Abstract
Phosphodiesterases (PDEs) are important enzymes that hydrolyze the cyclic nucleotides adenosine 3′5′-cyclic monophosphate (cAMP) and guanosine 3′5′-cyclic mono-phosphate (cGMP) to their inactive 5′ monophosphates. They are highly conserved across species and as well as their role in signal termination, they also have a vital role in intracellular localization of cyclic nucleotide signaling and integration of the cyclic nucleotide pathways with other signaling pathways. Because of their pivotal role in intracellular signaling, they are now of considerable interest as therapeutic targets in a wide variety diseases, including COPD where PDE inhibitors may have bronchodilator, anti-inflammatory and pulmonary vasodilator actions. This review examines the diversity and cellular localization of the isoforms of PDE, the known and speculative relevance of this to the treatment of COPD, and the range of PDE inhibitors in development together with a discussion of their possible role in treating COPD.
Cyclic nucleotide signaling
cAMP was the first “second messenger” to be identified (CitationSutherland 1970; CitationBeavo and Brunton 2002). It is now known that it transduces the intracellular effects of many hormones and neurotransmitters (CitationHabener 2001) and some of the effects of T-cell receptor activation (CitationLedbetter et al 1986). The level of intracellular cAMP is regulated by the balance of activity between adenyl cyclase (AC), which is responsible for its formation and cyclic nucleotide phosphodiesterase which is responsible for its inactivation. cAMP exerts its effects through activation of protein kinase A (PKA), the GTP-exchange protein EPAC and via cAMP gated ion channels in the cell membrane. Changes in cAMP levels can be extremely short lived, as in the rapid and brief rise in cAMP levels seen over milliseconds in olfactory neurons (CitationBreer, 1993) or more sustained, for example the changes over hours seen in the effects of LHRH on anterior pituitary cells (CitationBorgeat et al 1972).
Cyclic nucleotides, particularly cyclic AMP, have important regulatory roles in virtually all cell types involved in the pathophysiology of COPD. Elevation of intracellular cAMP levels suppresses the activity of immune and inflammatory cells (CitationBourne et al 1974; CitationKammer 1988; CitationMoore and Willoughby 1995) and elevation of both cAMP and cGMP leads to smooth muscle relaxation. cAMP may have an additional role in modulating airway smooth muscle hypertrophy and hyperplasia as it has cytostatic effects in many cell types (CitationPastan et al 1975; CitationFriedman et al 1976), and exerts an inhibitory effect influence on airway smooth muscle proliferation (CitationLew et al 1992; CitationTomlinson et al 1995).
In most cells and tissues, the capacity for hydrolysis of cyclic nucleotides by PDEs is an order of magnitude greater than the maximum rate of synthesis of cAMP and cGMP and thus small reductions in the activity of PDEs can produce large increases in the level of cyclic nucleotides and significant changes in the activity of cAMP-dependent protein kinase. There is growing evidence for sub-cellular compartmentalization of cAMP levels, allowing control of cAMP dependent signal transduction both spatially and temporally and PDE plays a crucial role in this sub-cellular localization by creating boundaries for cAMP diffusion (CitationMongillo et al 2004) and its role is more than simply a mechanism of terminating the signal.
This sub-cellular compartmentalization has been shown to be important in cardiac myocytes but its role in inflammatory cells and airway smooth muscle is still unclear.
PDE isoforms
Shortly after the identification of PDE it was realized that there was more than one isoform. PDEs with different chromatographic and kinetic properties, different substrate specificity and pharmacological properties were identified in extracts from brain and other tissues (CitationThompson and Appleman 1971). It is now realized that PDE forms a super family of enzymes containing at least eleven families. Three catalytic domains can hydrolyze the 3′ phosphate bond of cyclic nucleotides: the class I domain is shared by protozoa and metazoa, the class II domain is found in fungi, slime mould and amoebae and the class III domain has only been identified in the slime mould Dictostelium discoideum.
The catalytic domain in metazoa is highly conserved and is characterized by the metal binding domain H(X)3H(X)25–35(D/E), where H is histidine, D is aspartic acid, E is glutamic acid and X can be any amino acid. This domain is shared by a large superfamily of metal-dependent phosphohydrolases known as the HD-family and indicates that divalent cations are involved in cyclic nucleotide hydrolysis. Although PDEs are related to this superfamily they are distinct and have other conserved regions which they share with each other (CitationAravind and Koonin 1998).
Analysis of the human genome has identified 21 genes for cyclic nucleotide PDEs and the physiochemical and regulatory properties of the proteins they code for have been characterized (CitationConti and Jin 1999; CitationSoderling and Beavo 2000; CitationFrancis et al 2001). Based on their molecular sequence, kinetics, regulation and pharmacological characteristics mammalian PDEs can be classified into 11 families, denoted by an Arabic numeral 1–11. Some of these families have more than one member each of which is encoded by different genes and these are denoted by a capital letter after the numeral, eg, PDE4A, PDE4B, PDE4C, and PDE4D. To complicate matters further, most of the genes encoding PDEs have multiple promoters and the transcripts are subject to alternate splicing, resulting in nearly one hundred different PDE open reading frames (CitationConti and Beavo 2007). The splicing variant is denoted by a final Arabic numeral after the letter, eg, PDE4D3.
All PDEs contain three functional domains: a conserved catalytic core, a regulatory N-terminus and the C-terminus (CitationThompson 1991; CitationBolger 1994; CitationConti and Beavo 2007). The carboxyl terminus is similar in all the PDE families except PDE6, with 18%–46% sequence identity overall. Although there is some evidence that the C-terminal region of PDE4 may be involved in dimerization (CitationKovala et al 1997) and may also be a target for regulatory phosphorylation (CitationLenhard et al 1996) its physiological function remains unclear.
The N-terminal domain shows great diversity between PDE families () and understanding the functional relevance of the differences in the N-terminal domains is crucial to understanding the regulation and sub-cellular localization of different PDEs and to the development of drugs that modulate PDE activity.
Figure 1 Schematic diagram of the domain structure of the eleven PDE families. Copyright © 2007. Adapted from with permission CitationConti M, Beavo J. 2007. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem, 76:481–511.
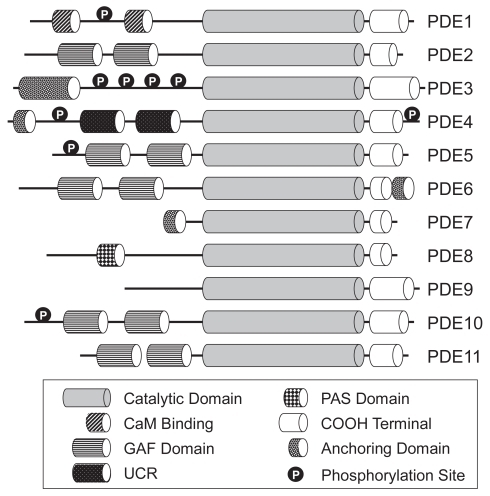
There are domains that are essential for ligand binding, PDE oligomerization domains and kinase recognition and phosphorylation domains which regulate PDE function. The regulatory domains include the calmodulin binding domain found in PDE1, the cGMP binding (GAF) domains found in PDE2, 5, 6, 10, and 11, and the so called upstream conserved regions 1 and 2 (UCR1 and UCR2) found in PDE4. The N-terminal domains also contain regions that determine intracellular localization.
The catalytic domain which contains about 270 amino acids shows a high degree (25%–49%) of amino acid conservation between the 11 PDE families; however, the families themselves and the isoforms within the respective family have varying substrate preferences for cAMP and cGMP. In mammals PDE4, PDE7 and PDE8 hydrolyze cAMP selectively, PDE5, PDE6, and PDE9 hydrolyse cGMP selectively and the remaining five PDEs (PDE1, 2, 3, 10, and 11) hydrolyze both cAMP and cGMP. Current evidence suggests that substrate specificity is conferred by the orientation of a single glutamine residue within the catalytic site which can either form hydrogen bonds with cAMP, cGMP or both depending on its fixed orientation or ability to rotate (CitationZhang et al 2004). The catalytic region comprises 17 α-helices divided into three subdomains (CitationXu et al 2000; CitationJeon et al 2005). There is an N-terminal cyclin-fold region, a linker region and a C-terminal helical bundle. The three sub-domains form a deep hydrophobic pocket and this contains four subsites: a metal-binding site (M site), a core pocket (Q pocket), a hydrophobic pocket (H pocket) and a lid region (L region) (CitationSung et al 2003; CitationJeon et al 2005). As discussed above, it appears that at least one of the metals binding at the M site which lies at bottom of the pocket is zinc and the other is likely to be magnesium.
It appears likely that PDEs function as dimers or oligomers in most cells and dimerization is an essential structural element that determines the regulatory properties and inhibitor sensitivities of PDE4 as discussed below (CitationRichter and Conti 2004). The spatial location of PDEs within cells is now also known to be important in determining their intracellular effects and this appears to be determined to some extent by the presence of different targeting domains in the N-terminal domain. One explanation for the multiple isoforms is targeting in different sub-cellular locations ().
Figure 2 Schematic representation of the some of the complexes in which phosphodiesterases (PDEs) are involved in a hypothetical cell. Reprinted from with permission CitationConti M, Beavo J. 2007. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem, 76:481–511. Copyright © 2007. Annual Reviews www.annualreviews.org.
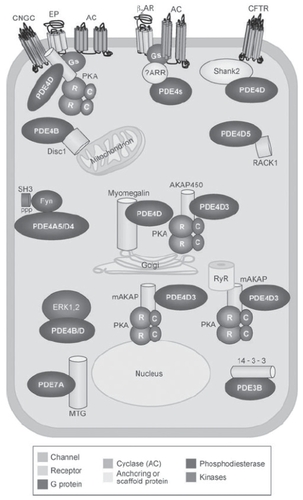
Scaffolding molecules such as A-kinase anchoring proteins (AKAP) dynamically assemble cAMP effector molecules, such as PKA, and PDEs into signaling complexes which regulate the temporal and spatial effects of cAMP (CitationMcConnachie et al 2006). In particular PDE4D3 and PKA have been shown to be associated with muscle mAKAP and phosphorylation of PDE4D3 by PKA in these complexes enhances its PDE activity thus forming a negative feedback control system to limit the activation of PKA and regulate local cAMP concentrations (CitationDodge et al 2001). Under resting conditions PDE4D3 maintains local cAMP concentrations below the threshold required for PKA activation and when cAMP levels rise following receptor stimulation, phosphorylation of PDE4D3 by activated PKA increases its activity returning cAMP levels to baseline (CitationDodge-Kafka et al 2005).
In some situations PDEs themselves may function as scaffolding for the assembly of macromolecular complexes which compartmentalize the effects of cAMP. PDE4D3 interacts with EPAC (a guanine nucleotide exchange factor for the Ras-like small GTPases Rap1 and Rap2 (CitationBos 2003)) and ERK5 (extracellular signal regulated kinase (CitationZhou et al 1995)). These intermolecular interactions facilitate the dissemination of distinct cAMP signals through each effector protein. ERK phosphorylation of PDE4D3 decreases the phosphodiesterase activity, thereby favoring local accumulation of cAMP and subsequent EPAC activation (CitationDodge-Kafka et al 2005).
