Abstract
Introduction
Intravenous augmentation therapy with purified intravenous alpha-1 antitrypsin replaces the deficient protein and is the only currently approved treatment for alpha-1 antitrypsin deficiency (AATD) related lung disease. While augmentation therapy has been available for more than 20 years, there are a limited number of studies evaluating the effect of augmentation on lung function.
Material and methods
We examined the decline in forced expiratory volume in one second (FEV1) in patients enrolled in the Alpha-1 Foundation DNA and Tissue Bank in relation to the use or not of alpha-1 antitrypsin augmentation therapy. For the purpose of our analysis we included 164 patients with AATD and PI ZZ genotype.
Results
Mean age of the patients was 60 years, 52% were females, 94% were white and 78% ex-smokers. The mean FEV1 at baseline was 1.7 L and the mean FEV1 % of predicted was 51.3%. The mean follow-up time was 41.7 months. A total of 124 (76%) patients received augmentation therapy (augmented group) while 40 patients (24%) did not received it (non-augmented group). When adjusted by age at baseline, sex, smoking status, baseline FEV1 % of predicted, the mean overall change in FEV1 was 47.6 mL/year, favoring the augmented group (ΔFEV1 10.6 ± 21.4 mL/year) in comparison with the non-augmented group (ΔFEV1 −36.96 ± 12.1 mL/year) (P = 0.05). Beneficial ΔFEV1 were observed in ex-smokers and the group with initial FEV1 % of predicted of <50%. No differences were observed in mortality.
Conclusions
In conclusion, augmentation therapy improves lung function in subjects with AATD when adjusted by age, gender, smoking status and baseline FEV1 % of predicted. The beneficial effects were noted in ex-smoker subjects with FEV1 below 50% of predicted.
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant inherited condition characterized by low levels of alpha-1 antitrypsin in serum and tissues. This protein, primarily produced in the liver, is the most prevalent protease inhibitor in serum and its main function is to inhibit neutrophil elastase, a proteolytic enzyme capable of destroying alveolar structures.Citation1–Citation3 The imbalance between proteases and antiproteases leads to alveolar destruction that in turn results in a rapid decline of the forced expiratory volume in one second (FEV1) and the development of emphysema at an early age.Citation4 Factors associated with a more rapid FEV1 decline include male gender, older age (specially after 50 years), low BMI, current smoking status, presence of bronchodilator response, frequent respiratory exacerbations and mid-range FEV1 % of predicted (actual percentage varied according to the studies from 35% to 80%).Citation5–Citation14
Augmentation therapy with purified intravenous alpha-1 antitrypsin replaces the deficient protein and is the only currently approved treatment for AATD. Few observational studies have demonstrated a beneficial effect of augmentation therapy with alpha-1 antitrypsin, in reducing the rapid decline in lung function seen in patients with this condition.Citation5,Citation15,Citation16 However, a randomized study in patients with moderate to severe emphysema demonstrated no changes in the annual FEV1 decline in the placebo versus the treatment arm.Citation17
We sought to examine the effect of alpha-1 antitrypsin augmentation therapy on FEV1 decline, in AATD patients enrolled in the Alpha-1 Foundation DNA and Tissue Bank study.
Material and methods
The Alpha-1 Foundation DNA and Tissue Bank collected, from across the United States, medical information and human tissue of individuals with AATD, immediate relatives of individuals with AATD and subjects without the disease who were interested in the project.
The Alpha-1 Foundation DNA and Tissue Bank project is sponsored by the Alpha-1 Foundation and is physically located at the University of Florida College of Medicine, Gainesville, FL, USA. The Alpha-1 Foundation DNA and Tissue Bank protocol and analysis of the data presented in this article were approved by the University of Florida Institutional Review Board.
Upon enrollment, individuals signed an informed consent and completed a registration form as well as an extensive medical questionnaire. The answers written in the introductory questionnaire were reviewed in detail and corroborated during an initial phone interview with the study participant. If the individuals indicated that they agreed to be contacted, they were called annually to update their medical information.
