Abstract
Aerosol delivery of Iloprost is a promising therapeutic approach. The aim of this study was to determine the output of an ultrasonic nebulizer in different ventilation set-ups at the tip of different endotracheal tubes.
Method
In set-up A, an ultrasonic nebulizer was connected directly to the endotracheal tube. In set-up B, the nebulizer was incorporated into the inspiratory limb of the ventilator circuit; a bypass arrangement allowed to selectively direct the exspiratory air discharged from the model lung. The test lungs were ventilated through a standard endotracheal tube (ET) and through a double-lumen tube (DLT). The nebulizer was filled with 5 ml of a Tc-99m 0.9%-NaCl solution. After nebulization, distribution of radioactivity was detected by gamma scintigraphy.
Results
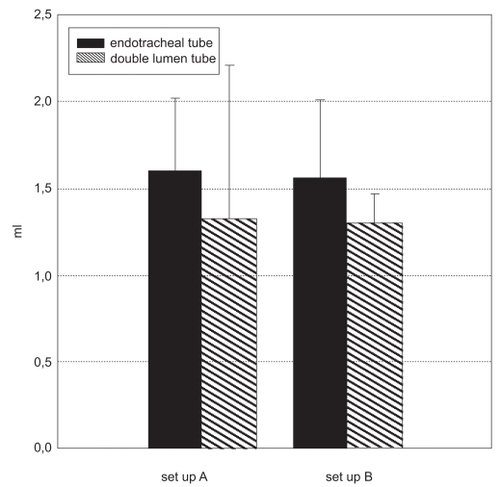
Set-up A, ventilation in volume-controlled mode (VCV) via an ET: Delivered dose (1.61 ± 0.41 ml), nebulization time 10.13 ± 1.71 min. Set-up A, pressure-controlled ventilation (PCV), via a DLT: Delivered dose (1.33 ± 0.88 ml), nebulization time 13.27 ± 2.58 min. Set-up B, VCV mode via an ET: Delivered dose (1.57 ± 0.44 ml), nebulization time (25.9 ± 3.8 min). Set-up B, PCV mode, via a DLT: Delivered dose (1.3 ± 0.17 ml), nebulization time (25.6 ± 4.0 min). Set-up B did not yield a significantly higher output (p < 0.05), but the nebulization time was significantly longer (p > 0.05) compared with set-up A.
Conclusion
Set-ups which involve connecting the nebulizer directly to an ET or a DLT exhibit sufficient output of aerosol and short nebulization times.
Keywords:
Introduction
Delivery of drugs in aerosolized form to the airways of patients with pulmonary diseases is a common clinical practice, and the use of nebulized drugs during mechanical ventilation is increasing. Numerous drugs have been investigated for possible delivery via aerosol. In most instances, the drug has a topical or direct effect on airway or lung tissue while keeping systemic exposure to a minimum (CitationShapiro and Peruzzi 2000; CitationAnderson 2005). Inhaled Iloprost, a stable long-acting prostacycline analogue, provides an effective therapy for patients with severe pulmonary hypertension (CitationOlschewski and Simonneau 2002). It has been shown that the employment of ultrasonic nebulizers offers more effective alveolar deposition of Iloprost in severe pulmonary hypertension (PHT), as compared with conventional jet nebulization devices (CitationGessler and Schmehl 2001). More recent studies have suggested that intraoperative inhalation of Iloprost is a therapy option in case of imminent right heart failure in patients with PHT (CitationLanger and Wilhelm 2003). Acute right heart failure is a life-threatening situation, and the drugs need to be administered quickly and efficiently.
Using mechanical ventilation in combination with aerosol administration of drugs is an established practice in intensive care units; the set-up is arranged such that only the inspiratory air flow passes through the nebulizer (CitationMiller and Amin 2003). The underlying assumption is that a significant amount of the drug contained in the volume of air common to the inspiratory and expiratory pathways will be lost on expiration. Modern anesthetic machines do not include a nebulization function. This disadvantage becomes particularly evident in intra-operative settings when connecting the nebulizer to the circle system of an anesthetic machine might prove laborious (CitationWilhelm and Grundmann 2004). The task is therefore to establish a convenient in vitro model that helps evaluate the amount of aerosol delivered through an ET tube under conditions similar to an intra-operative situation. We thus explored the influence that a quick attachment of an ultrasonic nebulizer to an endotracheal tube has on efficient drug output and nebulization time. The majority of patients who undergo lung operation are not ventilated through a standard tube, but through a double-lumen tube (DLT) that allows side-separated ventilation to a modified ventilation pattern. In addition, successful therapy with agents such as surfactant and prostaglandins requires targeting the aerosols to specific sites within the lung (CitationDhand 2003). Another interesting aspect was to find out whether or not different ventilator modes and types of endotracheal tubes influence aerosol delivery performance.
