Abstract
Diabetic cardiomyopathy (DCM), although a distinct clinical entity, is also a part of the diabetic atherosclerosis process. It may be independent of the coexistence of ischemic heart disease, hypertension, or other macrovascular complications. Its pathological substrate is characterized by the presence of myocardial damage, reactive hypertrophy, and intermediary fibrosis, structural and functional changes of the small coronary vessels, disturbance of the management of the metabolic cardiovascular load, and cardiac autonomic neuropathy. These alterations make the diabetic heart susceptible to ischemia and less able to recover from an ischemic attack. Arterial hypertension frequently coexists with and exacerbates cardiac functioning, leading to the premature appearance of heart failure. Classical and newer echocardiographic methods are available for early diagnosis. Currently, there is no specific treatment for DCM; targeting its pathophysiological substrate by effective risk management protects the myocardium from further damage and has a recognized primary role in its prevention. Its pathophysiological substrate is also the objective for the new therapies and alternative remedies.
Introduction
Cardiovascular disease is a common complication of diabetes responsible for 80% of the mortality in the diabetic population.Citation1 Coronary artery disease is the leading cause for the increased cardiovascular morbidity and mortality in diabetes, and atherosclerosis of the coronary vessels is its initial pathogenetic mechanism. Coronary artery disease and hypertension can account for most of the myocardial abnormalities (left ventricular [LV] hypertrophy and impaired contractility) that occur in diabetes. However, postmortem, experimental, and observational studies also provide evidence for a specific cardiomyopathy in diabetes, which may contribute to myocardial dysfunction in the absence of coronary artery atheroma.Citation2 This is also sustained by the fact that patients with diabetes, independently of the severity of coronary artery disease, have increased risk of heart failure in comparison with subjects without diabetes.Citation3,Citation4
In 1972 for the first time in medical history, the description of 4 patients with diabetes and heart failure but without arterial hypertension or coronary artery disease appeared. The anatomical dissection of their hearts revealed LV hypertrophy and fibrosis without evidence of coronary artery atheroma or another substrate pathology responsible for these findings. This clinical entity (disease) was considered as independent and was baptized “diabetic cardiomyopathy” (DCM).Citation5
The aim of this review is to provide up-to-date information on the pathophysiology, diagnosis, and management of DCM. We performed a comprehensive search in the PubMed and Embase databases up to February 2010 using the following keywords alone and in combination to retrieve available literature data: “diabetes mellitus”, “cardiomyopathy”, “cardiac myocytes,” “pathophysiology”, “hyperglycemia”, “insulin resistance”, “risk factors”, “myocardial performance”, “diastolic dysfunction”, “heart failure”, “diagnosis”, and “treatment”. All types of articles (randomized controlled trials, original studies, review articles, and case reports) in humans and animals published in English language were included. Publications were studied in detail.
Epidemiological data on DCM
Epidemiological evidence has demonstrated worldwide that macrovascular complications (coronary artery disease, peripheral vascular disease, and stroke) are more common (2–4 times) among diabetes patients in comparison with the nondiabetes subjects. This is valid for both type 1 and type 2 diabetes even when all other cardiovascular risk factors, such as hypertension, obesity, smoking, dyslipidemia, age, and sex, are taken into consideration.Citation6
The Framingham Study showed that the frequency of coronary artery disease is twice more common in diabetes patients of both sexes, since women with diabetes appear to lose their normal premenopausal protection against cardiovascular disease. Moreover, death from coronary artery disease is 3 times more common in diabetes patients compared with nondiabetes subjects matched by age and sex. In other words, a patient with diabetes is not only at high risk of developing coronary artery disease, but furthermore, he is more susceptible to a worse prognosis.Citation4 Atheromatous lesions have an earlier onset, faster development, and greater density in diabetes.Citation7 The risk of coronary artery disease increases rapidly after the age of 40 in patients with type 1 diabetes, and by the age of 55, 35% of the patients of both sexes have died because of a coronary artery event, compared with 8% of men and 4% of women in the general population.Citation8
It should be noted that in the last 30 years, the substantial decrease in cardiovascular mortality in men without diabetes (up to 36.4%) was not followed by a decrease in the cardiovascular mortality among the male diabetic population (up to 13.1% decline). Even more disappointing were the results among women. Although cardiovascular mortality in women without diabetes was reduced by 17%, it was increased by 23% in the female diabetic population.Citation9 This supports even further the loss of the premenopausal protection against coronary artery disease in diabetes seen in healthy women. The STENO-2 Study demonstrated that in patients with type 2 diabetes, even when all associated cardiovascular risk factors are treated and the incidence of cardiovascular events is decreased by 50%, the cardiovascular mortality still remains high.Citation10
In one prospective study that included a population of 1,059 patients with diabetes and 1,373 subjects without diabetes, who were followed up for at least 7 years, it was concluded that the frequency of mortality in patients with diabetes but without myocardial infarction was similar to that in subjects without diabetes but with myocardial infarction.Citation11 Moreover, in patients with diabetes and acute myocardial infarction, mortality during the first month is above 42%, whereas in subjects without diabetes, it is less than 20%.Citation12,Citation13 Some studies have demonstrated that good glycemic control is associated with higher survival rates after acute myocardial infarction.Citation14 In diabetes subjects with angiographically proven coronary artery disease, the survival rates are decreased by 30% compared with their nondiabetes counterparts.Citation15 Diabetic nephropathy, which often coexists, especially proteinuria, increases further the risk of cardiovascular death.Citation16 Moreover, a significant association between idiopathic cardiomyopathy and diabetes was recently described.Citation17
An important consequence of DCM is heart failure, which is more common in people with diabetes and complicates acute myocardial infarction more often than in the nondiabetic population. The increased percentage of patients with diabetes and heart failure in numerous multicenter epidemiological studies is in contrast to the total diabetic burden in the general population (6%–8%). In the Studies of Left Ventricular Dysfunction (SOLVD), the percentage of patients with diabetes and heart failure was up to 26%,Citation18 whereas in the Assessment Trial of Lisinopril and Survival (ATLAS) study, it was 19%Citation19 and in the Vasodilator-Heart Failure Trial II (V-HeFT II), it was up to 20%.Citation20
These findings are in agreement for the presence of an additional detrimental factor in the diabetic myocardium, which predisposes it to extensive damage followed by heart failure. Even more, they are supportive for the early treatment of diabetes patients with aspirin and other measures that are used for secondary prevention in the general population, even in the absence of clinically apparent macrovascular complications.
In summary, cardiovascular disease is 2–3 times more common, and survival is worse in subjects with diabetes in comparison with age-matched and sex-matched counterparts. The prevalence of heart failure with preservation of systolic function among patients with diabetes is 19%–26%.
Definition of DCM
DCM is defined as the cardiovascular damage present in diabetes patients, which is characterized by myocardial dilatation and hypertrophy, as well as a decrease in the systolic and diastolic function of the left ventricle, and its presence is independent of the coexistence of ischemic heart disease or hypertension. DCM may be subclinical for a long time, before the appearance of clinical symptoms or signs. According to the molecular theory of DCM, hyperglycemia is the main pathogenetic factor, which causes abnormalities at the cardiac myocyte level, eventually leading to structural and functional abnormalities.Citation21
The pathophysiological substrate of DCM
The anatomical damage of the myocardial substrate
Atheromatous plaques in patients with diabetes are not qualitatively different from those affecting the nondiabetic population. However, atheroma in patients with diabetes is more extensive, is more diffuse, and involves distal vessels in both the coronary and peripheral circulation.Citation22 Pathologic anatomical alterations of the small coronary vessels and the myocardial endothelium include increased cell adhesiveness, impaired relaxation, impaired fibrinolysis, and increased permability.Citation22 The early stages of DCM are dominated by the pathological alterations in the myocardial interstitium, ie, formation of nonenzymatic advanced glycation end products (AGEs), impaired compliance, and ischemia from the disease in the vasa vasorum. The morphology of the myocardial cells and small coronary vessels is anatomically preserved. These alterations lead to impaired myocardial contractility.Citation23 As the disease progresses, LV hypertrophy appears as a result of the hypertrophy of the myocardial cells, the interstitial and perivascular fibrosis, the greater thickening of the capillary basement membrane, and the formation of microaneurysms in small capillary vessels. This ultimate observation may be the actual pathophysiological connection between diabetes microvascular and macrovascular disease.Citation24
Structural alterations leading to ventricular dysfunction
Medial layers of arterioles throughout the cardiac circulation in diabetes show “hyaline” change, an amorphous, ground glass appearance, resulting from breakdown of structural proteins (largely thought to be collagen) and the uptake of glycated plasma proteins into the vessel wall. Similar changes are seen in older nondiabetes subjects as part of the aging process and in hypertension. The reduced blood supply resulting from microvascular disease and affecting the vasa vasorum in diabetes further damages the small and medium arterioles of the diabetic heart. A clear relationship between the loss of cardiac function and the histological damage in the diabetic blood vessels is very difficult to demonstrate. However, it is plausible that the structural changes and loss of elasticity contribute to hypertension and possibly to the greatly increased risk of macrovascular events in diabetes. The European Heart Association’s Guidelines (2009) have defined LV hypertrophy as an increase in LV mass index above 125 g/m2 for men and above 110 g/m2 for women. The Framingham Study demonstrated that the determination of LV hypertrophy electrocardiographically was not associated significantly with increased cardiovascular morbidity and mortality.Citation25 However, in the Framingham Study, when LV hypertrophy was assessed echocardiographically in a population of 3,222 patients, it was demonstrated that the relative risk for cardiovascular mortality for each 50 g/m2 increase of the LV mass above the normal limits was equal to 1.49 for men and 1.57 for women.Citation26
LV hypertrophy is significantly associated with an increase in markers of systemic inflammation, such as fibrinogen, C-reactive protein, and microalbuminuria. In one study including 1,299 patients with type 2 diabetes, microalbuminuria not only was a marker of endothelial dysfunction and increased risk of atherosclerosis but also was positively associated with LV mass.Citation27
Functional alterations of DCM
Uncomplicated reduction of systolic myocardial functioning is defined as the loss of the heart’s ability to pump arterial blood in the peripheral circulation. However, the elementary difference between the uncomplicated reduction of systolic myocardial function and systolic heart failure is that in the latter, the clinical symptoms and signs of heart attack appear as a result of the former. Thus, the main guidance point of systolic myocardial dysfunction is the reduction of the LV ejection fraction.
However, recent studies revealed that the classical 2-dimensional echocardiography (2DE) may not be able to detect and diagnose the early uncomplicated reduction of the LV systolic myocardial functioning. This is due to the fact that classical 2DE can determine only LV circumferential function and cannot estimate LV longitudinal functioning.Citation28
In DCM, progression and appearance of reduction of systolic function is slow, often when patients have already developed a significant reduction of diastolic myocardial functioning. The prognosis of patients with LV systolic myocardial dysfunction is poor, and the percentage of annual mortality is equal to 15%–20%.