FEV1 measured in liters and percentage of predicted were recorded at the time of the initial medical questionnaire and during annual phone interviews. For the purpose of the current analysis, patients were included if they had a proven PI ZZ genotype (by Taqman allelic discrimination) and at least two recorded postbronchodilator FEV1 measurements, 6 months apart or more. Patients not meeting the inclusion criteria or who underwent lung or liver transplant were excluded from the analysis. Great attention was put to assure high quality spirometric measurements following American Thoracic Society standards. Spirometry results that questioned the patient’s effort during the test were not recorded.
All the recorded FEV1 measurements were used to estimate the change in FEV1 (ΔFEV1). A change in FEV1 (ΔFEV1) is defined as the initial FEV1 in L/m minus the FEV1 obtained by random effects model. A positive ΔFEV1 represents an increase in FEV1 and a negative ΔFEV1 corresponds to a decrease in FEV1. We favored the use of ΔFEV1 instead of decline in FEV1 as we believe that a positive or negative ΔFEV1 is easier to understand than a positive or negative rate of decline in FEV1 or a positive or negative FEV1 slope (5). The overall change in FEV1 was defined as the difference in ΔFEV1 between the augmented and the non-augmented group.
Patients were divided into 2 groups: 1) “augmented” (patients who were receiving augmentation therapy at time of the inclusion in the study), 2) “nonaugmented“(patients who were not receiving augmentation therapy at the time of the inclusion in the study).
The decision to treat or not treat AATD patients with augmentation therapy was made by the patients’ physicians. The Alpha-1 Foundation DNA and Tissue Bank did not intervene in any way in the treatment plan of patients that participated in this study.
Patients were stratified in different groups according to the initial FEV1 percentage of predicted. One stratification divided the patients in 3 groups (FEV1 < 30%, 30% to 65% and >65% of predicted) using the most commonly described cut-off in the literature.Citation15,Citation16 In order to increase the numbers of patients in each group and provide more reliable conclusions, patients were also divided in 2 groups (FEV1 < 50% or ≥50% of predicted).Citation5 In addition we studied the group of patients with an initial FEV1 of 35% to 49% of predicted, because this group was the one that benefit the most from augmentation therapy in the Alpha-1-Antitrypsin Deficiency Registry Study Group.Citation5
Patients were also classified according to the smoking status. Current smoker was defined as the individual who was smoking at the time of the inclusion in the study. Ex-smoker was defined as a person who had not smoked for at least 3 months. Nonsmoker was defined as a subject who smoked less than 20 packs in his/her lifetime.
Statistical analysis
The decline in post-bronchodilator FEV1 per year was estimated separately for each group by random effects models, which included FEV1 as the outcome variable, FEV1 (% of predicted value) and age at baseline, sex and smoking status as fixed parameters, and the individual patients and follow-up time as random effects parameters. The estimated ΔFEV1 was compared between the augmented and the non-augmented groups, and the P-value was calculated using Z-test. The analysis was repeated after including 26 patients in whom the smoking history was initially missing. This information was retrieved from an earlier version of the study database. T-test and Chi-square test or Fisher’s exact test were used to compare continuous and categorical variables, respectively. The analysis of mortality at 5 years was performed with the use of logistic regression with age, gender, baseline FEV1, presence of COPD and smoking status as covariates. Values are expressed as mean (± standard error of the mean). A P-value < 0.05 was considered significant. All calculations were performed with the use of SAS software (version 6.10; SAS Institute Cary, NC, USA). Figures were created with Prism 5 for Windows version 5.00 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Baseline characteristics
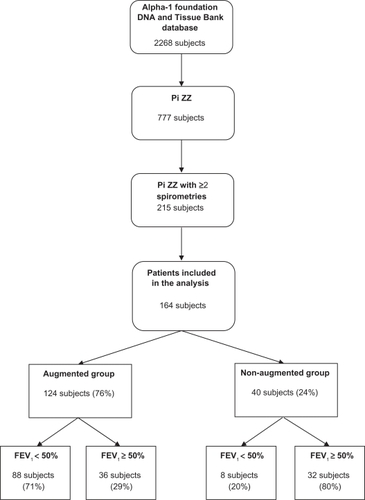
Out of a total of 2,268 patients included in the Alpha-1 Foundation DNA and Tissue bank from January 2001 to April 2009, 777 patients had a Pi ZZ genotype. Of the patients with Pi ZZ genotype only 215 had 2 or more measurements of FEV1 at least 6 months apart. We excluded 17 patients who had lung (n = 15) or liver transplantation (n = 2), and 8 patients who did not have the initial FEV1 % of predicted, which was used as a baseline covariate in the model. Twenty-six patients had missing smoking history. For the purpose of our analysis we included 164 patients, in whom all the variables included in the random effects model were available ().