PDE4 also forms another macromolecular signaling complex with β-arrestin to regulate cAMPs diffusion from activated receptors. Arrestins bind specifically to active (phosphorylated) G-protein coupled receptors (GPCRs), and arrest or reduce signaling by these receptors. β-arrestin binds to the β-adrenergic receptor G-protein and recruits PDE4. This both regulates local levels of cAMP and also controls phosphorylation of the β-receptor switching its predominant coupling from stimulatory guanine nucleotide regulatory protein (Gs) to inhibitory guanine nucleotide regulatory protein (Gi) thus controlling PKA activity at the membrane (CitationBaillie et al 2003).
PDE isoforms and COPD
Most interest in PDE isoforms from the perspective of COPD has centered on the PDE4 family, but PDE1, PDE3, PDE5, and PDE7 may also be of interest as therapeutic targets in COPD. This reflects the fact that COPD is now recognized as an inflammatory disease with pulmonary and systemic components (CitationNational Institute for Clinical Excellence [NICE] 2004; Global Initiative for Chronic Obstructive Pulmonary Disease).
Tobacco smoke induces an inflammatory response in the lungs that leads to the development of airflow obstruction, mucus hypersecretion, parenchymal destruction and systemic effects. There is inflammation in the conducting airways, the parenchyma and in the pulmonary vasculature, and increased levels of inflammatory mediators in peripheral blood. As the disease progresses small airways become occluded by inflammatory exudates containing mucus (CitationHogg et al 2004) and lung parenchymal tissue is destroyed. The systemic effects include skeletal muscle dysfunction, nutritional abnormalities and weight loss, cardiovascular, CNS and skeletal effects (CitationAgusti et al 2003; CitationWouters 2002; CitationHalpin, 2007).
The inflammation in COPD involves cytotoxic (CD8+) T cells, macrophages and neutrophils, and inflammatory mediators, including cytokines, chemokines, proteinases and oxidants. CD8+ T cells and macrophages infiltrate airway tissues while neutrophils are the predominant cells recovered from the airway lumen (CitationO’Shaughnessy et al 1997; CitationSaetta et al 1999). The inflammatory mediators and proteolytic enzymes released from these cells lead to mucus-hypersecretion in large airways, progressive obstructive changes in the small airways and destruction of lung parenchyma (CitationJeffery 2001a, Citationb; CitationAgostini et al 2003). Eosinophils may also play a role during exacerbations (CitationSaetta et al 1994) and mast cells may also be important as they have been found in increased numbers in the airway of smokers with bronchitis and airflow limitation (CitationGrashoff et al 1997). Recently, dendritic cells have also been shown to be important in the inflammatory process in COPD (CitationTsoumakidou et al 2008). Structural cells, such as epithelial cells, fibroblasts and airway smooth muscle cells are also thought to be important and have been shown to release inflammatory mediators, such as interleukin (IL)-8, tumor necrosis factor (TNF)-α, IL-10 and transforming growth factor (TGF)-β (CitationSaetta et al 2001; CitationJeffery 2001b) which can lead to subepithelial and peribronchiolar fibrosis (CitationLee et al 2001). These cells also express adhesion molecules which modulate interactions with lymphocytes and recruitment of neutrophils into the airway lumen.
PDE4 is the major regulator of cAMP levels in leukocytes and other inflammatory cells (CitationBarnette 1999). Inhibition of PDE4 increases intracellular cAMP concentrations which ultimately results in reduction of cellular inflammatory activity. Targeted inhibition of PDE4 has been considered as a way of reducing inflammation in patients with asthma or COPD (CitationBarnette 1999; CitationCompton et al 2001).
PDE3 is present in T lymphocytes, macrophages, monocytes as well as in airway smooth muscle and endothelial cells (CitationSchudt et al 1995; CitationTorphy 1998). Thus, in theory, inhibitors of PDE3 could act both as bronchodilators and anti-inflammatory drugs and may have synergistic effects with PDE4 inhibitors.
PDE1 accounts for more than 35% of the cyclic nucleotide hydrolytic activity in human airway smooth muscle (CitationTorphy et al 1993) and it has been implicated in human vascular smooth muscle proliferation (CitationRybalkin et al 1997; CitationRybalkin et al 2002a). It is not known whether or not it is also involved in airway smooth muscle proliferation but if it is, inhibitors of PDE1 could be of considerable benefit in treating airway remodeling in COPD.
PDE5 is also expressed in pulmonary vascular smooth muscle and airway smooth muscle (CitationYanaka et al 1998; CitationSebkhi et al 2003) and has a pivotal role in controlling regulation of smooth muscle tone by nitric oxide (NO), atrial natriuretic peptide (ANP), and other endogenous vasodilators. Inhibition of PDE5 in patients with COPD has the potential to reduce pulmonary vascular resistance and prevent vascular remodeling, as well as causing bronchodilatation and it may also have anti-inflammatory actions.
Finally, an isoform of PDE7 is also abundantly expressed in the airway smooth muscle, and in many pro-inflammatory and immune cells, including neutrophils recovered from induced sputum in patients with COPD. Theoretically inhibition of PDE7 may have an anti-inflammatory effect but so far this has not been demonstrated, although inhibition of PDE7 may augment the anti-inflammatory effects of PDE4 inhibition (CitationSmith et al 2004)
The structural and functional characteristics of these phosphodiesterase, as well as drugs which affect their activity will now be considered from the perspective of understanding their role and potential as therapeutic targets in COPD.
PDE4 isoforms
More than 20 isoforms of PDE4 are known at present. They are found in many cell types in the lung including airway epithelial cells (CitationDent et al 1998), airway and pulmonary vascular smooth muscle (Citationde Boer et al 1992; CitationPauvert et al 2002; CitationRabe et al 1993), and pulmonary vascular endothelium (CitationThompson et al 2002). They are also present in T lymphocytes (CitationTenor et al 1995; CitationGiembycz et al 1996), neutrophils (CitationNielson et al 1990), monocytes (CitationSeldon et al 1995), eosinophils (CitationDent et al 1994), and basophils (CitationPeachell et al 1992). Their function has been explored by the use of selective inhibitors and genetic manipulations including targeted gene knockout. The PDE4 isoforms have closely related kinetic properties and are all inhibited by rolipram.
Four human PDE4 subtypes (A, B, C, and D) have identified. The genes for these enzymes have been cloned and expressed. PDE4A and PDE4C are located on human chromosome 19 at 19p13.2 and 19p13.11, PDE4B is on chromosome 1p31 and PDE4D is on chromosome 5q12 (CitationBolger et al 1993; CitationMcLaughlin et al 1993; CitationMilatovich et al 1994; CitationEngels et al 1995; CitationHorton et al 1995; CitationSzpirer et al 1995; CitationNemoz et al 1996). The N-terminal domain of most members of the PDE4 family contains the two upstream conserved regions UCR1 and UCR2 mentioned above; however several truncated variants of PDE4B and PDE4D are formed via alternate splicing resulting in deletions of part or all of UCR1 (). The PDE4 isoforms can thus be divided into three groups on the basis of the structure of the N-terminal domain. Long isoforms contain both UCR1 and UCR2, short isoforms lack UCR1, and supershort isoforms lack UCR1 and have a truncated UCR2. UCR1 contains a PKA phosphorylation site which allows long forms to be activated by this enzyme (CitationSette and Conti 1996; CitationMacKenzie et al 2002), leading to local regulation of cAMP levels. All isoforms of PDE4 can be phosphorylated by ERK, but the effects of this on PDE4 activity depend on the presence of UCR1. Long isoforms are inhibited by ERK phosphorylation whereas short isoforms are activated and no effect on activity is seen in super short isoforms (CitationMacKenzie et al 2000). There are significant differences in the tissue distribution of the mRNA for the PDE4 isoforms (). PDE4A message is widely distributed in many tissues including pulmonary and inflammatory cells (CitationEngels et al 1994). PDE4C is absent from circulating inflammatory cells and there are conflicting reports about the distribution of PDE4B in some tissues (eg, liver, kidney or pancreas) (CitationMcLaughlin et al 1993, CitationEngels et al 1994) but general agreement that it is present in lung and inflammatory cells. PDE4D is present in some but not all inflammatory cells.
Table 1 Summary of the RT-PCR results with different human tissues and defined cell populations (After CitationEngels et al 1994)
Table 2 PDE4 inhibitors in current and discontinued clinical development
Figure 3 Schematic representation of human PDE4 subtypes and products of mRNA splice variants The number of amino acid (AA) residues in each protein appears to the right of the schematic diagram. Adapted and updated with permission from CitationTorphy TJ. 1998. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med, 157:351–70. Copyright © 2008 American Thoracic Society.
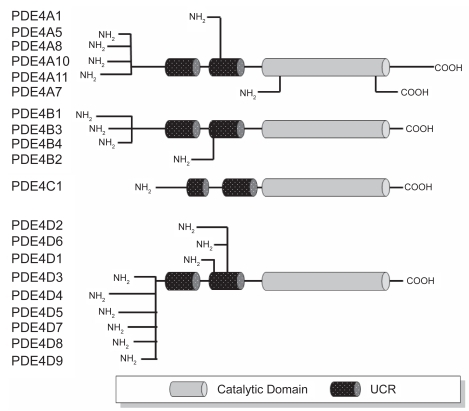
There is also evidence that the isoforms of PDE4 change as cells differentiate, suggesting that they are not simply redundant but have different intracellular roles. For example, differentiation of monocytes to macrophages is associated with a marked downregulation of PDE4D3 and PDE4D5, upregulation of PDE4B2 and induction of the PDE4A10 long isoform (CitationShepherd et al 2004). Changes in isoforms may occur in disease and it has been reported that PDE4A4B is upregulated in macrophages from smokers with COPD (CitationBarber et al 2004). In addition to their effect on enzyme activity the UCR domains are involved in interactions between PDE4 and scaffolding molecules such as myomegalin which localize components of the cAMP-dependent pathway to the Golgi/centrosomal region of the cell (CitationVerde et al 2001). Deletions in the N-terminal domain affect subcellular localization (CitationBeard et al 2002). For example, PDE4A1 is exclusively membrane-associated and is normally localized to the Golgi (CitationShakur et al 1995; CitationPooley et al 1997), but deletion of its unique N-terminal region of made it fully soluble and changed its location to the cytosol (CitationShakur et al 1993). The N-terminal domains of PDE4 isoforms also determine the ability of the enzyme to interact with other regulatory molecules such as the immunophilin XAP2 (CitationBolger et al 2003) and to recruit other proteins to form signaling cascades. For example, PED4A5 can bind to the SH3 domains which are found in a variety of cytoplasmic tyrosyl protein kinases as well as in cytoskeletal and adaptor proteins (CitationO’Connell et al 1996). The physiological significance of these interactions remains uncertain but they clearly demonstrate the complexity of intracellular signaling with cross-talk between different signaling cascades. An example of the importance of subcellular localization of PDE4 isoforms is their location in airway epithelial cells where it has been shown that they confine cAMP generated by apical adenosine A2B receptors to a microdomain that includes the target CFTR channels and prevents a rise in total cellular cAMP levels (CitationBarnes et al 2005). Furthermore, there is now evidence that although PDE4D is crucial in controlling the intracellular cAMP gradients and microdomains generated by stimulation of both the β1 and β2 adrenergic receptors, the PDE4D5 isoform is involved with β2 adrenergic receptor signaling and the PDE4D8 and PDE4D9 isoforms are associated with β1 adrenergic receptor signaling (CitationRichter et al 2008).