Diastole is defined as the period when the myocardial muscle generates effort and energy, while in mechanical (isovolumic deceleration and relaxation phase) and electrophysiological inactivity (automatic depolarization).Citation29 Reduction of diastolic myocardial functioning is determined by a delayed and extended diastolic phase. Diastolic dysfunction is also characterized electrophysiologically by the prolongation of the active dilatation and the augmented passive stiffness of the left ventricle, which portray the passive diastolic LV compliance in heart failure.Citation30 Impaired diastolic functioning of the left ventricle appears in 27%–69% of the cardiovascular asymptomatic population with diabetesCitation31 and is responsible for the 30% of the patients ending up with diabetes and clinical symptomatic heart failure.Citation32 Impaired diastolic compliance and maintenance of the systolic function is usually the initial heart lesion in the progression of DCM.Citation33 Failure of diastolic relaxation of the left ventricle leads to impaired filling and reduced cardiac reserve on exercise. In spite of the absence of systolic dysfunction, numerous clinical studies have demonstrated a decreased ejection fraction, which determines reduced cardiac residual reserve in asymptomatic patients with diabetes.Citation34 Subclinical DCM characterized by reduced cardiac residual reserve can develop into a significant clinical entity, especially when myocardial ischemia coexists or suddenly appears.
Diastolic myocardial function can be evaluated by cardiac catheterization for the evaluation of the isovolumic relaxation rhythm and the LV isovolumic contraction time.Citation35 This method, although highly accurate, for the evaluation of LV diastolic function is invasive and has increased possibilities of accidental injury. The alternative noninvasive techniques include classical 2DE and M-mode echocardiography with Doppler. LV diastolic functioning can also be evaluated by controlling the transmitral and pneumocardial blood flow.Citation36
The medical term “diastolic dysfunction” refers exclusively to the echocardiographic finding of an abnormal LV deceleration and relaxation time, without the coexistence of any clinical evidence suggestive of heart failure. However, diastolic heart failure is defined as the clinical syndrome of heart failure with preservation of a normal LV ejection fraction. Echocardiographic findings of isolated myocardial dysfunction, such as subclinical reduction of myocardial diastolic compliance and loading, are reliable prognostic markers of future chronic heart failure.Citation37 Moreover, patients with diastolic heart failure have increased cardiovascular mortality (equal to 5%–8%) compared with healthy subjects matched by age and sex (equal to 1%).Citation38–Citation40
The impact of diastolic dysfunction is associated with good glycemic control, as assessed by hemoglobin A1c (HbA1c) levels.Citation41 Possibly, the main cause for this relationship are the increased circulatory AGEsCitation42 and the increased production of free oxygen radicals,Citation43 resulting in an increased accumulation of collagen in the myocardium and in myocardial fibrosis.Citation44,Citation45
Coexistent hypertension accelerates the functional underlying process, leading to LV hypertrophy, systolic ventricular dysfunction, and greatly increased risk of congestive heart failure. Although, it is difficult to disentangle the effects of hypertension from the other facets of the pathophysiology of ventricular dysfunction in diabetes, LV hypertrophy has also been observed in nonhypertensive individuals with diabetes, and particularly in women.Citation46
In conclusion, DCM is characterized by LV hypertrophy and myocardial dilatation, which lead to LV diastolic and systolic dysfunction. These are related to diabetic fibrosis, inflammation, ischemia, and presence of microvascular complications. DCM is independent of the coexistence of ischemic heart disease or hypertension.
The molecular substrate of DCM
Hyperglycemia causes significant functional alterations to the cellular Na+–Ca2+ ionic channel, resulting in a decreased extrapolation and an increased intracellular concentration of ionic calcium. Moreover, in diabetes, a dysfunction of the cellular Na+–K+ ionic channel appears, which primarily causes an increase in the intracellular sodium concentration and secondarily an additional increase to the already augmented intracellular calcium concentration in the diabetic cardiac myocytes.Citation47
Dyslipidemia and increased production of free oxygen radicalsCitation48,Citation49 may cause metabolic alterations to the genetic transcription factors and coding.Citation50,Citation51 The result of these alterations is a modification in the translation of nucleoprotein genes, such as RAS and insulin-like growth factor 1 (IGF-1), responsible for the myocardial substrate and myocardial fiber structure and also for the maintenance of a normal myocardial compliance.Citation52,Citation53 Accordingly, increased expression of the early cardiopathology-related gene RAS predisposes to insulin resistance, whereas the IGF-1 increases myocardial contractility through an increase of the intracellular calcium accumulation and by intensifying the sensitivity of the cardiac myofibers to the circulatory calcium concentration. Citation54 IGF-1 is present in the cardiac myocytes, and its expression is augmented by decreased insulin concentration, increased mechanical stress, and increased peripheral vascular resistance. IGF-1 acts synergically with angiotensin II in promoting cellular development. As a result, this leads to myocardial hypertrophy of the diabetic heart ventricle mass even when blood pressure levels are within the normal range.Citation55
Thus, hyperglycemia, dyslipidemia, and increased production of free oxidant radicals are main metabolic pathogenetic risk factors leading to DCM.
The interplay of cardiac autonomic neuropathy in DCM
Cardiac autonomic neuropathy (CAN) is a common complication of diabetes, causing abnormalities in heart rate control and in central and peripheral vascular dynamics. CAN affects almost 17% of the patients with type 1 and 22% of those with type 2 diabetes. An additional 9% of patients with type 1 and 12% of those with type 2 diabetes have milder forms of autonomic dysfunction.Citation56
Several studies have confirmed that cardiac neuropathy has the propensity to cause a reduction in the cardiac ejection fraction, impair systolic dysfunction and decrease diastolic filling, thus having an important contribution to the deterioration of diastolic myocardial function and DCM. Abnormalities of LV diastolic filling are overstated in patients with diabetes and CAN in comparison with patients with diabetes but without this complication.Citation57 When LV function was assessed in a diabetic population by resting and exercise radionuclide ventriculography, an abnormal LV performance was revealed in 37% of the patients included in the study. CAN was present in 59% of the subjects with DCM, a significantly increased incidence compared with that of 8% in the patients without this complication. Moreover, there were significant reductions in the ventricular ejection fraction at rest and with maximal exercise in patients with CAN compared with those without this complication.Citation58
In a population of subjects with type 1 (insulin-dependent) diabetes, a decrease of the diastolic myocardial function to 21% was also demonstrated. None of the patients included in the study had clinical evidence of coronary artery disease, and diastolic myocardial dysfunction (impaired diastolic LV filling) was significantly associated with the presence of CAN.Citation59
CAN is portrayed by a significant reduction in heart rate variability and an alteration in the parasympathetic– sympathetic balance, in which a reduction in parasympathetic tone secondary to autonomic neuropathy leads to a relative overactivity of the sympathetic system. Patients with diabetes and mild CAN have been shown to have distal LV sympathetic denervation, whereas patients with severe CAN have a pattern of distal sympathetic denervation associated with proximal ventricular islands of hyperinnervation. These areas of denervation and hyperinnervation may cause unstable regions of electrical, vascular, or autonomic heterogeneity conducive to DCM.Citation60
Numerous studies have demonstrated a significant correlation between dysfunction of the parasympathetic nervous system activity and impaired myocardial function. One study evaluated the role of parasympathetic nervous system activity in cardiovascular performance on a group of clinically asymptomatic type 2 diabetes patients, ie, without any symptom, sign, or objective measurement of ischemia or cardiomyopathy. Although patients with CAN corresponded positively to the increase in oxygen uptake by an equivalent percent increase in cardiac output, this response was significantly reduced compared with that of patients without CAN. Thus, it was shown that impaired sympathetic and parasympathetic responses, which normally augment cardiac output and redirect peripheral blood flow to skeletal muscles during exertion, may limit exercise tolerance.Citation61
The relationship between parasympathetic nervous dysfunction and DCM was confirmed by an important decrease of heart rate variability during deep breathing maneuver in subjects with type 1 diabetes and by the presence of pathological echocardiographic findings of the LV filling rhythm.Citation62
Dysfunction of the sympathetic nervous system activity has been associated with a decrease in both systolic and diastolic myocardial function in subjects with type 2 diabetes.Citation63 A significant association between a decrease of the transmitral ratio of early to late peak mitral filling wave velocities and the augmentation of sympathetic myocardial neurosis has also been demonstrated.Citation64 This relationship has confirmed that in DCM, the abnormal cardiac sympathetic neurosis contributes to the abnormal LV filling. The transmitral ratio of early to late peak mitral filling has also been associated with orthostatic hypotension as a clinical manifestation of CAN.Citation46 Moreover, the same index of abnormal cardiac filling was associated with an abnormal expiration/inspiration ratio, ie, an index of parasympathetic dysfunction in patients with type 1 diabetes and CAN.Citation65 Finally, CAN is associated with a decrease of the elastic vascular properties and an increase of peripheral vascular resistance due to the augmentation of the sympathetic nervous tone.Citation66
Resting tachycardia reduces the heart’s ability to pump blood in the peripheral circulation and interferes with the ventricular chambers’ capacity to fill with blood properly. Ventricular tachycardia not only reduces the time of ventricular filling but also interferes with the timely atrial contraction. When the heart’s labor capacity is greatly reduced for a prolonged period, cardiac arrest and death are likely to happen.Citation67 Through predisposition to arrhythmias, CAN may lead to LV dysfunction followed by sudden death.Citation68 Recent studies revealed that classicalCitation69,Citation70 and newer markers of arrhythmogenicityCitation71 are increased in subjects with diabetes and CAN in comparison with subjects with diabetes but without this complication. Moreover, one recent study revealed that increased markers of spatial ventricular heterogeneity in type 2 diabetes subjects and CAN are associated with the structural and functional properties of the myocardium.Citation71
In conclusion, CAN is strongly and independently associated with the structural and functional pathogenetic substrate of DCM in both type 1 and type 2 diabetes patients. This is partially explained by the significant reduction in heart rate variability present in autonomic neuropathy, and an alteration in the parasympathetic–sympathetic balance of the heart, leading to parasympathetic reduction and sympathetic overactivity.
