Abstract
During the past decade, transdermal delivery systems (TDS) have become increasingly important for treating neurologic and psychiatric disorders. The rivastigmine patch was the first patch to be approved to treat Alzheimer’s disease (AD). The 9.5 mg/24 h patch has equal efficacy to the capsules and reduces gastrointestinal adverse events, such as nausea and vomiting, by two-thirds. This treatment is well tolerated by patients because drug delivery is even and continuous, reducing fluctuation in drug plasma level, and attenuating the development of centrally mediated cholinergic side effects. Furthermore, once-a-day application of the patch enables an easy treatment schedule, ease of handling, infrequent skin irritations, and a patient- and caregiver-friendly mode of administration. Improved compliance with a subsequent drug administration may contribute to better clinical efficacy, reduce caregiver burden, result in a slower rate of institutionalization, and lead to a decrease in healthcare and medical costs. Because of these advantages, the rivastigmine patch has enabled great progress in the treatment of AD, and represents an excellent alternative to the orally administered cholinesterase inhibitors.
Introduction
The first transdermal delivery system, a patch to treat sea sickness based on the agent scopolamine (Transderm Scop®), was approved in the 1970s. To date, various transdermal patches to treat neurological and psychiatric diseases have been approved, including methylphenidate (Daytrana™) to treat attention deficit hyperactivity disorder, rotigotine (Neupro®) to treat Parkinson’s disease, selegiline (Emsam®) to treat depression, and fentanyl (Durogesic®) for pain. In July 2007 the rivastigmine patch was approved to treat mild to moderate AD and Parkinson-associated dementia (CitationUS Food and Drug Administration 2008). TDS has several advantages. Continuous release, for example, enables a constant drug plasma level, which may be a benefit when treating Parkinson’s disease, assuming that a brief stimulation of the dopamine receptor in particular is responsible for the development of L-Dopa-associated motor complications (CitationFabbrini et al 2007). Because the drug absorption is independent of ingestion and gastrointestinal interactions, the incidence of adverse gastrointestinal effects may be reduced. The first-pass effect can also be circumvented (CitationOertel et al 2007). Furthermore, transdermal administration allows the application of drugs with a short half-life and a low therapeutic index. And in case of an accidental overdose, an effective disruption of transdermal administration is possible.
The easy employment of the patches, with usually only a once-daily change, increases patient compliance. Furthermore, the caregivers can inspect the application of the patch. Improved compliance of the patient, and thus intake of the agent, is a significant benefit in the treatment of AD (CitationSmall et al 2005; CitationOertel et al 2007). One disadvantage of the TDS is a possible skin reaction. In one clinical trial the application of the rotigotine patch caused local skin reactions in 44% of patients, although only 5% interrupted the treatment because of this side effect (CitationWatts et al 2007).
The aim of this article is to provide an overview on the relevance of the rivastigmine patch as a therapeutic option for AD.
Treatment of Alzheimer’s disease
Evidence shows that current treatment with acetylcholinesterase inhibitors (AchEIs) and memantine may have a beneficial effect on AD, encouraging early diagnosis and treatment (CitationFarlow et al 2000; CitationRaskind et al 2004; CitationFarlow and Cummings 2007). The American Psychiatric Association asks for an effect in four domains in the treatment of AD: improvement of cognitive functions, activities of daily living, exposure of the caregivers, and the global overall impression. Only the AchEIs, rivastigmine, galantamine, and donepezil, and the NMDA-receptor antagonist memantine, meet these criteria and are currently approved to treat AD. Possible benefits for behavioral disturbance, institutionalization, mortality, disease related quality of life, and the incidence of side effects should also be assessed.
Acetylcholinesterase inhibitors
The first AchEI, tacrine, a reversible, nonselective inhibitor, has shown an improvement in cognitive functions in patients suffering from AD (CitationSummers et al 1986) and was approved for the treatment of AD in 1993 by the FDA. Because of severe cholinergic effects, a pronounced hepatotoxicity, and a difficult dosing schedule, this drug is rarely prescribed today and has even been deregistered in some countries. The three drugs of the second generation, donezepil, rivastigmine, and galantamine, are characterized by fewer adverse effects and an easier dosing schedule.
The different and adverse effects of the three AchEIs are the result of unequal pharmacodynamic and pharmacokinetic characteristics ().