The mean age at the time of inclusion in the study was 60 (±0.73) years. The mean age at the time of diagnosis of AATD was 45.9 (±0.72) years. The majority of patients (94%) were white (the rest did not report race) and 51.8% were females. The greater part of patients were ex-smokers (n = 128, 78%). Nonsmokers and current smokers constituted 20.8% (n = 34) and 1.2% (n = 2) of the individuals, respectively. Of the patients that smoked and in whom data was available (n = 120), the mean number of years exposed to smoke was 18.9 (±0.74) years. The post-bronchodilator mean FEV1 at baseline was 1.7 (±0.07) liters and the mean initial FEV1 percentage of predicted was 51.3 (±2)%.
Follow-up
The mean (SEM; range) follow-up time was 41.7 (±2.6; range: 6 to 268) months. The number of spirometries was 2, 3, and 4 in 67%, 18% and 15% of patients, respectively (median of 2). During the follow-up 5.5% (n = 9) patients died.
Augmented versus non-augmented patients
Of the patients included in the analysis, 124 (76%) stated that they were receiving augmentation therapy (augmented group) and 40 patients (24%) declared that they were not receiving augmentation therapy (non-augmented group) at the time of the introductory questionnaire. In the augmented group, this treatment was initiated a mean of 69.6 (±6.7) months before the first FEV1 measurement available in the database. 20 patients initiated this therapy after the first and before the second FEV1 result. The augmentation therapy used was predominantly weekly intravenous Prolastin® (Talecris Biotherapeutics), in 88% of the patients. Less commonly the patients received Aralast® (Baxter) (10% of the patients) and Zemaira® (CSL-Behring) (2% of the patients). Data were available in only 48 patients (39%).
The group treated with augmentation therapy was older (61.3 versus 56.1 years, P = 0.014) and had higher percentage of ex-smokers individuals (84.7 versus 62.5%, P < 0.001). The presence of dyspnea, asthma and COPD (chronic obstructive pulmonary disease) was higher in the augmented group, leading to a higher use of inhaled bronchodilators and oxygen therapy (). The baseline post-bronchodilator FEV1 either in liters (1.41 versus 2.44 L, P < 0.001) or percentage of predicted (43 versus 77%, P < 0.001) was lower in the augmented group (). No difference between the groups were noted in the age at the time of diagnosis, gender, race, number of years of smoking, exposure to fumes or dust at work, history of bronchiectasis, hepatitis and cirrhosis, use of inhaled or systemic corticosteroids and theophylline. In addition no difference between the groups were found in duration of follow-up, number of spirometries, family history of AATD or emphysema, year when the first spirometry was performed, as well as in the 5-year mortality rate.