Risk factors for DCM
Hyperglycemia
Hyperglycemia could contribute to macrovascular disease through various mechanisms. Hyperglycemia causes significant functional alterations to the cellular Na+–Ca2+ ionic channel, resulting in a decreased extrapolation and an increased intracellular concentration of ionic calcium. Moreover, in diabetes, a dysfunction of the cellular Na+–K+ ionic channel appears, which primarily causes an increase in the intracellular sodium concentration and secondarily an additional increase to the already augmented intracellular calcium concentration in the diabetic cardiac myocytes.Citation47 However, the most convincing pathogenetic mechanism of hyperglycemia is via the formation of AGEs. AGEs cross-link vessel wall proteins, therefore causing thickening and leakage of the vasculature and forming irreversible and abnormal deposits of plasma-derived proteins in the myocardial subintimal arterial layers.Citation72 Furthermore, AGEs generate toxic reactive oxygen species (ROSs) that impair cellular interactions and damage myocardial vascular function, causing endothelial vasomotor dysfunction.Citation73
Some of the effects of AGEs are mediated through specific receptors (RAGE) expressed by vascular endothelial, smooth muscle cells and cardiac myocytes. In the cardiac endothelium, AGE–RAGE interaction leads to upregulation of procoagulant and adhesive proteins, including tissue factor, plasminogen activator inhibitor 1 (PAI-1), and vascular cell adhesion molecule 1 (VCAM-1). Expression of VCAM-1 results in an increase in monocyte adhesion, while monocytes expressing RAGE are recruited by chemotaxis to sites of AGE accumulation. There they infiltrate the myocardial subendothelium to form foam cells, an early step in atherogenesis. Other extracellular RAGE ligands are the RAGE- binding proteins that interact with RAGE on cardiac myocytes to induce expression of tumor necrosis factor α (TNF-α), VCAM-1, nuclear factor κB (NF-κB), and interleukin 1, thus modulating the inflammatory component of cardiovascular disease through the cytokine cascade.Citation74
A strong relationship between glycemic control and DCM has been demonstrated in major multicenter prospective studies. The Diabetes Conventional and Complications Trial (DCCT) showed that patients with diabetes who are conventionally treated have a nearly double incidence of DCM when compared with patients with diabetes who are intensively treated. Among the risks for DCM, beyond glycemic control, were increased levels of serum cholesterol and triglycerides.Citation75 For each 1% reduction in HbA1c, the United Kingdom Prospective Diabetes Study (UKPDS) demonstrated a 14% reduction in myocardial infarction.Citation76 Moreover, impaired glucose tolerance has been identified as a risk factor for DCM, suggesting that even mild hyperglycemia may contribute to DCM.Citation77
Insulin resistance and the metabolic syndrome
Insulin resistance and hyperinsulinemia have been identified as risk factors for DCM.Citation78,Citation79 In hyperinsulinemia and insulin resistance, an imbalance in the direct effects of insulin action occurs, accompanied by the confounding effects of other cardiovascular risk factors associated with the insulin resistance syndrome, ie, central obesity, hyperglycemia, hypertriglyceridemia, low high-density lipoprotein (HDL), and hypertension.Citation80 The clustering of these cardiovascular and atherothrombotic risk factors that accompany insulin resistance provides a plausible basis for DCM and is often associated with adverse cardiovascular effects.Citation81,Citation82 Hyperinsulinemia and insulin resistance are also associated with a cluster of thrombotic risk factors, such as elevated levels of PAI-1,Citation83,Citation84 factor VII, factor XII, and fibrinogen.Citation85–Citation87 Increased levels of factor VII and XII may also occur through activation by triglyceride-rich particles, whereas hyperfibrinogenemia may be attributed to smoking, increased body mass, and inflammatory responses.
Insulin resistance is often associated with a combination of established and emerging risk factors, including hypertension and C-reactive protein.Citation88,Citation89 C-reactive protein is an acute-phase inflammation marker that has been associated with an increased risk for DCM.Citation90,Citation91 Recent studies have confirmed the association between increased levels of C-reactive protein and worse LV myocardial performance, as well as risk factors for atherosclerosis and cardiovascular disease in subjects with metabolic syndrome (MetS).Citation92 MetS status per se was found to be the strongest predictor of cardiomyopathy, thus implying that the clustering of the metabolic disturbances has additional prognostic information than its individual components in terms of myocardial performance and may explain in part the excess cardiovascular risk present in the MetS.Citation92
In conclusion, the effects of insulin on the diabetic heart are related to both systemic metabolic abnormalities and the direct effects of insulin action on the vasculature. Although, the elevation and the imbalance of the cardiovascular and atherothrombotic metabolites and cytokines related to hyperinsulinemia and insulin resistance have a crucial contribution in the pathogenesis of DCM, it should be noted that the direct action of insulin on the vasculature has also been proposed to mediate a combination of proatherogenic and antiatherogenic effects related to endothelial function and smooth muscle cell growth.Citation93 The importance of insulin resistance and the MetS is further confirmed by the fact that insulin-resistant individuals who are either hyperinsulinemic and normoglycemicCitation79 or hyperinsulinemic with impaired glucose toleranceCitation94 show a similarly high-risk profile to those with overt diabetes, whereas others who are destined to develop diabetes, but lack insulin resistance, are not at increased risk.Citation81,Citation95
Dyslipidemia
Increased atherogenesis and insulin resistance in diabetes and MetS have been attributed in large part to a proathero-thrombotic dyslipidemia, related to hypertriglyceridemia, elevated very low-density lipoprotein, and decreased HDL cholesterol levels.Citation96,Citation97 Insulin resistance results in increased levels of triglyceride-enriched, small, dense low-density lipoprotein (LDL) particles, which have been associated with an increased risk of ischemic heart disease.Citation98 Moreover, the combined elevation of fasting insulin, apoprotein B, and small, dense LDL particles has been identified as a nontraditional risk factor cluster for coronary artery disease. Citation99 Dyslipidemia has been recently associated with worse myocardial performance in type 2 diabetes subjectsCitation100 and in the MetS.Citation92 Additional risk factors for DCM associated with dyslipidemia include increased lipoprotein(a)Citation101 and nonesterified fatty acids.Citation102
Hypertension
Hypertension commonly coexists with type 1 and type 2 diabetes. Hypertension per se is a major risk factor for vascular complications, ie, myocardial infarction and stroke.Citation103,Citation104 According to major multicenter studies, the majority (almost 50%) of patients with diabetes and no clinical evidence of atheromatous disease are hypertensive or are taking antihypertensive drugs. In diabetes subjects with DCM, the prevalence of hypertension is even higher.Citation105 Insulin resistance has been proposed to be the fundamental cause of hypertension and DCM in type 2 diabetes.Citation106 Acquired factors such as obesity and physical inactivity may also contribute. Hypertension is an important feature of the cluster of the cardiovascular risk factors included in the MetS. The relationship between these metabolic components is complex and in part largely unexplained. Insulin resistance could raise blood pressure by the loss of insulin’s normal vasodilator activity,Citation107 or through effects of the accompanying hyperinsulinemia. These include Na+ and water retention, increased intracellular Na+ concentrationsCitation108 that enhance the contractility and stimulate proliferation of vascular smooth muscle,Citation109 increase peripheral resistance, and cause sympathetic overactivity.Citation110 In other words, hypertension and DCM are synergic and share similar pathogenetic pathways. The characteristic dyslipidemia of insulin resistance that contributes to DCM may also contribute to hypertension, as small, dense LDL particles are especially susceptible to oxidation, and oxidized LDL can suppress endothelial nitric oxide production and so promote vasoconstriction; hypertriglyceridemia also impairs endothelium-dependent vasorelaxation.Citation111 Studies have shown that fibrates used to treat hypercholesterolemia also reduce the prevalence of hypertension up to 25%.Citation112
Obesity
Obesity predisposes to type 2 diabetes, hypertension, dyslipidemia, and ultimately atheroma, which is one of the major causes of premature death in obese people.Citation113 Obesity is recognized as an independent cardiovascular risk factor.Citation114 Central obesity is characterized by excess fat deposited both subcutaneously around the abdomen and within the visceral cavity and is commonly found in patients with type 2 diabetes and MetS. Central obesity is associated with insulin resistance and proinflammatory responses that cause glucose intolerance and a highly atherogenic-risk profile. Adipose tissue produces nonesterified fatty acids and cytokines such as TNF-α that may contribute to both insulin resistance and atherogenesis. Reduced levels of adiponectin, an insulin-sensitizing protein whose secretion by adipose tissue is paradoxically decreased in obesity, may also contribute to insulin resistance and DCM.Citation115 Indeed, in a recent study, central obesity, as part of the MetS, was significantly and independently associated with LV myocardial dysfunction.Citation116
The effect of diabetic nephropathy
Nephropathy is a common complication affecting almost one-third of patients with diabetes.Citation117 Both microalbuminuria (albumin excretion rate of 30–299 mg/24 h) and proteinuria (albumin excretion, >300 mg/24 h) are markers of increased cardiovascular risk.Citation118,Citation119 In type 1 diabetes, the relative risk of DCM is 1.2-fold in patients with microalbuminuriaCitation120 and 10-fold in patients with proteinuriaCitation121 compared with patients with normoalbuminuria. In type 2 diabetes, the risk of DCM is increased 2- to 3-fold in microalbuminuriaCitation122 and 9-fold in proteinuria.Citation123
The onset of microalbuminuria is associated with an excess of cardiovascular risk factors, including hyperglycemia, dyslipidemia, and numerous prothrombotic and atherogenic changes.Citation124,Citation125 Moreover, there is growing evidence that the genetic susceptibility influences the development of diabetic nephropathy and also affects the diabetic cardiovascular system.Citation126 Suggested mechanisms linking renal and cardiovascular disease include endothelial dysfunction, abnormalities of the renin–angiotensin system, and widespread vascular basement membrane defects.
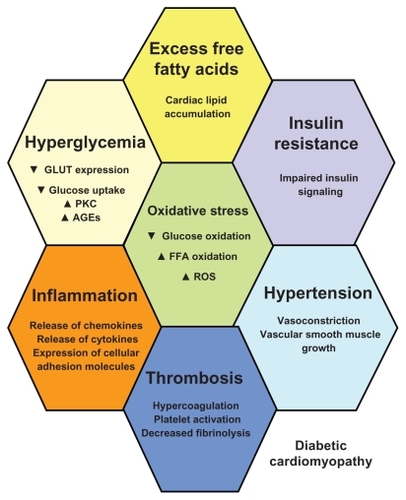
In conclusion, the pathogenic contribution to the development of DCM includes insulin resistance, increased formation of AGEs, MetS, hyperglycemia, dyslipidemia, obesity, and presence of microangiopathy. Their interplay is complex, since they can act in parallel and synergically and, at the same time, have a cause and effect result. Thus, they should be equally predicted and effectively treated. The contribution of the various factors in the development of DCM is depicted in .
Figure 1 The pathophysiological substrate of diabetic cardiomyopathy: in diabetes, hyperglycemia, excess free fatty acid (FFA) release, and insulin resistance, engender adverse metabolic events that affect the cardiac myocytes. Hyperglycemia is associated with decreased glucose transportation (GLUT), uptake, and oxidation, as well as increased formation of advanced glycation end products (AGEs) and increased activation of protein kinase C (PKC). Excess FFA release is followed by cardiac lipotoxicity, ie, increased cardiac lipid accumulation and increased generation of reduced reactive oxygen species (ROS) at the level of the electron transport chain. Together with insulin resistance and impaired insulin action and signaling, these metabolic paths augment vasoconstriction, produce and further aggravate arterial hypertension, increase inflammation with liberation of leukocyte-attracting chemokines, increase production of inflammatory cytokines, and augment expression of cellular adhesion molecules. Thrombosis is further promoted, together with platelet activation.
The diagnostic paths through DCM
Echocardiography is a reliable, noninvasive technique for the determination of LV hypertrophy and the reduction of both systolic and diastolic myocardial function. Moreover, it has a prognostic value for the early diagnosis of DCM.
