Abstract
Background:
Isolated fucoidans from brown marine algae have been shown to have a range of anti-inflammatory effects.
Purpose:
This present study tested a Maritech® extract formulation, containing a blend of extracts from three different species of brown algae, plus nutrients in an open label combined phase I and II pilot scale study to determine both acute safety and efficacy in osteoarthritis of the knee.
Patients and methods:
Participants (n = 12, five females [mean age, 62 ± 11.06 years] and seven males [mean age, 57.14 ± 9.20 years]) with a confirmed diagnosis of osteoarthritis of the knee were randomized to either 100 mg (n = 5) or 1000 mg (n = 7) of a Maritech® extract formulation per day. The formulation contained Maritech® seaweed extract containing Fucus vesiculosis (85% w/w), Macrocystis pyrifera (10% w/w) and Laminaria japonica (5% w/w) plus vitamin B6, zinc and manganese. Primary outcome was the average comprehensive arthritis test (COAT) score which is comprised of four sub-scales: pain, stiffness, difficulty with physical activity and overall symptom severity measured weekly. Safety measures included full blood count, serum lipids, liver function tests, urea, creatinine and electrolytes determined at baseline and week 12. All adverse events were recorded.
Results:
Eleven participants completed 12 weeks and one completed 10 weeks of the study. Using a multilevel linear model, the average COAT score was reduced by 18% for the 100 mg treatment and 52% for the 1000 mg dose at the end of the study. There was a clear dose response effect seen between the two treatments (P ≤ 0.0005) on the average COAT score and each of the four COAT subscales (pain, stiffness, difficulty with physical activity and overall symptom severity) (P ≤ 0.05). The preparation was well tolerated and the few adverse events were unlikely to be related to the study medication. There were no changes in blood parameters measured over the course of the study with the exception of an increase in serum albumin which was not clinically significant.
Conclusion:
The seaweed extract nutrient complex when taken orally over twelve weeks decreased the symptoms of osteoarthritis in a dose-dependent manner. It was demonstrated to be safe to use over the study period at the doses tested. The efficacy of the preparation now needs to be demonstrated in a phase III randomized controlled trial (RCT).
Australian and New Zealand Clinical Trials Register:
ACTRN12607000229471.
Introduction
Osteoarthritis (OA) is the most frequent cause of disability among adults in the developed world. Arthritis affects around three million people in Australia, representing about 15% of the population.Citation1 Similarly, more than 20 million people in the United States have the disease.Citation2 The lifetime risk of knee OA for males and females aged over 45 years in Johnston County is estimated at between 44.7% (nonobese) and 67% (obese) and these figures are believed to reflect the incidence of OA across the United States.Citation3 OA costs more than $60 billion per year in the USA and is second only to ischemic heart disease as a cause of work disability in men aged over 50 years.Citation4 The progressive deterioration of articular cartilage which occurs in OA results in pain, stiffness and difficulty with physical activities. The disease is managed rather than cured, with a focus on pain relief.Citation5 A number of herbal medicines have been found to have beneficial effects in alleviating the symptoms of OA in human clinical studies. These include advocado, soybean unsaponifiables,Citation6 lipids from green-lipped mussels,Citation7 calcified seaweed extracts,Citation8 and Pycnogenol (French maritime pine bark extract).Citation9 Boswellia serrata extracts have also show clinical promiseCitation10 as do preparations of Harpagophytum procumbens (Devil’s Claw).Citation11,Citation12 Polyphenols such as epigallocatetchin (from green tea) and phlorotannin-rich extracts of the seaweed Ecklonia cava have exhibited potential using in vitro models yet have generated little clinical evidence to date.Citation13
In the Western herbal medicine tradition, bladderwrack (Fucus vesiculosus) and other seaweeds in the form of topically applied liniments have been used as herbal approaches to the treatment of sore knees.Citation14 Seaweed extracts have been demonstrated to contain at least two major components with anti-inflammatory activity; fucoidans and polyphloroglucinols (algal polyphenols). Fucoidans are considered to be one of the main therapeutic components of brown algaeCitation15 and may constitute up to 25%–30% of the algal dry weight, depending on the specific seaweed species.Citation16 Although fucoidans are highly branched long chain polysaccharides, low levels of serum uptake of fucoidan were observed after Undaria fucoidan ingestionCitation17 indicating that there is potential for clinical activity. Fucoidan is a potent selectin blocker and has been used experimentally to prevent inflammatory damage after ischemic events.Citation18,Citation19
Polyphenolic fractions derived from seaweed also have profound antioxidant activityCitation20 which can also contribute to anti-inflammatory effects. Inhibition of oxalate damage to kidneys in animal models was attributed to the antioxidant qualities of Fucus-derived fucoidan.Citation21 Fucoidans have been shown to inhibit phospholipase A2Citation22 an important enzyme in the inflammatory cascade inhibited by corticosteroids.