Table 1 Comparative table of the patients receiving versus not receiving augmentation therapy
Overall change in FEV1 between the augmented versus the non-augmented group
When adjusted by age at baseline, sex, smoking status and baseline FEV1 % of predicted the augmented group had a mean increase in ΔFEV1 of 10.61 ± 21.4 mL/year. In comparison, the nonaugmented group had a mean decrease in ΔFEV1 of −36.96 ± 12.1 mL/year (P = 0.05), constituting an overall change in FEV1 (ΔFEV1 in augmented minus ΔFEV1 in the nonaugmented group) of 47.6 mL/year between the two groups (). When adding the 26 patients in whom the smoking history was initially missing, the difference between the augmented and non-augmented groups remained significant (ΔFEV1 in the augmented group: 8.92 ± 19.95 mL/year versus ΔFEV1 in the non-augmented group: −34.96 ± 9.24 mL/year, overall FEV1 change of 43.88 mL/year, P = 0.046).
Table 2 Annual ΔFEV1 for the augmented and the non-augmented groups
Subgroup analysis
When patients were divided in 3 subgroups according to the initial FEV1 % of predicted (<30%, 30% to 65% and >65%), a trend towards a beneficial effect of augmentation therapy was observed in the group with an initial FEV1 between 30% to 65% (ΔFEV1 augmented: 2.08 ± 24 mL/year versus ΔFEV1 nonaugmented: −51.92 ± 18.1 mL/year; for an overall change in FEV1 of 54 mL/year, P = 0.07) (). Of note is that the patients with FEV1 above 60% had higher rates of FEV1 decline if they received augmentation therapy (ΔFEV1 augmented: −108.7 ± 17.3 mL/year versus ΔFEV1 non-augmented: −29.2 ± 15.29 mL/year; for an overall change in FEV1 of 79.46 mL/year, P < 0.001) ().
Figure 2 ΔFEV1 (mL/year) in augmented and non-augmented patients by initial FEV1 % of predicted (>65%, 30% to 65% and <30%). *P = 0.0006, **P = 0.07.
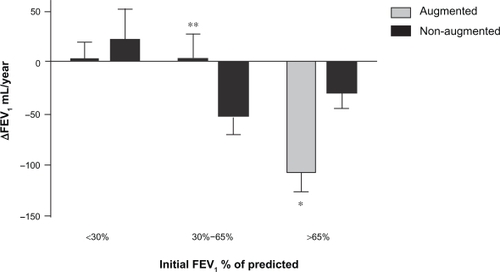
In the subset of patients with initial FEV1 % of predicted between 30% and 65%, one influential subject in the augmented group was identified using diagnostic statistics by PROC MIXED in SAS (SAS Institute). Omitting this subject the ΔFEV1 was 11.88 ± 23.96 mL/year in the augmented group (n = 78) and −51.92 ± 18.14 mL/year in the non-augmented group, P = 0.034.
The analysis was repeated after adding 26 patients in whom the smoking history was initially missing. The inclusion of these patients did not modify the results for the <30% and >65% subgroups, however it made the difference, in the subgroup of patients with initial FEV1% between 30% and 65%, statistically significant (ΔFEV1 in the augmented group (n = 89): 6.82 ± 24.07 mL/year versus ΔFEV1 in the non-augmented group: −49.44 ± 15.72 mL/year, for an overall change in FEV1 of 56.3 mL/year, P = 0.05).
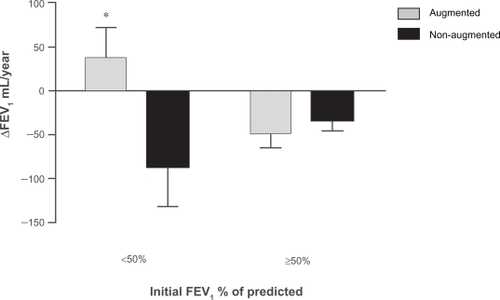
When patients were divided in 2 subgroups (<50% or ≥50%), only the subgroup with an initial FEV1 <50% of predicted benefited from augmentation treatment (ΔFEV1 augmented: 38.30 ± 33.7 mL/year versus ΔFEV1 nonaugmented: −86.73 ± 45.4 mL/year; for an overall change in FEV1 overall change in FEV1 of 125.03 mL/year, P = 0.03) (). The inclusion of the patients with missing smoking history did not alter the results of this particular analysis.