Binding of inhibitors to PDE4 is also influenced by the N-terminal domain structure. The binding kinetics of rolipram suggest that there two binding sites: a high affinity site (HPDE4) with a Ki approximately 50–1000 times greater than binding to the low affinity site (LPDE4). Deletion of the terminal 332 amino acids prevents high affinity binding of rolipram but has minimal effect on PDE4 catalytic activity. High affinity binding predominates in the CNS, while low affinity binding predominates in inflammatory cells leading to important clinical differences in the pharmacological properties of inhibitors. It was thought that high affinity binding was associated with unwanted CNS and gastric side effects of inhibitors and that low affinity binding was associated with the therapeutic effects of inhibitors (CitationTorphy 1998; CitationJeon et al 2005); however, this has subsequently proved to be too simplistic (CitationHouslay et al 2005) and cilomilast, which is a second generation inhibitor which targets low affinity binding, still produces adverse effects including emesis (CitationTorphy et al 1999).
Cilomilast and roflumilast are the most well characterized second generation PDE4 inhibitors but their clinical utility is still influenced by the fact that administration at therapeutically effective doses is limited by adverse effects. Studies in knockout mice have suggested that PDE4D is the main isoform associated with emesis (CitationRobichaud et al 2002), while PDE4B appears to be the main isoform responsible for mediating TNFα release (CitationJin and Conti 2002). This has led to the suggestion that PDE4B inhibitors may have anti-inflammatory properties in COPD which will not be limited by adverse effects. Alternative strategies to improve the therapeutic ratio of PDE4 inhibitors are to target isoforms that appear to be only expressed as part of the inflammatory process in COPD, such as PDE4A4, or to develop dual specificity inhibitors which inhibit PDE4 and either PDE1, PDE3, or PDE7 (CitationGiembycz 2005).
A number of molecules are currently under investigation as clinically useful PDE4 inhibitors () and some of these also have specificity for other PDE isozymes. The clinical development of many compounds has been discontinued because of lack of efficacy or because of unacceptable side effects. Most have been assessed as oral therapy but Arofylline (LAS-31025) was trialled as an inhaled therapy (CitationNewman et al 1997) although its development was halted in Phase III because of undesirable side effects. Some compounds, such as L-826,141 from Merck, have shown that it may be possible to differentially inhibit PDE4 subtypes (IC50 = 0.26 to 2.4 nM for catalytic domain activity of PDE4A, PDEB, PDEC, and PDED (CitationClaveau et al 2004) and such properties offer exciting opportunities to refine the effectiveness and clinical utility of PDE4 inhibitors.
PDE1 isoforms
PDE1 was first identified in rat brain tissue in 1970 (CitationKakiuchi and Yamazaki 1970). It is almost exclusively activated by changes in intracellular Ca2+ concentrations and it was shown that the Ca2+ sensitivity of the enzyme was enhanced by a protein factor subsequently called calmodulin (CaM) (CitationTeo and Wang 1973). An increase in intracellular calcium concentrations often leads to a fall in cAMP levels. In some cells this is mediated by Ca2+-inhibitable adenylyl cyclases (AC5 and AC6), while in others it is due to PDE1.
The PDE1 subfamily is as diverse as the PDE4 subfamily and is encoded by three genes giving the isoforms PDE1A, PDE1B, and PDE1C. These differ in their substrate specificity, regulatory properties and tissue distribution. PDE1A and PDE1B preferentially hydrolyze cGMP, whereas PDE1C degrades both cAMP and cGMP with high affinity.
The PDE1A gene has been located on chromosome 2q32 and currently nine splice variants have been described (CitationMichibata et al 2001). These include 3 splice variants at the N-terminal end and 3 at the C-terminal end. Different N-terminal domains are associated with different tissue distributions. PDE1A1, PDE1A4, and PDE1A8 have the N2 terminal sequence and are widely expressed in lung and other tissues, whilst PDE1A5, PDE1A6, and PDE1A9 have the N1 sequence and are expressed in brain tissue and PDE1A10 and PDE1A11 which have the N3 sequence are expressed in testis (CitationMichibata et al 2001). Differences in the C-terminal domain may affect the level of expression within tissues.
The PDE1B gene is located on chromosome 12q13. Two splice variants, PDE1B1 and PDE1B2, have been described (CitationFidock et al 2002). PDE1B expression is lower than other members of the PDE1 family. Neither form is found in lung tissue. Selective upregulation of PDE1B2 has been described on monocyte to macrophage differentiation (CitationBender et al 2005) and appears to have a role in determining the phenotype of the macrophage (CitationBender and Beavo 2006). PDE1B also appears to play an important role in T-cell activation and survival (CitationJiang et al 1996; CitationKanda and Watanabe 2001).
The PDE1C gene is located on chromosome 7p14.3. Five splice variants with differing tissue distributions have been described in mice (CitationYan et al 1996) and it appears that there may be at least as many human variants (CitationBingham et al 2006). PDE1C is the main cAMP hydrolytic activity in proliferating smooth muscle cells in the presence of Ca2+ It is localized to the nucleus and appears to be involved in regulating smooth muscle proliferation (CitationRybalkin et al 2002a; CitationDolci et al 2006). As well as regulating smooth muscle tone, cyclic nucleotides inhibit smooth muscle proliferation (CitationKoyama et al 2001)
PDE1 exists as a dimmer. In bovine brain, PDE1B1 and PDE1A2 homodimers have been purified but there is also evidence for the presence of a heterodimer containing PDE1B1 and PDE1A2. The relationship between structure and function of the PDE1 isoforms is complex. Some isoforms exhibit almost identical kinetic properties but are differentially regulated by CaM and Ca2+, whilst others show different kinetic properties (CitationSharma et al 2006). Despite the diversity of the isoforms, the overall structure of all PDE1s is similar. They all contain the common PDE catalytic domain and in addition they all have two CaM binding sites and a conserved inhibitory domain which holds the enzyme in a less active state in the absence of Ca2+ and CaM. For at least some of the isoforms it appears that CaM binding to just one of the CaM binding sites is sufficient to activate the enzyme (CitationSonnenburg et al 1995). It has been suggested that the presence of the second CaM binding sites may be important for intracellular targeting. As well as the CaM binding sites shared with other PDE1 isoforms, a PDE1 isoform purified from rabbit and bovine lung tissue also contains calmodulin as an integral subunit. Differences in the N-terminal domain can have profound influences on the regulation of the enzyme. PDE1A1 and PDE1A2 differ in only a single segment of 34 residues (CitationNovack et al 1991), yet CaM is a 10-fold more potent activator of PDE1A1 than PDE1A2 (CitationSonnenburg et al 1995). PDE1 isoforms are also regulated by phosphorylation. There are complex interactions between phosphorylation and the effects of CaM. For example, Ca2+/CaM can block phosphorylation of PDE1A2 and PDE1A1, whilst Ca2+/CaM stimulate phosphorylation of PDE1B1. Furthermore Ca2+/CaM can reverse phosphorylation of PDE1 isozymes through activation of CaM-dependent protein phosphatase (calcineurin) (CitationSharma et al 2006). A number of different phosphorylases are involved in the regulation of PDE1. PDE1A1 and PDE1A2 are phosphorylated by cAMP-dependent protein kinase (CitationSharma 1991; CitationSharma and Wang, 1985, CitationSharma et al 2002), while PDE1B1 is a substrate of CaM-dependent protein kinase II (CitationSharma and Wang 1986). Phosphorylation of PDE1 also changes the affinity of the isozymes for CaM. Thus, overall, the effect of CaM on PDE1 is complex since CaM activation is partly reversed by CaM-dependent phosphorylation, which, in turn, is opposed by the action of CaM-dependent phosphatase. In vivo these events may be temporally separated (CitationKakkar et al 1999).
Several drugs inhibit PDE1 including ginsenoides and selegeline (CitationSharma and Kalra 1993; CitationKakkar et al 1996). Amantadine selectively inhibits some but not all isoforms. It inhibits PDE1A1 but not PDE1A2 or PDE1B1 (CitationKakkar et al 1997) and it is thought that dihydropyridine calcium antagonists such as felodipine and nicardipine may also inhibit PDE1 isozymes (CitationSharma et al 1997). From a pulmonary perspective it has been shown that inhibition of PDE1 by vinpocetine augmented the pulmonary vasodilator response to nitric oxide in lambs (CitationEvgenov et al 2006) suggesting that PDE1 inhibition may have a role in treatment of pulmonary hypertension and given the role of PDE1 in smooth muscle proliferation it may have disease modifying potential.
PDE3 isoforms
Members of the PDE3 family are characterized by hydrophobic N-terminal membrane association domains. They exhibit high affinity for both cAMP and cGMP but the Vmax for cGMP hydrolysis is 4–10 times higher (CitationDegerman et al 1997). PDE3 differs from PDE4 in that it has high affinity for both cAMP and cGMP so that these substrates are mutually competitive and thus PDE3 hydrolysis of cAMP can be inhibited by cGMP. PDE4 is not inhibited by cGMP and thus originally PDE3 was called cGMP-inhibited PDE to distinguish it from PDE4. cGMP inhibition of PDE3 may be physiologically relevant to the elevation of cAMP levels in a variety of cells including human myocytes, where nitric oxide induced relaxation may be mediated by activation of guanylyl cyclase, elevation of intracellular cGMP levels and inhibition of PDE3 leading to elevated cAMP levels (CitationKirstein et al 1995). PDE3 is clinically significant because of its role in regulating vascular and cardiac smooth muscle as well as platelet aggregation.
Two genes encoding PDE3 have been isolated (CitationBeavo et al 1994). PDE3A is located on chromosome 12p12 and PDE3B is located on chromosome 11p15.1 (CitationMiki et al 1996; CitationKasuya et al 2000). PDE3A is found in myocardium, arterial, venous, bronchial, and gastrointestinal smooth muscle, while PDE3B is found in adipose tissue (CitationReinhardt et al 1995). In the lung, PDE3 activity is prominent in alveolar macrophages, endothelial cells, platelets, and airway smooth muscle cells. Three isoforms of PD3A have been identified in human myocardium. They are designated PDE3A-136, -118 and -94 because of the differing lengths of their N-terminal domains and appear to be generated by a combination of alternative transcriptional and post-transcriptional processing. PDE3a-136 appears to be located exclusively in microsomal fractions whilst the other two isoforms are present in both cytosolic and microsomal fractions (CitationSmith et al 1993). The isoforms have different regulatory properties, as result of loss of regulatory sites, and thus their different subcellular locations enables differential spatial regulation of cAMP levels (CitationHambleton et al 2005).