The toxicity of seaweed extracts has been investigated in both human and animal studies, although the source of the extracts has been different to the Fucus source used in this study. Previous human clinical studies using 3 g daily Undaria seaweed extracts with 75% fucoidan content indicated no clinically observed toxicity.Citation23 In a recent companion study on the coagulation effects of 3 g of the same Undaria extract, there was a significant change in clotting indices, but these remained within clinically normal parameters. Activated partial thromboplastin time increased from 28.41 to 34.01 s (n = 10; P = 0.01), thrombin time decreased from 18.62 to 17.55 s (n = 10, P = 0.04), and anti-thrombin-III increased from 113.5% to 117% (n = 10, P = 0.03).Citation24 There were no toxicological changes observed in rats given up to 300 mg/kg orally of fucoidan from Laminaria japonica. This dose is considerably higher that the 3000 mg per subject dose in the clinical studies. The absence of observations may be because of difference in the source species for the fucoidan, or the absorption of the fucoidan from the gut. Anticoagulant effects were observed at doses of 900 to 2,500 mg/kg, but no other signs of toxicity were observed.Citation25 In a similar study involving fucoidan extracted from Cladosiphon okamuranus, no significant toxicological changes were induced by fucoidan at a dose of 600 mg/kg of body weight/day in Wistar rats. However, with concentrations at and above 1,200 mg/kg of body weight/day, clotting time was significantly prolonged.Citation26 Overall, the dose levels used in this study (a total of either 100 mg and 1000 mg per day) would not be expected to produce changes outside of the clinically normal parameters, as they are many fold lower than the dose levels used in the animal studies, and a third of the dose used in the human clinical study. A polyphenol rich fraction of Fucus was also shown to lack acute toxic effects in rats after four weeks of oral dosing.Citation20 The latter extract was somewhat different to the extract used in this study as it contained a concentrated polyphenol fraction.
The present study tested a seaweed extract nutrient complex containing a blend of extracts from three different species of brown algae plus nutrients zinc, manganese and B6, using an open label design at two doses to determine effects in OA. This was a pilot scale combined phase I and II study aimed at providing data on acute safety and efficacy.
Material and methods
Research design
This trial was a pilot scale open label dosing study, with two different doses randomised to participants, conducted over 12 weeks (84 days) in Lismore New South Wales (Australia) and was conducted in 2008. The study was approved by the Human Research Ethics Committee of Southern Cross University (Ethics approval number: ECN-07-36). The research was conducted in compliance with Good Clinical Practices (GCP) and in accordance with the guidelines of the Australian National Health and Medical Research Council and the Declaration of Helsinki (as revised in 2004). The trial was registered with the Australian and New Zealand Clinical Trials Register (ACTRN12607000229471).
Participants
A convenience sample of healthy individuals aged between 18 and 65 years was recruited by email from staff and students at Southern Cross University, and from Lismore and surrounding areas through newspaper advertising, regional radio and television. All participants received a study information sheet outlining the study and signed an informed consent form agreeing to participate.
