Abstract
Biological therapies, such as monoclonal antibodies (mAbs) that target tumor-associated antigens have been considered an effective therapeutic approach in oncology. In considering Notch-1 receptor as a potential target, we performed immunohistochemistry on tissue microarrays to determine 1) whether the receptor is overexpressed in tumor cells as compared to their corresponding normal tissues and 2) the clinical significance of its expression levels in human breast, colorectal, lung and prostate cancers. We found that the expression of Notch-1 protein was overexpressed in primary colorectal adenocarcinoma and nonsmall cell lung carcinoma (NSCLC), but not in primary ductal breast carcinoma or prostate adenocarcinoma. Further analysis revealed that higher levels of Notch-1 protein expression were significantly associated with poorer differentiation of breast and prostate tumors. Strikingly, for NSCLC, the expression levels of Notch-1 protein were found to be inversely correlated with tumor differentiation and progression. For colorectal tumors, however, no correlation of Notch-1 protein expression was found with any tumor clinicopathological parameters, in spite of its overexpression in tumor cells. Our data demonstrated the complexity of Notch-1 protein expression in human solid tumors and further supported the notion that the roles of Notch-1 expression in tumorigenesis are highly context-dependent. The findings could provide the basis for development of distinct therapeutic strategies of Notch-1 mAbs for its applications in the treatment of suitable types of human cancers.
Introduction
The Notch family of receptors plays a crucial role in cell self-renewal, cell fate determination, cell division and apoptosis. Mammals have four Notch receptors (Notch-1, -2, -3, and -4) for five Notch ligands (DLL1, 3, 4 and Jagged 1, 2). The Notch receptors differ in the number of epidermal growth factor-like repeats present and the length of the intracellular domain. Notch signaling has also been shown to play a role in the regulation of remodeling of the vascular network.Citation1–Citation3 Tumor development is a cellular process that is partly driven by alterations in cell fate. Deregulated expression of Notch receptors and ligands is observed in a variety of solid tumors, including cervical, skin, pancreatic, ovarian, lung, prostate, and breast carcinomas.Citation4–Citation15 High-level expression of Notch-1 and Jagged-1 mRNAs is associated with both poor prognosis in breast cancer and metastasis in prostate cancer.Citation11–Citation13 Hodgkin’s lymphomas, anaplastic large-cell non-Hodgkin’s lymphomas, and some acute myeloid leukemias also show deregulated expression of Notch receptors or ligands.Citation16–Citation20 Interestingly, recent data suggests that Notch-1 is downregulated in late stage human papilloma virus-infected tumors.Citation21 Similar observations are also documented in small cell lung cancer and prostate adenocarcinomas.Citation14,Citation22 Therefore, the exact role of Notch signaling during tumorigenesis of various cancers remains controversial.
Targeted therapies have become a major focus for cancer research. Since the clinical application of imatinibCitation23 for the treatment of chronic myeloid leukemia, biological therapies that target tumor-associated antigens have raised hopes for improved survival outcomes in many cancers.Citation24 Many tumor antigens recognized by monoclonal antibodies (mAbs) are expressed not only by malignant cells, but also by normal cells.Citation24,Citation25 Therefore, the best target for a therapeutic antigen is one that is highly expressed by tumor cells while only minimally expressed by normal cells from which tumors arise, such as the epithelial cells. For instance, the tyrosine-kinase receptor HER-2 is strongly expressed (determined by immunohistochemical analysis) in 30% of diagnosed breast cancer patients, while only weakly expressed in the luminal epithelial cells of normal breast tissue determined by immunohistochemical analysis.Citation26 This overexpression is associated with an unfavorable prognosis of the breast cancer patients.Citation27 Breast cancer patients whose tumors overexpress the HER-2 receptor derive most clinical benefits from trastuzumab, a therapeutic mAb targeting the HER-2 receptor.Citation28
To assess whether the Notch-1 receptor is a candidate for the development of targeted therapeutic antibodies, we sought to systemically investigate the expression profiles of the Notch-1 protein in tumor cells and its correlation with the cancer patient’s prognosis.