Figure 3 ΔFEV1 (mL/year) in augmented and non-augmented patients by initial FEV1 % of predicted (<50% and ≥50%).*P = 0.026.
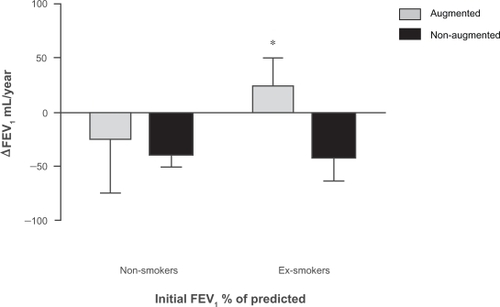
When patients were divided by the smoking status, the beneficial effects of augmentation therapy were noted only in the group of patients classified as ex-smokers (ΔFEV1 augmented: 24.24 ± 25.7 mL/year versus ΔFEV1 non-augmented: −41.19 ± 22.5 mL/year; for an overall change in FEV1 of 65.4 mL/year, P = 0.05) (). The patients that quit smoking did so 21.9 (±1.02) years before the inclusion in the study (range: 3.5 to 48.9 years). The inclusion of the patients with missing smoking history did not alter the results of this particular analysis.
Mortality
During the follow up, one patient died in the nonaugmented group and eight patients died in the augmented group. The 5-year mortality rate in this group of patients was 2.5% and 4% for the nonaugmented and the augmented group, respectively (P = 0.581, by logistic regression). Of note is that patients in the augmented group were older, and more commonly had COPD with lower FEV1 and higher number of individuals required oxygen therapy.
Comparison with patients with 1 versus 2 or more spirometries
Patients that had one FEV1 measurement (n = 296) had a higher mortality rate (9.8%) when compared to patients that had two or more FEV1 recorded in the database (4.1%) (P = 0.012), despite the shorter follow-up time (one third less) in the former group of patients. This suggest a possible “survivor effect” on the rate of FEV1 decline in patients who had 2 or more FEV1 measurements.
Discussion
In the group of patients with AATD studied, augmentation therapy improved lung function, manifested as an increase in the FEV1, when results were adjusted by age, gender, smoking status and baseline FEV1 percentage of predicted. The increase in FEV1 was only observed in ex-smokers and patients with FEV1 < 50% of predicted.
The FEV1 rate of decline in the non-augmented group is consistent with the one observed in previous observational studies.Citation8,Citation10 It is unclear why we found an unusual increase in FEV1 instead of a reduction in the FEV1 decline as reported in previous studies.Citation5,Citation15,Citation16 Possible explanations include anti-inflammatory effects of treatment with favorable effects over potential reversible processes such us bronchoconstriction and/or the use of different spirometry equipments. The first possible reason is supported by a higher incidence of asthma (25% versus 10%) and use of inhaled bronchodilators (86% versus 55%) in the group of patients receiving augmentation therapy. The use of different spirometry equipments, may have introduced variability in the results recorded. We cannot completely exclude the possibility of an increase in FEV1 associated with a submaximal expiratory effort (unfortunately peak flow was not recorded). To the best of our knowledge the centers in which the spirometries were performed followed the American Thoracic Society standards and the best effort was made to insure the best quality and reliability of spirometry results. It is interesting to note that in the Alpha-1-Antitrypsin Deficiency Registry Study Group, some patients had a positive FEV1 slope (in our case positive ΔFEV1) when individual rates of FEV1 were shown.Citation5
The increase in FEV1 in augmented patients was observed only in ex-smokers and patients with an initial FEV1 percentage of predicted <50%. Interestingly, augmented patients with an initial FEV1 > 65% of predicted had a significant larger FEV1 decline than nonaugmented patients, probably due to selection bias, as it is more likely to provide augmentation treatment to patients who have FEV1 > 65% and an accelerated FEV1 decline. Another possible explanation is based on the unusually low rate of FEV1 decline in patients with FEV1 > 65% who did not received augmentation therapy (ΔFEV1−29.24 mL/year). This low value could have accentuated the differences in this subgroup of patients. A less likely possibility is a deleterious effect of augmentation therapy in AATD patients with FEV1 > 65%.