Participants were included if they had both X-ray and clinical evidence of osteoarthritis of the knees; if they had a baseline comprehensive osteoarthritis test (COAT) score between 3 and 7; were otherwise healthy (had no other acute nor chronic medical condition); and if they were willing to discontinue their current OA treatment for the duration of the study. Participants were excluded if they had a history of trauma associated with the affected joint; if they had rheumatoid arthritis or other inflammatory joint conditions (including gout); if they used corticosteroids (intra-articular or systemic) within four weeks prior to baseline and throughout the study; if they used anti-inflammatory agents or anti-arthritic complementary medicines three weeks prior to baseline and during the duration of the study; had liver function tests greater than three times the upper limit of normal at baseline; if they had a history of alcohol or substance abuse; were female participants who were lactating, pregnant or planning to become pregnant; if they had participated in another clinical trial in the last 30 days; if they were unwilling to have blood taken three times during the study; or if they were unwilling to comply with the study protocols.
Outcome measurements
The primary outcome measurement in this study were the safety measures and the average COAT score, a validated measurement instrument for the assessment of the symptoms of osteoarthritis.Citation27 The COAT score is composed of four subscales: pain, stiffness, difficulty with physical activity and overall symptom severity. Secondary outcomes included the individual COAT sub-scales, TNF-alpha measurements, serum lipids and paracetamol usage over the 12 week study.
Baseline COAT measures were recorded and participants with visual analog scale (VAS) scores between 3 and 7 out of a possible 10 who agreed to wash out from their current osteoarthritis treatment were admitted to the study. Wash out commenced four weeks prior to the start of the trial and participants did not take their current osteoarthritis medications for the duration of the study. The participants were supplied with study diaries and asked to record their COAT scores and paracetamol use daily for four weeks. They were requested not to take narcotic analgesics and those containing codeine for seven days before the next clinic and until the end of the study. Study staff contacted the participants each week at the same time to ensure compliance and provide support.
COAT scores included joint pain, stiffness, difficulty with physical activities and overall symptom score. The participants completed a baseline COAT score under the supervision of the clinic staff by making a mark on a 10 cm VAS with a single vertical line to show the severity of each descriptor over the past twenty-four hours. The descriptors were none to extreme with a numerical score of 0 to 10. The scores were converted to a numerical grade through measurement with a ruler placed on the mark. Daily diaries were issued along with identical rulers (manufactured by Celco) and study staff contacted the participants each day at the same time to collect scores, ensure compliance and provide support. These instructions complied with the methods used with the validated tool. Participants were screened four weeks prior to the trial, they returned two weeks prior to ensure that scores remained between 3 and 7, and then returned at baseline. If the scores remained between 3 and 7 at baseline, the participant was randomised to a study medication.
Safety was assessed by actively monitoring adverse events. Subjects were questioned weekly about adverse events which were then recorded. Participants attended three clinics during which weight, blood pressure, pulse rate, and concomitant medication use was recorded and fasting blood samples collected at baseline, week 4, and week 12. Safety measurements undertaken by an independent accredited laboratory were full blood count, liver function tests and determination of urea, creatinine, electrolytes, cholesterol and triglyceride concentrations to assess toxicity to the hemopoietic, hepatic and renal systems; and assess the impact on fasting lipids. The body mass index (BMI) was calculated at the start and conclusion of the study.
Subjects returned all remaining capsules at each clinic visit and these were counted as a measure of compliance. The investigator maintained an inventory record of all capsules received and dispensed. It was assumed that capsules not returned were taken.
Participants were asked at the end of the study to evaluate the study medication by answering a question indicating their satisfaction, dissatisfaction, or neither satisfaction nor dissatisfaction with the study medication.