The classical echocardiographic markers of DCM: advantages and disadvantages
Early deteriorations of myocardial function are characterized by a normal ejection fraction and a decrease in ventricle filling during the initial phase of diastole. Prolongation of isovolumic myocardial relaxation and an increase in atrial filling further confirm a decrease in diastolic myocardial function. The Framingham study showed an increase of LV mass in women with diabetes, independent of the effects of other traditional risk factors.Citation46 Subsequent studies conf irmed these results in both genders, highlighting associations of both diabetes and glucose intolerance with LV structure abnormalities, independent of the influence of relevant covariates, such as arterial blood pressure levels and treatment with angiotensin-converting enzyme (ACE) inhibitors.Citation43,Citation127–Citation130 The same was true for patients with type 1 diabetes.Citation131 Early echocardiographic studies revealed that half of the diabetic population with decreased myocardial diastolic function remain undiagnosed with the classical echocardiographic techniques. This is due to the fact that the echocardiographic subestimation of the degree of diastolic dysfunction with the classical echocardiographic markers in the subjects with normal arterial blood pressure levels is equal to 60%.Citation132,Citation133
The newest echocardiographic markers: their performance structure and role as diagnostic tools
Total ejection isovolumic (TEI) index is a myocardial performance index and a method for the global estimation of the LV performance.Citation134 The TEI index is not influenced by the increased left atrial loading pressure often present in the advanced stages of diastolic heart failure, which is a key advantage compared with the classical echocardiographic methods.Citation135,Citation136 The isolated estimation of the decrease in diastolic myocardial function has been recently questioned owing to the presence of reduced systolic function in patients with normal ejection fraction. Therefore, evaluation of TEI index may become a valuable tool for the early diagnosis of upcoming myocardial ischemia.Citation137
An important limitation of the single use of TEI index for the estimation of myocardial dysfunction is that it does not allow the evaluation of the pathogenetic substrate of myocardial dysfunction because it cannot evaluate the myocardial pressure levels during diastolic filling or the changes in systolic preload.Citation138 However, the clinical significance of TEI index as a diagnostic tool for New York Heart Association Grade I–III heart failure was recently successfully demonstrated and was identical when compared with the results of invasive techniques.Citation139 Moreover, TEI index was demonstrated as a significant prognostic marker of cardiovascular morbidity and mortality in elderly patients, independent of the coexistence of the classical cardiovascular risk factors.Citation140
In a recent study in type 2 diabetes patients without macrovascular complications, TEI index was significantly and independently associated with the presence of DCM.Citation100 Moreover, in type 2 diabetes patients with DCM, higher values of the TEI index were associated with increased ventricular arrhythmogenicity, suggesting a significant association between the underlying LV diastolic dysfunction and the LV electrophysiological activity of the diabetic heart. This association was further supported by the findings of another study in a nondiabetic population with MetS, where worse myocardial function, assessed by higher TEI index values, was associated with increased ventricular arrhythmogenicity. Citation92 This study concluded that the electrophysiological abnormalities present in the MetS may be due to the direct effects of hyperinsulinemia on myocardial membrane activity or the electrophysiological alterations that accompany the biochemical and functional abnormalities caused by the shift in the myocardial substrate utilization.Citation141
MetS was also associated with worse myocardial performance assessed by the TEI index in a population without diabetes, hypertension, or other macrovascular complications. This association was confirmed for the MetS per se, the sum of its components and its particular components. Additionally, it was demonstrated that factors associated with conditions commonly associated with MetS, such as insulin resistance, low-grade inflammation, and microalbuminuria, were also associated with worse LV myocardial performance.Citation116
Other modern echocardiographic techniques, such as transmitral pulsed Doppler and its applications (strain and strain rate), which are less susceptible to the alterations of the cardiac loading conditions, have recently demonstrated 63% sensitivity against the 50% of the pulsed Doppler in diagnosing DCM.Citation137,Citation142–Citation144 shows the main echocardiographic findings and the incidence of DCM in major and recent studies.
Table 1 Main and recent echocardiographic, population-based studies on diabetic cardiomyopathy
Treatment strategies of DCM: risk factors, management and lessons from studies
Lifestyle
Regular physical exercise and limitation of fat and total energy intake remain the cornerstone of the management of overweight diabetes patients who currently represent 80% of type 2 and at least 25% of type 1 diabetes patients worldwide. Patients with diabetes should be encouraged to undertake moderate aerobic activity, ie, walking for 30–45 min everyday. Patients with angina or breathlessness on exertion should exercise within their limitations, aiming gradually to increase their activity. In obese patients with a body mass index (BMI) > 30 kg/m2, an initial target should be 10% weight loss; in those who are not obese, a target BMI of ≤25 kg/m2 may be reasonable.
One prospective, randomized, controlled study evaluated the effects of diet and exercise on the reduction of the overall incidence of DCM in patients with type 2 diabetes for a follow-up period of 6 years. Subjects were randomized either to a control group or to 1 of the 3 active treatment groups, ie, diet only, exercise only, or diet plus exercise. The cumulative incidence of DCM at 6 years was 67.7% in the control group compared with 43.85% in the diet group, 41.1% in the exercise group, and 46% in the diet plus exercise group. Each of the active intervention groups differed significantly from the control group (P < 0.05). In a proportional hazards analysis comparing the 3 intervention groups to the control group, there was a reduction in the cardiovascular risk of 33% in the diet group (P < 0.03), 47% in the exercise group (P < 0.0005), and 38% in the diet plus exercise group (P < 0.005). In conclusion, lifestyle changes with diet and/or exercise interventions led to a significant decrease in the incidence of DCM over a 6-year period among subjects with type 2 diabetes.Citation145
Another randomized, blinded study with a mean duration of 3.2 years evaluated the feasibility and effects of a program of lifestyle changes designed to prevent or delay the onset of type 2 diabetes in 523 middle-aged, overweight (BMI ~31.3 kg/m2) subjects with impaired glucose tolerance and the incidence of DCM in patients with type 2 diabetes. Citation146 The intervention group consisted of individualized counseling aimed at reducing weight and total fat intake and increasing fiber intake and physical activity (at least 30 min per day). During the trial, the risk of diabetes was reduced by 58% in the intervention group and was directly associated with changes in lifestyle. The changes in lifestyle also reduced (by 56% in both men and women) the magnitude of DCM incidence.Citation147
Cigarette smoking is an obvious and important modifiable risk factor for cardiovascular disease. Smoking cessation confers immediate and lasting benefits that are likely to be even greater in people with diabetes, whose vascular risk is further increased by smoking. In the Multiple Risk Factor Intervention Study (MRFIT), the absolute risk of cardiovascular death over 12 years was much higher for diabetes subjects compared with the nondiabetes participants and was significantly associated with reported number of cigarettes/day (P < 0.0001). Together with dyslipidemia and hypertension, cigarette smoking was a dominant predictor of cardiovascular morbidity (increased risk for DCM by 53% in both sexes) and cardiovascular mortality (overall 3 times higher risk of death from heart failure).Citation103,Citation148
Optimizing glycemic control
This is an important treatment aim in the management of DCM. A prospective cohort study analyzed the relative risk of macrovascular disease in patients with newly diagnosed type 2 diabetes and determined whether an intensive approach to dietary management, besides satisfactory glycemic control, would ameliorate the long-term cardiovascular risk and reduce the incidence of DCM and cardiovascular mortality. The study compared dietary management alone to oral hypoglycemic therapy (tolbutamide 500 mg or metformin twice a day) with continued diet and finally insulin regimen. At the end of the 10-year follow-up period, the survival rate was 75%, whereas 26% of the patients suffered myocardial infarction, of which 41% was fatal. The analysis of baseline data showed a relative risk equal to 1.04 for myocardial infarction for each 1 mmol/L increase in fasting plasma glucose. The risk of myocardial infarction while on insulin or oral treatment was not significantly elevated relative to dietary treatment. The study concluded that fasting plasma glucose is a significantly independent predictor of diabetic macrovascular disease, and the type of antidiabetic treatment does not prevent long-term cardiovascular disease.Citation149
The epidemiological analysis of a prospective, multicenter, population-based study of patients with newly diagnosed type 2 diabetes demonstrated that the incidence of myocardial infarction was 15.2% and of these 19.2% died. The results showed that good glycemic control was associated with lower risk not only for myocardial infarction (15.2% vs 42%) but also for DCM (15% vs 32%). Good glycemic control was associated with a lower incidence of DCM (27% vs 9%), whereas postprandial blood glucose levels were among the independent predictors for cardiovascular morbidity and mortality.Citation150,Citation151 The UKPDS evaluated the relationship between exposure to glycemia over time and the development of DCM in patients with type 2 diabetes. For each 1% reduction in mean HbA1c, there was a 14% associated decrease in risk for myocardial infarction and a 16% decrease in risk for heart failure.Citation152
Management of dyslipidemia
A double-blind, randomized, placebo-controlled, multicenter clinical trial assessed the effect of cholesterol lowering with simvastatin on mortality and the risk of coronary heart disease in patients with diabetes and DCM. Treatment of dyslipidemia significantly decreased the risk of coronary heart disease (recurrent myocardial infarction) by 55% (P = 0.002) in subjects with type 2 diabetes. Since in diabetes subjects with DCM the case fatality is very high, aggressive lipid lowering should be extended to diabetes subjects who have not yet experienced a clinical event (primary and secondary prevention).Citation153
Another multicenter, randomized, double-blind, placebo-controlled study determined if treatment with gemfibrozil would reduce the incidence of major cardiovascular events and outcomes in patients with type 2 diabetes. DCM was reduced by 22% with gemfibrozil treatment, and major cardiovascular events were reduced by 24%. For every 5 mg/dL increase in HDL cholesterol, there was an 11% reduction in cardiovascular events.Citation154 The effect of lipidlowering treatment at various levels of glucose tolerance or hyperinsulinemia was also evaluated by the same group, and the association between diabetes status, glucose–insulin levels and cardiovascular risk was examined. In patients with diabetes, a fasting plasma insulin level ≥ 271 pmol/L was associated with a 31% increased risk of cardiovascular events. Gemfibrozil was effective in patients with diabetes, and the reduction in DCM was 53%, whereas in cardiovascular mortality, it was 41%.Citation155–Citation157
Hypertension management and renoprotection
A randomized, double-blind, active-controlled clinical trial reached to determine whether the combined incidence of fatal coronary heart disease and nonfatal myocardial infarction differs between diuretic treatment (chlorthalidone) and 3 alternative therapies: calcium channel blockers (amlodipine), ACE inhibitors (lisinopril), and α-blockers (doxazosin). The incidence of DCM was higher in the group under therapy with α-blockers compared with diuretics (relative risk equal to 1.25 at a 4-year rate). No significant differences were observed between calcium channel blockers, ACE inhibitors, and diuretics for the primary outcome or for all-cause mortality. Amlodipine had a higher 6-year rate of heart failure (10.2% vs the diuretic group) and of hospitalized or fatal heart failure (8.4%). Lisinopril was associated with a 10% higher risk of DCM and a 19% higher risk for heart failure. Five-year systolic blood pressure was significantly higher in the amlodipine group (0.8 mm Hg; P = 0.03), and lisinopril (2 mm Hg; P < 0.001) groups, compared with chlorthalidone. Thus, thiazide-type diuretics should be considered first for antihypertensive treatment and for patients who cannot be treated with diuretics; calcium channel blockers and ACE inhibitors could be considered as alternative choices.Citation158
A double-blind, randomized, 2 × 2 factorial study, the Heart Outcomes Prevention Evaluation (HOPE) study, compared an ACE inhibitor, ie, ramipril, vs placebo in preventing death from cardiovascular causes, myocardial infarction, or all-cause death. The study concluded that ramipril significantly reduced the rates of death (6.1% compared with 8.1% in the placebo group), myocardial infarction (9.9% vs 12.3%), and all-cause mortality (10.4% vs 12.2%). Moreover, ramipril therapy significantly decreased the rate of heart failure (9% vs 11.5% in the placebo group) and of DCM (6.4% vs 7.6%; P = 0.03). Ramipril achieved a significant reduction in the new cases with diabetes in the high-risk population included in the study, with unknown myocardial dysfunction (low ejection fraction) or heart failure.Citation159
The association between major cardiovascular events and the achievement of different blood pressure targets during antihypertensive therapy, as well as the addition of low doses of acetylsalicylic acid was assessed in another randomized trial. Felodipine (5 mg/d) was given as baseline therapy, and other agents were added according to a 5-step regimen. The lowest incidence of DCM and major cardiovascular morbidity and mortality occurred in the blood pressure target group of ≤135/80 mm Hg. DCM was reduced by 51%, and the addition of acetylsalicylic acid further achieved a 15% reduction in major cardiovascular morbidity and a 36% reduction in myocardial infarction. The study demonstrated that active lowering of blood pressure is particularly beneficial in patients with diabetes and that addition of a small dose of acetylsalicylic acid, provided that blood pressure is well controlled and the risk of gastrointestinal and nasal bleeding is carefully assessed, further reduces the risk of acute myocardial infarction.Citation160
A double-masked, randomized, parallel-controlled group trial determined the effectiveness of losartan, a selective angiotensin II type 1 (AT1) receptor antagonist in comparison with the β-blocker atenolol in reducing cardiovascular morbidity and mortality in diabetes patients with hypertension and LV hypertrophy. Losartan was more effective than atenolol in reducing cardiovascular morbidity and mortality (76% reduction of the relative risk of a primary cardiovascular end point). The greater cardiovascular protection of losartan was attributed mainly to the result of a more pronounced protection against the detrimental effects of angiotensin II.Citation161
A randomized controlled trial, further determined the effectiveness of tight blood pressure control (,150/85 mm Hg) with the use of an ACE inhibitor (captopril) or a β-blocker (atenolol) as main treatment in the reduction of cardiovascular morbidity and mortality. Reduction in cardiovascular risk by 24% in all end points related to diabetes was achieved with tight blood pressure control (32% in deaths). In addition, there was a 56% reduction in the risk of heart failure.Citation162
Microalbuminuria heralds a further increase in risk of myocardial infarction and necessitates even stricter blood pressure control. A randomized control trial determined that blood pressure lowering with captopril and atenolol is equally effective and safe in reducing the risk of fatal and nonfatal macrovascular and microvascular complications in patients with type 2 diabetes.Citation163 ACE inhibitors largely remain in the antihypertensive class of choice, as their use delays the progression of nephropathy.Citation164
Medical management of cardiovascular disease in diabetes
Heart failure is also conventionally managed with diuretics and ACE inhibitors. Certain β-blockers, such as carvedilol, are among the drugs of choice for patients with diabetes, due to the fact that it has a beneficial effect on insulin resistance and a neutral activity on the lipid profile of patients with diabetes.Citation165,Citation166 The results of the intervention studies for the prevention of DCM are summarized in .