Table 1 Characteristics of the three cholinesterase inhibitors
The three AchEIs compensate for the cholinergic deficit in the synaptic clefts and increase cholinergic neurotransmission, which is reduced in patients with AD (CitationScarpini et al 2003). While donezepil only selectively and reversibly inhibits acetylcholinesterase, the selective and reversible AchEI galantamine has an allosterical stake on the nicotinergic acetylcholine receptor, and thus enhances the effect of acetylcholine receptor agonists on cholinergic transmission (Scott and Goa 2000). In addition to a pseudo-irreversible inhibition, rivastigmine also affects the activity of butyrylcholinesterase (BchE) (CitationXie et al 2000), which forms up to 10% of the cholinesterase in the human brain. This enzyme is mainly found in glial cells, but also occurs in neurons, endothelial tissue, and synaptic clefts. BchE can compensate for the AchE-associated effect after deletion of the AchE gene in mice (CitationXie et al 2000). During the course of AD the activity of AchE decreases, while the BchE activity remains constant or increases (CitationPerry et al 1978), which suggests that rivastigmine also has a role in the treatment of an advanced stage of the disease. Moreover, there is evidence that BchE plays a major role in certain cognitive functions. The results of one multiple-dose study have shown that the inhibition of BchE activity, but not that of AchE, in the cerebrospinal fluid correlated significantly with an improvement in attention, speed, and memory. Another crucial difference between rivastigmine and the other AchEIs, is the priority selective effect of the G1-subtype of AchE, which occurs particularly in the amygdala, hippocampus, and cortex regions of the brain, which are especially involved in AD (CitationPolinsky 1998). The G1-subtype of AchE seems to have an exceptional role in the development of AD. In contrast, donezepil and galantamine primarily inhibit the G2- and G4-subtype of AchE, which are usually found in the basal ganglia and outside the central nervous system. This difference could be responsible for the fewer extrapyramidal-motoric, cardiac, and muscular side effects of rivastigmine.
In accordance with current studies, the German Institute for Quality and Efficiency in Health Care (IQWiG) reached the conclusion that donezepil, rivastigmine, and galantamine lead to an improvement of cognition, activities of daily living, and global overall impression (CitationInstitute for Quality and Efficiency in Health Care 2007). The annual report of the IQWiG for galantamine notes evidence of an improvement in behavioral disturbance, quality of life of caregivers, and mentoring effort. Because of negative study results or the lack of well-designed studies, there is no evidence of a positive influence on the institutionalization, mortality, and disease related quality of life for one of the three drugs (CitationInstitute for Quality and Efficiency in Health Care 2007). Significant improvement of the cognitive functions measured on the Alzheimer’s Disease Assessment Scale (ADAS-cog) (CitationRosen et al 1984) by patients suffering from mild to moderate AD, was supported in two randomized, placebo-controlled trials (CitationCorey-Bloom et al 1998; CitationRosler et al 1999). Both trials found a significant improvement in only the high-dose groups, with a dosage of 6 to 12 mg rivastigmine daily, compared with the placebo group (CitationCorey-Bloom et al 1998: ADAS-cog p < 0.01; Rosler et al: p = 0.011 [CitationInstitute for Quality and Efficiency in Health Care 2007]). To evaluate daily living activity, the Progressive Deterioration Scale (PDS) was used in both trials. In the Corey-Bloom et al clinical trial, a significant improvement in activities of daily living was observed in the high dosage group (6–12 mg daily), while in the low dose group (1–4 mg daily) no significant difference, compared with the placebo group, was noticed. In the Rosler et al trial, only an insignificant benefit of rivastigmine was detected in the high dose group (p = 0.07) (CitationRosler et al 1999).
In both trials, global functioning under treatment with rivastigmine was measured with the Clinican’s Interview Based Impression of Change Plus Caregiver Input (CIBIC-Plus). In the Rosler et al trial, a significant increase in patients with improved global function was observed in 37% of the high dose group, and 30% of the low dose group, compared with the placebo-treated group. The Corey-Bloom et al trial registered a pronounced improvement in global function in the low and high dose groups (CitationCorey-Bloom et al 1998).
Under the guidelines of the National Institute for Health and Clinical Excellence (NICE), the studies found a significant improvement in cognition and global outcomes after treatment with donezepil. The evidence for an effect on the quality of life, and on behavioral disturbance, was less conclusive. Only a short-term benefit was suggested for the functional outcome. According to the NICE guidelines, the studies found evidence for an improvement in cognition, functional outcomes, and activities of daily living for treatment with galantamine, but after the Assessment Group had pooled the studies, an improvement in global outcome was not approved. One study showed a significant improvement in behavior with a higher galantamine dosage (16 mg/day). For rivastigmine, the trials suggest an improvement in cognition and global outcome, while no significant benefit for behavior, and a less conclusive effect on functional outcomes, was reported (CitationNICE 2007). To date, no placebo-controlled, randomized trials have been published, which show a significant difference between rivastigmine and placebo for the caregiver burden, or in behavioral disturbance of patients, institutionalization, mortality, and the health-related quality of life (CitationInstitute for Quality and Efficiency in Health Care 2007).