Study medication and dose
The study medication used Maritech® extract (Marinova Pty Ltd, Hobart, Australia). These are fucoidan-rich extracts of seaweeds (Maritech® extracts) which are manufactured using a proprietary aqueous process that produces fucoidan fractions. Nothing is added during the process. Insoluble matter and salts are removed as part of the process. They are ‘whole plant’ extracts which contain fucoidan and seaweed polyphenols (polyphloroglucinols) associated with the fucoidan molecule. In this study we used a blend of three Maritech® extracts from different species of brown seaweeds; Maritech® Fucus vesiculosis (85% w/w), Maritech® Macrocystis pyrifera (10% w/w) and Maritech® Laminaria japonica (5% w/w). In addition, Vitamin B6, zinc sulfate and manganese sulfate were included in the formulation, as detailed in . The study medication was manufactured as 100 mg and 250 mg capsules (Gel Caps) under the code of good manufacturing practice (GMP). The total fucoidan concentration in the 100 mg and 250 mg capsules was 75 mg and 187.5 mg respectively. Fucoidan content is assessed using a validated spectrophotometric method based on a modified Dubois method.Citation28
Table 1 Contents of Maritech® capsules
Subjects were randomized to either a100 mg or 1000 mg dose group using the research randomizer website (http://www.randomizer.org/). Seven sets of two numbers per set, using a range of one to two, were generated. These numbers were used to assign the participants to either the 100 mg or 1000 mg dose. The 100 mg dose was delivered in one 100 mg gel capsule daily (taken in the morning) to five participants. The 1000 mg dose was delivered as four 250 mg capsules daily (two capsules taken twice daily) to seven participants. The capsules were self-administered orally by the participants after food.
TNF alpha assays
After collection serum was stored at −70 °C until analyzed. Serum tumor necrosis factor-α (TNF-α) was measured using a sandwich ELISA assay (Cayman Chemical Company, Denver, CO, USA). Thawed serum was supplemented with 5% mouse serum and 9 mM dithiothreitol in order to minimise nonspecific binding to the mouse anti-TNF-α. Serum was assayed without further dilution. TNF-α standards were prepared in TNF-α free human serum and similarly supplemented with mouse serum and dithiothreitol. Each sample was assayed in duplicate on two separate microplates (n = 4). Four samples had a single outlier removed. The standard curves for both plates had R2s of 0.99 over a concentration range of 0–250 pg/mL.
Statistical methods
A multilevel repeated measures analysis with auto-correlated time was conducted on the average COAT score and each of the four COAT subscales over the 85 measurement occasions from baseline (day 0) to completion (day 84) using SPSS (version 17.0; SPSS Inc, Chicago, IL, USA) with the Mixed procedure with a first order autoregressive (AR1) covariance modeled on the daily repeated measurements. The model presented fitted a two-piece linear response over timeCitation29 with a change point at day 21, and included the effects of treatment, 2-piece time, and 2-piece time by treatment interaction.
Results
Study participants
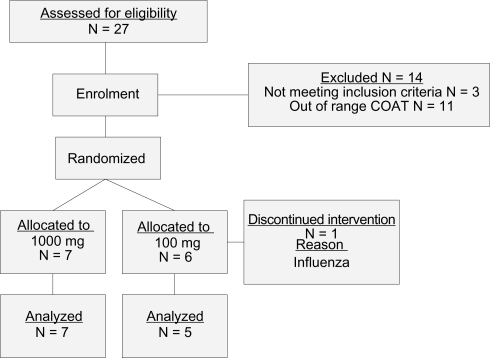
The study screened volunteers and subsequently enrolled thirteen people who met the inclusion/exclusion criteria and were comprised of six females (mean age [±SD] 62.16 [±9.04] years) and seven males (mean age [±SD] 57.14 [±9.20] years). One subject withdrew a week prior to the start of the baseline measure due to an acute respiratory tract infection. Eleven participants completed 12 weeks of the study. One participant completed 10 weeks of the study due to overseas travel for the final 2 weeks and their final blood measurement was taken at week 10. Data was analysed for these 12 participants. Five participants received the formulation in doses of 100 mg Maritech® extract and seven received 1000 mg Maritech® extract. There was 99.2% compliance at four weeks and 99.6% at twelve weeks with participants taking 100 mg daily and 97.4% at four weeks and 95.9% compliance at twelve weeks in individuals taking 1000 mg per day. Please see for a flow diagram of the trial. The study medication was well tolerated as all 12 participants were satisfied with the effects of taking the study medication (compared with dissatisfied; or neither satisfied or dissatisfied).