Table 2 Major studies on treatment strategies of diabetic cardiomyopathy
Struggling toward additional knowledge
Targeting the pathophysiological substrate: new experimental therapies, regimens, and remedies
Recently, direct correlations between apelin plasma levels and the structural and functional alterations, ie, LV hypertrophy and pressure overload, were demonstrated in humans and diabetic rats with DCM. A parallel study was conducted in streptozotocin-induced diabetic rats, where pressure overload was established by suprarenal aortic banding, as well as in patients with diabetes, where during surgery for aortic and mitral stenosis, LV function and structure were evaluated by 2DE and LV endomyocardial biopsies were procured. In both situations, the myocardial expression of apelin was significantly downregulated by pressure overload and apelin plasma levels were accordingly elevated. Since activation of the apelinergic system has a direct positive inotropic and vasodilatation effect, it was concluded that elevation in apelin plasma levels may represent a compensatory mechanism to maintain inotropism and cardiac output during pressure overload conditions, such as DCM.Citation167
The kinins are vasoactive peptides and part of the kallikrein–kinin system (KKS). Recent studies have demonstrated that they are involved in different aspects of remodeling, inflammation, and angiogenesis. It is increasingly recognized that the KKS is involved in the inflammatory processes of the heart. Evidence shows that the KKS has beneficial effects in myocardial diseases by protecting from inflammation, fibrosis, and apoptosis. KKS also shows a proinflammatory character by increasing the production of cytokines and stimulating the migration of immune cells. Novel important actions of the KKS contribute to neovascularization and recruitment of endothelial progenitor cells in ischemic areas and endothelial dysfunction. All these evidence support that the KKS could constitute a potential therapeutic target in the treatment of myocardial ischemia and DCM.Citation168
As previously discussed,Citation47 a significant role in the myocardial dysfunction of DCM is supported by the reduction of the sarcoplasmic calcium ATPase myocardial expression (SERCA2). Resveratrol, a potent activator of the cardiac function and the SERCA2 in type 1 diabetes were recently administered to adult male streptozotocin-induced diabetic mice, where the induced diabetes produced a progressive decline in cardiac function associated with markedly reduced SERCA2 and increased collagen deposition. Resveratrol treatment to these mice had a tremendous beneficial effect both in terms of improving SERCA2 expression and cardiac function. The data demonstrate that in DCM, where the expression of SERCA2 is reduced, resveratrol enhances the expression of the SERCA2 and improves cardiac function.Citation169
Cardiac fibrosis and protein O-glycosylation are elevated in DCM.Citation22,Citation23 One in vitro study in rat cardiac fibroblasts recently demonstrated that long-term glucose load significantly increases collagen expression. Treatment with glucosamine not only induced the collagen expression but also increased the O-glycosylated protein. These results suggest that O-glycosylation of protein modifies collagen expression and, therefore, contributes to DCM.Citation170 ROSs play an important role in the development of DCM.Citation42,Citation43 Matrix metalloproteinase 2 (MMP-2) is activated by ROS and contributes to the acute loss of myocardial contractile function by targeting and cleaving susceptible proteins including troponin I and α-actinin. Using the streptozotocin-induced diabetic rat model, one study evaluated the 4-week administration effect on heart function of a daily in vivo administration of sodium selenate (0.3 mg/kg) or a pure ω-3 fish oil with antioxidant vitamin E (ω-3E, 50 mg/kg). Both treatments prevented the diabetes-induced functional changes; however, the improvement in myocardial function was greater with sodium selenate compared with that of the omega-3E. Moreover, these treatments reduced the diabetes-induced increase in myocardial oxidized protein sulfhydryl and nitrite concentrations. The diabetes-induced changes in myocardial levels of MMP-2 were associated with a significant reduction in troponin I and α-actinin protein levels in both treatment groups. These results suggest that diabetes-induced alterations in MMP-2 contribute to myocardial contractile dysfunction by targeting troponin I and α-actinin and that sodium selenate or omega-3E could have therapeutic benefits in DCM.Citation171
Another study also aimed at the suppression of mitochondrial oxidative stress and mitochondrion-dependent myocardial apoptosis with the administration of antioxidant α-lipoic acid (α-LA) to prevent evolution of DCM. In a streptozotocin-induced diabetic rat model, α-LA was administered (100 mg/kg intraperitoneally per day) for 12 weeks. Antioxidant α-LA effectively attenuated mitochondriondependent cardiac apoptosis and exerted a protective role against the development of DCM. The ability of α-LA to suppress mitochondrial oxidative damage was concomitant with an enhancement of manganese superoxide dismutase activity in the myocardial mitochondria and an increase in the glutathione content of myocardial mitochondria.Citation172
The diabetic heart is resistant to ischemic preconditioning because of diabetes-associated impairment of phosphatidylinositol 3-kinase (PI3K)-Akt signaling. One study examined the hypothesis that phosphorylation of Janus kinase 2 (Jak2) upstream of PI3K is impaired in diabetic hearts by an AT1 receptor-mediated mechanism. Thus, in a type 2 diabetic rat model, myocardial infarct was produced. Erythropoietin (EPO) administration activated the Jak2-mediated signaling and limited the infarct size in experimental rats. Where EPO failed to induced infarct size limitation, blockade of the AT1 receptor by valsartan or losartan treatment for 2 weeks restored the myocardial response to EPO. Furthermore, treatment with valsartan normalized the calcineurin activity in diabetic rats. These results suggest that the diabetic heart is refractory to protection by Jak2-activating ligands because of AT1 receptor-mediated upregulation of calcineurin activity.Citation173
The β isoform of protein kinase C (PKC-β) is a target implicated in diabetic complications. To investigate the influence of breviscapine on the cardiac structure and function in rats with DCM, as well as the expression of PKC and Ca2+ cycling proteins, type 2 diabetic rats were treated with breviscapine for 6 weeks, and invasive cardiac function and echocardiographic parameters were measured, while heart tissue was obtained for electron microscope study. Treatment with breviscapine reversed the cardiac dysfunction and structure changes in rats with DCM, decreased the expression of PKC, and increased the expression of calcium handling regulators, ie, protein phosphatase inhibitor-1, phospholamban, and SERCA2. This study showed that breviscapine has a protective effect on DCM, which is dose related.Citation174 Breviscapine also prevented cardiac hypertrophy in diabetic rats by inhibiting the expression of PKC, which may have a protective effect in the pathogenesis of DCM via the PKC/NF-κB/c-fos signal transduction pathway.Citation175
Using a hemodynamically validated rodent model of diabetic diastolic heart failure, the (mRen-2)27 transgenic rat another study further determined the selective inhibition of PKC-β. Preservation of cardiac function and reduction of structural injury were evaluated. Diabetic rats were randomized to receive either vehicle or the PKC-β inhibitor, ruboxistaurin (20 mg/kg/day) and followed for 6 weeks. Compared with untreated animals, ruboxistaurin-treated diabetic rats demonstrated preserved systolic and diastolic function. Collagen deposition and cardiomyocyte hypertrophy were both reduced in diabetic animals treated with ruboxistaurin as was phosphorylated-Smad2, an index of transforming growth factor β (TGF-β) activity. PKC-β inhibition attenuated diastolic dysfunction, myocyte hypertrophy, and collagen deposition and preserved cardiac contractility. PKC-β inhibition may represent a novel therapeutic strategy for the prevention of diabetes-associated cardiac dysfunction.Citation176
Studies have shown that caffeic and ellagic acid, 2 phenolic acids naturally occurring in many plant foods, can provide triglyceride-lowering, anticoagulatory, antioxidative, and anti-inflammatory protection in cardiac tissue of diabetic mice. Thus, the supplement of these agents might be helpful for the prevention or attenuation of DCM.Citation177
Total aralosides of Aralia elata (Miq) Seem (TASAES) from the Chinese traditional herb Longya Aralia chinensis L. has been demonstrated to improve cardiac function. A recent study determined the protective effects of TASAES on DCM in streptozotocin-induced diabetic rats. Diabetic rats were treated with TASAES for 8 weeks. Cardiac function was evaluated by in situ hemodynamic measurements. TASAES showed a significant protection against diabetes-induced cardiac dysfunction and enhanced the amplitude of Ca2+ channel currents. Moreover, histological staining indicated a significant inhibition of diabetes-caused pathological changes and upregulation of profibrotic factor, and connective tissue growth factor, and expression. The results suggest that TASAES prevents diabetes-induced cardiac dysfunction and pathological damage.Citation178
The effects of Ginkgo biloba extract (EGb 761), a radical scavenger, against diabetes-induced myocardial interstitium and microvasculature damage and against additional ischemia or reperfusion injury in spontaneously diabetic BioBreeding/Ottawa Karlsburg (BB/OK) rats modeling diabetic cardiac infarction were also investigated as an alternative antioxidative therapy. Pretreatment of diabetic myocardium with EGb resulted in an improvement of impaired endothelial-dependent vasodilatation in diabetes and additional ischemia or reperfusion, diminished mast cell and substance P accumulation, and better preserved myocardial ultrastructure compared with unprotected myocardium. EGb may act as a potent therapeutic adjuvant in patients with diabetes with respect to ischemic myocardial injury and may contribute to preventing late complications in DCM.Citation179 Another study, also demonstrated EGb as a promising adjuvant therapeutic drug in patients with diabetes with respect to ischemic myocardium injury. It concluded that it may contribute to the prevention of late diabetic complications in DCM.Citation180
Natural medicine for the treatment of DCM was further recognized by the demonstration of grape seed proanthocyanidin extracts (GSPEs) as therapeutic agents against DCM in streptozotocin-induced diabetic rats. GSPEs decreased AGEs and ameliorated glycation-associated cardiac damage.Citation181
