Abstract
Recombinant human follicle stimulating hormone (rFSH) and luteinizing hormone (LH), also known as follitropin alpha and lutropin alpha, are manufactured by genetic engineering techniques which ensure high quality and batch to batch consistency. Follitropin alpha can be used for controlled ovarian hyperstimulation in assisted reproduction, ovulation induction for WHO group I and II anovulatory infertility and in men with hypogonadotrophic hypogonadism (HH) or idiopathic oligo-asthenospermia. Current evidence suggests superiority of urinary human menopausal gonadotropin (HMG) over follitropin alpha in controlled ovarian hyperstimulation for IVF in terms of live birth rate per couple. Addition of lutropin to follitropin alpha in an unselected IVF population does not appear to confer any benefit; however, it may have a role in ovulation induction in women with hypothalamic hypogonadism. Urinary HMG preparations (especially currently available highly purified preparations) are more cost effective than rFSH in terms of cost per ongoing pregnancy. However, women using rFSH injection pen devices have higher levels of satisfaction as compared to those using urinary HMG by means of conventional syringes.
Introduction
The pivotal role of the pituitary gland in reproductive function was established in the 20th century, when it became clear that it secreted two key hormones – follicle stimulating hormone (FSH) and luteinizing hormone (LH).Citation1,Citation2 This discovery allowed clinicians to treat infertile couples by means of pituitary extracts.Citation2,Citation3 Animal pituitary extracts of follicle stimulating hormone (FSH) were the first commercially available gonadotropins in the 1930s while the first use of cadaveric human gonadotropins for induction of ovulation was reported in 1958.Citation4,Citation5 Human pituitary gonadotropins (HPG) continued to be used in clinical practice for a number of decades before being withdrawn following reports of an association between its use and cases of Creutzfeld-Jakob disease (CJD).Citation5–Citation7 Meanwhile, increasing demands for gonadotropins, which could not be met from cadaver specimens, led to the extraction and isolation of human menopausal gonadotropin (HMG) from urine in 1950.Citation3 Human menopausal preparations contain both FSH and LH activity in a ratio of 1:1, though some of the LH activity was achieved by addition of human chorionic gonadotropin (HCG).Citation8,Citation9 Subsequently the development of advanced immunopurification and fractionation techniques using specific monoclonal antibodies led to the introduction of highly purified urinary preparations.Citation3,Citation5,Citation10,Citation11 More recently, use of genetic engineering technology led to the development of the recombinant human gonadotropins preparations.Citation12,Citation13 Follitropin alpha was the world’s first recombinant human FSH preparation and lutropin alpha the first recombinant human luteinizing hormone (LH).Citation14,Citation15 A mixture of follitropin alpha and lutropin alpha (follitropin alpha/lutropin alpha 150 IU/75 IU) has been recently commercially available in a single product called Pergoveris™ (Merck Serono).Citation16 Biopatency studies have confirmed that the new drug is treated in the body similarly as if each product were administered separately.Citation14,Citation16 This combination could be of value for the stimulation of follicular development in infertile women with severe endogenous FSH and LH deficiency, using a single daily injection.Citation17
Pharmacology
Structure
Follitropin alpha and lutropin alpha are glycoproteins which are structurally similar to endogenous FSH and LH. They possess similar alpha but different specific beta subunits.Citation18,Citation19 The nomenclature “alpha” differentiates it from another recombinant human FSH product which was marketed later as follitropin beta.Citation20
Isoforms
Endogenous gonadotropins exist in a number of different isoforms which have similar amino acid sequences but differ in their terminal silaic acid content.Citation21–Citation24 Different isoforms can vary in their biophysical characters; but their clinical roles have yet to be determined.Citation19,Citation25,Citation26 An isoform isolated at any particular time in the human body can be affected by gender, age, source of the sample, endocrine state and phase of menstrual cycle.Citation27–Citation29 Follitropin alpha is similar to the natural FSH isoform detected at mid cycle while Follitropin beta resembles that detected in the early follicular phase.Citation30 Recombinant FSH preparations differ from urinary HMG in their silaic acid content and have a shorter half life as they are more basic.Citation9,Citation31 Currently, lutropin alpha is the only commercially available recombinant LH preparation with a consistent isoform profile.Citation32
Biological and specific activity
Biological activity of an agent is related to its effect on living tissue while specific activity represents its activity per unit mass. Follitropin alpha has a specific activity of 10,000 IU/mg which is similar to the urinary highly purified urofollitropin but is 100 times higher than that of other urinary derived FSH products.Citation8,Citation33 Follitropin alpha has been shown to induce follicular growth on its own without the addition of LH in most cases.Citation34,Citation35 However, the role of LH for optimal follicular development has been recently described – especially in profoundly LH deficient women with hypogonadotrophic hypogonadism.Citation36,Citation37 Although a minimum level of serum LH is required for optimum growth, excess LH can cause follicular growth arrest and prevent growing follicles from reaching the late antral stage.Citation38,Citation39 Follitropin alpha administration has been associated with a significant increase in serum levels of estradiol level, inhibin A and inhibin B.Citation40,Citation41 Significant increases in follicular levels of insulin and growth hormone have been detected in follitropin stimulated women.Citation20
Pharmacokinetics
The pharmacokinetic properties of follitropin alpha and lutropin alpha are similar to those of urinary derived FSH and LH, respectively.Citation20,Citation42 Both are eliminated by means of the liver and kidney.Citation42,Citation43 Although subcutaneous (SC) administration is recommended, both products can also be administrated by the intramuscular route (IM).Citation7,Citation44 Subcutaneous administration had been found to produce shorter absorption half life and time to maximum plasma concentration. Following a single subcutaneous 150 IU dose, follitropin alpha has a terminal half-life of about 37 hours, bioavailability of 74%, mean peak serum drug concentration (Cmax) 3 IU/L and the time to maximum plasma concentration (tmax) was 16 hours.Citation45
Lutropin alpha has a one compartment first-order process.Citation42 Following subcutaneous administration of 150 IU lutropin alpha, a mean Cmax of 1.1 IU/L is reached after 6 hours (tmax).Citation42 Lutropin alpha has a terminal half-life of about 18 hours and a bioavailability of 56% (following a single subcutaneous 10,000 IU dose).Citation44
Manufacturing
Both follitropin alpha and lutropin alpha are manufactured by recombinant DNA technology.Citation12,Citation32 The gene encoded for the bio formation of each hormone is incorporated into a genetically engineered Chinese hamster cell line.Citation20,Citation46 The products of this cell line are then extracted and purified by means of a series of immunochromatograpic techniques.Citation46,Citation47 which help to maintain quality assurance and batch to batch consistency.Citation3,Citation48,Citation49 The current manufacturing process permits the follitropin alpha active ingredient to be quantified by its protein content (mass in μg); a technique called filled by mass (FbM) rather than the conventional method which relied on a product’s biological activity (bioassay).Citation50,Citation51 It has been suggested that the use of follitropin alpha filled by mass (FbM) may lead to more consistent ovarian stimulation, less need to dose adjustment and fewer cycle cancellations.Citation51,Citation52 The biological activity of lutropin alpha is determined by bioassay.Citation53
Safety
General
Clinical trials have shown that follitropin alpha and lutropin alpha are very well-tolerated by patients. Ovarian hyperstimulation syndrome and multiple pregnancy are the most serious side-effects linked to gonadotropin use.Citation54 Recombinant FSH is not believed to increase the risk of miscarriage.Citation55 No fetal effects had been reported after accidental first trimester exposure to follitropin alpha.Citation56 Headache, nausea, abdominal pain, breast pain, ovarian cyst formation are the most common side-effects of both follitropin alpha and lutropin alpha, while ovarian hyperstimulation is a serious, albeit rare side-effect.Citation57