The mean ages, and the baseline means for blood pressure, weight and pulse rate for the 100 mg and 1000 mg arms were not statistically different (data not presented).
COAT scores
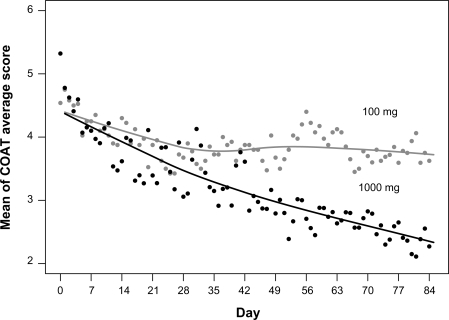
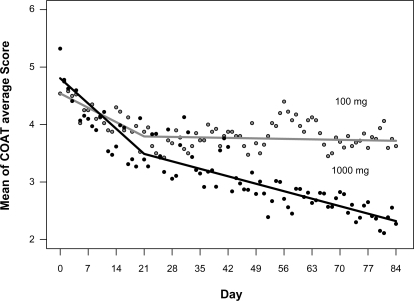
Subjects taking either dose experienced reduction in COAT scores over the 12 weeks of the study. Means for the main outcome, the average of the COAT score subscales (average COAT), at baseline and on every seventh measurement occasion are provided for each treatment in to summarize the response. A more complete description showing the daily mean average COAT scores is provided graphically in to which an empirical Loess curve was fitted to summarize the underlying response profiles. On observing , two-piece linear functions with the break points at days 21 and 28 were tested in the statistical model for the Average COAT score, with the day 21 break point displaying superior fit in terms of the −2 log likelihood fit statistics (2007.9 at 21 days and 2010.7 at 28 days). reports the parameter estimates for the average COAT model and shows the estimated mean average COAT profiles by time by treatment. There was a highly significant treatment by time interaction effect (likelihood ratio test, chi-square = 15.155, df = 2, P = 0.0005), which was largely located after day 21 as indicated by the tests of the interaction parameters in . The parameter estimates are not reported for the pain, stiffness, physical difficulties and overall symptoms sub-scales but were similar to those obtained for the average COAT scale as would be expected from highly correlated variables. reports the estimated means for each of the COAT scales at baseline and completion for both treatments, and the results of a one-tailed test of the hypothesis that the reduction from baseline to completion was significantly greater in the 1000 mg than the 100 mg treatment. This hypothesis was confirmed at P < 0.05 for the average COAT (0.043) scale, and the physical difficulties (0.010) and overall symptoms (0.044) sub-scales, but failed to reach significance for the pain (0.088) and stiffness (0.089) subscales. As shown in , mean average COAT reduced from 4.54 to 3.72 (18%) in the 100 mg treatment and from 4.81 to 2.32 (52%) in the 1000 mg treatment.
Table 2 Average COAT score: mean, standard deviation (SD), minimum and maximum at the weekly measurement occasions by treatment
Table 3 Average COAT scores: parameter estimates with their standard errors and tests of significance
Table 4 COAT scales: estimated baseline and completion means with 95% confidence intervals (CI) and tests of the difference in the reduction from baseline to completion between treatments
Adverse events
Six adverse events were noted. The first event, influenza occurred prior to the commencement of the trial and the participant withdrew. Two participants had hypertension, one baseline and one at week 4. Both participants had a history of hypertension. One participant had a chest infection at week 12, one had root canal work at week 12, and one participant had hyperacidity at week 12 with a history of gastric acidity at baseline. All events were considered to be unlikely to be related to the study medication.