Materials and methods
Tissue microarrays (TMAs)
TMAs of four major types of tumors (breast, colorectum, lung, and prostate) and their corresponding normal tissues were purchased from multiple TMA suppliers including Accurate Chemical and Scientific Corp. (West Bury, NY, USA); Biochain Institute, Inc. (Hayward, CA, USA); US Biomax, Inc. (Rockville, MD, USA); BioVintage, Inc. (San Diego, CA, USA); and Imgenex Corp. (San Diego, CA, USA). We also obtained normal human organ TMA (Catalog # FDA993; US Biomax) with 33 types of organs taken from 3 normal human individuals including 30 types recommended by the US Food and Drug Administration.
Breast
The 408 breast tumor samples were obtained from 4 men and 404 women, ranging in age from 23 to 87 years (median at 48 years) at surgery. Histologically, there were 367 cases of invasive ductal carcinoma (90%), 29 cases of invasive lobular carcinoma (7%), and 11 cases of other tumor types (3%). There were a total of 79 normal breast samples from 1 man and 78 women, ranging in age from 18 to 71 years (median at 43 years). Thirty-three (42%) were cancer adjacent normal breast tissue and 46 (58%) were from noncancer individuals.
Colorectum
The 511 colorectal tumor samples were collected from 328 men and 183 women with median age at surgery of 58 years (ranging from 20 to 87 years). Overall, 462 patients (90%) had colon cancer and 49 (10%) had rectal cancer. The vast majority (499 cases) were histologically adenocarcinoma (98%), the remaining 12 cases (2%) being mucinous carcinomas. Additionally, 194 normal colorectal tissues were obtained from 132 men and 62 women with median age of 56 (ranging from 13 to 87 years); of which, 141 (73%) samples were cancer-adjacent normal colorectal tissues and 53 (27%) were colorectal tissues from noncancer individuals.
Lung
The 429 lung tumor samples were biopsied from 311 men and 118 women ranging from 28 to 89 years (median at 59 years) at the time of surgery. The most common histological type was nonsmall cell lung cancer (NSCLC) (92%, 395 samples), including 195 adenocarcinoma (49%) and 200 squamous cell carcinoma (51%). The remaining 34 cases were small cell lung carcinoma (SCLC, 8%). There were 68 normal lung tissues collected from 53 men and 15 women ranging from 21 to 81 years (median at 58 years); of which 58 (85%) samples were cancer adjacent normal lung tissues and 10 (15%) were noncancer individual lung tissues.
Prostate
The age of 227 prostate cancer patients ranged from 20 to 89 years (median age of 66 years). Almost all tumors (225) were adenocarcinma (99%), with only 2 tumors being transitional cell carcinoma (1%). The 128 normal prostate tissues were made up of 66 (52%) cancer adjacent normal prostate tissues and 62 (48%) prostate tissues from noncancer men. Their ages spanned from 19 to 86 yeas with median age of 64 years.
Generation of Flp-In™ T-REx™ 293 cell line stably expressing human Notch-1
The full-length Notch-1 coding region tagged with C-terminal end FLAG peptide was chemically synthesized at DNA 2.0 (Menlo Park, CA, USA). Flanking sequences contained 5′ HindIII and 3′ NotI which were used for directional restriction cloning into pcDNA™ 5/FRT/TO (Invitrogen Corporation, Carlsbad, CA, USA) expression vector. Flp-In™ T-REx™ 293 cells (Invitrogen) contain a single genomic FRT (Flp [Flippase] recombinase target) site and express the Tet repressor which allows for the construction of isogenic recombinants under tetracycline control. Tetracycline-inducible stable expression cell lines were generated by cotransfecting pcDNA5/FRT/TO-HN1 +FLAG and pOG44 (Invitrogen), a plasmid encoding FLP recombinase, in a 1:9 ratio into Flp-In 293 T-REx using FuGENE™ 6 (Roche Diagnostics Corporation, Indianapolis, IN, USA) transfection reagent as per manufacturer’s recommendations. Cell lines with stably integrated cDNAs were then selected with hygromycin B (100 μg/mL) (Mediatech, Inc., Manassas, VA, USA) and were expanded. Expression levels of the Notch proteins were assessed by immunohistochemical (IHC) staining, Western blotting, and flow cytometry.