No difference in survival between the non-augmented and augmented group were observed when the 5-year mortality was adjusted for age, gender, smoking status, presence of COPD and baseline FEV1. As the overall sample is small and few patients died during the 3.5 year follow-up period, our study is underpowered to detect any difference in survival between the groups.
Few studies have compared the ΔFEV1 in patients receiving versus not receiving alpha-1 antitrypsin augmentation therapy. Seersholm et al compared the ΔFEV1 in AATD, among 97 Danish ex-smoker patients who did not received augmentation therapy, versus 198 German ex-smoker patients who received this therapy. The authors showed a significant slower rate of decline in patients treated with augmentation therapy (mean ΔFEV1 of 22 mL/year, P = 0.02). When stratified by the initial FEV1% of predicted, the authors showed a greater reduction in FEV1 decline among patients with initial FEV1 between 31% and 65% of predicted (mean overall change in FEV1 of 22 mL/year, P = 0.04).Citation15
The Alpha-1-Antitrypsin Deficiency Registry Study Group included 927 AATD patients and found no differences in ΔFEV1 in patients receiving versus patients not receiving augmentation therapy (ΔFEV1 4 mL/year, P = 0.4). However, a significant lower FEV1 decline was observed in patients receiving augmentation therapy who had an initial FEV1 value of 35% to 49% predicted (overall change in FEV1 of 27 mL/year, P = 0.03) or of 30% to 64% predicted (overall change in FEV1 of 18 mL/year, P = 0.03).Citation5
Wencker et al analyzed the ΔFEV1 in 96 patients with severe before and after the institution of augmentation therapy. Overall, there was a significant lower decline in ΔFEV1 when patients received augmentation therapy in comparison with no treatment (ΔFEV1 15 mL/year, P = 0.019). Patients with FEV1 < 30% before initiation of therapy showed a significant reduction of the FEV1 decline (overall change in FEV1 of 31.3 mL/year p < 0.0001). No significant changes were noted in the group of patients with FEV1 30% to 65% or >65% of predicted.Citation16
Dirksen et al randomized 26 Danish and 30 Dutch patients with AATD to receive albumin versus alpha-1 antitrypsin augmentation therapy. No significant difference in the annual FEV1 change was noted between the groups (overall change in FEV1 of −19.8 mL/year, P = 0.25).Citation17 The same authors recently reported the results of a randomized trial that included 77 patients with AATD that received alpha-1 antitrypsin augmentation therapy or placebo. They explored the effect of augmentation treatment on computed tomography lung density. A trend suggestive of treatment benefit in the group receiving augmentation therapy was noted when change in lung density by computed tomography was used as outcome. No differences were observed in the mean annual FEV1 decrease between the treatment and placebo groups.Citation18
Selection bias at the time of the inclusion and “survivor effect” may help explain some of the discrepancies among the subgroup of patients that benefited the most when treated with alpha-1 antitrypsin augmentation therapy. When stratified by the initial FEV1 percentage of predicted the group of patients that benefited the most were the ones with FEV1 30% to 65%Citation1,Citation6 or FEV1 < 30%.Citation16 In our study, the subgroup of patients with an initial FEV1 35% to 65% of predicted showed a trend to benefit from augmentation therapy (P = 0.07). This difference became statistically significant after adding 26 patients in whom the smoking history was initially missing (P = 0.05) or when an influential subject in the augmented group was omitted from the analysis (P = 0.02). The studies by Seersholm et al and Alpha-1-Antitrypsin Deficiency Registry Study Group observed a beneficial effect of augmentation therapy in the subgroup of patients with an initial FEV1 30% to 65% of predicted.Citation5,Citation15
The majority of patients included in the previous studies were ex-smokers, especially in the group of patients that received augmentation therapy.Citation5 Similarly in our study, 78% of the patients were ex-smokers and this percentage increase to 85% when only considering the augmented patients. Only two patients were current smokers at the time of inclusion. A significant overall change in FEV1 was only noted in the group of patients that were ex-smokers. This difference is not attributable to a reduction in the rate of FEV1 decline due to discontinuation of tobacco products as ex-smoker patients quit smoking an average of 21.9 years before the inclusion in the study (all ex-smoker patients quit smoking at least 3 years before their inclusion).