Blood safety measures
There were no toxicity issues observed over the period of the study hemopoietic, hepatic and renal systems. Due to the small numbers of participants in this study it is not possible to consider gender differences. presents the mean, standard deviation (SD) and sample size for each blood safety measure at baseline and completion in each treatment and a test of the significance of the change. There were no statistically significant changes in either treatment over time although there was a statistically significant but not clinically significant increase in albumin over time for both treatments combined (P = 0.034).
Table 5 Blood safety measures: mean, standard deviation (SD) at baseline and completion 12 together with mean, SD, P-values for differences between baseline and completion by treatment
Paracetamol use
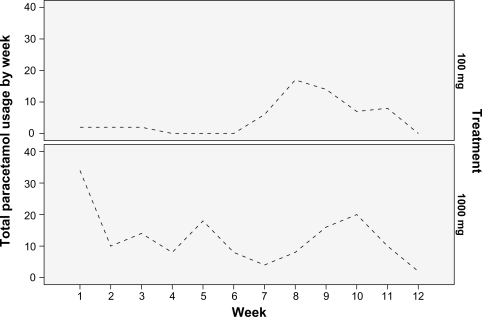
Descriptive statistics for paracetamol use are reported in together with Mann–Whitney tests comparing the treatments on use at baseline and total use over the duration of the trial. Weekly total paracetamol use (500 mg tablets) is plotted by treatment in . None of subjects in the 100 mg treatment took any paracetamol on the first day of treatment while, of the seven subjects in the 1000 mg treatment, four took no paracetamol with the remaining three taking eight 500 mg tablets between them. The subjects in the 100 mg treatment took a total of 58 tablets (each 500 mg) over the duration of the trial with individual consumption ranging from 0 to 40 tablets, while the subjects in the 1000 mg treatment took a total of 152 tablets with individual consumption ranging from 0 to 62 tablets. Mann–Whitney comparisons of the two treatments showed no significant difference in consumption between the two groups either at baseline or over the duration of the trial.
Table 6 Paracetamol usage: mean, standard deviation (SD), and mean rank by treatment at baseline and over the duration of the trial with the Mann–Whitney P-values
TNF-α
Descriptive statistics for TNF-α are given in . There were no changes in TNF-alpha serum levels over time (P = 0.170), nor any differences between treatment groups (P = 0.227).
Table 7 Tumor necrosis factor alpha levels: means, standard deviation (SD), minima and maxima by treatment arm and measurement occasion
Cholesterol and serum lipids
Repeated measures analysis of variance was conducted on the baseline and 12 week values for serum lipids. Descriptive data is given in . One subject in the 100 mg group did not have a recordable low-density lipoprotein (LDL) in week 12. There were no differences found between the doses.
Table 8 Cholesterol, HDL, LDL, ratio and triglycerides: means, SD, minima and maxima by treatment and measurement
Discussion
This study investigated the effects on the symptoms of osteoarthritis using two doses of a complex containing a blend of Maritech® extracts from three brown algae species (Fucus vesiculosis, Macrocystis pyrifera, Laminaria japonica.) plus vitamin B6, zinc and manganese in an open label design. There was a clear dose dependent effect on symptoms of osteoarthritis as assessed using the COAT index. The preparation was found to be safe at the doses used in the study population over 12 weeks.
After 12 weeks, the 100 mg dose reduced the average COAT score by 18% and the 1000 mg dose by 52%. The decrease in OA symptoms demonstrated by the 1000 mg dose is marked. Whilst there is no direct comparator in this study, results expected from nonsteroid anti-inflammatory drugs (NSAIDS) produce similar reductions in OA symptoms.Citation30 Osteoarthritis studies often have large placebo responses and a limitation of this early investigation was a lack of a placebo arm. The significant difference between the two doses in a dose-dependent progression increases the likelihood that the 1000 mg dose is superior to a placebo.