Paraffin embedment of Notch-1-transfected cells
Actively growing Notch-1 stably transfected Flp-In™ T-REx™-293 cells were induced by doxycycline (2 μg/mL) for 48 hours and harvested through trypsinization. The cell pellets were then fixed in 10% neutral buffered formalin (ThermoFisher, Pittsburgh, PA, USA) for 16–24 hours. The cells were processed with 70% ethanol, 95% ethanol, 100% ethanol, xylenes, and paraffin in a tissue processor (Sakura, Torrance, CA, USA). The cells were then embedded in paraffin, cut into 5 μm sections, and mounted onto Superfrost® Plus glass slides (ThermoFisher).
Western blotting
Cell lysates from full-length Notch-1-transfected Flp-In™ T-REx™-293 cells, with or without induction of doxycycline (2 μg/mL) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 3%–8% Tris-Acetate gel (Invitrogen) and transferred to nitrocellulose membrane using the iBlot system (Invitrogen). The blots were blocked overnight (16–20 hours) at 4°C in a 1% bovine serum albumin-containing 1X Tris-Tween buffered saline (TTBS) solution. After two rinses with 1X TTBS, the blots were incubated with rabbit anti-Notch-1 polyclonal antibody (Cat. # sc-6014-R; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at a concentration of 1 μg/mL for 2 hours. After six 5-minute washes in 1X TTBS, the blots were incubated with goat-anti-rabbit-HRP (R&D Systems, Inc., Minneapolis, MN, USA) at a 1:2000 dilution for 1 hour, followed by six more 5-minute washes with 1X TTBS. Antibody binding was detected by an enhanced chemiluminescence kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Immunohistochemistry
Paraffin embedded cell or tissue sections were deparaffinized by incubation at 60°C overnight followed by immersion in two changes of xylene (ThermoFisher) and two changes of absolute ethanol (Pharmco Products Inc., Brookfield, CT, USA), and rehydrated in distilled water. Antigen retrieval was performed in a calibrated steam pressure cooker (Decloaking Chamber, BioCare Medical, Walnut Creek, CA, USA) for 30 seconds at 125°C in 1X Target Retrieval Solution (Dako-Cytomation, Carpinteria, CA, USA). All additional steps (unless specified) were done at room temperature in a hydrated chamber. Endogenous peroxidase was blocked with peroxidase block from an EnVision™ + Rabbit Kit (DakoCytomation) for 10 minutes. The slides were also immersed in 5% nonfat dry milk for 30 minutes to minimize nonspecific binding due to hydrophobic interaction. The slides were then incubated with the rabbit anti-Notch-1 polyclonal antibody overnight at 4°C followed by labeled polymer from an EnVision + Rabbit Kit for 30 minutes. The slides were developed with diaminobenzidine and hydrogen peroxidase for 3 minutes and counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO, USA).
Scoring system and statistical analysis
The semi-quantitative method of staining intensity of Notch-1 protein on normal and tumor cells was manually scored as negative (–, no staining), weak (+), moderate (++), and strong (+++) by two of the investigators (YL and JB) according to a prespecified intensity scale, with discrepancies agreed upon by consensus. Representative examples for the different scores are provided in .
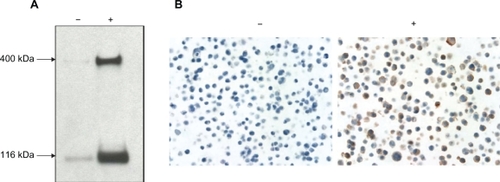
Figure 1 Specificity of the anti-Notch-1 antibody. A) Western blots. The cell lysates from full-length Notch-1-transfected Flp-In™ · T-REx™ 293 cells, both doxycycline (DOX)-uninduced (−) and induced (+) were used to test the specificity of the rabbit anti-Notch-1 antibody. The cross-reactive protein of Notch-1 transmembrane protein of full-length (300 KD) and truncated (120 KD) forms were visualized by enhanced chemiluminescence. B) Immunocytochemistry. Paraffin sections of DOX-uninduced (−) and induced (+) Notch-1-transfected Flp-In T-REx-293 cells were stained with antibody showing the membranous and cytoplasmic staining of Notch-1 in DOX-induced cells.