Side effects have been reported in 46.5% of patients who used follitropin alpha alone and 42.4% women receiving follitropin alpha/lutropin alpha. These include headache, nausea, mastalgia, fatigue, abdominal pain and development of functional ovarian cysts.Citation6,Citation20,Citation33,Citation53,Citation57–Citation59
A case report has described subclavian deep vein thrombosis and mild ovarian hyperstimulation associated with treatment with recombinant FSH.Citation60 Bar et al have suggested diminished platelet aggregation in women using urinary FSH compared to rFSH.Citation61 Local skin reaction including mild irritation, pain, erythema and pruritus has been reported in 1.8% of a total of 1093 follitropin alpha injections.Citation62 Subcutaneous injection of lutropin alpha was not associated with any adverse local skin reactions in almost 90% of the injected cases,Citation36 only 3.4% women reported anything more than a mild skin reaction after SC injections with recombinant LH.Citation37 No antibodies to follitropin alpha have been discovered in women receiving any of these preparations.Citation33,Citation37,Citation57 There were two case reports describing two separate IVF cycles where follitropin alpha was used successfully in inducing follicular growth in the absence of any allergic reactions in two women with severe allergic reactions to urinary FSH.Citation63,Citation64 Data from randomized trials and case series suggest that follitropin alpha is associated with better local tolerance and fewer injection site side effects than follitropin beta.Citation65–Citation67
OHSS
Severe ovarian hyperstimulation syndrome (OHSS) is a serious and a life-threatening complication with an incidence of about 0.5% to 2%.Citation68,Citation69 Polycystic ovarian syndrome (PCOS), previous episodes of OHSS and high doses of exogenous gonadotropins are known to increase the risk of developing OHSS.Citation70–Citation72 There is some evidence that individual sensitivity to FSH stimulation may be more important than the total amount of gonadotropins used.Citation73 The incidence of OHSS in women in women using recombinant FSH in IVF treatment has been reported in two recent systematic reviews to range between 0% to 4.6% when rFSH was used for controlled ovarian hyperstimulation (COH) in IVF.Citation74–Citation76 The overall incidence was 2.6% after pooling results from 9 studies including a total of 1454 women in the rFSH group.Citation68 There was no difference in the rate of OHSS between women on rFSH versus urinary FSH.Citation77,Citation78
After pooling results from 4 randomized trials including 381 participants undergoing IVF in GnRH agonist down regulated cycles, the reported incidence of OHSS was 2.8% when lutropin alpha was co-administrated with rFSH.Citation79 There was no difference in the incidence of OHSS between women who received rLH plus rFSH and those who received rFSH alone.Citation79
Multiple pregnancy
Gonadotropin stimulation of the ovaries in assisted reproduction leads to multifollicular growth.Citation80,Citation81 Unlike IVF, where the number of embryos replaced determines the incidence of multiple gestations,Citation81 the release of more than one oocyte in ovulation induction or superovulation with IUI could potentially increase chances of multiple pregnancy.Citation82 Phase III trials of follitropin alpha show a multiple birth rate of 20% when the drug is used for ovulation induction and 35% when it is used in IVF.Citation53
A number of different strategies have been proposed to decrease the chance of multiple births after ovulation induction but their impact has been limited.Citation54 The two systematic reviews of trials comparing rFSH to urinary HMG in IVF showed no difference in multiple pregnancy rates between the two treatment groups.Citation77,Citation78
Clinical efficacy
FSH and LH have significant roles in ovarian follicle differentiation, selection and survival.Citation83 Exogenous gonadotropin administration has been suggested as an effective means of treatment in WHO group I and II anovulation, and in males with hypogonadotrophic hypogonadism.Citation59,Citation84,Citation85 In normogonadotropic women, COH is an essential prerequisite for successful in vitro fertilization (IVF) treatment.Citation86 The aim in IVF is to stimulate multiple follicular growth in order to enhance the increase the yield of oocytes.Citation87 Superovulation is also used in conjunction with intrauterine insemination even in absence of evidence of anovulation, though the rationale for this intervention has been challenged recently.Citation88,Citation89
Different protocols for COH had been described. In IVF, these protocols usually involve pituitary suppression by GnRH agonists or antagonists.Citation90–Citation92 Variable long, short or ultrashort protocols for GnRH agonists have been suggested.Citation90,Citation93 The commonest is the long luteal phase protocol where GnRH agonists are started in the luteal phase of the cycle preceding the IVF cycle.Citation94,Citation95
Efficacy in IVF/ICSI
Follitropin alpha
Recently, two systematic reviews of randomized trials comparing recombinant FSH (rFSH) and urinary HMG (uHMG) in unselected subfertile women undergoing IVF/ICSI (intracytoplasmic sperm injection of eggs), have been published.Citation77,Citation78
In the first, results from a meta-analysis of 12 randomized controlled trials (RCTs), with a total of 2937 participants have shown an overall live birth rate of 21.8% in the rFSH group compared to 24.9% in the uHMG group.Citation77 The second systematic review included only 7 RCTs which compared rFSH and HMG in IVF cycles where a long pituitary down-regulation protocol was used.Citation78 The pooled results based on a total of 1259 women showed that the live birth rate per woman treated with rFSH was 21.6% compared to 25.4% in the HMG group ().Citation78
Figure 1 Meta-analysis of randomized trials of hMG versus rFSH following a long down-regulation protocol for the outcome of live births. Adapted with permission from Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van Wely M. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IV F or ICSI treatment: a systematic review and meta-analysis. Hum Reprod. 2008;23(2):310–315.Citation78 Copyright © 2008 Oxford University Press.
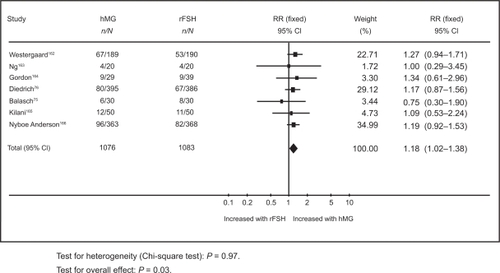
Al-Inany et alCitation77 found live birth rate per woman in the HMG group to be significantly higher than in the rFSH group (odds ratio [OR] 1.20, 95% confidence interval [CI] 1.01 to 1.42).Citation77 Coomarasamy et alCitation78 showed live birth rate per woman in the uHMG group to be significantly higher than the rFSH group (OR 1.18, 95% CI 1.02 to 1.38).Citation78 These results are based on a general population of women undergoing IVF. It has been suggested that specific groups of patients such as older women or poor responders might benefit from rFSH.Citation96,Citation97 However, there is a need for relevant evaluative studies in order to elucidate the role of rFSH in those women. Neither systematic review has shown any differences in rates of multiple pregnancy rates, ovarian hyperstimulation or miscarriage.Citation77,Citation78 Unlike Coomarasamy and his colleagues, Al-Inany et al reported a significant reduction in dose and duration of stimulation and available embryos in the rFSH group.Citation77
Traditional uHMG preparations contained FSH and LH in a ratio of 1:1, while more recent highly purified FSH (HP FSH) products using monoclonal antibody techniques for extraction and purification of FSH contain negligible amounts of LH (P < 0.001%).Citation8 In their systematic review,Citation77 Al-Inany et al examined the effect of the type of HMG (purified versus conventional) compared with rFSH on IVF outcome. They performed a subgroup analysis to compare HP HMG with rFSH and showed a similar outcomes in the HP-HMG group in terms of live birth rate (OR 1.21, 95% CI 1.02 to 1.44) and clinical pregnancy rate (OR 1.26, 95% CI 1.04 to 1.53).Citation77 Two recent RCTs, one using a long downregulation protocol along with a GnRH agonist and the other a GnRH antagonist, failed to demonstrate any significant difference in pregnancy rates between HPFSH and rFSH.Citation98,Citation99
Follitropin alpha and follitropin beta represent two isoforms of the same molecule.Citation20 Although some authors have suggested a difference in clinical efficacy between the two molecules,Citation13,Citation100 live birth rates and clinical pregnancy rates have been shown to be comparable in four randomized controlled trials.Citation65,Citation101–Citation103
