptomsClin Ther20032544445812749506
- BettiniRGrossiERapazziniPGiardinaGDiclofenac sodium versus acetylsalicylic acid: a randomized study in febrile patientsJ Int Med Res198614951003516755
- RobertHBSaraNNMarcNRHowardASAntipyretic effectiveness of intravenous ketorolac tromethamineJ Emerg Med20042640741015093845