The chi-square test was used to determine the differentiation of Notch-1 expression between cancer patients and normal population. It was also used in analyzing the clinicopathological correlations for parameters with two categories. When evaluating their associations to tumor stage and grade, a chi-square test for trend is utilized. A P-value of less than 0.05 was considered to indicate statistical significance. All tests were performed using GraphPad Prism (version 5.01 for Windows; GraphPad Software, San Diego, CA, USA).
Results
Characterization of Notch-1 antibody
We first tested the specificity of commercially available Notch-1 antibody on Western blots containing whole cell protein extracts from Notch-1-transfected Flp-In™ T-REx™-293 cells with or without doxycycline induction. Immunostaining with the polyclonal rabbit antibody against the cytoplasmic domain of Notch-1 revealed a strong cross-reactive protein of the expected size of Notch-1 receptor, especially in the cell protein extracts from the cells stimulated with doxycycline (). The specificity of the antibody was further confirmed by immunohistochemical staining on the cells embedded in paraffin, showing membranous as well as cytoplasmic staining of Notch-1 receptor on the transfected Flp-In™ T-REx™-293 cells induced by doxycycline ().
Expression of Notch-1 protein was significantly upregulated in primary colorectal and lung but not in breast or prostate tumors
To determine whether Notch-1 protein is overexpressed in breast, colorectal, lung, and prostate tumors in comparison with their corresponding normal tissues, immunohistochemistry was performed on a number of tissue microarrays consisting of a large number of tumor and corresponding normal tissues. We compared the expression profiles of Notch-1 protein between tumor cells and the luminal epithelial cells of adjacent normal tissues to see whether the receptor was overexpressed in tumor cells. We found that Notch-1 protein was detected in the majority of breast tumors (largely consisted of ductal breast carcinomas) and normal breast tissues (ductal epithelial cells) ( and ). However, there was no difference in Notch-1 protein expression levels between breast tumor and normal tissues (P = 0.2896). Unlike breast tumors, the overall expression of Notch-1 protein was significantly higher in colorectal adenocarcinoma as compared to the normal colorectal epithelial cells (P = 0.0005). Noticeably, there were 61 (12%) of colorectal adenocarcinoma versus 6 (3%) of normal colorectum with moderate to strong expression of Notch-1 protein (). Similarly, the overall Notch-1 protein expression was significantly higher in primary lung tumors (consisting mainly of NSCLC) than in normal lung tissues (bronchial luminal epithelial cells) (). Notch-1 protein was detected in 74% of lung tumors while only in 47% of normal lung tissues. In addition, more lung tumor tissues showed stronger reactivity (moderate staining intensity) to Notch-1 protein than normal lung tissues did (12% vs 0%). Notch-1 protein expression was also detectable in the majority of prostate adenocarcinoma and adjacent normal prostate tissues. However, there was no significant up-regulation of Notch-1 protein in prostate tumors (P =0.1309; ).
Figure 2 Representative images of Notch-1 protein expression in breast, colorectal and lung tumors and their adjacent noncancer lung tissues. Negative: no staining; weak: weak staining; moderate: moderate staining. Original magnification, 400X.