Lutropin alpha in IVF
It has been reported that high LH levels in the follicular phase of the IVF cycle could have a detrimental effect on the outcome of IVFCitation104,Citation105 but a minimum threshold serum concentration of LH is required for optimum folliculogenesis.Citation38 According to Loumaye and colleagues, the effect of LH on the growing cohort of follicles demonstrates a ceiling effect and exceeding a certain threshold can compromise follicular development.Citation38
Results of a recent Cochrane review do not confirm an increase in live birth rates associated with the addition of rLH to rFSH in GnRH agonist downregulated IVF cycles compared to rFSH only stimulated cycles (two trials: OR 1.51, 95% CI 0.79 to 2.87).Citation79
Meta-analyses of RCTs where GnRH antagonists (rather than GnRH agonist) were used for pituitary suppression also failed to find any significant differences in terms of clinical pregnancy rates, as none of the studies included reported live birth.Citation79
There was no difference in the risk of early miscarriage between women on rFSH who were co-treated with rLH (eight trials: OR 0.59, 95% CI 0.35 to 1.02) compared to women who were treated with rFSH alone.Citation79 However, after exclusion of a single trial that used a flare up GnRH protocol, a trend towards reduced miscarriage rates (of borderline significance) was found in women co-treated rLH (seven trials: OR 0.57, 95% CI 0.33 to 1.00). There was a significant difference in live birth rate in favor of rLH supplementation in poor responders (three trials: OR 1.85, 95% CI 1.10 to 3.11). There were no differences in other IVF outcomes such as OHSS, number of oocytes retrieved, amount of rFSH used, serum estradiol level on the day of HCG administration and miscarriage rate.Citation79
These findings are in accordance with results from a previous meta-analysis of results from 4 RCTs examining the effect of adding rLH to rFSH in GnRH agonist down-regulated IVF cycles.Citation106
An RCT which included 84 participants found no significant difference in pregnancy rate between poor responders treated with either rFSH alone or rLH and FSH in an GnRH agonist flare up protocol.Citation107
In a systematic review where trials using GnRH agonists and antagonist cycles were pooled, live birth rates and clinical pregnancy rates were similar regardless of whetherrLH was co-administrated with rFSH or not.Citation108
Although some clinicians have reported that rLH administration prior to rFSH in IVF cycles increased the number of antral follicles, this did not translate into improved rates of live birth pregnancy.Citation109 Thus, there is no evidence at the present time that co-administration of rLH to rFSH, in controlled ovarian hyperstimulation for IVF, has a beneficial effect in IVF.
In the European Union, a combination of follitropin alpha and lutropin alpha (Pergoveris™) is currently available for single subcutaneous injection.Citation17 The ratio of follitropin alpha to lutropin alpha in that preparation is 2:1, respectively. A randomized crossover trial had demonstrated bioequivalence between follitropin alpha and lutropin alpha administered alone or in this fixed 2:1 combination.Citation110
Use of follitropin alpha in ovulation induction
Hypogonadotrophic hypogonadism
WHO group I anovulation is a rare condition that can be caused by a hypothalamic or pituitary defect due to congenital or acquired causes ().Citation111 Management options include exogenous replacement of gonadotropins and pulsatile GnRH agonist administration.Citation112 In women with intact pituitary function, pulsatile gonadotropin releasing hormone (GnRH) therapy can be used.Citation113 The advantages of pulsatile GnRH compared with gonadotropins are that there is a lower risk of hyperstimulation and multiple pregnancies and the need for monitoring is minimal.Citation36 Exogenous gonadotropins administration is the alternative therapeutic option in hypothalamic dysfunction and the first line treatment if the defect is primary pituitary failure.Citation113,Citation114
Table 1 Classification of disorders of ovulation
Currently available evidence indicates that rFSH alone may not be sufficient to promote optimum follicular growth in severely gonadotropin deficient women.Citation37 It has been suggested that a minimum threshold of serum LH is required to re-establish meiosis and final stages of growth of antral follicles. Meanwhile, follicular growth arrest might occur, should LH exceed that threshold, in what is called an LH ceiling. Antral follicle growth arrest (at 10 mm diameter) has been observed in LH deficient cycles.Citation38
A dose finding trial included 38 WHO type I anovulatory patients, who were randomly assigned to receive either 0, 25, 75, or 225 IU rLH once daily in addition to 150 IU follitropin alpha once daily for up to 20 days. None of the 8 patients who received follitropin alpha alone ovulated in the absence of rLH. Fourteen percent of patients who received follitropin alpha and 25 IU/L rLH ovulated compared to 66% and 80% of those who received 75 IU/L and 225%, respectively.Citation37 Significant dose dependent increases in the rate of optimal follicular growth were observed in women receiving follitropin alpha with different doses of rLH varying from 0 to 225 IU/day.Citation37,Citation84 Another randomized trial has shown significantly higher rates of optimum follicular growth in severely deficient LH women taking follitropin alpha plus lutropin alpha than those who were taking follitropin alpha with placebo.Citation115 A case series from Spain included 38 hypogonadotrophic anovulatory (WHO group I) women undergoing 84 ovulation induction cycles where patients received 150 IU/day rFSH and 75 IU/day rLH. Sufficient follicular growth was observed in 79 (94%) out of 84 initiated cycles. The 75 IU rLH dose was found to be effective in 94% of the treatment cycles.Citation36 The cumulative pregnancy rate following three cycles of stimulation with follitropin alpha and lutropin alpha was 39.5%.Citation36 Clinical pregnancy occurred in 16 out of the 31 women received lutropin alpha with follitropin alpha in an extension phase of the randomized trial published by O’Dea et al in 2000 on severely hypogonadotrophic women.Citation116 Two case reports documented pregnancies in 2 women with Kallman syndrome (amenorrhea, anosmia and hypogonadotrophic hypogonadism) and empty sella syndrome who received follitropin alpha and rLH.Citation117,Citation118
WHO group II anovulation
It had been estimated that 90% of women in women in WHO type II anovulation would be expected to have polycystic ovarian syndrome (PCOS).Citation119 According to the Rotterdam consensus criteria, PCOS should be considered when two of three features are diagnosed; ovarian dysfunction, features of hyperandrogenism (clinical or biochemical) and PCO morphology.Citation120 Although serum LH is not included as a diagnostic feature, the large majority of women with PCOS would have excess elevated LH concentrations when measured at the appropriate time.Citation121 This may justify the potential advantages in preparations devoid of LH activity as follitropin alpha. Currently, there is no role of lutropin alpha in the management of women with PCOS.
The first baby born after ovulation induction by follitropin in a clomiphene resistant PCOS patient was reported in 1992.Citation122 Randomized trials comparing follitropin alpha to other gonadotropins preparation or other ovulatory medications, in infertile women with WHO type II anovulation, have reported a live birth rate of 17% to 20%.Citation59,Citation123,Citation124 The rate of successful ovulation has been reported to be between 57% and 85%.Citation33,Citation125–Citation127 The pooled ovulation rate per cycle after rFSH in clomiphene citrate resistant PCOS women has been calculated to be 71% in a Cochrane review ().Citation128 Recent randomized trials have reported higher ovulation rates from 85% and up to 97% in this group of women.Citation59,Citation103,Citation127 with comparable clinical pregnancy rates per woman ranging from 17% to 20% after one cycle,Citation59,Citation103,Citation126 and a cumulative clinical pregnancy rate per woman of 42%.Citation128 A similar cumulative live birth rate of 43% was reported by a subsequent RCT.Citation129
Figure 2 Meta-analysis of randomized trials of hMG versus rFSH for the outcome of pregnancy rate per patient in women undergoing ovulation induction for subfertility associated with polycystic ovarian syndrome. Bayram N, van Wely M, van Der Veen F. Recombinant FSH versus urinary gonadotrophins or recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database Syst Rev. 2001;2(2):CD002121.Citation128 Copyright © Cochrane Collaboration, reproduced with permission.