Materials and methods
To assess the aerosol output of a nebulizer in different artificial ventilation set-ups, an ultrasonic nebulizer (Multisonic Schill GmbH, Probstzella, Germany) was connected to a Centiva/5 Ventilator (Datex Ohmeda, Duisburg, Germany). Methods to integrate the nebulizer into the ventilation circuit fell into two different approaches.
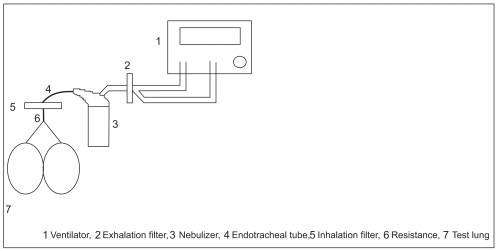
The first method encompassed a mainstream set-up (A), in which the nebulizer is placed between the endotracheal tube and the ventilator circuit. Consequently, inhalation and exhalation gases flow into and out of the nebulizer that is connected directly to the tube. At the end of the tube, an inhalation heat and moisture exchanger (Pall filter BB50TE), as well as a resistance of 5 kPa/l/s (10 kPa/l/s with the double-lumen tube) were placed between the tube and a test lung (2.3 l with Y-piece). The nebulizer air inlet was connected to the Centiva/5 tubing via an exhalation heat and moisture exchanger (Pall filter BB50TE, Pall GmbH, Dreieich, Germany) (See ).
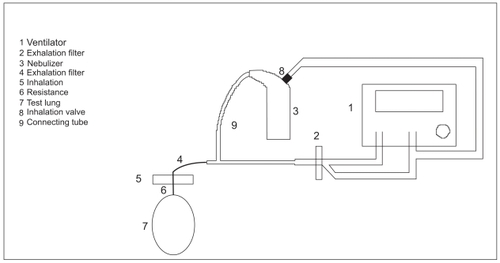
The second set-up comprised a bypass set-up (B), in which the nebulizer was placed between endotracheal tube and respirator. This arrangement allowed only the inspiratory air flow to pass through the nebulizer.
This set-up involved connecting the upper part of the nebulizer to the endotracheal tube via a tube and a T-piece. Centiva/5 tubing and an exhalation heat and moisture exchanger (Pall Filter BB50TE, Pall GmbH, Dreieich, Germany) were attached to the other side of the T-piece. Another T-piece was inserted into the inhalation path of the Centiva/5 tubing. As a result it became possible to connect an additional 20 mm tube to the air inlet of the nebulizer. The nebulizer inhalation valve remained in its original position. At the end of the endotracheal tube, an inhalation heat and moisture exchanger (Pall filter BB50TE, Pall GmbH, Dreieich, Germany), as well as a resistance of 10 kPa/l/s, was placed between the endotracheal tube and a test lung (2.3 l) (See ).
Aerosol nebulizer output was measured in two different ventilation set-ups at the tip of two different endotracheal tubes.
The test lung was managed by volume control ventilation (FiO2 21%, tidal volume 700 ml, ventilation rate 12/min, I:E 1:2; peak pressure max. 30 cm H2O, PEEP 3 cm H20, flow 30 l/min) via a standard endotracheal tube (7.5 ID single lumen, Portex, Kirchseeon/Eglharting, Smiths Medical Germany).
Pressure-controlled ventilation was maintained in all tests involving the use of a double-lumen tube (Broncho Cath 37FR, Tyco Healthcare Deutschland GmbH, Neustadt, Germany) (FiO2 21%, ventilation rate 12/min, I:E 1:2; Bi-level ramp 0.2 seconds; Peak pressure inspiratory 30 cm H2O, PEEP 3 cm H20, trigger sensitivity 20 l/min).