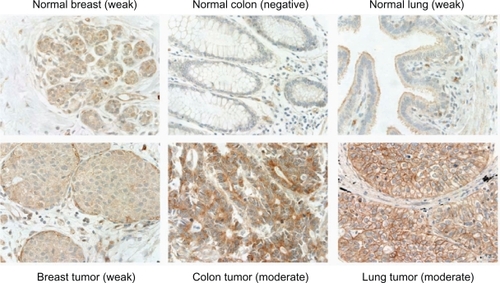
Table 1 Expression of Notch-1 in primary tumors and corresponding noncancer tissues
Notch-1 protein was highly overexpressed in squamous cell lung carcinoma of NSCLC
Among the lung cancer samples available for this study, we found that the most common histological type was NSCLC (92% of 429 samples), consisting of 195 adenocarcinoma and 200 squamous cell carcinoma. The remaining 34 samples were SCLC (8% of 429 samples). SCLC showed higher number of negative samples than normal samples (68% in SCLC vs 53% in normal tissues), suggesting that SCLC patients might not benefit from Notch-1 antibody-based therapies. In contrast, we found remarkably that 77% of NSCLC compared to 47% of normal tissues and only 32% of SCLC were positive for Notch-1 protein (P < 0.001; ). Moreover, within the NSCLC tumor samples, higher expression of Notch-1 protein was recorded in 22% of the squamous cell carcinoma group but not the adenocarcinoma group that showed only 3% of the samples displaying stronger reactivity. The difference was highly statistically significant (P < 0.0001; ).
Figure 3 Significant overexpression of Notch-1 protein in lung tumors. Notch-1 protein was overexpressed in lung tumors (mainly in nonsmall cell lung carcinoma [NSCLC] but not small cell lung carcinoma [SCLC]). The overexpression was predominantly observed in squamous cell carcinoma (SCC), but not in adenocarcinoma (AC).
![Figure 3 Significant overexpression of Notch-1 protein in lung tumors. Notch-1 protein was overexpressed in lung tumors (mainly in nonsmall cell lung carcinoma [NSCLC] but not small cell lung carcinoma [SCLC]). The overexpression was predominantly observed in squamous cell carcinoma (SCC), but not in adenocarcinoma (AC).](/cms/asset/a6fbfb92-9d3f-420c-b187-4e1f3db3088e/dbtt_a_11021_f0003_c.jpg)
Correlation of Notch-1 expression levels with tumor clinicopathological parameters
To understand the clinical significance of Notch-1 protein expression in tumors, we sought to determine whether the levels of Notch-1 protein expression correlate with tumor clinicopathological parameters. We categorized the staining intensities into high and low expression groups. High expression group represented strong and/or moderate staining and low expression group represented weak and/or negative staining. The expression profiles of Notch-1 protein in tumors were directly analyzed with these parameters to see whether there was a correlation. For breast tumors, the higher levels of Notch-1 protein expression were significantly correlated with poorly-differentiated and estrogen receptor-positive breast tumors (P = 0.0067 and 0.0134, respectively). No statistical differences were observed with age, pathological stages, or progesterone receptor status (). For colorectal tumors, the levels of Notch-1 protein expression were not associated with age, gender, pathological stages or tumor grades. For lung tumors, the levels of Notch-1 protein expression were significantly higher in male patients than in female patients (P = 0.0526). Remarkably, the levels of Notch-1 protein expression were inversely correlated with tumor grades and pathological stages (). Lower levels of Notch-1 protein expression was significantly associated with poorly-differentiated tumors and later stages of tumors with more advanced local invasion, regional lymph node, and distal metastasis (P = 0.0047 and 0.0019, respectively; ). For prostate tumors, higher levels of Notch-1 protein expression were significantly associated with higher Gleason scores (P = 0.0081). No correlation was observed with age or pathological stages ().