The structural organization of PDE3A and PDE3B proteins is similar. The catalytic domain of PDE3 contains a 44 amino acid insert not found in other PDEs and which differs between the isoforms. This insert interrupts the first of the Zn2+ binding domains present in the catalytic domains but whether it is involved in its interaction with substrates or inhibitors remains unclear. There are significant differences in the amino acid sequence of the N-terminal regions of PDE3A and PDE3B, but they both contain two hydrophobic regions involved in intracellular targeting termed N-terminal hydrophobic regions (“NHRs”) and sites for phosphorylation and activation by PK-B (CitationDegerman et al 1997). NHR1 contains transmembrane helices, whereas NHR2 appears to be involved in the binding of PDE3 to other currently unidentified proteins. The three regulatory phosphorylation sites which are phosphorylated by PK-B and PK-A lie between NHR1 and NHR2 (CitationRascon et al 1994; CitationKitamura et al 1999; CitationRondinone et al 2000). Phosphorylation of PDE3 is important in activating PDE3 in response to a variety of extracellular signals in cardiac, vascular and airway smooth muscle, as well as a number of other cells, including adipocytes and platelets (CitationDegerman et al 1997). It has recently been shown that phosphorylation allows the binding of 14-3-3 proteins to PDE3 () (CitationPozuelo Rubio et al 2005). These proteins bind to specific phosphorylated sites on diverse target proteins, and appear to either force conformational changes that cannot be induced by phosphorylation alone or act as double ended “adapters” induce interactions between their targets and other molecules. Their importance as integrators of the specificity and strength of signaling is just being recognized (CitationMackintosh 2004; CitationPozuelo Rubio et al 2004).
PDE3 can be inhibited by a number of drugs, including cilostamide, enoximone, and lixazinone. PDE3 inhibitors enhance myocardial contractility and induce vascular and airway smooth muscle relaxation (CitationLeeman et al 1987; CitationMyou et al 1999; CitationFujimura et al 1995). Siguazodan, a PDE3 inhibitor, potentiated relaxation induced by rolipram suggesting an interaction between PDE3 inhibition and PDE4 inhibition (CitationTorphy et al 1993). Similarly, although PDE3 inhibitors do not appear to have direct anti-inflammatory actions, they appear to augment the anti-inflammatory actions of PDE4 inhibitors (CitationRobicsek et al 1991; CitationSchudt et al 1995; CitationGiembycz et al 1996).
PDE5 isoforms
PDE5 has become of considerable clinical interest as it is the target of sildenafil, the treatment for erectile dysfunction. PDE5 was first identified in rat lung tissue (CitationLincoln et al 1976) and its enzyme activity has subsequently been identified in many other tissues. The enzyme has been purified and cloned (CitationFrancis et al 1980; CitationThomas et al 1990a; CitationMcAllister-Lucas et al 1993) and the human gene identified on chromosome 4q26 (CitationLoughney et al 1998; CitationYanaka et al 1998). It has subsequently been shown that there are three splice variants designated PDE5A1, PDE5A2, and PDE5A3 (CitationKotera et al 1998; CitationLoughney et al 1998; CitationLin et al 2000). PDE5A1 and A2 isoforms are expressed in a wide variety of tissues, including lung, heart, skeletal muscle, brain, kidney, and liver, but the A3 isoform is confined to tissues with a smooth muscle or cardiac muscle component (CitationLin et al 2000). Whether the splice variants affect sub-cellular localization of PDE5 activity is not yet known, but there are preliminary reports that PDE5 activity is associated with cytoplasmic vesicles and in the centrosomal area of human myometrial cells (CitationDolci et al 2006).
PDE5 exists as a homodimer, and as well as the conserved catalytic site, it contains highly cGMP-specific allosteric (non-catalytic) cGMP-binding sites and a phosphorylation site in the N-terminal domain (CitationCorbin and Francis 1999). The protein sequence of the allosteric binding sites is conserved across PDE2, PDE5, PDE6, and PDE10 and these tandem homologous repeats of 110 amino acids each in the regulatory domain are termed GAF domains (a and b) because of their presence in cGMP-binding cyclic nucleotide PDEs, Anabaena adenylyl cyclase, and the bacterial transcription factor FhlA (CitationThomas et al 1990a; CitationMcAllister-Lucas et al 1995; CitationAravind and Ponting 1997). Allosteric binding of cGMP to PDE5 regulatory domain increases affinity of the catalytic site for cGMP, thereby stimulating the rate of cGMP hydrolysis (CitationThomas et al 1990b; CitationMullershausen et al 2001; CitationOkada and Asakawa 2002; CitationCorbin et al 2003; CitationRybalkin et al 2003). cGMP binding to the regulatory domain also stimulates phosphorylation of PDE5 by cGMP-dependent protein kinase (CitationThomas et al 1990b; CitationWyatt et al 1998; CitationMullershausen et al 2001; CitationMurthy 2001; CitationRybalkin et al 2002b). cGMP binding to these sites must be preceded by occupation of the catalytic site by cGMP (CitationFrancis et al 1980; CitationThomas et al 1990b) and it appears that cGMP binding to the regulatory domain produces a conformational change in PDE5 that exposes Ser-92 allowing phosphorylation which increases affinity of the regulatory domain for cGMP and increases catalytic activity as well (CitationCorbin et al 2000). Thus, when intracellular cGMP levels rise, cGMP breakdown is enhanced both by increased activity at the catalytic site, followed by enhanced cGMP binding at the allosteric sites, leading to increased enzymatic activity as a result of phosphorylation. Classical PDE5 inhibitors and selected cyclic nucleotide analogs compete with cGMP at the catalytic site but do not interact with the cGMP-binding allosteric sites (CitationBlount et al 2004).
As a major regulator of cGMP in smooth muscle cells, PDE5 controls the cGMP-dependent protein kinase (PKG) regulation of smooth muscle tone by nitric oxide (NO), atrial natriuretic peptide (ANP), and other endogenous vasodilators, especially under conditions of low calcium. PKG phosphorylates the regulatory myosin-binding subunit of myosin phosphatase (CitationSurks et al 1999), calcium-activated maxi K+ (BKCa) channels (CitationFukao et al 1999), and IRAG (IP3 receptor associated cGMP kinase substrate)(CitationSchlossmann et al 2000), leading to a reduction of intracellular Ca2+ concentration or reduction in sensitivity to Ca2+ and thereby decreased smooth muscle tone (CitationSchlossmann et al 2003).
PDE7 isoforms
PDE7 was first identified as part of a human DNA screening programme (CitationMichaeli et al 1993). It is a high affinity cAMP-specific PDE and it is insensitive to potent selective inhibitors of other PDE families such as rolipram and milrinone. Two genes encoding PDE7 have been identified in humans (CitationGardner et al 2000 CitationHetman et al 2000; CitationSasaki et al 2000). PDE7A is on chromosome 8q13 (CitationHan et al 1998) and PDE7B is on chromosome 6q23–q24. Three splice variants of PDE7A have been described (PDE7A1, PDE7A2, and PDE7A3) but in humans, unlike mice, only one form of PDE7B has been described. Compared with PDE7A1, PDE7A2 has an additional 20 amino acid hydrophobic region in the N-terminal domain which is thought to be responsible for membrane tethering.
mRNA for PDE7A1 and PDE7A2 can be detected in airway and vascular smooth muscle cells, airway epithelial cells, CD4 and CD8 T-cells, neutrophils, macrophages and monocytes, while PDE7A3 is expressed in T-cells (CitationBloom and Beavo 1996; CitationHan et al 1997; CitationGlavas et al 2001; CitationSmith et al 2003). It appears that PDE7A1 expression is low in naïve T cells but increases on activation by CD3 X CD28 costimulation (CitationLi et al 1999). PDE7B is expressed predominantly in the brain but is also found in a number of other tissues including liver, heart, thyroid and skeletal muscle but not leukocytes (CitationGardner et al 2000; CitationHetman et al 2000; CitationSasaki et al 2000). Despite the expression of PDE7A2 mRNA in smooth muscle and leukocytes no protein could be detected in these cells, suggesting that if expressed at all, the amount of PDE7A2 present is very low and the amount of PDE7A1 protein is greater in T-cells than in airway and vascular smooth muscle cells (CitationSmith et al 2003). Alternative splicing of PDE7A determines the subcellular localization of its protein products without affecting their kinetic properties. The hydrophobic N-terminus of PDE7A2 leads to membrane association (CitationHan et al 1997), while PDE7A1 appears to be associated with the Golgi in T cells through associations with the AKAP scaffolding protein myeloid translocation gene (MTG) () (CitationAsirvatham et al 2004), which also targets PKA to the Golgi in T cells (CitationSchillace et al 2002). The N-terminal domain of PDE7A1 also contains two copies of a PKA pseudosubstrate site which bind to and inhibit the activity of the catalytic subunit of PKA (CitationHan et al 2006). Thus, in addition to cAMP hydrolysis, PDE7A1 can terminate cAMP signaling by direct interaction with the catalytic subunit of PKA.
Given the distribution of PDE7A1 in pro-inflammatory and airway cells there has been considerable interest in whether inhibition of PDE7A may be anti-inflammatory and of therapeutic benefit in COPD. However, there is conflicting evidence in mice about the effects of inhibiting PDE7A on T cell proliferation and IL-2 production (CitationLi et al 1999; CitationYang et al 2003). This may reflect the methods used to inhibit PDE7A activity which used different molecular genetic techniques to block PDE7A activity. A few selective inhibitors of PDE7 have now been identified (CitationGiembycz 2005) and using these it is becoming clear that inhibition of PDE7A can regulate proinflammatory and immune cell function when used in associated with PDE4 inhibitors. For example, although inhibition of PDE7A with BRL 50481 does not itself affect T cell proliferation or TNF-α production by macrophages, it does augment the effects of rolipram on these cells (CitationSmith et al 2004).
Clinical effects of PDE inhibitors in COPD
As a result of greater knowledge about isoforms of PDE and their role in regulating inflammatory cells and airway and vascular smooth muscle there has been considerable interest in the clinical effects of PDE inhibitors in patients with COPD. Most interest has centered on inhibition of PDE4 and three drugs have reached phase III clinical trials in patients with COPD: rolipram, cilomilast, and roflumilast.
Unfortunately the use of rolipram was limited by significant side effects: particularly nausea, vomiting and gastric acid secretion (CitationHorowski and Sastre-Y-Hernandez 1985) due to inhibition of PDE4 in the CNS and gastric parietal glands. These findings led to the development of the second generation PDE4 inhibitors roflumilast and cilomilast which have better therapeutic ratios (CitationTorphy et al 1999).
Roflumilast has proven anti-inflammatory properties in vitro and in animal models (CitationBundschuh et al 2001; CitationHatzelmann and Schudt 2001). Preliminary clinical data suggested that roflumilast could improve lung function while being well tolerated in patients with COPD when given orally once daily (CitationLipworth 2005) and a 24-week clinical trial showed that it improved lung function and reduced exacerbations compared with placebo (CitationRabe et al 2005). A subsequent, year-long trial has again shown that it improved lung function, but in this study there was no effect on health status or exacerbation rates (CitationCalverley et al 2007).