In all experiments, the nebulizer was filled with 5 ml of radio labelled 0.9%-NaCl solution and placed into the setup as described above. Said Tc-99m 0.9%-NaCl solution (Tyco Healthcare, Germany) had a specific activity of app. 1.25 MBq (Mega Bequerel) per ml. 6 samples of 1 ml each of this solution were placed into a scintillation counter that determined the specific activity of the solution. The nebulizer switched off automatically as soon as the medicament was used up, and/or as soon as the nebulizer′s residual volume of 0.6 ml was reached. The Centiva/5 was switched on. After the Centiva/5 had reached steady state, the nebulizer was switched on. Nebulization was performed until the nebulizer switched off automatically. The nebulization time was recorded.
The set-up was dismounted, and the deposits were analyzed using a gamma scintillation counter (Perkin Elmer, Germany). The deposits stemmed from the inhalation heat and moisture exchanger, endotracheal tube, residue in nebulizer, upper part of nebulizer, exhalation heat, and moisture exchanger. In set-up B, the material deposit that had accumulated inside the tube leading to the nebulizer was analyzed. Ambient conditions (humidity, temperature) were recorded before each measurement.
Data evaluation
In order to determine the amount of aerosol present on each heat and moisture exchanger, the activity measured in the sample heat and moisture exchangers was divided by the specific activity values. Similar calculations were performed for the other parts of the set up.
Statistical analysis
The data are presented as mean ± standard deviation; a two-group comparison was done with two – sample t-test and Mann-Whitney U-test. p-values < 0.05 were considered statistically significant. All statistical analyses were carried out using SPSS version 14.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
Results
Each measurement was performed three times. The mass balance record was determined from all measurements. Recovery rates ranged from 95.9% to 99.6%. All measurements were made at ambient temperatures ranging from 22 °C to 24 °C and relative humidities between 56% and 61%.
In the test lung that received volume-controlled ventilation (VCV) via a standard endotracheal tube (set-up A, mainstream set-up), the mean dose delivered amounted to 1.61 ± 0.41 ml. Mean aerosol loss resulting from impaction on the tubings: 1.53 ± 0.02 ml, mean residual volume left in the nebulizer: 1.7 ± 0.07 ml, mean nebulization time: 10.13 ± 1.71 min (See ).
Table 1 Set up A. Volume-controlled ventilation (VCV)
In the lung model that received pressure-controlled ventilation (PCV) via a double-lumen tube (set-up A, mainstream set up), the mean delivered dose amounted to 1.33 ± 0.88 ml. Mean aerosol loss resulting from impaction on the tubings: 1.62 ± 0.17 ml, mean residual volume left in the nebulizer: 1.89 ± 0.14 ml, mean nebulization time: 13.27 ± 2.58 min (See ).
Table 2 Set-up A. Pressure-controlled ventilation (PCV)
The set-up B (bypass set-up) lung model that was managed by volume-controlled ventilation (VCV) via a standard endotracheal tube showed the following results: mean dose delivered was 1.57 ± 0.44 ml, mean residual volume left in the nebulizer: 1.87 ± 0.1 ml, mean nebulization time: 25.9 ± 3.8 min (see ).
Table 3 Set-up B. Volume-controlled ventilation (VCV)
The set-up B (bypass set up) test lung that received pressure-controlled ventilation (PCV) via a double-lumen tube exhibited the following results: mean dose delivered: 1.30 ± 0.17 ml, mean aerosol loss resulting from impaction in tubing: 1.38 ± 0.21 ml, mean residual volume left in the nebulizer: 2.2 ± 0.15 ml, mean nebulization time: 25.6 min ± 4.07 min (See ).
Table 4 Set-up B. Pressure-controlled ventilation (PCV)
After nebulization, no significant differences were seen in the amounts of aerosol trapped in the set-up A inhalation heat and moisture exchanger (mainstream set up) and that of set-up B (bypass set-up) (1.5 ± 0.2 ml vs. 1.4 ± 0.3 ml) (p = 0.82); (p = 0.63; u-test) ().
Set-up A (mainstream set up), however, offered a significantly reduced nebulization time compared to set-up B (11.7 ± 2.6 min vs. 25.8 ± 3.5 min) (p = 0.0001); (p = 0.004; u-test).