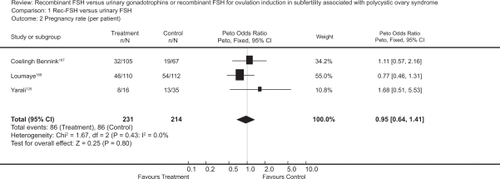
There were no significant differences in ovulation rates, pregnancy rates or live birth rates between follitropin alpha and highly purified FSHCitation59,Citation103,Citation125 However, a small RCT showed more favorable pregnancy rates with rFSH compared to urinary FSH.Citation130 Two protocols have been suggested for ovulation induction with gonadotropins in this group of patients.Citation131 In the step-up protocol the FSH dose is increased by ≤75 IU every 5 to 7 days, while in the low-dose regimen, it is administrated at a low dose for 14 days followed by small incremental dose increases (when necessary), at intervals not shorter than 7 days, until follicular development is initiated.Citation66,Citation112,Citation132 The type of the protocol, has not been shown to affect ovulation or pregnancy rates in studies using follitropin alpha.Citation59,Citation62 However, the low dose protocol significantly reduces the incidence of OHSS and multiple pregnancy.Citation69,Citation132
Male subfertility
FSH and LH are gonadotropins and have an important role in the process of spermatogenesis, though the actual mechanism of action is poorly understood.Citation133 LH may stimulate testosterone secretion from the Leydig cells of the testicle, while FSH stimulates Sertoli cells to facilitate germ cell differentiation.Citation134 Follitropin alpha alone or with human chorionic gonadotropin (HCG) had been used to improve sperm parameters in male factor infertility.Citation135 A Cochrane review included RCTs that compared pregnancy rates (spontaneous and after ART) following treatment of couples with idiopathic male factor infertility with urinary or recombinant gonadotropins (compared to placebo or no treatment), showed a significantly higher spontaneous pregnancy rate per couple randomized within three months of completing gonadotropin therapy (OR 4.17, 95% CI 1.30 to 7.09). However, there were only three trials with a total of 234 participants and the authors concluded that more studies were needed to confirm this finding.Citation133 The two RCTs included in this meta-analysis, where follitropin alpha was administered, showed no significant difference in pregnancy rates between groups which received rFSH injections compared to those which received placebo or no treatment.Citation136,Citation137 Treatment of azospermic men with rFSH for 10 months prior to ICSI may lead to detection of sperms in the ejaculate and spare these men surgical sperm retrieval procedures.Citation138 As age of the female partner is considered the single most important factor in predicting success of other interventions such as ICSI, the benefit of this relatively long period of treatment may need to be weighed up against the expected advancement in maternal age, especially in women above 35.
There are few data on the use of lutropin alpha in male factor infertility. Due to its structural similarity, purified HCG may be an effective substitute for LH as the two hormones act through the same Leydig cell receptor.Citation139 In normal men, a single IV injection of 150 IU lutropin induces a 25% rise in plasma testosterone levels by comparison with placebo.Citation140 We are not aware of any published randomized trials investigating the effect of lutropin alpha for male factor infertility.
In males with hypogonadotrophic hypogonadism presented by azospermia or sever oligoasthenoteratospermia, rFSH may be effective in achieving spermatogenesis when combined with HCG.Citation141–Citation144 Combined analysis of data from four clinical trials shows that HCG and rFSH induced spermatogenesis in 84% of men with hypogonadotrophic hypogonadism.Citation145 A number of baseline factors, including mean testicular volume, body mass index, age of disease onset and response to previous therapy, has been shown to influence the response.Citation145,Citation146
Patient satisfaction
Recombinant FSH can be used either as subcutaneous or intramuscular injection. Both follitropin alpha and beta are currently available in prefilled pen like devices for self injection. This delivery system has been shown to improve patient compliance and satisfaction.Citation82,Citation147,Citation148 A randomized trial comparing follitropin alpha in a pen device to the conventional syringe has shown that the former is associated with significantly higher rates of self-administration and satisfaction, with significantly less pain and local reactions at the injection site.Citation149 A questionnaire based study on ease-of-use, safety and efficacy of two follitropin injection pens found the follitropin alpha pen to be effective, well tolerated with higher patient and nurse acceptance than the follitropin beta pen.Citation150
Economic evaluation
A number of economic analyses comparing rFSH versus uHMG have been published.Citation151–Citation154 Two studies compared highly purified FSH with rFSH.Citation155,Citation156 Results from these studies, which were supported directly or indirectly by pharmaceutical companies, were conflicting. One of these analysesCitation156 was based on data from a large randomized trial comparing the use of HP HMG to rFSH in IVF treatment.Citation73 The results have shown urine-derived highly purified HMG to be a cost-effective alternative to follitropin alpha. The median cost per live birth was significantly lower in the HP HMG group than in the rFSH group (£8893 and £11741, respectively, P < 0.001).Citation156
An economic analysis based on data from a meta-analysis of 8 RCTs, comparing rFSH to uHMG, has estimated an average cost of an ongoing pregnancy at 13,946 Egyptian pounds (EGP) for a HMG cycle versus 18,721 (EGP) for a rFSH cycle.Citation157,Citation158 This economic analysis was based on the prices of rFSH and uHMG in the Egyptian market (150 Egyptian pounds for 75 IU rFSH, and 50 Egyptian pounds for 75 IU uHMG). The cost was calculated on the base of the fees charged by the authors’ IVF center. Al-Inany et al showed that a 60% reduction in the cost of rFSH would be needed in order to provide a cost per ongoing IVF pregnancy similar to that achieved with uHMG.Citation158 HMG use would result in 4565 more pregnancies in a hypothetical model based on 100,000 IVF cycles. Wechowski et alCitation156 estimated that the savings associated with HP-HMG (as opposed to rFSH) would fund one additional IVF cycle in every 10 cycles while Lloyd et alCitation155 projected a 13% increase in the number of cycles possible with the same budget.Citation155,Citation156
The use of uHMG for ovulation induction in anovulatory women can lead to 9.4% reduction in the cost per live birth.Citation124 Two separate economic analyses have demonstrated that uHMG is more cost-effective than rFSH in superovulation with IUI.Citation159,Citation160
Conclusion
Follitropin alpha and lutropin alpha are human rFSH and rLH, respectively. They are manufactured by genetic engineering techniques which ensure high quality and batch to batch consistency. Current evidence suggested superiority of uHMG over follitropin alpha in controlled ovarian hyperstimulation for IVF in terms of live birth rate per couple. Currently, there is no evidence to recommend the routine use of lutropin to follitropin alpha in an unselected IVF population. The use of follitropin alpha is comparable to HP FSH for ovulation induction in WHO group II anovulation. There is evidence that uHMG preparations (especially currently available highly purified preparations) are more cost effective than rFSH in terms of cost per ongoing pregnancy. However, patient satisfaction and quality of life in women using rFSH injection pen devices are higher than those using the conventional syringes for uHMG.
Disclosures
The authors declare no conflicts of interest.