Recent studies in mice experimental models with acute ischemic injury revealed that EPO has numerous tissue-protective effects in the heart, brain, and kidneys. One study explored the tissue-protective properties of chronic EPO treatment in an experimental model of the db/db mouse with diabetic heart injury. Continuous erythropoietin receptor activator (CERA)-treated mice had a significant reduction in TGF-β1 and collagen I expression in cardiac tissue. Atrial natriuretic peptide expression was increased in CERA-treated mice. Chronic treatment with CERA protects cardiac tissue in diabetic animals by inhibiting molecular pathways of cardiac fibrosis, and the effects are dose dependent.Citation182
Cell-based myocardial regenerative therapy
Cell-based myocardial regenerative therapy can be potentially applied in the treatment of DCM in order to limit the consequences of decreased contractile function and compliance of damaged ventricles, owing to ischemic and nonischemic myocardial areas. A variety of myogenic and angiogenic cell types have been proposed, such as skeletal myoblasts, mononuclear and mesenchymal bone marrow cells, circulating blood-derived progenitors, adipose-derived stromal cells, induced pluripotent stem cells, umbilical cord cells, endometrial mesenchymal stem cells, adult testis pluripotent stem cells, and embryonic cells. It is important to note that stem cells are not an alternative to heart transplantation; selected patients should be in an early stage of heart failure, as the goal of this regenerative approach is to avoid or delay organ transplantation. Since the cell niche provides crucial support needed for stem cell maintenance, the most interesting and realistic perspectives include the association of intramyocardial cell transplantation with tissue-engineered scaffolds and multisite cardiac pacing in order to transform a passive regenerative approach into a “dynamic cellular support,” a promising method for the creation of “bioartificial myocardium”.Citation183
Conclusion
DCM is not uncommon and is associated with increased morbidity and mortality in patients with diabetes. Metabolic pathway deregulation in diabetes adversely affects the cardiac myocytes, as well as every cellular element within the vascular walls. Other pathogenetic mechanisms involved in decreasing myocardial contractility in DCM are impaired calcium homeostasis, renin–angiotensin system upregulation, increased oxidative stress, altered substrate metabolism, and mitochondrial dysfunction.Citation184 Continued research has provided novel insights into underlying molecular and pathophysiological mechanisms that increase the vulnerability of the diabetic heart to failure.Citation185 The choice of antidiabetic medication may influence the cardiovascular outcome.Citation186,Citation187 However, intensive treatment of the gamut of metabolic abnormalities associated with diabetes beyond hyperglycemia reduces the rates of DCM, myocardial infarction, and associated cardiovascular death. As this high-risk population increases, elucidation of the mechanisms responsible for DCM will further motivate the generation of novel therapies tailored to decrease the cardiovascular morbidity and mortality in diabetes. Strategies to encourage the aggressive use of targeted medical therapies will further reduce the magnified rates of the diabetic heart failure burden.
Disclosure
The authors report no conflicts of interest in this work.
References
- AmosAFMcCartyDJZimmetPThe rising global burden of diabetes and its complications: estimates and projections to the year 2010Diabet Med199714Suppl 1S1S859450510
- FisherBMGillenGLindopGBDargieHJFrierBMCardiac function and coronary arteriography in asymptomatic type-1 (insulin-dependent) diabetic patients: evidence for a specif ic diabetic heart diseaseDiabetologia1986297067123803744
- KannelWBHjortlandMCastelliWPRole of diabetes in congestive heart failure: the Framingham StudyAm J Cardiol19763429344835750
- KannelWBMcGeeDLDiabetes and cardiovascular disease: the Framingham StudyJAMA197924120352038430798
- RublerSDlugashJYuceogluYZKumralTBranwoodAWGrishmanANew type of cardiomyopathy associated with diabetic glomerulosclerosisAm J Cardiol1972305956024263660
- ZimmetPAlbertiKGMMSwanJGlobal and social implications of the diabetes epidemicNature200141478278711742409
- BerensonGSSrinivasanSRBaoWNewmanWPIIITracyREWattigneyWAAssociation between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart StudyN Engl J Med1998338165016569614255
- KròlewskiASKosinskiEJWarramJHMagnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitusAm J Cardiol1987597507553825934
- Barrett-ConnorEWingardDLSex differences in ischemic heart disease mortality in diabetics: a prospective population based studyAm J Epidemiol19831184894966637976
- GaedePVedelPParvingHHPedersenOIntensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the STENO type 2 randomized studyLancet199935361762210030326
- HaffnerSMLebtoSRoenemaaTPyöräläKLaaksoMMortality from coronary heart disease in subjects with type 2 diabetes and in non diabetic subjects with and without prior myocardial infarctionN Engl J Med19983392292349673301
- MalmbergKRydenLMyocardial infarction in patients with diabetes mellitusEur Heart J19889256264
- HerlitzJMalmbergKKarlssonBRydénLHjalmarsonAMortality and morbidity during a five year follow up of diabetics with myocardial infarctionActa Med Scand19882431383046232
- NorhammarATenerzANilssonGGlucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective studyLancet20023592140214412090978
- BarzilayJIKronmalRABittnerVEakerEEvansCFosterEDCoronary artery disease and coronary artery bypass grafting in diabetic subjects aged ≥65 yearsAm J Cardiol1994743343398059694
- Borch-JohnsenKAndersenPKDeckertTThe effect of proteinuria on relative mortality in type-1 (insulin-dependent) diabetes mellitusDiabetologia1985285905964054448
- BertoniAGTsaiAKasperEKBrancatiFLDiabetes and idiopathic cardiomyopathyDiabetes Care2003262791279514514581
- ShindlerDMKostisJBYusufSDiabetes Mellitus, a predictor of morbidity and mortality in the studies of the left ventricular dysfunction (SOLVD) trials and registryAm J Cardiol199677101710208644628
- RydenLArmstrongPWClelandJGEfficacy and safety of highdose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trialEur Heart J2000211967197811071803
- CohnJNJohnsonGZiescheSA comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failureN Engl J Med19913253033102057035
- HayatSAPatelBKhattarRSMalikRADiabetic cardiomyopathy: mechanisms, diagnosis, and treatmentClin Sci200410753955715341511
- VigoritaVJMorreGWHutchensSMAbsence of correlation between coronary arterial atherosclerosis and severity or duration of diabetes mellitus of adult onsetAm J Cardiol1980465355427416013
- FactorSMMinaseTSonnenblickEHClinical and morphological features of human hypertensive-diabetic cardiomyopathyAm Heart J1980994464586444776
- FactorSMBhanRMinaseTWolinskyHSonnenblickEHHypertensive diabetic cardiomyopathy in the rat: an experimental model of human diseaseAm J Pathol19811022192287468768
- KawaguchiMTechigawaraMIshihataTA comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with and without diabetes mellitus with and without hypertensionHeart Vessels1997122672749860193
- LevyDGarrisonRJSavageDDKannelWBCastelliWPPrognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart StudyN Eng J Med199032215611566
- PalmieriVBellaJNTracyRPRelationship of left ventricular hypertrophy to inflammation and albuminuria in adults with type 2 diabetes. The Strong Heart StudyDiabetes Care2003262764276914514577
- PetrieMCCaruanaLBerryCMcMurrayJJDiastolic heart failure caused by subtle left ventricular systolic dysfunctionHeart200287293111751660
- BrutsaertDLHousmansPRGoethalsMADual control of relaxation. Its role in the ventricular function in the mammalian heartCirc Res1980476376526106532
- ZileMRBaicuCFGaaschWHDiastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricleN Engl J Med20043501953195915128895
- RaevDCWhich left ventricular dysfunction is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type 1 diabetic patientsDiabetes Care1994176336397924771
- RiggsTWTransueDDoppler echocardiographic evaluation of left ventricular diastolic dysfunction in adolescents with diabetes mellitusAm J Cardiol1990658999022321540
- MargonatoAGerundiniPVicedominiGGilardiMCPozzaGFazioFAbnormal cardiovascular response to exercise in young asymptomatic diabetic patients with retinopathyAm Heart J19861125545603751866
- MirskyIAssessing diastolic function: suggested methods and future considerationsCirculation1984698368416697466
- OmmenSRNishimuraRAA clinical approach to the assessment of left ventricular diastolic function by Doppler echocardiography: updateHeart200389182312482783
- AurigemmaGPGottdienerJSShemanskiLGardinJKitzmanDPredictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the Cardiovascular Health StudyJ Am Coll Cardiol2001371042104811263606
- VasanRLarsonMGBenjaminEJEvansJCReissCKLevyDCongestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohortJ Am Coll Cardiol1999331948195510362198
- JudgeKWPawitanYCaldwellJGershBJKennedyJWCongestive heart failure in patients with preserved left ventricular systolic function: analysis of the CASS registryJ Am Coll Cardiol1991183773821856405
- BroganWCIIIHillisLDFloresEDLangeRAThe natural history of isolated left ventricular diastolic dysfunctionAm J Med1992926276301605144
- IribarrenCKarterAJGoASGlycaemic control and heart failure among adult patients with diabetesCirculation20011032668267311390335
- AvendanoGFAgarwalRKBasheyRIEffects of glucose intolerance on myocardial function and collagen-linked glycationDiabetes1999481443144710389851
- SinghRBardenAMoriTBeilinLAdvanced glycation end products: a reviewDiabetologia20014412914611270668
- DevereuxRBRomanMJParanicasMImpact of diabetes on cardiac structure and function: The Strong Heart StudyCirculation20001012271227610811594
- YoungMEMcnultyPTaegtmeyerHAdaptation and maladaptation of the heart in Diabetes: part II Potential mechanismsCirculation20021051861187011956132
- CandidoRForbesJMThomasMCA breaker of advanced glycation end products attenuates diabetes induced myocardial structural changesCirc Res20039278579212623881
- GalderisiMAndersonKMWilsonPWFLevyDEchocardiographic evidence for the existence of a distinct diabetic cardiomyopathy. The Framingham Heart StudyAm J Cardiol19916885892058564
- RodriguesBCamMCMcNeillJHMetabolic disturbances in diabetic cardiomyopathyMol Cell Biochem199818053579546630
- ZhouYGrayburnPKarimALipotoxic heart disease in obese rats: implications for human obesityProc Natl Acad Sci U S A20009717941789
- AsifMEganJVasanSAn advanced gylcation end product crosslink breaker can reverse age-related increases in myocardial stiffnessProc Natl Acad Sci U S A2000972809281310706607
- LiangQCarlsonECDonthiRVOverexpression of metallothionein reduces diabetic cardiomyopathyDiabetes20025117418111756338