Table 2 Correlation of expression levels of Notch-1 protein with tumor clinicopathological parameters
Notch-1 protein expression was widely detectable in endothelial cells of vasculature
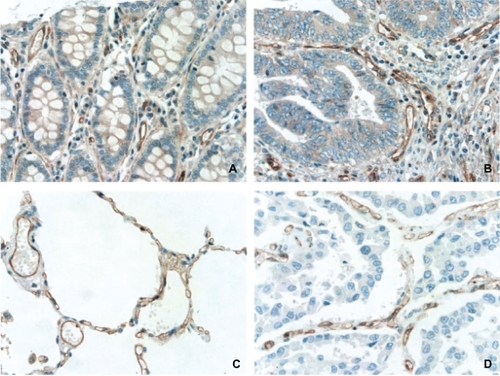
Notch signaling plays a role during vascular development as well as during maintenance of vessel homeostasis in the adult.Citation3 It was found that Notch-1, -3, and -4 are all expressed in arteries.Citation3 Endothelial cells express Notch-1 and -4 where as pericytes express Notch-1 and -3.Citation29 In the current study, we surveyed 33 types of human normal tissues/organs to determine the distribution pattern of Notch-1 protein expression. We found that Notch-1 protein expression was essentially detectable in all tissues, predominantly in vasculature, consistent with previous reports.Citation1,Citation2 Further analysis showed that Notch-1 protein was mainly expressed on the endothelial cells of vasculature as well as on capillaries, such as in lung tissue ( and ). Moreover, Notch-1 protein expression was widely detected on the endothelial cells of vasculature within tumors ( and ).
Discussion
Accumulating preclinical and clinical evidence supports a pro-oncogenic function for Notch signaling in several solid tumors, particularly in breast cancer.Citation30–Citation35 A high level of Notch-1 mRNA expression has been linked with a poorer prognosis for breast cancer patients.Citation11,Citation12 Notch-1 protein expression in breast cancer tissues generally exhibits a higher and more widespread levels of Notch-1 protein than in the noncancer breast tissue (luminal ductal epithelial cells).Citation11,Citation35,Citation36 However, these observations were largely based on the limited numbers of breast tumor and non-cancer breast samples. We compared the expression profiles of Notch-1 protein in a larger number of breast tumor and noncancer breast tissue samples (408 and 79, respectively). We were surprised to find that there was no evidence of overexpression of Notch-1 protein in breast tumor cells as compared to the luminal epithelial cells of noncancer breast tissue. However, further analysis of correlation between Notch-1 protein expression and tumor clinicopathological parameters revealed that the higher levels of Notch-1 protein expression was actually associated with poorly-differentiated tumors. Similarly, no overexpression of Notch-1 protein was found in prostate tumors, but higher levels of Notch-1 protein were significantly associated with prostate tumors with poorer prognosis. Our findings suggest that over-expression of Notch-1 protein is not essential for the onset and maintenance of breast or prostate cancers. Instead, the activation status of Notch-1 receptor may play a more important role. It is possible that the activation levels of Notch-1 receptor are higher in tumor cells than in the luminal epithelial cells of noncancer breast and prostate tissues. Further studies examining the protein expression levels of the downstream Notch signaling components, such as Hes-1, may be useful to verify this hypothesis.
Notch signaling components are widely distributed in adult gut tissues and Notch signaling is considered a gatekeeper of intestinal progenitor cells.Citation37–Citation39 It has been demonstrated that a highly conserved expression pattern exists between colon crypt cell progenitors and colorectal cancer cells, suggesting a role of Notch activation in proliferating adenoma cells.Citation40 This hypothesis was confirmed by a study in which treatment of multiple intestinal neoplasia mice carrying a heterozygous mutation of the APC tumor suppressor gene with γ-secretase inhibitors induces goblet cell differentiation and reduces proliferation in such adenoma.Citation41 However, the exact role of Notch-1 protein expression remains unclear in human colorectal cancer tumorigenesis. In the present study, we found that the Notch-1 protein was overexpressed in colorectal adenocarcinoma as compared to noncancer colorectal tissue. Unexpectedly, further analysis revealed that the expression levels of Notch-1 protein did not correlate with the tumor clinicopathological parameters used in this study, such as tumor grades and pathological stages. The findings suggest that in colorectal tumor cells, Notch-1 signaling is probably not physiologically activated, or that its activation has minimal impact on clinicopathological parameters. It is also possible that other Notch signaling components (such as Jagged-1, Jagged-2, or Notch-2 receptor) play more important roles in colorectal tumorigenesis.Citation38
The role of Notch signaling in human lung cancer still remains unclear although it has been shown that it plays an important role in fetal lung development by regulating airway epithelial development.Citation42 Notch-1 mutation in transgenic mice proved to be embryonic lethal at day 11.Citation43 Several in vitro studies have suggested that Notch-1 activation may play the tumor growth promotion and inhibition roles for NSCLC and SCLC tumorigenesis, respectively.Citation22,Citation44 Our data showed that Notch-1 protein was significantly overexpressed in NSCLC (mainly in squamous cell carcinoma) but only weakly expressed in SCLC, consistent with other’s observations.Citation42 However, we were surprised to find that the elevated levels of Notch-1 protein expression were associated with well-differentiated tumors as well as tumors with favorable prognosis (mainly in NSCLC). This inverse correlation suggests that like SCLC, high levels of Notch-1 protein expression may play a tumor growth inhibitive role in tumor differentiation, as well as metastasis of NSCLC.