Development of other therapy options for Alzheimer’s disease
In the past few years, knowledge of the pathophysiology of AD has sharply increased, opening doors for positive research on new therapeutic options.
It is generally accepted that the cerebral accumulation of the amyloid-β peptide (Aβ) plays an important role in the pathogenesis of AD. Aβ is inherently neurotoxic in vitro and is indirectly responsible for neuronal death (CitationCummings 2004), because of a secondary initiation of inflammation, oxidation, hyperphosphorylation of tau protein, and glutama-tergic excitotoxicity (CitationHardy and Selkoe 2002). Accordingly, the prevention of the production and aggregation of Aβ could be an effective target for drugs. With the aim of avoiding the production of Aβ, the development of secretase inhibitors, which retard the processing of the amyloid precursor protein, have become a promising subject of research (CitationDewachter and Van Leuven 2002). Short synthetic peptides have been devised with the aim of stopping the aggregation of amyloid plaques, which prevent the connection of Aβ (CitationPermanne et al 2002; CitationSoto et al 2000).
Another auspicious target for future treatment of AD is passive and active immunization against Aβ. In older transgenic mice, research showed that an active immunization with an intraperitonal application of Aβ 1–42 reduced the burden of amyloid plaques, and that this was linked to the development of Aβ antibodies (CitationSchenk et al 1999). However, a clinical trial using Aβ1–42 as antigen has ceased, because 6% of the patients developed encephalitis (CitationOrgogozo et al 2003). There is evidence that passive immunization, using antibodies against Aβ reduced the amyloid plaques (CitationDodel et al 2003). Both active and passive immunizations led to a cognitive recovery in mouse models (CitationMorgan et al 2000; CitationDodart et al 2002).
Furthermore, treatment with statins seems to prevent AD (CitationPuglielli et al 2003). By reduction of the intracellular cholesterol, statins might inhibit the accumulation of amyloid plaques, and may also ameliorate amyloid plaque-associated inflammation (CitationCrisby et al 2002).
Studies have shown that long-term use of certain NSAIDs may reduce the incidence of AD (CitationMcGeer et al 1996, Citationin t’Veld et al 2001), but are unable to improve the symptoms in patients suffering from AD (Citationvan Gool et al 2003). The protective effect of certain NSAIDs such as indomethacine could be linked to an inhibition of cyclooxygenase and to the processing of β-amyloid precursor protein (CitationWeggen et al 2001).
Pre-clinical efficacy of the rivastigmine patch
Patch structure
Patches could be either topical, for a local effect only, or based on TDS for transdermal systemic release of the drug. TDS in particular have become important in the treatment of neurological diseases in recent years. In contrast to conventional reservoir patches, matrix patches with a polymer layer enable admission of a larger amount of the drug, and therefore, production of smaller patches.
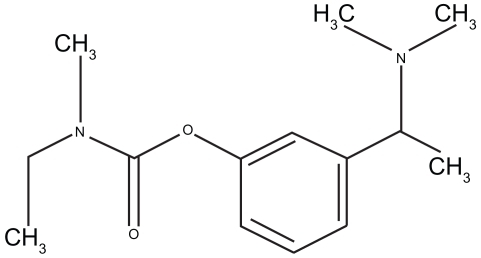
Rivastigmine is a 2.6-dioxo-4-phenyl-piperidine-3-carbonitrile. Its chemical formula is shown in . The small molecular weight of 250.34 Da, the lipophil, and the hydrophil characteristics, along with the potent effect of even very small portions, established the explicit aptitude of the drug for application with TDS.
Figure 1 Chemical structure of rivastigmine (adapted from CitationCummings and Winblad 2007).
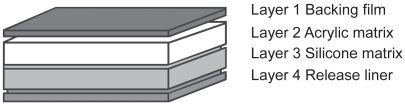
The rivastigmine patch is composed of four layers (). The highest layer, the backing film, is colored and has a protective function against mechanical, extraneous causes. In the second layer, the drug is incorporated into an acrylic matrix, which ensures effective storage of rivastigmine (CitationOertel et al 2007). The next coating, a silicone matrix layer with a silicone polymer, provides good adhesion of the patch to the skin. Directly on the skin, a release liner guarantees continuous dispensing of the drug through the skin, providing smooth delivery into the bloodstream. This layer also minimizes skin reactions.