Cilomilast is another second generation PDE4 inhibitor which shows anti-inflammatory effects in both pre-clinical and clinical studies (CitationBarnette et al 1998; CitationGriswold et al 1998; CitationTorphy et al 1999; CitationGamble et al 2003; CitationProfita et al 2003). Bronchial biopsies, taken from COPD patients, randomized to receive either cilomilast orally or placebo for 12 weeks showed that cilomilast significantly reduced the number of infiltrating tissue CD8+T cells and CD68+ macrophages by 48% (p < 0.01) and 47% (p < 0.001), respectively (CitationGamble et al 2003) and a clinical trial in patients with COPD showed that it improved lung function and health status compared with placebo and reduced exacerbations over 24 weeks (CitationRennard et al 2006)
Both cilomilast and roflumilast cause nausea, diarrhea, headache, abdominal pain and dizziness. GI side effects appear more common in patients taking cilomilast than roflumilast (CitationRennard et al 2006; CitationRabe et al 2005; CitationCalverley et al 2007). In preclinical trials in rats and dogs PDE4 inhibitors have been associated with the development of arteritis/periarteritis (CitationGiembycz 2005). These species appear particularly susceptible to this toxic effect but some PDE4 inhibitors may also cause this effect in primates: arteritis has been reported to have been produced in cynomolgus monkeys (CitationLosco et al 2004) and Merck discontinued the development of a PDE4 inhibitor because of the occurrence of colitis (CitationGiembycz 2005). To date, no PDE4 inhibitor has received a license from the FDA or EMEA.
Future directions
The side effects of PDE4 inhibitors reflect the ubiquitous distribution of PDE4 isozymes and their importance in a wide variety of cellular processes. The therapeutic ratio of PDE4 inhibitors may be improved by using specific inhibitors targeting those isozymes in airway smooth muscle or inflammatory cells or which are relatively inactive against PDE4C, the isoform that predominates in the CNS. Developing such inhibitors presents a considerable challenge given the conserved nature of the catalytic site and the similarity of the overall sequence of the four subtypes. Nevertheless, dual PDE4A/D and specific PDE4D inhibitors have been developed and tested in in vitro models (CitationMuller et al 1996; CitationManning et al 1999; CitationGiovannoni et al 2007) but they have not yet been assessed in clinical trials.
An alternative strategy to improve the tolerability of PDE4 inhibitors is to develop dual specificity inhibitors that inhibit both PDE4 and either PDE1, PDE3 or PDE7. In this way the anti-inflammatory properties may be augmented whilst reducing PDE4 related side-effects in other organs. Although inhibitors of PDE3 have little intrinsic anti-inflammatory action they do appear to augment the anti-inflammatory properties of PDE4 inhibitors (CitationRobicsek et al 1991; CitationGiembycz et al 1996; CitationSchudt et al 1995). Similarly, there is some in vitro evidence that inhibition of PDE7 may augment the effects of PDE4 inhibition (CitationSmith et al 2004), although PDE7A inhibitors have little or no anti-inflammatory properties themselves (CitationGiembycz 2005). Dual specificity PDE inhibitors have been developed, but none of have reached phase III trials and there are still concerns about the toxic effects of inhibiting members of other PDE families (CitationGiembycz 2005).
Although there are no selective PDE1 inhibitors available for clinical use at present, PDE1 inhibition offers theoretical anti-inflammatory benefits in COPD through inhibition of T-cell activation and inducing apoptosis, and prevention of the development of pulmonary hypertension as a result of augmenting pulmonary vasodilatation and inhibiting vascular smooth muscle proliferation. Thus, dual specificity PDE inhibitors that inhibit PDE1 may offer greater anti-inflammatory potential if combined with anti-PDE4 activity, or enhanced and sustained effects on pulmonary hypertension if combined with anti-PDE5 activity.
Given their tissue distribution, in particular their presence in pulmonary and vascular smooth muscle, it is possible that inhibition of PDE3 and PDE5 could be of benefit in patients with COPD. Selective PDE3 inhibitors promote bronchodilatation in humans (CitationLeeman et al 1987; CitationFujimura et al 1995; CitationMyou et al 1999) and inhibition of PDE5 with sildenafil reduces pulmonary vascular resistance in subjects with hypoxic-induced pulmonary hypertension and in patients with severe pulmonary hypertension (CitationZhao et al 2001; CitationGhofrani et al 2004; CitationSastry et al 2004). There is also some preliminary evidence that PDE5 inhibitors may also have anti-inflammatory properties similar to the PDE4 inhibitors. Sildenafil pre-treatment inhibited LPS-induced airway hyperreactivity, leukocyte influx, and NO generation in a guinea pig model of airways disease as a result of inhibition of PDE5 rather than via effects on NO synthase (CitationToward et al 2004) and a single case report of 2 patients with COPD taking sildenafil for erectile dysfunction, described improvements in FEV1 (CitationCharan 2001). Further randomized studies are required to investigate this effect further.
Conclusion
There are currently nearly 100 members of the PDE superfamily divided into 11 families, with multiple isoforms in most families based on different genes and alternate splicing of gene products. PDEs control the duration and localization of intracellular cyclic nucleotide signaling and mediate interactions with other signaling cascades including the MAP kinase and Ca2+ pathways. The activity of PDEs is regulated by phosphorylation and interaction with other signaling molecules, some of which are assembled into macromolecular complexes with PDE by scaffolding proteins. Inhibition of cyclic nucleotide hydrolysis leads to elevation of their levels with consequent effects on cellular proliferation, smooth muscle relaxation and inhibition of inflammatory cells. Inhibition of PDE4 is currently the most tested strategy for patients with COPD but in clinical practice the benefits have been modest and its use has been limited by adverse effects. As understanding of the roles of PDE4 isoforms and other members of the PDE superfamily grows, these problems may be overcome by using isoform specific inhibitors as well as dual specificity inhibitors.
Abbreviations
| AC | = | adenyl cyclase |
| AC5 | = | Ca2+-inhibitable adenylyl cyclase 5 |
| AC6 | = | Ca2+-inhibitable adenylyl cyclase 6 |
| AKAP | = | A-kinase anchoring proteins |
| ANP | = | atrial natriuretic peptide |
| BKCa | = | calcium-activated maxi K+ channels |
| CaM | = | calmodulin |
| cAMP | = | adenosine 3′5′-cyclic monophosphate |
| cGMP | = | guanosine 3′5′-cyclic monophosphate |
| EPAC | = | GTP-exchange protein |
| ERK5 | = | extracellular signal regulated kinase |
| GAF | = | cGMP binding domains |
| GPCRs | = | G-protein coupled receptors |
| H pocket | = | catalytic region hydrophobic pocket |
| HPDE4 | = | PDE4 high affinity rolipram binding site |
| IL-2 | = | interleukin 2 |
| IL-8 | = | interleukin 8 |
| IL-10 | = | interleukin 10 |
| IRAG | = | IP3 receptor associated cGMP kinase substrate |
| L region | = | catalytic region lid region |
| LHRH | = | luteinizing hormone releasing hormone |
| LPDE4 | = | PDE4 low affinity rolipram binding site |
| M site | = | catalytic region metal-binding site |
| MTG | = | AKAP scaffolding protein myeloid translocation gene |
| NHRs | = | N-terminal hydrophobic regions |
| NO | = | nitric oxide |
| PDEs | = | phospodiesterases |
| PKA | = | protein kinase A |
| Q pocket | = | catalytic region core pocket |
| TGF-β | = | transforming growth factor β |
| TNF-α | = | tumour necrosis factor α |
| UCR1 | = | upstream conserved region 1 |
| UCR2 | = | upstream conserved region 2 |
| XAP2 | = | hepatitis B virus X-associated protein 2 |
Disclosures
The author has no conflicts of interest to disclose.
References
- AgostiniCTrentinLAdamiF2003Chronic obstructive pulmonary disease (COPD): new insights on the events leading to pulmonary inflammationSarcoidosis Vasc Diffuse Lung Dis203712737274
- AgustiAGNogueraASauledaJ2003Systemic effects of chronic obstructive pulmonary diseaseEur Respir J213476012608452
- AlexanderRPWarrellowGJEatonMA2002CDP840. A prototype of a novel class of orally active anti-inflammatory phosphodiesterase 4 inhibitorsBioorg Med Chem Lett121451612031318
- AokiMKobayashiMIshikawaJ2000A novel phosphodiesterase type 4 inhibitor, YM976 (4-(3-chlorophenyl)-1,7-diethylpyrido [2,3-d]pyrimidin-2(1H)-one), with little emetogenic activityJ Pharmacol Exp Ther2952556010991987
- AravindLKooninEV1998The HD domain defines a new superfamily of metal-dependent phosphohydrolasesTrends Biochem Sci23469729868367
- AravindLPontingCP1997The GAF domain: an evolutionary link between diverse phototransducing proteinsTrends Biochem Sci2245899433123
- AsirvathamALGalliganSGSchillaceRV2004A-kinase anchoring proteins interact with phosphodiesterases in T lymphocyte cell linesJ Immunol17348061415470020
- BaillieGSSoodAMcpheeI2003beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to GiProc Natl Acad Sci U S A100940512552097
- BarberRBaillieGSBergmannR2004Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokersAm J Physiol Lung Cell Mol Physiol287L3324315047569
- BarnesAPLiveraGHuangP2005Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epitheliumJ Biol Chem2807997800315611099
- BarnetteMS1999Phosphodiesterase 4 (PDE4) inhibitors in asthma and chronic obstructive pulmonary disease (COPD)Prog Drug Res5319322910616299
- BarnetteMSChristensenSBEssayanDM1998SB 207499 (Ariflo), a potent and selective second-generation phosphodiesterase 4 inhibitor: In vitro anti-inflammatory actionsJ Pharmacol Exp Ther28442069435206
- BaumerWHoppmannJRundfeldtC2007Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasisInflamm Allergy Drug Targets6172617352685
- BeardMBHustonECampbellL2002In addition to the SH3 binding region, multiple regions within the N-terminal noncatalytic portion of the cAMP-specific phosphodiesterase, PDE4A5, contribute to its intracellular targetingCell Signal144536511882390
- BeavoJABruntonLL2002Cyclic nucleotide research – still expanding after half a centuryNat Rev Mol Cell Biol3710812209131
- BeavoJAContiMHeaslipRJ1994Multiple cyclic nucleotide phosphodiesterasesMol Pharmacol463994057935318
- BeletaJBouJMiralpeixM1996LAS 31025, a new compound with selective phosphodiesterase IV inhibitory activityThird International Conference on Cyclic Nucleotide Phosphodiesterases: From Genes to TherapiesGlasgow