Non-smokers had a non significant difference in the rate of FEV1 decline, probably due to a reduced effect of therapy, in patients with slower FEV1 decline, or smaller sample size. Observational studies have shown no significant difference in the rate of FEV1 decline when ex-smokers were compared with non-smokers.Citation8
The majority of patients included in our study received augmentation therapy (76%). Reasons for not receiving therapy included: 1) not recommended by physician (n = 10), 2) recent diagnosis, 3) waiting to begin therapy, 4) financial reasons and 5) personal desire. The mean FEV1 % of predicted for the patients who did not received therapy by physician recommendation was 95 (±7)%, meanwhile for the rest of the patients in the nonaugmented group the mean (SE) FEV1 % of predicted was 48 (±2)%, (P < 0.001).
A similar percentage of AATD patients treated with augmentation therapy (70%) was reported by the Alpha-1-Antitrypsin Deficiency Registry. The main reasons for not receiving therapy in the registry were 1) not indicated or recommended by physician, 2) high cost, 3) receipt or anticipation of a lung transplant and 4) medical contraindication. Patients who did not receive augmentation therapy had higher FEV1, less pulmonary symptoms, lower family income and were less likely to have insurance coverage (5).
Limitations of our study included 1) observational non-randomized study; 2) a possible selection bias, as only patients who had all the variables of interest recorded and who had two spirometries at least 6 months apart were included in the analysis (patients had a higher mortality rate if they only had one FEV1 measurement in comparison with two or more, leading to an underestimation of the rate of FEV1 decline, the so called “healthy survivor effect”); 3) spirometries were performed in different centers that used diverse equipments; 4) patients were divided in augmented and non-augmented group at the time of the inclusion in the study (some patients may have started or stopped therapy afterwards); 5) the criteria for starting treatment and compliance with therapy were not recorded, 6) the length of follow-up was relatively short (3.5 years), 7) other risk factors that may influence the rate of FEV1 decline, such us bronchodilator response and respiratory exacerbation rates were not investigated.Citation10,Citation12,Citation19–Citation22
In spite of the previous limitations, our study has a different research design, and the analysis of the data still supports the findings of previous studies, that showed an improvement in lung function in AATD patients receiving augmentation therapy. This study demonstrated that ex-smokers with FEV1 < 50% benefited the most from alpha-1 antitrypsin augmentation therapy. In addition it provides “everyday practice” information about the characteristics of patients with AATD that received versus the ones that did not receive alpha-1 antitrypsin augmentation therapy.
Conclusion
Augmentation therapy improves lung function in patients with AATD when adjusted by age, gender, smoking status and baseline FEV1 % of predicted. The beneficial effects were noted in ex-smoker patients with FEV1 below 50% of predicted.
Acknowledgements
The authors acknowledge funding from the Alpha-1 Foundation.
Disclosures
The authors declare no conflicts of interest.