Taken together, our data demonstrate the complexity of Notch-1 protein expression in four major types of tumors and further support the notion that the roles of Notch-1 expression in human solid tumor pathogenesis are highly context-dependent. These results, thus, may be able to assist in the design, development and selection of tumor types for targeting of Notch-1 therapeutic mAbs. For instance, the Notch-1 antagonist mAbs may be more suitable for breast and prostate cancers with high levels of Notch-1 protein expression. On the other hand, the agonist mAbs could be useful for the treatment of lung cancers in both NSCLC and SCLC. It is worth noting, that unlike DLL4 mAbs, Notch-1 mAbs may present some challenges for targeted therapies to diseased tissues as we, along with others,Citation1–Citation3,Citation45 observed a widespread expression of Notch-1 protein on endothelial cells of vasculatures in both tumor and noncancer tissues.
Acknowledgements
We would like to thank Timothy Toner for expert technical assistance on Western blots; Jon Stek for his careful language editing of the manuscript. Merck & Co., Inc. sponsored and funded this study.
Disclosure
Employees of the sponsor (indicated on the title page) may own stock or stock options in the company. The report was primarily drafted by Yuan Li and Zhi-Qiang Zhang. The sponsor formally reviewed a penultimate draft. All coauthors approved the final version of the manuscript.
References
- LindnerVBoothCPrudovskyISmallDMaciagTLiawLMembers of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interactionAm J Pathol200115987588311549580
- LiuZJShirakawaTLiYRegulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesisMol Cell Biol200323142512482957
- SullivanDCBicknellRNew molecular pathways in angiogenesisBr J Cancer20038922823112865906
- BuchlerPGazdharASchubertMThe Notch signaling pathway is related to neurovascular progression of pancreatic cancerAnn Surg200524279180016327489
- FanXMikolaenkoIElhassanINotch1 and Notch2 have opposite effects on embryonal brain tumor growthCancer Res2004647787779315520184
- HerlynMLiuZJPinnixCBalintKNotch signaling in angiogenesis and melanoma progressionClin Cancer Res200511S9170
- LiuZJXiaoMBalintKNotch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expressionCancer Res2006664182419016618740
- MassiDTarantiniFFranchiAEvidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanomaMod Pathol20061924625416341148
- NickoloffBJOsborneBAMieleLNotch signaling as a therapeutic target in cancer: A new approach to the development of cell fate modifying agentsOncogene2003226598660814528285
- ParkJTLiMNakayamaKNotch3 gene amplification in ovarian cancerCancer Res2006666312631816778208
- ParrCWatkinsGJiangWGThe possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancerInt J Mol Med20041477978615492845
- ReedijkMOdorcicSChangLHigh-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survivalCancer Res2005658530853716166334
- SantagataSDemichelisFRivaAJAGGED1 expression is associated with prostate cancer metastasis and recurrenceCancer Res2004646854685715466172
- ShouJRossSKoeppenHde SauvageFJGaoWQDynamics of notch expression during murine prostate development and tumorigenesisCancer Res2001617291729711585768
- ZagourasPStifaniSBlaumuellerCMCarcangiuMLArtavanis-TsakonasSAlterations in Notch signaling in neoplastic lesions of the human cervixProc Natl Acad Sci U S A199592641464187604005
- HoudeCLiYSongLOverexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell linesBlood20041043697370415292061