References
- SmithMGOn the interruption of pregnancy in the rat by the injection of ovarian follicular extractBull Johns Hopkins Hosp192639420321412255683
- FevoldSLHisawFLLeonardSLThe gonad-stimulating and the luteinizing hormones of the anterior lobe of the hypophysisAm J Physiol193197291301
- LunenfeldBHistorical perspectives in gonadotrophin therapyHum Reprod Update200410645346715388674
- GemzellCADiczfalusyETillingerGClinical effect of human pituitary follicle-stimulating hormone (FSH)J Clin Endocrinol Metab195818121333134813611018
- LunenfeldBDevelopment of gonadotrophins for clinical useReprod Biomed Online20024Suppl111117
- DumbleLJKleinRDCreutzfeldt-Jakob legacy for Australian women treated with human pituitary gonadotropinsLancet199234088238478481357261
- CochiusJIBurnsRJBlumbergsPCMackKAldermanCPCreutzfeldt-Jakob disease in a recipient of human pituitary-derived gonadotrophinAust NZ J Med1990204592593
- StrowitzkiTBiotechnological drugs for reproductive disorders: a review of developmentsBioDrugs19978536037018020526
- GordonKNew developments in gonadotrophin pharmacologyReprod Biomed Online20025325926412470523
- GiudiceLCInsulin-like growth factor family in Graafian follicle development and functionJ Soc Gynecol Investig200181 Suppl ProceedingsS26S29
- WolfensonCGroismanJCoutoASBatch-to-batch consistency of human-derived gonadotrophin preparations compared with recombinant preparationsReprod Biomed Online200510444245415901450
- HowlesCMGenetic engineering of human FSH (Gonal-F)Hum Reprod Update1996221721919079412
- OlijveWde BoerWMuldersJWvan WezenbeekPMMolecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon)Mol Hum Reprod1996253713829238705
- AlperMMeyerRDekkersCEzcurraDSchertzJKellyEAssessment of the biopotency of follitropin alfa and lutropin alfa combined in one injection: a comparative trial in Sprague-Dawley ratsReprod Biol Endocrinol20086313618647398
- Saz-ParkinsonZLópez-CuadradoTBouzaCAmateJMOutcomes of new quality standards of follitropin alfa on ovarian stimulation: meta-analysis of previous studiesBioDrugs2009231374219344190
- AgostinettoRAdministration of follitropin alfa and lutropin alfa combined in a single injection: a feasibility assessmentReprod Biol Endocrinol20097485219450267
- http://www.emea.europa.eu/humandocs/PDFs/EPAR/pergoveris/H-714-en1.pdf
- PierceJGFaithMRGiudiceLCReeveJRStructure and structure-function relationships in glycoprotein hormonesCiba Found Symp197641225250780075
- GordonKPrinsMSchullerKLewinJOne million and countingReprod Biomed Online200613331331416984754
- GoaKLWagstaffAJFollitropin alpha in infertility: a reviewBioDrugs19989323526018020563
- WideLBakosOMore basic forms of both human follicle-stimulating hormone and luteinizing hormone in serum at midcycle compared with the follicular or luteal phaseJ Clin Endocrinol Metab19937648858898473400
- PapandreouMJAsteriaCPetterssonKRoninCBeck-PeccozPConcanavalin A affinity chromatography of human serum gonadotropins: evidence for changes of carbohydrate structure in different clinical conditionsJ Clin Endocrinol Metab1993764100810138473374
- GreenEDBaenzigerJUAsparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormonesJ Biol Chem1988263136443121612
- DahlKDStoneMPFSH isoforms, radioimmunoassays, bioassays, and their significanceJ Androl199213111221551802
- AndersenANDevroeyPArceJCClinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trialHum Reprod200621123217322716873892
- LambertARodgersMMitchellRIn-vitro biopotency and glycoform distribution of recombinant human follicle stimulating hormone (Org 32489), Metrodin and Metrodin-HPHum Reprod1995107192819358583012
- MitchellRBauerfeldCSchaeferFScharerKRobertsonWRLess acidic forms of luteinizing hormone are associated with lower testosterone secretion in men on haemodialysis treatmentClin Endocrinol (Oxf)199441165738050133
- ChappelSCHeterogeneity of follicle stimulating hormone: control and physiological functionHum Reprod Update199554794879080221
- ZambranoEOlivaresAMendezJPDynamics of basal and gonadotropin-releasing hormone-releasable serum follicle-stimulating hormone charge isoform distribution throughout the human menstrual cycleJ Clin Endocrinol Metab1995805164716567745013
- AnobileCJTalbotJAMcCannSJPadmanabhanVRobertsonWRGlycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal stateMol Hum Reprod1998476316399701785
- BishopLANguyenTVSchofieldPRBoth of the beta-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potencyEndocrinology19951366263526407750487
- DhillonSKeatingGMLutropin alfaDrugs200868111529154018627209
- LoumayeEEngrandPHowlesCMO’DeaLAssessment of the role of serum luteinizing hormone and estradiol response to follicle-stimulating hormone on in vitro fertilization treatment outcomeFertil Steril19976758898999130895
- WestonAMZelinski-WootenMBHutchisonJSStoufferRLWolfDPDevelopmental potential of embryos produced by in-vitro fertilization from gonadotrophin-releasing hormone antagonist-treated macaques stimulated with recombinant human follicle stimulating hormone alone or in combination with luteinizing hormoneHum Reprod19961136086138671277
- Zelinski-WootenMBHutchisonJSHessDLWolfDPStoufferRLFollicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeysHum Reprod1995107165816668582957
- BurguesSSpanish Collaborative Group on Female Hypogonadotrophic Hypogonadism. The effectiveness and safety of recombinant human LH to support follicular development induced by recombinant human FSH in WHO group I anovulation: evidence from a multicentre study in SpainHum Reprod200116122525253211726569
- Recombinant LH Study GroupDoes the addition of recombinant LH in WHO group II anovulatory women over-responding to FSH treatment reduce the number of developing follicles? A dose-finding studyHum Reprod200520362963515618252
- LoumayeEOvarian stimulation: is exogenous LH necessary in all patients?Gynecol Obstet Fertil2002301189089512476696
- LoumayeEEngrandPShohamZHillierSGBairdDTClinical evidence for an LH ceiling?Hum Reprod200318122719272014645197
- LockwoodGMMuttukrishnaSGroomeNPKnightPGLedgerWLCirculating inhibins and activin A during GnRH-analogue down-regulation and ovarian hyperstimulation with recombinant FSH for in-vitro fertilization-embryo transferClin Endocrinol (Oxf)19964567417489039341
- FischBAvrechOMPinkasHSuperovulation before IVF by recombinant versus urinary human FSH (combined with a long GnRH analog protocol): a comparative studyJ Assist Reprod Genet199512126317580006
- le CotonnecJYLoumayeEPorchetHCBeltramiVMunafoAPharmacokinetic and pharmacodynamic interactions between recombinant human luteinizing hormone and recombinant human follicle-stimulating hormoneFertil Steril19986922012099496329
- KarlssonMOWadeJRLoumayeEMunafoAThe population pharmacokinetics of recombinant- and urinary-human follicle stimulating hormone in womenBr J Clin Pharmacol199845113209489588
- le CotonnecJYPorchetHCBeltramiVMunafoAClinical pharmacology of recombinant human luteinizing hormone: Part I. Pharmacokinetics after intravenous administration to healthy female volunteers and comparison with urinary human luteinizing hormoneFertil Steril19986921891949496327
- le CotonnecJYPorchetHCBeltramiVKhanAToonSRowlandMClinical pharmacology of recombinant human follicle-stimulating hormone (FSH). I. Comparative pharmacokinetics with urinary human FSHFertil Steril19946146696788150109
- BaerGLoumayeEComparison of recombinant human luteinising hormone (r-hLH) and human menopausal gonadotropin (hMG) in assisted reproductive technologyCurr Med Res Opin2003192838812740151
- SieboldBPhysicochemical characterization of recombinant human follicle stimulating hormoneHum Reprod199611 Suppl11091115 discussion 117–1198671400