- BenderATBeavoJA2006PDE1B2 regulates cGMP and a subset of the phenotypic characteristics acquired upon macrophage differentiation from a monocyteProc Natl Acad Sci U S A103460516407168
- BenderATOstensonCLWangEH2005Selective up-regulation of PDE1B2 upon monocyteto-macrophage differentiationProc Natl Acad Sci U S A10249750215625104
- BillahMMCooperNMinnicozziM2002Pharmacology of N-(3,5-dichloro-1-oxido-4-pyridinyl)-8-methoxy-2-(trifluoromethyl)-5-quino line carboxamide (SCH 351591), a novel, orally active phosphodiesterase 4 inhibitorJ Pharmacol Exp Ther3021273712065709
- BinghamJSudarsanamSSrinivasanS2006Profiling human phosphodiesterase genes and splice isoformsBiochem Biophys Res Commun350253216987497
- BloomTJBeavoJA1996Identification and tissue-specific expression of PDE7 phosphodiesterase splice variantsProc Natl Acad Sci U S A9314188928943082
- BlountMABeasleyAZoraghiR2004Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulationMol Pharmacol661445215213306
- BolgerGMichaeliTMartinsT1993A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugsMol Cell Biol136558718413254
- BolgerGB1994Molecular biology of the cyclic AMP-specific cyclic nucleotide phosphodiesterases: a diverse family of regulatory enzymesCell Signal685197718405
- BolgerGBPedenAHSteeleMR2003Attenuation of the activity of the cAMP-specific phosphodiesterase PDE4A5 by interaction with the immunophilin XAP2J Biol Chem278333516312810716
- BootJDDe HaasSLVan GervenJM2008MK-0873, a PDE4 inhibitor, does not influence the pharmacokinetics of theophylline in healthy male volunteersPulm Pharmacol Ther In press
- BorgeatPChavancyGDupontA1972Stimulation of adenosine 3′:5′-cyclic monophosphate accumulation in anterior pituitary gland in vitro by synthetic luteinizing hormone-releasing hormoneProc Natl Acad Sci U S A692677814341705
- BosJL2003Epac: a new cAMP target and new avenues in cAMP researchNat Rev Mol Cell Biol4733814506476
- BourneHRLichtensteinLMMelmonKL1974Modulation of inflammation and immunity by cyclic AMPScience18419284131281
- BreerH1993Second messenger signalling in olfactionCiba Found Symp17997109 discussion 109–14, 147–98168385
- BuckleyGCooperNDykeHJ20007-Methoxybenzofuran-4-carboxamides as PDE 4 inhibitors: a potential treatment for asthmaBioorg Med Chem Lett1021374010999488
- BundschuhDSEltzeMBarsigJ2001In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitorJ Pharmacol Exp Ther2972809011259555
- BurnoufCAuclairEAvenelN2000Synthesis, structure-activity relationships, and pharmacological profile of 9-amino-4-oxo-1-phenyl-3,4,6,7-tetrahydro[1,4]diazepino[6, 7,1-hi]indoles: discovery of potent, selective phosphodiesterase type 4 inhibitorsJ Med Chem4348506711123995
- CalverleyPMSanchez-TorilFMcIvorA2007Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1761546117463412
- CharanNB2001Does sildenafil also improve breathing?Chest120305611451855
- ChenJCChenJQXieQM2004Selective inhibition of purified human phosphodiesterase 4A expressed in yeast cell GL62 by ciclami-last, piclamilast, and rolipramActa Pharmacol Sin251171515339393
- ChihiroMNagamotoHTakemuraI1995Novel thiazole derivatives as inhibitors of superoxide production by human neutrophils: synthesis and structure-activity relationshipsJ Med Chem3835387830278
- ChristensenSBGuiderAForsterCJ19981,4-Cyclohexanecarboxylates: potent and selective inhibitors of phosophodiesterase 4 for the treatment of asthmaJ Med Chem41821359526558
- ClaveauDChenSLO’KeefeS2004Preferential inhibition of T helper 1, but not T helper 2, cytokines in vitro by L-826,141 [4-[2-(3,4-Bisdifluromethoxyphenyl)-2-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxy propan-2-yl)-phenyl]-ethyl]3-methylpyridine-1-oxide], a potent and selective phosphodiesterase 4 inhibitorJ Pharmacol Exp Ther3107526015082748
- ComptonCHGubbJNiemanR2001Cilomilast, a selective phosphodiesterase-4 inhibitor for treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging studyLancet3582657011498212
- ContiMBeavoJ2007Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signalingAnnu Rev Biochem7648151117376027
- ContiMJinSL1999The molecular biology of cyclic nucleotide phosphodiesterasesProg Nucleic Acid Res Mol Biol6313810506827
- CorbinJDBlountMAWeeksJL2nd2003[3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMPMol Pharmacol6313647212761347
- CorbinJDFrancisSH1999Cyclic GMP phosphodiesterase-5: target of sildenafilJ Biol Chem274137293210318772
- CorbinJDTurkoIVBeasleyA2000Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activitiesEur J Biochem2672760710785399
- De BoerJPhilpottAJVan AmsterdamRG1992Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitorsBr J Pharmacol1061028341393276
- DegermanEBelfragePManganielloVC1997Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3)J Biol Chem272682369102399
- DentGGiembyczMAEvansPM1994Suppression of human eosinophil respiratory burst and cyclic AMP hydrolysis by inhibitors of type IV phosphodiesterase: interaction with the beta adrenoceptor agonist albuterolJ Pharmacol Exp Ther2711167747996422
- DentGWhiteSRTenorH1998Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: characterization of isoenzymes and functional effects of PDE inhibitorsPulm Pharmacol Ther1147569802963
- Dodge-KafkaKLSoughayerJPareGC2005The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathwaysNature437574816177794
- DodgeKLKhouangsathieneSKapiloffMS2001mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling moduleEMBO J2019213011296225
- DolciSBelmonteASantoneR2006Subcellular localization and regulation of type-1C and type-5 phosphodiesterasesBiochem Biophys Res Commun3418374616455054
- DuplantierAJBachertELChengJB2007SAR of a series of 5,6-dihydro-(9H)-pyrazolo[3,4-c]-1,2,4-triazolo[4,3-alpha]pyridines as potent inhibitors of human eosinophil phosphodiesteraseJ Med Chem50344917228876
- EneferS2005Inflammation 2005 – Seventh World Congress. Highlights IIDrugs87889016254791
- EngelsPFichtelKLubbertH1994Expression and regulation of human and rat phosphodiesterase type IV isogenesFEBS Lett35029158070581
- EngelsPSullivanMMullerT1995Molecular cloning and functional expression in yeast of a human cAMP-specific phosphodiesterase subtype (PDE IV-C)FEBS Lett358305107843419
- EvgenovOVBuschCJEvgenovNV2006Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertensionAm J Physiol Lung Cell Mol Physiol290L723L916284211
- FidockMMillerMLanfearJ2002Isolation and differential tissue distribution of two human cDNAs encoding PDE1 splice variantsCell Signal14536011747989
- FrancisSHLincolnTMCorbinJD1980Characterization of a novel cGMP binding protein from rat lungJ Biol Chem25562066153179
- FrancisSHTurkoIVCorbinJD2001Cyclic nucleotide phosphodiesterases: relating structure and functionProg Nucleic Acid Res Mol Biol6515211008484
- FriedmanDLJohnsonRAZeiligCE1976The role of cyclic nucleotides in the cell cycleAdv Cyclic Nucleotide Res769114188318
- FujimuraMKamioYSaitoM1995Bronchodilator and broncho-protective effects of cilostazol in humans in vivoAm J Respir Crit Care Med15122257812559
- FukaoMMasonHSBrittonFC1999Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072J Biol Chem274109273510196172
- FuruieHNakagawaSKawashimaM2003Suppressive effect of novel phosphodiesterase4 (PDE4) inhibitor ONO-6126 on TNF-α release was increased after repeated oral administration in healthy Japanese subjectsEur Respir J22Suppl 45395s
- GaleDDLandellsLJSpinaD2002Pharmacokinetic and pharmaco-dynamic profile following oral administration of the phosphodiesterase (PDE)4 inhibitor V11294A in healthy volunteersBr J Clin Pharmacol544788412445026
- GambleEGrootendorstDCBrightlingCE2003Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1689768212816740
- GardnerCRobasNCawkillD2000Cloning and characterization of the human and mouse PDE7B, a novel cAMP-specific cyclic nucleotide phosphodiesteraseBiochem Biophys Res Commun2721869210872825
- GhofraniHAVoswinckelRReichenbergerF2004Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective studyJ Am Coll Cardiol4414889615464333
- GiembyczMA2005Phosphodiesterase-4. Selective and dual specificity inhibitors for the therapy of chronic obstructive pulmonary diseaseProc Am Thorac Soc232633316267357
- GiembyczMACorriganCJSeyboldJ1996Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2Br J Pharmacol1181945588864528
- GiovannoniMPCesariNGrazianoA2007Synthesis of pyrrolo[2,3-d]pyridazinones as potent, subtype selective PDE4 inhibitorsJ Enzyme Inhib Med Chem223091817674813
- GlavasNAOstensonCSchaeferJB2001T cell activation up-regulates cyclic nucleotide phosphodiesterases 8A1 and 7A3Proc Natl Acad Sci U S A9863192411371644
- Global Initiative for Chronic Obstructive Pulmonary Disease2006Global strategy for the diagnosis, management and prevention of chronicobstructive pulmonary disease, Executive Summary URL: www.goldcopd.com
- GrashoffWFSontJKSterkPJ1997Chronic obstructive pulmonary disease: role of bronchiolar mast cells and macrophagesAm J Pathol1511785909403729
- GriswoldDEWebbEFBadgerAM1998SB 207499 (Ariflo), a second generation phosphodiesterase 4 inhibitor, reduces tumor necrosis factor alpha and interleukin-4 production in vivoJ Pharmacol Exp Ther287705119808700
- GrootendorstDCGauwSABenschopN2003Efficacy of the novel phosphodiesterase-4 inhibitor BAY 19-8004 on lung function and airway inflammation in asthma and chronic obstructive pulmonary disease (COPD)Pulm Pharmacol Ther16341714580925
- GutkeHJGuseJHKhobzaouiM2005AWD-12-281 (inhaled) (elbion/GlaxoSmithKline)Curr Opin Investig Drugs6114958
- HabenerJFDegrooLJJamesonJL2001The cyclic AMP second messenger signaling pathwayEndocrinologyPhiladelphiaWB Saunders
- HalpinDMG2007Systemic Effects of COPDExpert Rev Resp Med17584
- HambletonRKrallJTikishviliE2005Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP hydrolytic activity in subcellular fractions of human myocardiumJ Biol Chem280391687416172121
- HanPFletcherCFCopelandNG1998Assignment of the mouse Pde7A gene to the proximal region of chromosome 3 and of the human PDE7A gene to chromosome 8q13Genomics4827569521885
- HanPSonatiPRubinC2006PDE7A1, a cAMP-specific phosphodiesterase, inhibits cAMP-dependent protein kinase by a direct interaction with CJ Biol Chem28115050716556600
- HanPZhuXMichaeliT1997/Alternative splicing of the high affinity cAMP-specific phosphodiesterase (PDE7A) mRNA in human skeletal muscle and heartJ Biol Chem2721615279195912
- HatzelmannASchudtC2001Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitroJ Pharmacol Exp Ther2972677911259554
- HeaslipRJLombardoLJGolankiewiczJM1994Phosphodiesterase-IV inhibition, respiratory muscle relaxation and bronchodilation by WAY-PDA-641J Pharmacol Exp Ther268888968114002