A recent randomised controlled study on a mineral based supplement derived from seaweed undertaken over 12 weeks demonstrated changes in the symptoms of osteoarthritis when measured by WOMAC.Citation8 Although derived from a seaweed, the mineral-based supplement was substantially different from the soluble fucoidan-rich preparations used in this study, and cannot be directly compared.
Individuals with OA show raised levels of TNF-α,Citation31 and we hypothesized that the seaweed formulation would demonstrate a dose-dependent reduction over time as the fucoidans and polyphenols have been identified as anti-inflammatory agents. In this study, the TNF-α levels showed no change at either dosage perhaps indicating that the effect observed is not mediated via a TNF-α-associated cascade. However the study is not sufficiently powered to rule out a reduction in TNF-α, and further studies should include the measure.
Fucoidan is a major component of Maritech® seaweed extracts. It is not a precursor to mammalian tissue formation, and is generally assumed to be impervious to mammalian enzyme breakdown. Fucoidans have profound selectin-blocking activity,Citation18 and serum uptake of fucoidan has been demonstrated previously.Citation17 It is possible that, in this clinical trial, selectin-blocking effects may reduce leukocyte accumulation in the osteoarthritis affected areas, reducing the COAT scores via a generalized inhibition of inflammation. However paradoxically others have argued that selectin blockade may actually reduce pain relief.Citation32 Further clinical trials and laboratory investigations are necessary to elucidate its mechanism of action.
The preparation was shown to be safe at the dosages consumed by the study population and any adverse effects were mild and self limiting. There were no changes in the cholesterol, liver function, renal function, and hemopoietic function that were of any clinical significance during the course of the study.
The most significant limitation of this study was; being a pilot scale open label combined phase I and II trial it was subject to potential bias that would be reduced by the use of randomization to placebo or active with appropriate blinding. This study aimed to determine if this preparation had any potential in the treatment of the symptoms of osteoarthritis. The study achieved this aim and the significant dose responsiveness demonstrated provides evidence that these results are unlikely to be due to chance and deserve further exploration.
Conclusion
A seaweed extract nutrient complex when taken orally over twelve weeks significantly reduced the symptoms of osteoarthritis in a phase I and II open label study. The effect was highly dose-dependent with final reduction in COAT score of 18% and 52% respectively for the 100 mg and 1000 mg doses. The preparation was demonstrated to be safe to use over the study period at the doses tested in the study population. The observed pharmacological benefits now need to be demonstrated in a phase III randomized controlled trial.
Acknowledgments/disclosures
The study was sponsored by Marinova Pty Ltd under contract to Southern Cross University and performed independently by NatMed-Research. Dr Fitton is employed by Marinova Pty Ltd. While she was involved in the study design, interpretation of results and preparation of the manuscript, she had no interaction with any study participant, nor was she involved in the day to day running or management of the clinical trial. Marinova Pty Ltd paid the article-processing charge associated with the publication of this paper. We thank Catherine Avila, Gareth Vanderhope and Airdre Grant at NatMed-Research who provided clinical research assistance; Dion Thompson who performed the TNF-α testing; the Northern Rivers Pathology Unit who undertook the safety measurements; and the participants who made it possible.