- DriebergenRBaerGQuantification of follicle stimulating hormone (follitropin alfa): is in vivo bioassay still relevant in the recombinant age?Curr Med Res Opin2003191414612661779
- LispiMBassettRCrisciCComparative assessment of the consistency and quality of a highly purified FSH extracted from human urine (urofollitropin) and a recombinant human FSH (follitropin alpha)Reprod Biomed Online200613217919316895630
- LassAMcVeighEUK Gonal-f FbM PMS GroupRoutine use of r-hFSH follitropin alfa filled-by-mass for follicular development for IVF: a large multicentre observational study in the UKReprod Biomed Online20049660461015670403
- WiklandMHuguesJNHowlesCImproving the consistency of ovarian stimulation: follitropin alfa filled-by-massReprod Biomed Online200612666366816792840
- KeckCBassettRLudwigMFactors influencing response to ovarian stimulationReprod Biomed Online200511556256916409704
- http://www.fda.gov/cder/foi/label/2004/21322/lbl.pdf
- DickeyRPStrategies to reduce multiple pregnancies due to ovulation stimulationFertil Steril200991111718973894
- Van LoonKSafety of high doses of recombinant human growth hormoneHorm Res199849# Suppl 27881
- HuguesJNRecombinant human follicle-stimulating hormone: a scientific step to clinical improvementReprod Biomed Online200121546412537825
- Recombinant Human FSH Study GroupClinical assessment of recombinant human follicle-stimulating hormone in stimulating ovarian follicular development before in vitro fertilizationFertil Steril199563177867805928
- BerghCHowlesCMBorgKRecombinant human follicle stimulating hormone (r-hFSH; Gonal-F) versus highly purified urinary FSH (Metrodin HP): results of a randomized comparative study in women undergoing assisted reproductive techniquesHum Reprod19971210213321399402268
- BalenAPlatteauPAndersenANDevroeyPHelmgaardLArceJCfor the Bravelle Ovulation Induction (BOI) Study GroupHighly purified FSH is as efficacious as recombinant FSH for ovulation induction in women with WHO Group II anovulatory infertility: a randomized controlled non-inferiority trialHum Reprod20072271816182317449511
- Loret de MolaJRKiwiRAustinCGoldfarbJMSubclavian deep vein thrombosis associated with the use of recombinant follicle-stimulating hormone (Gonal-F) complicating mild ovarian hyperstimulation syndromeFertil Steril20007361253125610917745
- BarJOrvietoRLahavJHodMKaplanBFischBEffect of urinary versus recombinant follicle-stimulating hormone on platelet function and other hemostatic variables in controlled ovarian hyperstimulationFertil Steril20048261564156915589861
- HedonBHuguesJNEmperaireJCA comparative prospective study of a chronic low dose versus a conventional ovulation stimulation regimen using recombinant human follicle stimulating hormone in anovulatory infertile womenHum Reprod19981310268826929804214
- HarrisonSWolfTAbuzeidMIAdministration of recombinant follicle stimulating hormone in a woman with allergic reaction to menotropin: a case reportGynecol Endocrinol200014314915210923273
- PhippsWRHoldenDSheehanRKUse of recombinant human follicle-stimulating hormone for in vitro fertilization-embryo transfer after severe systemic immunoglobulin E-mediated reaction to urofollitropinFertil Steril19966611481508752627
- BrinsdenPAkagbosuFGibbonsLMA comparison of the efficacy and tolerability of two recombinant human follicle-stimulating hormone preparations in patients undergoing in vitro fertilization-embryo transferFertil Steril200073111411610632423
- OrvietoRNahumRRabinsonJAshkenaziJAntebyEYMeltcerSFollitropin-alpha (Gonal-F) versus follitropin-beta (Puregon) in controlled ovarian hyperstimulation for in vitro fertilization: is there any difference?Fertil Steril2009914 Suppl1522152518851846
- FriedGHarlinJWramsbyHRecombinant FSH – clinical experience with two different preparationsFertil Steril199870Suppl 1S112
- EgbasePESevere OHSS: how many cases are preventable?Hum Reprod200015181010611179
- AboulgharMEversJHAl-InanyHIntravenous albumin for preventing severe ovarian hyperstimulation syndrome: a Cochrane reviewHum Reprod200217123027303212456597
- BuyalosRPLeeCTPolycystic ovary syndrome: pathophysiology and outcome with in vitro fertilizationFertil Steril19966511108557121
- MacDougallMJTanSLBalenAJacobsHSA controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilizationHum Reprod1993822332378473426
- FormanRGFrydmanREganDRossCBarlowDHSevere ovarian hyperstimulation syndrome using agonists of gonadotropin-releasing hormone for in vitro fertilization: a European series and a proposal for preventionFertil Steril19905335025092106456
- FauserBCVan HeusdenAMManipulation of human ovarian function: physiological concepts and clinical consequencesEndocr Rev1997181711069034787
- BalaschJFabreguesFPenarrubiaJOutcome from consecutive assisted reproduction cycles in patients treated with recombinant follitropin alfa filled-by-bioassay and those treated with recombinant follitropin alfa filled-by-massReprod Biomed Online20048440841315149563
- BalaschJPenarrubiaJFabreguesFOvarian responses to recombinant FSH or HMG in normogonadotrophic women following pituitary desensitization by a depot GnRH agonist for assisted reproductionReprod Biomed Online200371354212930572
- European and Israeli Study Group on Highly Purified Menotropin versus Recombinant Follicle-Stimulating HormoneEfficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trialFertil Steril200278352052812215327
- Al-InanyHGAbou-SettaAMAboulgharMAMansourRTSerourGIEfficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta-analysisReprod Biomed Online2008161818818252052
- CoomarasamyAAfnanMCheemaDvan der VeenFBossuytPMvan WelyMUrinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysisHum Reprod200823231031518056719
- MochtarMHVan derVZiechMvan WelyMRecombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cyclesCochrane Database Syst Rev20072CD00507017443569
- TimevaTMilachichTAntonovaIArabajiTShterevAOmarHACorrelation between number of retrieved oocytes and pregnancy rate after in vitro fertilization/intracytoplasmic sperm infectionScientific WorldJournal20066686690
- van RumsteMMCustersIMvan der VeenFvan WelyMEversJLMolBWThe influence of the number of follicles on pregnancy rates in intrauterine insemination with ovarian stimulation: a meta-analysisHum Reprod Update200814656357018687698
- SedbonEWainerRPervesCQuality of life of patients undergoing ovarian stimulation with injectable drugs in relation to medical practice in FranceReprod Biomed Online200612329830316569316
- PalermoRDifferential actions of FSH and LH during folliculogenesisReprod Biomed Online200715332633717854533
- O’DeaLO’BrienFCurrieKHemseyGFollicular development induced by recombinant luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: evidence of a threshold effectCurr Med Res Opin200824102785279318727841
- KlieschSBehreHMNieschlagERecombinant human follicle-stimulating hormone and human chorionic gonadotropin for induction of spermatogenesis in a hypogonadotropic maleFertil Steril1995636132613287750608
- ArslanAAGuYZeleniuch-JacquotteAReproducibility of serum pituitary hormones in womenCancer Epidemiol Biomarkers Prev20081781880188318708375
- DhontMOnghenaACoetsierTDe SutterPProspective randomized study of clomiphene citrate and gonadotrophins versus goserelin and gonadotrophins for follicular stimulation in assisted reproductionHum Reprod19951047917967650122
- SteuresPvan der SteegJWHompesPGIntra-uterine insemination with controlled ovarian hyperstimulation compared to an expectant management in couples with unexplained subfertility and an intermediate prognosis: a randomised studyNed Tijdschr Geneeskd2008152271525153118681363
- BensdorpAJCohlenBJHeinemanMJVandekerckhovePIntra-uterine insemination for male subfertilityCochrane Database Syst Rev200744CD00036017943739
- BarlowDHGnRH agonists and in vitro fertilizationJ Reprod Med1998433 Suppl2452519564657