- HetmanJMSoderlingSHGlavasNA2000Cloning and characterization of PDE7B, a cAMP-specific phosphodiesteraseProc Natl Acad Sci U S A97472610618442
- HoggJCChuFUtokaparchS2004The nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med35026455315215480
- HorowskiRSastre-Y-HernandezM1985Clinical effects of the neurotropic selective cAMP phosphodiesterase inhibitor rolipram in depressed patients: global evaluation of the preliminary reportsCurr Ther Res38239
- HortonYMSullivanMHouslayMD1995Molecular cloning of a novel splice variant of human type IVA (PDE-IVA) cyclic AMP phosphodiesterase and localization of the gene to the p13.2–q12 region of human chromosome 19 [corrected]Biochem J308683917772058
- HouslayMDSchaferPZhangKY2005Keynote review: phosphodiesterase-4 as a therapeutic targetDrug Discov Today1015031916257373
- HuangZLiuSZhangL2006Preferential inhibition of human phosphodiesterase 4 by ibudilastLife Sci782663816313925
- JefferyPK2001aLymphocytes, chronic bronchitis and chronic obstructive pulmonary diseaseNovartis Found Symp23414961 discussion 161–811199094
- JefferyPK2001bRemodeling in asthma and chronic obstructive lung diseaseAm J Respir Crit Care Med164S283811734464
- JeonYHHeoYSKimCM2005Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug developmentCell Mol Life Sci62119822015798894
- JiangXLiJPaskindMEpsteinPM1996Inhibition of calmodulin-dependent phosphodiesterase induces apoptosis in human leukemic cellsProc Natl Acad Sci U S A9311236418855339
- JinSLContiM2002Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responsesProc Natl Acad Sci U S A9976283312032334
- KakiuchiSYamazakiR1970Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3′,5′-nucleotide phosphodiesterase (3)Biochem Biophys Res Commun411104104320714
- KakkarRRajuRVRajputAH1996Inhibition of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase isozymes by deprenylLife Sci5933741
- KakkarRRajuRVRajputAH1997Amantadine: an antiparkinsonian agent inhibits bovine brain 60 kDa calmodulin-dependent cyclic nucleotide phosphodiesterase isozymeBrain Res74929049138729
- KakkarRRajuRVSharmaRK1999Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1)Cell Mol Life Sci5511648610442095
- KammerGM1988The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune responseImmunol Today922292855581
- KandaNWatanabeS2001Regulatory roles of adenylate cyclase and cyclic nucleotide phosphodiesterases 1 and 4 in interleukin-13 production by activated human T cellsBiochem Pharmacol6249550711448460
- KasuyaJLiangSJGokoH2000Cardiac type cGMP-inhibited phosphodiesterase (PDE3A) gene structure: similarity and difference to adipocyte type PDE3B geneBiochem Biophys Res Commun2688273410679291
- KirsteinMRivet-BastideMHatemS1995Nitric oxide regulates the calcium current in isolated human atrial myocytesJ Clin Invest957948027860763
- KitamuraTKitamuraYKurodaS1999Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase AktMol Cell Biol1962869610454575
- KoteraJFujishigeKAkatsukaH1998Novel alternative splice variants of cGMP-binding cGMP-specific phosphodiesteraseJ Biol Chem27326982909756948
- KovalaTSanwalBDBallEH1997Recombinant expression of a type IV, cAMP-specific phosphodiesterase: characterization and structure-function studies of deletion mutantsBiochemistry362968769062127
- KoyamaHBornfeldtKEFukumotoS2001Molecular pathways of cyclic nucleotide-induced inhibition of arterial smooth muscle cell proliferationJ Cell Physiol18611011147803
- KucharekovaMHornixMAshikagaT2003The effect of the PDE-4 inhibitor (cipamfylline) in two human models of irritant contact dermatitisArch Dermatol Res295293212709818
- LedbetterJAParsonsMMartinPJ1986Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppressionJ Immunol13732993053021852
- LeeCGHomerRJZhuZ2001Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1)J Exp Med1948092111560996
- LeemanMLejeunePMelotC1987Reduction in pulmonary hypertension and in airway resistances by enoximone (MDL 17,043) in decompensated COPDChest9166262952467
- LenhardJMKasselDBRocqueWJ1996Phosphorylation of a cAMP-specific phosphodiesterase (HSPDE4B2B) by mitogen-activated protein kinaseBiochem J31675188670148
- LewDBNebigilCMalikKU1992Dual regulation by cAMP of beta-hexosaminidase-induced mitogenesis in bovine tracheal myocytesAm J Respir Cell Mol Biol761491333245
- LiLYeeCBeavoJA1999CD3- and CD28-dependent induction of PDE7 required for T cell activationScience283848519933169
- LinCSLauATuR2000Expression of three isoforms of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in human penile cavernosumBiochem Biophys Res Commun2686283510679255
- LincolnTMHallCLParkCR1976Guanosine 3′,5′-cyclic mono-phosphate binding proteins in rat tissuesProc Natl Acad Sci U S A732559638775
- LipworthBJ2005Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary diseaseLancet3651677515639300
- LoherFSchmallKFreytagP2003The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in miceJ Pharmacol Exp Ther3055495612606674
- LoscoPEEvansEWBaratSA2004The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeysToxicol Pathol3229530815204971
- LoughneyKHillTRFlorioVA1998Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′,5′-cyclic nucleotide phosphodiesteraseGene216139479714779
- MacdonaldEVan Der LeeHPocockD2007A novel phosphodiesterase type 4 inhibitor, HT-0712, enhances rehabilitation-dependent motor recovery and cortical reorganization after focal cortical ischemiaNeurorehabil Neural Repair214869617823313
- MackenzieSJBaillieGSMcPheeI2000ERK2 mitogen-activated protein kinase binding, phosphorylation, and regulation of the PDE4D cAMP-specific phosphodiesterases. The involvement of COOH-terminal docking sites and NH2-terminal UCR regionsJ Biol Chem275166091710828059
- MackenzieSJBaillieGSMcPheeI2002Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1)Br J Pharmacol1364213312023945
- MackintoshC2004Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processesBiochem J3813294215167810
- ManningCDBurmanMChristensenSB1999Suppression of human inflammatory cell function by subtype-selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4BBr J Pharmacol1281393810602317
- McAllister-LucasLMHaikTLColbranJL1995An essential aspartic acid at each of two allosteric cGMP-binding sites of a cGMP-specific phosphodiesteraseJ Biol Chem2703067198530505
- McAllister-LucasLMSonnenburgWKKadlecekA1993The structure of a bovine lung cGMP-binding, cGMP-specific phosphodiesterase deduced from a cDNA cloneJ Biol Chem26822863738226796
- McConnachieGLangebergLKScottJD2006AKAP signaling complexes: getting to the heart of the matterTrends Mol Med123172316809066
- McLaughlinMMCieslinskiLBBurmanM1993A low-Km, rolipram-sensitive, cAMP-specific phosphodiesterase from human brain. Cloning and expression of cDNA, biochemical characterization of recombinant protein, and tissue distribution of mRNAJ Biol Chem268647068384210
- MichaeliTBloomTJMartinsT1993Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP phosphodiesterase-deficient Saccharomyces cerevisiaeJ Biol Chem26812925328389765
- MichibataHYanakaNKanohY2001Human Ca2+/calmodulin-dependent phosphodiesterase PDE1A: novel splice variants, their specific expression, genomic organization, and chromosomal localizationBiochim Biophys Acta15172788711342109
- MikiTTairaMHockmanS1996Characterization of the cDNA and gene encoding human PDE3B, the cGIP1 isoform of the human cyclic GMP-inhibited cyclic nucleotide phosphodiesterase familyGenomics36476858884271
- MilatovichABolgerGMichaeliT1994Chromosome localizations of genes for five cAMP-specific phosphodiesterases in man and mouseSomat Cell Mol Genet207586
- MongilloMMcSorleyTEvellinS2004Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterasesCirc Res95677515178638
- MooreARWilloughbyDA1995The role of cAMP regulation in controlling inflammationClin Exp Immunol10138797664483
- MullerTEngelsPFozardJR1996Subtypes of the type 4 cAMP phosphodiesterases: structure, regulation and selective inhibitionTrends Pharmacol Sci1729488810876
- MullershausenFRusswurmMThompsonWJLiu2001Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzymeJ Cell Biol155271811604422
- MurthyKS2001Activation of phosphodiesterase 5 and inhibition of guanylate cyclase by cGMP-dependent protein kinase in smooth muscleBiochem J36019920811696008
- MyouSFujimuraMKamioY1999Bronchodilator effect of inhaled olprinone, a phosphodiesterase 3 inhibitor, in asthmatic patientsAm J Respir Crit Care Med1608172010471602
- National Institute for Clinical Excellence (Nice)2004Chronic obstructive pulmonary disease. National clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary careThorax59Suppl 11232
- NemozGZhangRSetteCContiM1996Identification of cyclic AMP-phosphodiesterase variants from the PDE4D gene expressed in human peripheral mononuclear cellsFEBS Lett384971028797812
- NewmanSPRiveroXLuriaXHooperGPitcairnGR1997A scintigraphic study to evaluate the deposition patterns of a novel anti-asthma drug inhaled from the Cyclohaler dry powder inhalerAdv Drug Deliv Rev26596710837533
- NielsonCPVestalRESturmRJ1990Effects of selective phosphodiesterase inhibitors on the polymorphonuclear leukocyte respiratory burstJ Allergy Clin Immunol8680181977788
- NovackJPCharbonneauHBentleyJK1991Sequence comparison of the 63-, 61-, and 59-kDa calmodulin-dependent cyclic nucleotide phosphodiesterasesBiochemistry30794071651112
- O’connellJCMccallumJFMcPheeI1996The SH3 domain of Src tyrosyl protein kinase interacts with the N-terminal splice region of the PDE4A cAMP-specific phosphodiesterase RPDE-6 (RNPDE4A5)Biochem J318255618761480
- O’ShaughnessyTCAnsariTWBarnesNC1997Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1Am J Respir Crit Care Med15585279117016
- OkadaDAsakawaS2002Allosteric activation of cGMP-specific, cGMP-binding phosphodiesterase (PDE5) by cGMPBiochemistry419672912135389
- OuaguedMMartin-ChoulyCABrinchaultG2005The novel phosphodiesterase 4 inhibitor, CI-1044, inhibits LPS-induced TNF-alpha production in whole blood from COPD patientsPulm Pharmacol Ther18495415607127
- PastanIHJohnsonGSAndersonWB1975Role of cyclic nucleotides in growth controlAnnu Rev Biochem44491522166606
- PauvertOSalvailDRousseauE2002Characterisation of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary arteryBiochem Pharmacol6317637212007579
- PeachellPTUndemBJSchleimerRP1992Preliminary identification and role of phosphodiesterase isozymes in human basophilsJ Immunol1482503101373172
- PooleyLShakurYRenaG1997Intracellular localization of the PDE4A cAMP-specific phosphodiesterase splice variant RD1 (RNPDE4A1A) in stably transfected human thyroid carcinoma FTC cell linesBiochem J321177859003417
- Pozuelo RubioMCampbellDGMorriceNA2005Phosphodiesterase 3A binds to 14-3-3 proteins in response to PMA-induced phosphorylation of Ser428Biochem J3921637216153182