- ZhangFLiGDingWScreening and analysis of early cardiopathology-related gene in type 2 diabetes mellitusZhonghua Nei Ke Za Zhi20024153053312421500
- MonkemannHde VrieseASBlomHJEarly molecular events in the development of the diabetic cardiomyopathyAmino Acids20022333133612373555
- MizushigeKYaoLNomaTAlteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of type II diabetic modelCirculation200010189990710694530
- RenJSampsonWKSowersJRInsulin-like growth factor 1 as a cardiac hormon: physiological and pathophysiological implications in heart diseaseMoll Cell Cardiol19993120492061
- AiraksinenKKostinenJAkaheimoMHuikuriHAugmentation of atrial contraction to LV filling in IDDM subjects as assessed by Doppler echocardiographDiabetes Care1989121591612702899
- ZieglerDCardiovascular autonomic neuropathy: clinical manifestations and measurementDiabetes Rev19997300315
- KahnKZolaBJuniJEVinikAIRadionuclide assessment of LV diastolic filling pressures in diabetes mellitus with and without cardiac autonomic neuropathyJ Am Coll Cardiol19867130313093011872
- ZolaBKahnJKJuniJEVinikAIAbnormal cardiac function in diabetic patients with autonomic neuropathy in the absence of ischemic heart diseaseJ Clin Endocrinol Metab1986632082143711260
- UusitupaMMustonenJLaaksoMImpairment of diastolic function in middle aged type 1 and type 2 diabetic patients free of cardiovascular diseaseDiabetologia1988317837913234632
- RathmannWZieglerDJahnkeMHaastertBGriesFAMortality in diabetic patients with cardiovascular autonomic neuropathyDiabet Med1993108208248281726
- RoyTMPetersonHRSniderHLAutonomic influence on cardiovascular performance in diabetic subjectsAm J Med1989873823882801728
- AnnonuAKFattahAAMokhtarMSGhareebSElhendyALV systolic and diastolic functional abnormalities in asymptomatic patients with non-insulin-dependent diabetes mellitusJ Am Soc Echocardiogr20011488589111547274
- MustonenJMantysaariMKuikkaJDecreased myocardial 123I-metaiodobenzylguanidine uptake is associated with disturbed LV diastolic filling in diabetesAm Heart J19921238048051539538
- MonteagudoPTMoisesVAKohlmannOJrRibeiroABLimaVCZanellaMTInfluence of autonomic neuropathy upon LV dysfunction in insulin-dependent diabetic patientsClin Cardiol20002337137510803447
- WillenheimerRBErhardtLRNilssonHLiljaBJuul-MöllerSSundkvistGParasympathetic neuropathy associated with left ventricular diastolic dysfunction in patients with insulin-dependent diabetes mellitusScand Cardiovasc J19983217229536501
- di CarliMFBianco-BatllesDLandaMEEffects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitusCirculation199910081381910458716
- BalkauBJouvenXDucimetiérePEschwègeEDiabetes as a risk factor for sudden deathLancet19993541968196910622302
- EwingDJBolandONeilsonJMChoCGClarkeBFAutonomic neuropathy, QT interval lengthening, and unexpected deaths in male diabetic patientsDiabetologia1991341821851884890
- TentolourisNKatsilambrosNPapazachosGCorrected QT interval in relation to the severity of diabetic autonomic neuropathyEur J Clin Invest199727104910549466135
- PoirierPBogatyPPhilipponFPreclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetesMetabolism2003521056106112898473
- VoulgariCTentolourisNMoyssakisIThe spatial QRS-T angle is increased in patients with type 2 diabetes mellitus and cardiac autonomic neuropathyDiabetologia200951Suppl 1S500S500
- ChappetODosquetCWautierMPAdvanced glycation end products, oxidant stress and vascular lesionsEur J Clin Invest199727971089061302
- BrownleeMAdvanced protein glycation in diabetes and agingAnn Rev Med1995462232347598459
- BanCRTwiggSMFibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markersVasc Health Risk Manag2008457559618827908
- Effect of intensive management on macrovascular events and risk factors in Diabetes Control and Complications TrialAm J Cardiol1995758949037732997
- StrattonIMAdlerAINeilHAAssociation of glycaemia with macrovascular and microvascular complications in type 2 diabetes (UKPDS 35): prospective observational studyBMJ200032140541210938048
- HaffnerSMImpaired glucose tolerance, insulin resistance, and cardiovascular diseaseDiabet Med199714Suppl 3S12S189272608
- DespresJPLamarcheBMauriegePHyperinsulinemia as an independent risk factor for ischemic heart diseaseN Engl J Med19963349529578596596
- ZavaroniIBonoraEPagliaraMRisk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose toleranceN Engl J Med19893207027062646537
- ReavenGMBanting lecture 1988. Role of insulin resistance in human diseaseDiabetes198837159516073056758
- HaffnerSMMykkanenLFestaAInsulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic stageCirculation200010197598010704163
- KoivistoVAStevensLKMattockMCardiovascular disease and its risk factors in IDDM in Europe. EURODIAB IDDM Complications Study GroupDiabetes Care1996196896978799621
- KohlerHPGrantPJMechanisms of disease: plasminogen activator inhibitor-1 and coronary artery diseaseN Engl J Med20003421792180110853003
- Juhan-VagueIAlessiMCVaguePIncreased plasma plasminogen activator inhibitor 1 levels: a possible link between insulin resistance and atherothrombosisDiabetologia1991344574621916049
- MansfieldMWHeywoodDGrantPJCirculating levels of factor VII, fibrinogen, and non Willebrand factor and features of insulin resistance in first degree relatives of patients with NIDDMCirculation199694217121768901668
- HeywoodDMMansfieldMWGrantPJFactor VII gene polymorphisms, factor VII: C levels and features of insulin resistance in non-insulin-dependent diabetes mellitusThromb Haemost1996754014068701397
- KohlerHPCarterAMSticklandMHGrantPJLevels of activated FXII in survivors of myocardial infarction: association with circulating risk factors and extent of coronary artery diseaseThromb Haemost19987914189459314
- FestaAD’AgostinoRJHowardGMykkänenLTracyRPHaffnerSMChronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS)Circulation2000102424710880413
- SchlkwijkCGPolandDCvan DijkWPlasma concentrations of C-reactive protein are increased in type 1 diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammationDiabetologia19994235135710096789
- KoenigWSundMFrohlichMC-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992Circulation1999992372429892589
- RidkerPMHennekensCHBuringJERifaiNC-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in womenN Engl J Med200034283684310733371
- VoulgariCTentolourisNPapadogiannisDIncreased left ventricular arrhythmogenicity in metabolic syndrome and relationship with myocardial performance, risk factors for atherosclerosis, and low-grade inflammationMetabolism20105915916519766273
- FeenerEPKingGLVascular dysfunction in diabetes mellitusLancet1997350S19113
- ZavaroniIDall’AglioEBonoraEAlpiOPasseriMReavenGMEvidence that multiple risk factors for coronary artery disease exist in persons with abnormal glucose toleranceAm J Med1987836096123674049
- HaffnerSMD’AgostinoRJrMykkanenLInsulin sensitivity in subjects with type 2 diabetes: relationship to cardiovascular risk factors. The insulin Resistance Atherosclerosis StudyDiabetes Care19992256256810189532
- GrundySMHypertriglyceridemia, atherogenic dyslipidemia and the metabolic syndromeAm J Cardiol19888118B25B
- AustinMAEdwardsKLSmall dense low density lipoproteins, the insulin resistance syndrome, and non-insulin dependent diabetesCurr Opin Lipidol199671671718818515
- LamarcheBTchernofAMauriegePSmall, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular StudyCirculation19979569758994419
- LamarcheBTchernofAMoorjaniSFasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart diseaseJAMA1998279195519619643858
- VoulgariCHTentolourisNMoyssakisISpatial QRS-T angle: association with diabetes and left ventricular performanceEur J Clin Invest20063660861316919042
- HiragaTKobayashiTOkuboMProspective study of lipoprotein (a) as a risk factor for atherosclerotic cardiovascular disease in patients with diabetesDiabetes Care1995182412447729305
- ForsblomCMSaneTGroopPHRisk factors for mortality in type II (non-insulin-dependent) diabetes: evidence for a role for neuropathy and a protective effect of HLA-DR4Diabetologia199841125312629833930
- StamlerJVaccaroONeatonJDDiabetes, other risk factors and 12 year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention TrialDiabetes Care1993164344448432214
- LichtensteinMJShipleyMJRoseGSystolic and diastolic blood pressures as predictors of coronary heart disease mortality in the Whitehall studyBMJ19852912432453926137
- TurnerRCMillinsHNeilHAWRisk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS)BMJ19983468238289549452
- deFronzoRFerranniniEInsulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic diseaseDiabetes Care1991141731942044434
- deFronzoRAGoldbergMAgusZSThe effects of glucose and insulin on renal electrolyte transportJ Clin Invest1976588390932211
- HallJESummersRLBrandsMWKeenHAlonso-GaliciaMResistance to metabolic actions of insulin and its role in hypertensionAm J Hypertens199477727887986471
- CapronLJarnetJKazandjianSHoussetEGrowth-promoting effects of diabetes and insulin on arteries: an in vivo study of rat aortaDiabetes1986359739783527827
- AndersonEABalonTWHoffmanRPSinkeyCAMarkALInsulin increases sympathetic activity but not blood pressure in borderline hypertensive humansHypertension1992196216271592458
- GoodeGKMillerJPHeagertyAMHyperlipidemia, hypertension and coronary heart diseaseLancet19953453623647845119
- Comitte of Prinicipal InvestigatorsA co-operative trial in the primary prevention of ischaemic heart disease using clofibrateBr Heart J19784010691118361054
- CalleEEThunMJPetrelliJMRodriguezCHeathCWJrBody mass index and mortality in a prospective cohort of US adultsN Engl J Med19993411097110510511607
- Barret-ConnerELObesity, atherosclerosis, and coronary heart diseaseAnn Intern Med1985103101010193904565
- YamauchiTKamonJWakiHThe fat-derived hormone adiponectin reverses insulin resistance associated both lipoatrophy and obesityNat Med2001794194611479627
- VoulgariCMoyssakisIPapazafiropoulouAThe impact of metabolic syndrome on left ventricular myocardial performanceDiabetes Metab Res Rev20102612112720131336
- MogensenCEMicroalbuminuria predicts clinical proteinuria and early mortality in maturity onset diabetesN Engl J Med19843103563606690964
- ManskeCLWilsonRFWangYThomasWPrevalence of, and risk factors for, angiographically determined coronary artery disease in type 1 diabetic patients with nephropathyArch Intern Med19921522450024505
- YudkinJSForrestRDJacksonCAMicroalbuminuria as a predictor of vascular disease in non-diabetic subjectsLancet198825305332900920
- DeckertTYokoyamaHMathiesenECohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetesBMJ19963128718748611873
- Borch-JohnsenKKreinerSProteinuria: value as predictor of cardiovascular mortality in insulin-dependent diabetes mellitusBMJ1989294165116543113569
- DinneenSFGersteinHCThe association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: a systematic overview of the literatureArch Intern Med1997157141314189224218