References
- BrantlyMNukiwaTCrystalRGMolecular basis of alpha-1-antitrypsin deficiencyAm J Med19888413313289385
- GadekJEFellsGAZimmermanRLRennardSICrystalRGAntielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysemaJ Clin Invest1981688898986169740
- StockleyRAThe pathogenesis of chronic obstructive lung diseases: implications for therapyQJM1995881411467704565
- American Thoracic SocietyEuropean Respiratory SocietyAmerican Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1-antitrypsin deficiencyAm J Respir Crit Care Med200316881890014522813
- The Alpha-1-Antitrypsin Deficiency Registry Study GroupSurvival and FEV1 decline in individuals with severe deficiency of alpha-1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study GroupAm J Respir Crit Care Med199815849599655706
- EvaldTDirksenAKeittelmannSViskumKKok-JensenADecline in pulmonary function in patients with alpha-1-antitrypsin deficiencyLung19901685795852117166
- SeersholmNKok-JensenADirksenADecline in FEV1 among patients with severe hereditary alpha-1-antitrypsin deficiency type PiZAm J Respir Crit Care Med1995152192219258520756
- PiitulainenEErikssonSDecline in FEV1 related to smoking status in individuals with severe alpha-1-antitrypsin deficiency (PiZZ)Eur Respir J19991324725110065663
- JanusEDPhillipsNTCarrellRWSmoking, lung function, and alpha-1-antitrypsin deficiencyLancet198511521542857224
- DawkinsPADawkinsCLWoodAMNightingalePGStockleyJAStockleyRARate of progression of lung function impairment in alpha-1-antitrypsin deficiencyEur Respir J2009331338134419164359
- WuMCErikssonSLung function, smoking and survival in severe alpha 1-antitrypsin deficiency, PiZZJ Clin Epidemiol198841115711653264848
- PiitulainenETornlingGErikssonSEffect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with alpha 1-antitrypsin deficiency (PiZZ)Thorax1997522442489093340
- DowsonLJGuestPJStockleyRALongitudinal changes in physiological, radiological, and health status measurements in alpha(1)-antitrypsin deficiency and factors associated with declineAm J Respir Crit Care Med20011641805180911734427
- EdenEHammelJRouhaniFNAsthma features in severe alpha-1-antitrypsin deficiency: experience of the National Heart, Lung, and Blood Institute RegistryChest200312376577112628876
- SeersholmNWenckerMBanikNDoes alpha-1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients with severe hereditary alpha-1-antitrypsin deficiency?Eur Respir J199710226022639387950
- WenckerMFuhrmannBBanikNKonietzkoNLongitudinal follow-up of patients with alpha(1)-protease inhibitor deficiency before and during therapy with IV alpha(1)-protease inhibitorChest200111973774411243951
- DirksenADijkmanJHMadsenFA randomized clinical trial of alpha(1)-antitrypsin augmentation therapyAm J Respir Crit Care Med19991601468147210556107
- PiitulainenETornlingGErikssonSEnvironmental correlates of impaired lung function in non-smokers with severe alpha-1-antitrypsin deficiency (PiZZ)Thorax19985393994310193391
- LiebermanJAugmentation therapy reduces frequency of lung infections in antitrypsin deficiency: a new hypothesis with supporting dataChest20001181480148511083705
- SilvermanEKProvinceMARaoDCPierceJACampbellEJA family study of the variability of pulmonary function in alpha-1-antitrypsin deficiency. Quantitative phenotypesAm Rev Respir Dis1990142101510212240821
- SilvermanEKProvinceMACampbellEJPierceJARaoDCVariability of pulmonary function in alpha-1-antitrypsin deficiency: residual family resemblance beyond the effect of the Pi locusHum Hered1990403403552083948
- DirksenAPiitulainenEParrDGExploring the role of CT densitometry: a randomised study of augmentation therapy in alpha-1-antitrypsin deficiencyEur Respir J2009331345135319196813