Set-up A had a significantly larger amount of aerosol in the exhalation heat and moisture exchanger than set-up B (1.47 ± 0.08 ml vs. 0.26 ± 0.23 ml) (p = 0.0001); (p = 0.004; u-test).
A large residual amount (0.89 ± 0.16 ml) remained in the bypass tube of set-up B.
Discussion
This was the first ever study aimed at measuring both the output and the nebulization time from an ultrasonic nebulizer which was connected directly to a standard endotracheal tube and to a double-lumen tube with no change in ventilator settings. These data were compared with data achieved in a bypass set-up involving a nebulizer placed between endotracheal tube and respirator in such a way that it was incorporated only into the inspiratory limb of the ventilator circuit; a bypass line was in place for selectively directing the exspiratory air discharged from the test lungs.
Surprisingly enough, the bypass set-up (B) was not clearly superior to the mainstream set-up (A) with respect to the total output at the tip of the endotracheal tube. The reasons for this are that the advantage provided by the bypass circuit, namely that the nebulizer releases aerosol only during inhalation, was almost eliminated because a large amount of aerosol accumulated inside the additional tubing. The nebulization time for the bypass set-up (B) was more than twofold greater than that of the mainstream set-up (A). In the intra-operative situation particularly, attaching the nebulizer device directly to the endotracheal tube should be the preferred method. The shortcoming of this method, namely that a somewhat lower amount of aerosol is provided, is outweighed by the advantage of faster treatment. Moreover, shorter nebulization times result in reduced influences from other factors affecting aerosol performance during nebulization, such as changes in temperature, concentration, surface tension, viscosity, and saturated vapor pressure of the nebulizing solution (CitationSteckel and Eskandar 2003). According to expectations, the use of a double-lumen tube was associated with a somewhat higher loss of aerosol in the tube as compared with a standard endotracheal tube; this can be explained by the fact that it provides a greater surface area of contact.
The mainstream set-up (A) comprising an ultrasonic nebulizer also has advantages over nebulizers which require an additional source of gas. Alterations in VT are avoided, and flow-measurement components that can be found in some ventilator circuit systems are not allowed to get damaged (CitationHess 1994).
The method chosen for our study, ie, to connect the nebulizer directly to an ET or DLT without using supplemental gas to drive the nebuliser, yielded a significantly higher aerosol output (Set up A 32%, Set up B 36%) compared with results of studies conducted by CitationO′Doherty and Thomas (1992, 1993) using several ultrasonic and jet nebulizer devices.
These studies reported maximum output levels of 10%. Only one nebulizer could provide an 8% increase through a filling volume that totalled six times that of the others (CitationO’Doherty and Thomas 1992; CitationThomas and O’Doherty 1993).
The Servo 945 nebulizer driver had a profound influence on the output of some of the aerosol delivery devices.
A variety of new devices that deliver drugs to the lung with high efficiency can be employed for drug delivery during mechanical ventilation (CitationDhand 2004). But most of the data relating to drug dosage have been obtained from spontaneously breathing patients (CitationGeller 2005). Data from such in vitro assays are very useful in guiding aerosol therapy during mechanical ventilation. Several studies have recently been conducted to determine the suitability of various nebulizer devices (CitationDi Paolo and Pannatier 2005; CitationVecellio and Guerin 2005). Ultrasonic-nebulizer aided delivery of aerosolized Iloprost into the lungs of ventilated patients with pulmonary hypertension is becoming increasingly important. Iloprost aerosol administration offers the advantage of avoiding the effects of an intravenous therapy on systemic circulation. As for the nebulization of Iloprost, ultrasonic nebulizers provide far superior efficiency compared to customary compressed-air nebulizer devices (CitationGessler and Schmehl 2001). A particularly noteworthy feature is that the aerosol particles generated are so small that they can migrate into the deeper regions of the lung (CitationKohler and Sollich 2003).
The ultrasonic nebulizer device used in the present study generates particles in the size range of 3.2 μg to 3.5 μg.
Limitations
Although only one ultrasonic nebulizer was investigated in this study, the findings allow the conclusion that the loss in all the various ultrasonic nebulizers will be of the same order of magnitude as the loss encountered in the present bypass arrangement.