- Pozuelo RubioMGeraghtyKMWongBH200414-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and traffickingBiochem J37939540814744259
- ProfitaMChiapparaGMirabellaF2003Effect of cilomilast (Ariflo) on TNF-alpha, IL-8, and GM-CSF release by airway cells of patients with COPDThorax58573912832668
- RabeKFBatemanEDO’DonnellD2005Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trialLancet3665637116099292
- RabeKFTenorHDentG1993Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterizationAm J Physiol264L458647684572
- RasconADegermanETairaM1994Identification of the phosphorylation site in vitro for cAMP-dependent protein kinase on the rat adipocyte cGMP-inhibited cAMP phosphodiesteraseJ Biol Chem2691196268163498
- ReinhardtRRChinEZhouJ1995Distinctive anatomical patterns of gene expression for cGMP-inhibited cyclic nucleotide phosphodiesterasesJ Clin Invest951528387706458
- RennardSISchachterNStrekM2006Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4Chest129566616424413
- RichterWContiM2004The oligomerization state determines regulatory properties and inhibitor sensitivity of type 4 cAMP-specific phosphodiesterasesJ Biol Chem279303384815131123
- RichterWDayPAgrawalR2008Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4Embo J273849318188154
- RobichaudAStamatiouPBJinSL2002Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesisJ Clin Invest11010455212370283
- RobicsekSABlanchardDKDjeuJY1991Multiple high-affinity cAMP-phosphodiesterases in human T-lymphocytesBiochem Pharmacol42869771651080
- RondinoneCMCarvalhoERahnT2000Phosphorylation of PDE3B by phosphatidylinositol 3-kinase associated with the insulin receptorJ Biol Chem27510093810744689
- RybalkinSDBornfeldtKESonnenburgWK1997Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotypeJ Clin Invest1002611219366577
- RybalkinSDRybalkinaIBeavoJA2002aCyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferationCirc Res90151711834707
- RybalkinSDRybalkinaIGFeilR2002bRegulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cellsJ Biol Chem2773310711723116
- RybalkinSDRybalkinaIGShimizu-AlbergineM2003PDE5 is converted to an activated state upon cGMP binding to the GAF A domainEmbo J224697812554648
- SaettaMBaraldoSCorbinoL1999CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med160711710430750
- SaettaMDi StefanoAMaestrelliP1994Airway eosinophilia in chronic bronchitis during exacerbationsAm J Respir Crit Care Med1501646527952628
- SaettaMTuratoGMaestrelliP2001Cellular and structural bases of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1631304911371392
- SasakiTKoteraJYuasaK2000Identification of human PDE7B, a cAMP-specific phosphodiesteraseBiochem Biophys Res Commun2715758310814504
- SastryBKNarasimhanCReddyNK2004Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover studyJ Am Coll Cardiol4311495315063421
- SchillaceRVAndrewsSFLibertyGA2002Identification and characterization of myeloid translocation gene 16b as a novel a kinase anchoring protein in T lymphocytesJ Immunol16815909
- SchlossmannJAmmendolaAAshmanK2000Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase IbetaNature40419720110724174
- SchlossmannJFeilRHofmannF2003Signaling through NO and cGMP-dependent protein kinasesAnn Med3521712693609
- SchudtCTenorHHatzelmannA1995PDE isoenzymes as targets for anti-asthma drugsEur Respir J81179837589403
- SebkhiAStrangeJWPhillipsSC2003Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertensionCirculation1073230512796132
- SeldonPMBarnesPJMejaK1995Suppression of lipopolysaccharide-induced tumor necrosis factor-alpha generation from human peripheral blood monocytes by inhibitors of phosphodiesterase 4: interaction with stimulants of adenylyl cyclaseMol Pharmacol48747577476903
- SetteCContiM1996Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activationJ Biol Chem27116526348663227
- ShakurYPrydeJGHouslayMD1993Engineered deletion of the unique N-terminal domain of the cyclic AMP-specific phosphodiesterase RD1 prevents plasma membrane association and the attainment of enhanced thermostability without altering its sensitivity to inhibition by rolipramBiochem J292677867686364
- ShakurYWilsonMPooleyL1995Identification and characterization of the type-IVA cyclic AMP-specific phosphodiesterase RD1 as a membrane-bound protein expressed in cerebellumBiochem J30680197702577
- SharmaRK1991Phosphorylation and characterization of bovine heart calmodulin-dependent phosphodiesteraseBiochemistry30596381646004
- SharmaRKDasSBLakshmikuttyammaA2006Regulation of calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1): reviewInt J Mol Med189510516786160
- SharmaRKKalraJ1993Ginsenosides are potent and selective inhibitors of some calmodulin-dependent phosphodiesterase isozymesBiochemistry32497588388250
- SharmaRKSeitzDPSinghB2002Localization and regulation of bovine eye calmodulin-dependent cyclic nucleotide phosphodiesterase by cyclic AMP-dependent protein kinaseInt J Mol Med10172312060846
- SharmaRKWangJH1985Differential regulation of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase isoenzymes by cyclic AMP-dependent protein kinase and calmodulin-dependent phosphataseProc Natl Acad Sci U S A82260372986124
- SharmaRKWangJH1986Calmodulin and Ca2+-dependent phosphorylation and dephosphorylation of 63-kDa subunit-containing bovine brain calmodulin-stimulated cyclic nucleotide phosphodiesterase isozymeJ Biol Chem261132283944089
- SharmaRKWangJHWuZ1997Mechanisms of inhibition of calmodulin-stimulated cyclic nucleotide phosphodiesterase by dihydropyridine calcium antagonistsJ Neurochem69845509231746
- ShepherdMCBaillieGSStirlingDI2004Remodelling of the PDE4 cAMP phosphodiesterase isoform profile upon monocyte-macrophage differentiation of human U937 cellsBr J Pharmacol1423395115066910
- SmithCJKrallJManganielloVC1993Cytosolic and sarcoplasmic reticulum-associated low Km, cGMP-inhibited cAMP phosphodiesterase in mammalian myocardiumBiochem Biophys Res Commun190516218381278
- SmithSJBrookes-FazakerleySDonnellyLE2003Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cellsAm J Physiol Lung Cell Mol Physiol284L2798912388353
- SmithSJCieslinskiLBNewtonR2004Discovery of BRL 50481 [3-(N,N-dimethylsulfonamido)-4-methyl-nitrobenzene], a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytesMol Pharmacol6616798915371556
- SoderlingSHBeavoJA2000Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functionsCurr Opin Cell Biol12174910712916
- SonnenburgWKSegerDKwakKS1995Identification of inhibitory and calmodulin-binding domains of the PDE1A1 and PDE1A2 calmodulin-stimulated cyclic nucleotide phosphodiesterasesJ Biol Chem2703098910008537356
- SturtonGFitzgeraldM2002Phosphodiesterase 4 inhibitors for the treatment of COPDChest121192S6S12010850
- SungBJHwangKYJeonYH2003Structure of the catalytic domain of human phosphodiesterase 5 with bound drug moleculesNature4259810212955149
- SurksHKMochizukiNKasaiY1999Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase IalphaScience2861583710567269
- SutherlandEW1970On the biological role of cyclic AMPJAMA214128184320556
- SzpirerCSzpirerJRiviereM1995Chromosomal localization of the human and rat genes (PDE4D and PDE4B) encoding the cAMP-specific phosphodiesterases 3 and 4Cytogenet Cell Genet691147835077
- TenorHStaniciuLSchudtC1995Cyclic nucleotide phosphodiesterases from purified human CD4+ and CD8+ T lymphocytesClin Exp Allergy25616248521180
- TeoTSWangJH1973Mechanism of activation of a cyclic adenosine 3′,5′-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding proteinJ Biol Chem248595054353626
- ThomasMKFrancisSHCorbinJD1990aCharacterization of a purified bovine lung cGMP-binding cGMP phosphodiesteraseJ Biol Chem26514964701697584
- ThomasMKFrancisSHCorbinJD1990bSubstrate- and kinase-directed regulation of phosphorylation of a cGMP-binding phosphodiesterase by cGMPJ Biol Chem2651497182168396
- ThompsonWJ1991Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and functionPharmacol Ther5113331663250
- ThompsonWJApplemanMM1971Characterization of cyclic nucleotide phosphodiesterases of rat tissuesJ Biol Chem2463145504324892
- ThompsonWJAshikagaTKellyJJ2002Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4)Biochem Pharmacol6379780711992650
- TomlinsonPRWilsonJWStewart1995Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: relationship to elevated cAMP levelsBiochem Pharmacol491809197598743
- TorphyTJ1998Phosphodiesterase isozymes: molecular targets for novel antiasthma agentsAm J Respir Crit Care Med157351709476844
- TorphyTJBarnetteMSUnderwoodDC1999Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinicPulm Pharmacol Ther12131510373396
- TorphyTJUndemBJCieslinskiLB1993Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscleJ Pharmacol Exp Ther2651213238389856
- TowardTJSmithNBroadleyKJ2004Effect of phosphodiesterase-5 inhibitor, sildenafil (Viagra), in animal models of airways diseaseAm J Respir Crit Care Med1692273414597480
- TsoumakidouMDemedtsIKBrusselleGG2008Dendritic cells in COPD: new players in an old gameAm J Respir Crit Care Med In press
- UkitaTSugaharaMTerakawaY1999Novel, potent, and selective phosphodiesterase-4 inhibitors as antiasthmatic agents: synthesis and biological activities of a series of 1-pyridylnaphthalene derivativesJ Med Chem4210889910090791
- VerdeIPahlkeGSalanovaM2001Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesteraseJ Biol Chem276111899811134006
- WoutersEF2002Chronic obstructive pulmonary disease. 5: systemic effects of COPDThorax5710677012454303
- WrightKFTurnerCRJayasinghe-BeckR1997Differential in vivo and in vitro bronchorelaxant activities of CP-80,633, a selective phosphodiesterase 4 inhibitorCan J Physiol Pharmacol75100189360015
- WyattTANaftilanAJFrancisSH1998ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cellsAm J Physiol274H448559486247
- XuRXHassellAMVanderwallD2000Atomic structure of PDE4: insights into phosphodiesterase mechanism and specificityScience2881822510846163
- YanCZhaoAZBentleyJK1996The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific mannerJ Biol Chem271256997068810348
- YanakaNKoteraJOhtsukaA1998Expression, structure and chromosomal localization of the human cGMP-binding cGMP-specific phosphodiesterase PDE5A geneEur J Biochem25539199716380
- YangGMcintyreKWTownsendRM2003Phosphodiesterase 7A-deficient mice have functional T cellsJ Immunol17164142014662840
- ZhangKYCardGLSuzukiY2004A glutamine switch mechanism for nucleotide selectivity by phosphodiesterasesMol Cell152798615260978
- ZhaoLMasonNAMorrellNW2001Sildenafil inhibits hypoxia-induced pulmonary hypertensionCirculation104424811468204
- ZhouGBaoZQDixonJE1995Components of a new human protein kinase signal transduction pathwayJ Biol Chem2701266597759517