- JundtFAnagnostopoulosIForsterRMathasSSteinHDorkenBActivated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphomaBlood2002993398340311964309
- NefedovaYChengPAlsinaMDaltonWSGabrilovichDIInvolvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell linesBlood20041033503351014670925
- TohdaSNaraNExpression of Notch1 and Jagged1 proteins in acute myeloid leukemia cellsLeuk Lymphoma20014246747211699411
- WengAPFerrandoAALeeWActivating mutations of NOTCH1 in human T cell acute lymphoblastic leukemiaScience200430626927115472075
- TaloraCCialfiSSegattoOConstitutively active Notch1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathwaysExp Cell Res200530534335415817159
- SriuranpongVBorgesMWRaviRKNotch signaling induces cell cycle arrest in small cell lung cancer cellsCancer Res2001613200320511306509
- Imatinib [package insert]East Hanover, NJNovartis Pharmaceuticals Corporation2001
- HarrisMMonoclonal antibodies as therapeutic agents for cancerLancet Oncol2004529230215120666
- WeinbergRAThe Biology of CancerNew York, NYGarland Science, Taylor and Francis Group, LLC2007
- LebeauADeimlingDKaltzCHer-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridizationJ Clin Oncol20011935436311208826
- LebeauAHER2 testing in breast cancer: Opportunities and challengesBreast Care200616976
- SlamonDJLeyland-JonesBShakSUse of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med200134478379211248153
- ArmulikAAbramssonABetsholtzCEndothelial/pericyte interactionsCirc Res20059751252316166562
- CallahanRRaafatANotch signaling in mammary gland tumorigenesisJ Mammary Gland Biol Neoplasia20016233611467450
- DievartABeaulieuNJolicoeurPInvolvement of Notch1 in the development of mouse mammary tumorsOncogene1999185973598110557086
- KlinakisASzabolcsMPolitiKKiarisHArtavanis-TsakonasSEfstratiadisAMyc is a Notch1 transcriptional target and a requisite for Notch1-induced mammary tumorigenesis in miceProc Natl Acad Sci U S A20061039262926716751266
- RizzoPMiaoHSiziopikouKNotch signaling is altered in breast cancer and is a potential therapeutic targetBreast Cancer Res Treat200594119120
- RizzoPOsipoCForemanKGoldeTOsborneBMieleLRational targeting of Notch signaling in cancerOncogene2008275124513118758481
- WeijzenSRizzoPBraidMActivation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cellsNat Med2002897998612185362
- StylianouSClarkeRBBrennanKAberrant activation of notch signaling in human breast cancerCancer Res2006661517152516452208
- RadtkeFCleversHRiccioOFrom gut homeostasis to cancerCurr Mol Med2006627528916712475
- SanderGRPowellBCExpression of notch receptors and ligands in the adult gutJ Histochem Cytochem20045250951615034002
- WilsonARadtkeFMultiple functions of Notch signaling in self-renewing organs and cancerFEBS Lett20065802860286816574107
- van de WeteringMSanchoEVerweijCThe beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cellsCell200211124125012408868
- van EsJHvan GijnMERiccioONotch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cellsNature200543595996315959515
- CollinsBJKleebergerWBallDWNotch in lung development and lung cancerSemin Cancer Biol20041435736415288261
- MorimuraTGoitsukaRZhangYSaitoIRethMKitamuraDCell cycle arrest and apoptosis induced by Notch1 in B cellsJ Biol Chem2000275365233653110967117
- SriuranpongVBorgesMWStrockCLNotch signaling induces rapid degradation of achaete-scute homolog 1Mol Cell Biol2002223129313911940670
- RehmanAOWangCYNotch signaling in the regulation of tumor angiogenesisTrends Cell Biol20061629330016697642