The data generated from the experiments described above refer only to the nebulized amount determined directly at the tip of the endotracheal tube.
In this study, we have not evaluated in vivo the actual amount of aerosol deposition within the alveoli after nebulization.
Information about how much aerosol is able to go deeper into the lungs cannot reliably be derived from these data.
The in vitro experiments described above were done primarily to assess the suitability of different ultrasonic nebulizer arrangements without exposing patients to unnecessary radiation. Respiratory tract deposition data of aerosol particles after nebulization are well known from other authors (CitationStahlhofen and Gebhart 1980; CitationMorrow 1986; CitationKohler and Sollich 2003).
Conclusion
A set-up in which the nebulizer is connected directly to an ET or DLT achieved higher aerosol output and shorter nebulization times than a bypass set-up. The ideal nebulization conditions remain to be established. However, we have shown that the method involving the use of an ultrasonic nebulizer to intra-operatively administer a nebulized drug directly to a standard endotracheal tube or a double-lumen tube yields convincing results.
Acknowledgment
The authors want to thank Karl Cornelius-Lorenz (Technologie Institut Medizin Gmbh Göttingen, Germany) and Knut Sommer (Inamed Research GmbH Gauting, Germany) for conducting the analyses. The laboratory analyses are supported by Schill, Probstzella, Germany. The authors declare no conflicts of interest.
References
- AndersonPJ2005History of aerosol therapy: liquid nebulization to MDIs to DPIsRespir Care911395016122398
- ShapiroBAPeruzziWTMillerRD2000Respiratory CareAnesthesia5Churchill LivingstoneLondon240342
- DhandR2003aAerosol therapy during mechanical ventilation: getting ready for prime timeAm J Respir Crit Care Med1681148914607822
- DhandR2004bNew frontiers in aerosol delivery during mechanical ventilationRespir Care496667715165301
- Di PaoloERPannatierACottingJ2005In vitro evaluation of bronchodilator drug delivery by jet nebulization during pediatric mechanical ventilationPediatr Crit Care Med6462915982436
- GellerDE2005Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhalerRespir Care5013132216185367
- GesslerTSchmehlTHoeperMM2001Ultrasonic versus jet nebulization of iloprost in severe pulmonary hypertensionEur Respir J1714911307743
- HessD1994Inhaled bronchodilators during mechanical ventilation: delivery techniques, evaluation of response, and cost-effectivenessRespir Care391052210145961
- KohlerESollichVSchuster-WonkaR2003Lung deposition in cystic fibrosis patients using an ultrasonic or a jet nebulizerJ Aerosol Med16374612737683
- LangerFWilhelmWTschollD2003Intraoperative inhalation of the long-acting prostacyclin analog iloprost for pulmonary hypertensionJ Thorac Cardiovasc Surg126874514502175
- MillerDDAminMMPalmerLB2003Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluationAm J Respir Crit Care Med1681205912893644
- MorrowPE1986Factors determining hygroscopic aerosol deposition in airwaysPhysiol Rev66330763515373
- O’DohertyMJThomasSHPageCJ1992Delivery of a nebulized aerosol to a lung model during mechanical ventilation. Effect of ventilator settings and nebulizer type, position, and volume of fillAm Rev Respir Dis14638381489128
- OlschewskiHSimonneauGGalieN2002Aerosolized Iloprost Randomized Study Group. Inhaled iloprost for severe pulmonary hypertensionN Engl J Med347322912151469
- StahlhofenWGebhartJHeyderJ1980Experimental determination of the regional deposition of aerosol particles in the human respiratory tractAm Ind Hyg Assoc J41385987395752
- SteckelHEskandarF2003Factors affecting aerosol performance during nebulization with jet ultrasonic nebulizersEur J Pharm Sci194435512907295
- ThomasSHO’DohertyMJPageCJ1993Delivery of ultrasonic nebulized aerosols to a lung model during mechanical ventilationAm Rev Respir Dis14887278214941
- VecellioLGuerinCGrimbertD2005In vitro study and semiempirical model for aerosol delivery control during mechanical ventilationIntens Care Med318716
- WilhelmWGrundmannU2004Iloprost. Pharmacology and clinical application during surgeryAnaesthesist53745715241524