- SbraciaMColabianchiJGiallonardoACetrorelix protocol versus gonadotropin-releasing hormone analog suppression long protocol for superovulation in intracytoplasmic sperm injection patients older than 40Fertil Steril20099151842184718501900
- CocciaMEComparettoCBraccoGLScarselliGGnRH antagonistsEur J Obstet Gynecol Reprod Biol2004115Suppl 1S44S5615196716
- KingslandCTanSLBickertonNMasonBCampbellSThe routine use of gonadotropin-releasing hormone agonists for all patients undergoing in vitro fertilization. Is there any medical advantage? A prospective randomized studyFertil Steril19925748048091555691
- BroekmansFJBernardusREBerkhoutGSchoemakerJPituitary and ovarian suppression after early follicular and mid-luteal administration of a LHRH agonist in a depot formulation: decapeptyl CRGynecol Endocrinol1992631531611442160
- AbdallaHIAhujaKKLeonardTMorrisNNHonourJWJacobsHSComparative trial of luteinizing hormone-releasing hormone analog/human menopausal gonadotropin and clomiphene citrate/human menopausal gonadotropin in an assisted conception programFertil Steril19905334734782106453
- De PlacidoGAlviggiCMolloAStrinaIVarricchioMTMolisMRecombinant follicle stimulating hormone is effective in poor responders to highly purified follicle stimulating hormoneHum Reprod2000151172010611181
- MohamedMASbraciaMPacchiarottiAUrinary follicle-stimulating hormone (FSH) is more effective than recombinant FSH in older women in a controlled randomized studyFertil Steril20068551398140316600226
- HompesPGBroekmansFJHoozemansDASchatsRFIRM groupEffectiveness of highly purified human menopausal gonadotropin vs recombinant follicle-stimulating hormone in first-cycle in vitro fertilization-intracytoplasmic sperm injection patientsFertil Steril20088961685169317681325
- BoschEVidalCLabartaESimonCRemohiJPellicerAHighly purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonists – a randomized studyHum Reprod200823102346235118583332
- ShohamZInslerVRecombinant technique and gonadotropins production: new era in reproductive medicineFertil Steril19966621872018690100
- TulppalaMAhoMTuuriTComparison of two recombinant follicle-stimulating hormone preparations in in-vitro fertilization: a randomized clinical studyHum Reprod199914112709271510548606
- HarlinJCsemiczkyGWramsbyHFriedGRecombinant follicle stimulating hormone in in-vitro fertilization treatment-clinical experience with follitropin alpha and follitropin betaHum Reprod200015223924410655291
- PlatteauPAndersenANBalenAMenopur Ovulation Induction (MOI) Study GroupSimilar ovulation rates, but different follicular development with highly purified menotrophin compared with recombinant FSH in WHO Group II anovulatory infertility: a randomized controlled studyHum Reprod20062171798180416571641
- KolibianakisEZikopoulosKAlbanoCReproductive outcome of polycystic ovarian syndrome patients treated with GnRH antagonists and recombinant FSH for IVF/ICSIReprod Biomed Online20037331331814653891
- WatsonHKiddyDSHamilton-FairleyDHypersecretion of luteinizing hormone and ovarian steroids in women with recurrent early miscarriageHum Reprod1993868298338345070
- OliveiraJBMauriALPetersenCGRecombinant luteinizing hormone supplementation to recombinant follicle-stimulation hormone during induced ovarian stimulation in the GnRH-agonist protocol: a meta-analysisJ Assist Reprod Genet2007242–3677517195098
- BarrenetxeaGAgirregoikoaJAJimenezMRde LarruzeaALGanzabalTCarboneroKOvarian response and pregnancy outcome in poor-responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cyclesFertil Steril200889354655317531989
- KolibianakisEMKalogeropoulouLGriesingerGAmong patients treated with FSH and GnRH analogues for in vitro fertilization, is the addition of recombinant LH associated with the probability of live birth? A systematic review and meta-analysisHum Reprod Update200713544545217586849
- DurnerinCIErbKFlemingRLuveris Pretreatment GroupEffects of recombinant LH treatment on folliculogenesis and responsiveness to FSH stimulationHum Reprod200823242142618084048
- PicardMRossierCPapasouliotisOLuganIBioequivalence of recombinant human FSH and recombinant human LH in a fixed 2:1 combination: two phase I, randomised, crossover studiesCurr Med Res Opin20082441199120818348746
- LachelinGCYenSSHypothalamic chronic anovulationAm J Obstet Gynecol19781307825831147631
- HomburgROvulation inductionExpert Opin Pharmacother20034111995200414596653
- FilicoriMFlamigniCCognigniGDellaiPMichelacciLArnoneRIncreased insulin secretion in patients with multifollicular and polycystic ovaries and its impact on ovulation inductionFertil Steril19946222792858034073
- The ESHRE Capri Workshop GroupAnovulatory infertilityHum Reprod1995106154915537593533
- ShohamZSmithHYekoTO’BrienFHemseyGO’DeaLRecombinant LH (lutropin alfa) for the treatment of hypogonadotrophic women with profound LH deficiency: a randomized, double-blind, placebo-controlled, proof-of-efficacy studyClin Endocrinol (Oxf)200869347147818485121
- KaufmannRDunnRVaughnTRecombinant human luteinizing hormone, lutropin alfa, for the induction of follicular development and pregnancy in profoundly gonadotrophin-deficient womenClin Endocrinol (Oxf)200767456356917692110
- KoustaEWhiteDMPiazziALoumayeEFranksSSuccessful induction ovulation and completed pregnancy using recombinant human luteinizing hormone and follicle stimulating hormone in a woman with Kallmann’s syndromeHum Reprod199611170718671160
- CampoSCampoVLanzoneATwin pregnancy using recombinant gonadotropins in a woman with hypogonadotropic hypogonadismGynecol Endocrinol2002161273211915578
- BroekmansFJFauserBCDiagnostic criteria for polycystic ovarian syndromeEndocrine200630131117185786
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop GroupRevised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndromeFertil Steril20048111925
- HendriksMLBrouwerJHompesPGHomburgRLambalkCBLH as a diagnostic criterion for polycystic ovary syndrome in patients with WHO II oligo/amenorrhoeaReprod Biomed Online200816676577118549684
- DonderwinkelPFSchootDCCoelingh BenninkHJFauserBCPregnancy after induction of ovulation with recombinant human FSH in polycystic ovary syndromeLancet199234088259831357394
- PlatteauPAndersenANBalenAOvulation Induction (MOI) Study Group. Similar ovulation rates, but different follicular development with highly purified menotrophin compared with recombinant FSH in WHO Group II anovulatory infertility: a randomized controlled studyHum Reprod20062171798180416571641
- RevelliAPosoFGennarelliGMoffaFGrassiGMassobrioMRecombinant versus highly-purified, urinary follicle-stimulating hormone (r-FSH vs HP-uFSH) in ovulation induction: a prospective, randomized study with cost-minimization analysisReprod Biol Endocrinol200643816848893
- YaraliHBukulmezOGurganTUrinary follicle-stimulating hormone (FSH) versus recombinant FSH in clomiphene citrate-resistant, normogonadotropic, chronic anovulation: a prospective randomized studyFertil Steril199972227628110438995
- YaraliHZeynelogluHBGonadotrophin treatment in patients with polycystic ovary syndromeReprod Biomed Online20048552853715151714
- FulghesuAMApaRBelosiCRecombinant versus urinary follicle-stimulating hormone in the low-dose regimen in anovulatory patients with polycystic ovary syndrome: a safer and more effective treatmentHorm Res200155522422811740143
- BayramNvan WelyMvan Der VeenFRecombinant FSH versus urinary gonadotrophins or recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndromeCochrane Database Syst Rev200122CD00212111406034
- LopezEGunbyJDayaSParrillaJJAbadLBalaschJOvulation induction in women with polycystic ovary syndrome: randomized trial of clomiphene citrate versus low-dose recombinant FSH as first line therapyReprod Biomed Online20049438239015511336