- FullerJHStevensLKWangSLWHO Multinational Study GroupRisk Factors for cardiovascular mortality and morbidity: the WHO multinational study of vascular disease in diabetesDiabetologia200144Suppl 2S54S6411587051
- GrudenGCavallo-PerinPBazzanMStellaSVuoloAPaganoGPAI-1 and factor VII activity are higher in IDDM patients with microalbuminuriaDiabetes1994434264298314015
- BrunoGCavallo-PerinPBargeroGBorraMD’ErricoNPaganoGAssociation of fibrinogen with glycemic control and albumin excretion rate in patients with non-insulin-dependent diabetes mellitusAnn Intern Med19961256536578849150
- EarleKSWalkerJHillCVibertiGFamilial clustering of cardiovascular disease in patients with insulin-dependent diabetes and nephropathyN Engl J Med19923256736771736105
- LeeMGardinJMLynchJCDiabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health StudyAm Heart J199713336439006288
- PalmieriVBellaJNArnettDKEffect of Type 2 Diabetes Mellitus on Left Ventricular Geometry and Systolic Function in Hypertensive Subjects: Hypertension Genetic Epidemiology Network (HyperGEN) StudyCirculation200110310210711136693
- IlercilADevereuxRBRomanMJRelationship of impaired glucose tolerance to left ventricular structure and function: the Strong Heart StudyAm Heart J200114199299811376315
- StruthersADMorrisADScreening for and treating left ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deathsLancet20023591430143211978359
- KimballTRDanielsSRKhouryPRMagnottiRATurnerAMDolanLMCardiovascular status in young patients with insulin dependent diabetes mellitusCirculation1994903573618026019
- DumesnilJGGaudreaultGHonosGNKingmaJGJrUse of Valsalva maneuver to unmask left ventricular diastolic function abnormalities by Doppler echocardiography in patients with coronary artery disease or systemic hypertensionAm J Cardiol1991685155191872280
- GarneauCDiastolic dysfunction in normotensive men with well controlled type 2 diabetes mellitus. Importance of manoeuvres in echocardiographic screening for preclinical diabetic cardiomyopathyDiabetes Care20012451011194240
- TeiCNew non-invasive index for combined systolic and diastolic ventricular functionJ Cardiol1995261351367674144
- TeiCLingLHHodgeDONew index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function: a study in normal and dilated cardiomyopathyJ Cardiol1995263573668558414
- PoulsenSHNielsenJCAndersenHRThe influence of heart rate on the Doppler-derived myocardial performance indexJ Am Soc Echocardiogr20001337938410804435
- VinereanuDNicolaidesETweddelACSubclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobinClin Sci200310559159912831396
- GillebertTCvan de VeireNde BuyzereMLde SutterJTime intervals and global cardiac function. Use and limitationsEur Heart J2004252185218615589634
- BruchCSchmermundAMarinDTei-Index in patients with mild-to-moderate congestive heart failureEur Heart J2000211888189511052862
- ArnlövJLindLAndrénBRisérusUBerglundLLithellHA Doppler-derived index of combined left ventricular systolic and diastolic function is an independent predictor of cardiovascular mortality in elderly menAm Heart J200514990290715894975
- SzczepaniakLSVictorRGOrciLUngerRHForgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in AmericaCirc Res200710175976717932333
- FangZYYudaSAndersonVShortLCaseCMarwickTHEchocardiographic detection of early diabetic myocardial diseaseJ Am Coll Cardiol20034161161712598073
- FangZYNajos-ValenciaOLeanoRMarwickTHPatients with early diabetic heart disease demonstrate a normal myocardial response to dobutamineJ Am Coll Cardiol20034244645312906970
- KosmalaWKucharskiWPrzewlocka-KosmalaMMazurekWComparison of left ventricular function by tissue doppler imaging in patients with diabetes mellitus without systemic hypertension vs diabetes mellitus with systemic hypertensionAm J Cardiol20049439539915276118
- Guang-weiLYing-huaHWen-yingYEffects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes complications: the Da Qing IGT and diabetes studyDiabetes Res Clin Pract20025819320012413779
- TuomilehtoJLindstrőmJErikssonJGfor the Finnish Diabetes Prevention Study GroupPrevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose toleranceN Engl J Med20013441343135011333990
- HuGTuomilehtoJSilventoinenKBarengoNJousilahtiPJoint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and womenEur Heart J2004252212221915589638
- KemplerPLearning from large cardiovascular clinical trials: classical cardiovascular risk factorsDiabetes Res Clin Pract200568Suppl 1S43S4715955374
- HaddenDRPattersonCCAtkinsonABMacrovascular disease and hyperglycemia: 10-year survival analysis in type 2 diabetes mellitus: the Belfast Diet StudyDiabet Med1997146636729272593
- HanefeldMFischerSJuliusUfor the DIS groupRisk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-upDiabetologia199639157715838960845
- MeierMHummelMCardiovascular disease and intensive glucose control in type 2 diabetes mellitus: moving practice toward evidence-based strategiesVasc Health Risk Manag2009585987119898642
- UK Prospective Diabetes Study GroupAssociation of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational studyBMJ200032140541210938048
- HaffnerSMThe Scandinavian Simvastatin Survival Study (4S) subgroup analysis of diabetic subjects: implications for the prevention of coronary heart diseaseDiabetes Care1997204694719096961
- RobinsSJCollinsDWittesJTfor the VA-HIT groupRelation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trialJAMA20012851585159111268266
- RubinsHBRobinsSJCollinsDfor the VA-HIT study groupDiabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs High-density lipoprotein intervention Trial (VA-HIT)Arch Intern Med20021622597260412456232
- RobinsSJCollinsDMcNamaraJRBloomfieldHEBody weight, plasma insulin, and coronary events with gemfibrozil in the Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT)Atherosclerosis2008196284985517335828
- JonesPHExpert perspective: reducing cardiovascular risk in metabolic syndrome and type 2 diabetes mellitus beyond low-density lipoprotein cholesterol loweringAm J Cardiol200810212A41L47L
- The ALLHAT officers and coordinators for the ALLHAT collaborative research studyMajor outcomes in high-risk hypertensive patients randomized to ACE inhibitors or calcium channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (ALLHAT)JAMA20022882981299712479763
- The Heart Outcomes Prevention Evaluation Study InvestigatorsEffects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patientsN Engl J Med200034214515310639539
- HanssonLZachettiACarruthersSGfor the HOT study groupEffects of intensive blood-pressure lowering and lowdose aspirin in patients with hypertension; principal results of the Hypertension Optimal Treatment (HOT) randomized trialLancet1998351175517629635947
- LindholmLHIbsenHDalhofBfor the LIFE Study groupCardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomized trial against atenololLancet20023591004101011937179
- UK Prospective Diabetes Study GroupTight blood pressure control and risk of macrovascular complications in type 2 diabetes: UKPDS 38BMJ19983177037119732337
- HolmanRTurnerRStrattonIUK Prospective Diabetes Study GroupEfficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39BMJ19983177137209732338
- LewisEJHunsickerLGClarkeWRfor the collaborative study groupRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med200134585186011565517
- BristowMRBeta-adrenergic receptor blockade in chronic heart failureCirculation200010155856910662755
- The CURE Study InvestigatorsThe Clopidogrel in Unstable Angina to prevent Recurrent Events Study. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial InvestigatorsN Engl J Med200134549450211519503
- Falcão-PiresIGonçalvesNGavinaCCorrelation between plasma levels of apelin and myocardial hypertrophy in rats and humans: possible target for treatmentExpert Opin Ther Targets20101423124120055692
- SavvatisKWestermannDSchultheissHPTschöpeCKinins in cardiac inflammation and regeneration: insights from ischemic and diabetic cardiomyopathyNeuropeptides201044211912520036002
- SulaimanMMattaMJSundaresanNRResveratrol, an activator of SIRT1 up-regulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathyAm J Physiol Heart Circ Physiol2010298H83384320008278
- KohdaYKanematsuMKonoTTerasakiFTanakaTProtein O-glycosylation induces collagen expression and contributes to diabetic cardiomyopathy in rat cardiac fibroblastsJ Pharmacol Sci200911144645019966509
- Aydemir-KoksoyABilginogluASariahmetogluMSchulzRTuranBAntioxidant treatment protects diabetic rats from cardiac dysfunction by preserving contractile protein targets of oxidative stressJ Nutr Biochem200910.1016/j.jnutbio.2009.06.006
- LiCJZhangQMLiMZZhangJYYuPYuDMAttenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathyChin Med J (Engl)20091222580258619951573
- HottaHMiuraTMikiTAngiotensin II type 1 receptor-mediated upregulation of calcineurin activity underlies impairment of cardio-protective signaling in diabetic heartsCirc Res201010612923219910577
- WangMZhangWBZhuJHFuGSZhouBQBreviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca(2+)-cycling proteins in streptozotocin-induced diabetic ratsActa Diabetol200910.1007/s00592-009-0164-x
- WangMZhangWBZhuJHFuGSZhouBQBreviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathwayActa Pharmacol Sin20093081081109119597526
- ConnellyKAKellyDJZhangYInhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathyCirc Heart Fail2009212913719808328
- ChaoPCHsuCCYinMCAnti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic miceNutr Metab (Lond)200963319678956
- XiSZhouGZhangXZhangWCaiLZhaoCProtective effect of total aralosides of Aralia elata (Miq) Seem (TASAES) against diabetic cardiomyopathy in rats during the early stage, and possible mechanismsExp Mol Med20094153854719381071
- SchneiderRWeltKAustWLösterHFitzlGCardiac ischemia and reperfusion in spontaneously diabetic rats with and without application of EGb 761: II. Interstitium and microvasculatureHistol Histopathol20092458759819283667
- SchneiderRWeltKAustWLösterHFitzlGCardiac ischemia and reperfusion in spontaneously diabetic rats with and without application of EGb 761: I. cardiomyocytesHistol Histopathol20082380781718437679
- ChengMGaoHQXuLLiBYZhangHLiXHCardioprotective effects of grape seed proanthocyanidins extracts in streptozocin induced diabetic ratsJ Cardiovasc Pharmacol20075050350918030059
- ShushakovaNParkJKMenneJFliserDChronic erythropoietin treatment affects different molecular pathways of diabetic cardiomyopathy in mouseEur J Clin Invest20093975576019614950
- ChachquesJCCellular cardiac regenerative therapy in which patientsExpert Rev Cardiovasc Ther2009791191919673669
- BoudinaSAbelEDDiabetic cardiomyopathy revisitedCirculation20071153213322317592090
- AnejaATangWHBansilalSGarciaMJFarkouhMEDiabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic optionsAm J Med200812174875718724960
- KhavandiKKhavandiAAsgharODiabetic cardiomyopathy – a distinct diseaseBest Pract Res Clin Endocrinol Metab20092334736019520308
- AsgharOAl-SunniAKhavandiKDiabetic cardiomyopathyClin Sci (Lond)200911674176019364331