References
- MarchLMBaggaHEpidemiology of osteoarthritis in AustraliaMed J Aust20041805 SupplS6S1014984356
- IssaSNSharmaLEpidemiology of osteoarthritis: an updateCurr Rheumatol Rep20068171516515759
- MurphyLSchwartzTAHelmickCGLifetime risk of symptomatic knee osteoarthritisArthritis Rheum20085991207121318759314
- BuckwalterJASaltzmanCBrownTThe impact of osteoarthritis: implications for researchClin Orthop Relat Res2004427SupplS6S1515480076
- FajardoMDi CesarePEDisease-modifying therapies for osteoarthritis: current statusDrugs Aging200522214116115733021
- FrechTMCleggDOThe utility of nutraceuticals in the treatment of osteoarthritisCurr Rheumatol Rep200791253017437663
- AmeyeLGCheeWSOsteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidenceArthritis Res Ther200684R12716859534
- FrestedtJLWalshMKuskowskiMAZenkJLA natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized controlled pilot trialNutr J20087918279523
- CisarPJanyRWaczulikovaIEffect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritisPhytother Res20082281087109218570266
- SenguptaKAlluriKVSatishARA double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the kneeArthritis Res Ther2008104R8518667054
- BrienSLewithGTMcGregorGDevil’s Claw (Harpagophytum procumbens) as a treatment for osteoarthritis: a review of efficacy and safetyJ Altern Complement Med2006121098199317212570
- HuangTHTranVHDukeRKHarpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-kappa B activationJ Ethnopharmacol20061041–214915516203115
- ShinHCHwangHJKangKJLeeBHAn antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cavaArch Pharm Res200629216517116526282
- GrieveMA Modern HerbalHarmondsworth, Middlesex, EnglandPenguin Books Ltd1977
- LiBLuFWeiXZhaoRFucoidan: structure and bioactivityMolecules20081381671169518794778
- KusaykinMBakuninaISovaVStructure, biological activity, and enzymatic transformation of fucoidans from the brown seaweedsBiotechnol J20083790491518543244
- IrhimehMRFittonJHLowenthalRMKongtawelertPA quantitative method to detect fucoidan in human plasma using a novel antibodyMethods Find Exp Clin Pharmacol2005271070571016395421
- CumashiAUshakovaNAPreobrazhenskayaMEA comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweedsGlycobiology200717554155217296677
- RitterLSCopelandJGMcDonaghPFFucoidin reduces coronary microvascular leukocyte accumulation early in reperfusionAnn Thorac Surg199866620632071discussion 20729930494
- ZaragozaMCLopezDMPSToxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extractsJ Agric Food Chem200856177773778018683949
- VeenaCKJosephineAPreethaSPVaralakshmiPPhysico-chemical alterations of urine in experimental hyperoxaluria: a biochemical approach with fucoidanJ Pharm Pharmacol200759341942717331346
- AnguloYLomonteBInhibitory effect of fucoidan on the activities of crotaline snake venom myotoxic phospholipases A(2)Biochem Pharmacol200366101993200014599557
- IrhimehMRFittonJHLowenthalRMFucoidan ingestion increases the expression of CXCR4 on human CD34+ cellsExp Hematol200735698999417533053
- IrhimehMRFittonJHLowenthalRMPilot clinical study to evaluate the anticoagulant activity of fucoidanBlood Coagul Fibrinolysis2009207607610
- LiNZhangQSongJToxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar ratsFood Chem Toxicol20054342142615680677
- GideonTPRengasamyRToxicological evaluation of fucoidan from Cladosiphon okamuranusJ Med Food200811463864219053854
- BrooksLORolfeMICherasPAMyersSPThe comprehensive osteoarthritis test: a simple index for measurement of treatment effects in clinical trialsJ Rheumatol20043161180118615170933
- DuboisMGKHamiltonJKRebersPASmithFColorimetric methods for determination of sugars and related substancesAnal Chem1956283350356
- SnijdersTABBoskerRJMultilevel AnalysisAn Introduction to Basic and Advanced Multilevel ModellingLondon, UKSage Publications1999
- OngCKLirkPTanCHSeymourRAAn evidence-based update on nonsteroidal anti-inflammatory drugsClin Med Res200751193417456832
- HulejovaHBaresovaVKlezlZPolanskaMAdamMSenoltLIncreased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral boneCytokine200738315115617689092
- MachelskaHCabotPJMousaSAZhangQSteinCPain control in inflammation governed by selectinsNat Med1998412142514289846582