- SzilagyiABartfaiGManfaiAKoloszarSPalASzaboILow-dose ovulation induction with urinary gonadotropins or recombinant follicle stimulating hormone in patients with polycystic ovary syndromeGynecol Endocrinol2004181172215106360
- CristelloFCelaVArtiniPGGenazzaniARTherapeutic strategies for ovulation induction in infertile women with polycystic ovary syndromeGynecol Endocrinol200521634035216390783
- LeaderAMonofollicular Ovulation Induction Study GroupImproved monofollicular ovulation in anovulatory or oligo-ovulatory women after a low-dose step-up protocol with weekly increments of 25 international units of follicle-stimulating hormoneFertil Steril20068561766177316759926
- AttiaAMAl-InanyHGGonadotrophins for idiopathic male factor subfertilityCochrane Database Syst Rev200744CD00507117943837
- McLachlanRIThe endocrine control of spermatogenesisBaillieres Best Pract Res Clin Endocrinol Metab200014334536211097780
- MiyagawaYTsujimuraAMatsumiyaKTakaoTTohdaAKogaMOutcome of gonadotropin therapy for male hypogonadotropic hypogonadism at university affiliated male infertility centers: a 30-year retrospective studyJ Urol20051732072207515879837
- KamischkeABehreHMBergmannMSimoniMSchaferTNieschlagERecombinant human follicle stimulating hormone for treatment of male idiopathic infertility: a randomized, double-blind, placebo-controlled, clinical trialHum Reprod19981335966039572419
- ForestaCSeliceRFerlinAGarollaARecombinant FSH in the treatment of oligozoospermiaExpert Opin Biol Ther20099565966619379121
- BakirciogluMEErdenHFCirayHNBayazitNBahceciMGonadotrophin therapy in combination with ICSI in men with hypogonadotrophic hypogonadismReprod Biomed Online200715215616017697490
- LeeKHDiekmanMAMossGEAllrichRDPituitary gonadotropins, hypothalamic gonadotropin-releasing hormone, and testicular traits of boars exposed to natural or supplemental lighting during pubertal developmentBiol Reprod1987365116411693113500
- Cailleux-BounacerAReznikYCauliezBMenardJFDuparcCKuhnJMEvaluation of endocrine testing of Leydig cell function using extractive and recombinant human chorionic gonadotropin and different doses of recombinant human LH in normal menEur J Endocrinol2008159217117818495695
- BoulouxPWarneDWLoumayeEEfficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotrophic hypogonadismFertil Steril20027727027311821082
- LiuPYTurnerLRushfordDMcDonaldJBakerHWConwayAJEfficacy and safety of recombinant human follicle stimulating hormone (Gonal-F) with urinary human chorionic gonadotrophin for induction of spermatogenesis and fertility in gonadotrophin-deficient menHum Reprod1999141540154510357972
- MatsumotoAMSnyderPBhasinSMartinKWeberTWintersSStimulation of spermatogenesis with recombinant human follicle-stimulating hormone (follitropin alfa; GONAL-f) long-term treatment in azoospermic men with hypogonadotropic hypogonadism (HH)Fertil Steril2008 [Epub ahead of print]
- OkadaHon behalf of the Japanese MHH study groupRecombinant human follicle-stimulating hormone (r-hFSH, follitropin alfa) is efficacious for induction of spermatogenesis and pregnancy in Japanese men with hypogonadotropic hypogonadismFertil Steril200584Suppl 1S222
- WarneDOkadaHYanoYKoideNHowlesCA combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropinFertil SterilIn press2009
- LiuPYGebskiVJTurnerLConwayAJWishartSMHandelsmanDJPredicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile menHum Reprod20021762563311870114
- PangSKaplanBKarandeVFollistim Pen Trade Mark COH Study GroupAdministration of recombinant human FSH (solution in cartridge) with a pen device in women undergoing ovarian stimulationReprod Biomed Online20037331932614653893
- PangSCA pen injection device for self-administration of recombinant follicle-stimulating hormone for fertility treatmentsExpert Rev Med Devices200521273216293025
- AghssaMMAzargoonARamezanzadehFBagheriMA comparison of the efficacy, tolerability, and convenience of two formulations of follitropin-alpha in Iranian woman undergoing intracytoplasmic sperm injection cyclesFertil Steril20089041043104818053995
- PorterRKisselCSaundersHKeckCPatient and nurse evaluation of recombinant human follicle-stimulating hormone administration methods: comparison of two follitropin injection pensCurr Med Res Opin200824372773518230195
- DayaSLedgerWAurayJPCost-effectiveness modelling of recombinant FSH versus urinary FSH in assisted reproduction techniques in the UKHum Reprod200116122563256911726575
- HatoumHTKeyeWRJrMarrsRPWaltonSMMarshallDCA Markov model of the cost-effectiveness of human-derived follicle-stimulating hormone (FSH) versus recombinant FSH using comparative clinical trial dataFertil Steril200583380480715749525
- SykesDOutHJPalmerSJvan LoonJThe cost-effectiveness of IVF in the UK: a comparison of three gonadotrophin treatmentsHum Reprod200116122557256211726574
- ForestaCSeliceRFerlinAGarollaARecombinant FSH in the treatment of oligozoospermiaExpert Opin Biol Ther20099565966619379121
- LloydAKennedyRHutchinsonJSawyerWEconomic evaluation of highly purified menotropin compared with recombinant follicle-stimulating hormone in assisted reproductionFertil Steril20038051108111314607557
- WechowskiJConnollyMMcEwanPKennedyRAn economic evaluation of highly purified HMG and recombinant FSH based on a large randomized trialReprod Biomed Online200715550050618028739
- Al-InanyHAboulgharMAMansourRTProctorMRecombinant versus urinary gonadotrophins for triggering ovulation in assisted conceptionHum Reprod20052082061207316024539
- Al-InanyHGAbou-SettaAMAboulgharMAMansourRTSerourGIHMG versus rFSH for ovulation induction in developing countries: a cost-effectiveness analysis based on the results of a recent meta-analysisReprod Biomed Online200612216316916478578
- GerliSCasiniMLUnferVCostabileLBiniVDi RenzoGCRecombinant versus urinary follicle-stimulating hormone in intra-uterine insemination cycles: a prospective, randomized analysis of cost effectivenessFertil Steril200482357357815374698
- GerliSBiniVDi RenzoGCCost-effectiveness of recombinant follicle-stimulating hormone (FSH) versus human FSH in intrauterine insemination cycles: a statistical model-derived analysisGynecol Endocrinol2008241182318224540
- Shetty A Disorders of ovulationTempletonAAManagement of Infertility for the MRCOG and BeyondLondonRCOG press2001
- WestergaardLGErbKLaursenSBRexSRasmussenPEHuman menopausal gonadotropin versus recombinant follicle-stimulating hormone in normogonadotropic women down-regulated with a gonadotropin-releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized studyFertil Steril200176354354911532479
- NgEHLauEYYeungWSHoPCHMG is as good as recombinant human FSH in terms of oocyte and embryo quality: a prospective randomized trialHum Reprod200116231932511157827
- GordonUDHarrisonRFFawzyMHennellyBGordonACA randomized prospective assessor-blind evaluation of luteinizing hormone dosage and in vitro fertilization outcomeFertil Steril200175232433111172834
- KilaniZDakkakAGhunaimSA prospective, randomized, controlled trial comparing highly purified hMG with recombinant FSH in women undergoing ICSI: ovarian response and clinical outcomesHum Reprod20031861194119912773445
- Nyboe AndersenADevroeyPArceJ-Cfor the MERIT GroupClinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trialHum Reprod200621123217322716873892
- Coelingh BenninkHJFauserBCOutHJRecombinant follicle-stimulating hormone (FSH; Puregon) is more efficient than urinary FSH (Metrodin) in women with clomiphene citrate-resistant, normogonadotropic, chronic anovulation: a prospective, multicenter, assessor-blind, randomized, clinical trial. European Puregon Collaborative Anovulation Study GroupFertil Steril199869119259457926
- LoumayeEMartineauIPiazziAClinical assessment of human gonadotrophins produced by recombinant DNA technologyHum Reprod199611 Suppl195107 discussion 117–119