Dosage and patch application
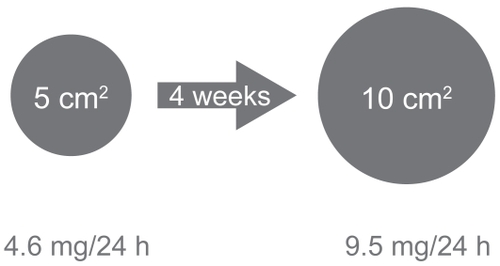
Two different patches are available. The smaller 5 cm2 patch contains a total dose of 9 mg rivastigmine and releases 4.6 mg/day rivastigmine. The 10 cm2 patch has a capacity of 18 mg rivastigmine, and releases 9.5 mg/day. Therapy should begin with the small patch of 4.6 mg/24 h and, if well tolerated, the dose could be increased after a minimum of 4 weeks to the 9.5 mg/24 h patch ().
Figure 3 Dosage of the rivastigmine patch (adapted from CitationCummings and Winblad 2007).
If the drug cannot be tolerated because of side effects such as nausea, therapy can be interrupted, or the dosage reduced. If the patch application is discontinued for more than seven days, the treatment should start with the 4.6 mg/24 h patch. Oral treatment may be directly switched for the transdermal application (oral dosage <6 mg to the 4.6 mg/24 h patch, oral dosage from 6 to 12 mg to the 9.5 mg/24 h patch). No dosage reduction is necessary in patients with renal or hepatic disturbance.
However, the rivastigmine patch should be applied only on uninjured, clean, dry, and hairless skin. The upper back, chest or the forearm are especially well suited for application. The skin should be cleaned only with water, and soap residues should be avoided. Swimming and sports are also possible, although heat and sun exposure should be avoided for long periods of time (CitationNovartis 2007).
Pharmacokinetics
Three placebo-controlled trials have investigated the different pharmacokinetic profile of the patch and the capsule formulation (CitationLefevre et al 2007; CitationLefevre et al 2008; CitationMercier et al 2007). The rivastigmine patch provides a continuous and smooth release of the drug. After application of the first patch, the absorption of the drug begins after a time-lag of 0.5 to 1 h (CitationLefevre et al 2008). The maximum plasma level of rivastigmine is reached after a mean of 8 h. After this point, drug concentration reduces only slowly, so that fluctuation of the plasma level in transdermal administered rivastigmine is low. At a steady state, the trough level of the drug after transdermal application is 60%–80% of the maximum plasma level. In contrast, after oral administration with the capsule formulation for all doses, the plasma peak is reached after just 1 h, followed by a rapid decline in plasma levels (CitationHossain et al 2002). The maximum concentration after a 3 mg capsule twice daily is approximately 70% higher than after using the 9.5 mg/24 h patch. Therefore, fluctuating plasma concentration after oral administration is accelerated more sharply than after patch application (fluctuation index: 6.2 for twice daily 3 mg and 4.0 for twice daily 1.5 mg oral administration; 0.6 for the 4.6 mg/24 h patch and 0.8 for the 9.5 mg/24 h patch) () (CitationLefevre et al 2008).
Figure 4 Drug concentration over the time after administration of the patch or twice-daily capsules (adapted from CitationCummings and Winblad 2007).
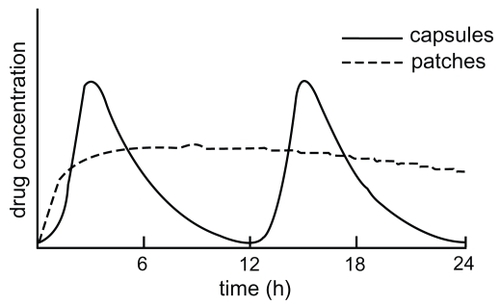
The measured drug exposure after application of the 9.5 mg/24 h patch is close to rivastigmine exposure after administration of 3 mg twice daily. The drug delivery rate of the two patches amounts to approximately 50% (CitationLefevre et al 2008). The delivery rate and the maximum drug concentration are dependent on the region of application of the patch. Compared with the upper back, the relative bioavailability of the drug is 100% for the chest, 92% for the upper arm, 80% for the abdomen, and 71% for the thigh (CitationLefevre et al 2007). Thus the highest exposure rate of plasma concentration can be reached on the chest, upper back, and upper arm.
Rivastigmine binds low (approximately 40%) to the plasma proteins and has an apparent distribution volume of 1.8 to 2.7 L/kg. Following transdermal administration, rivastigmine is metabolized by AchE and BchE-mediated hydrolysis to the principal metabolite NAP226-90. In vitro, NAP226-90 inhibits AchE to a minimal extent (<10%) (CitationPolinsky 1998). There is evidence that rivastigmine is present in only a very small amount metabolized by the hepatic cytochrome P-450 system, which could be the reason for the minimal interaction potential of the drug (CitationPolinsky 1998). After transdermal administration, less NAP226-90 is formed compared with oral administration, indicating that metabolization of transdermally administered rivastigmine is lower than after intake of the capsules. This difference is attributed to the distinct hepatic first-pass effect after oral administration, which is responsible for the low bioavailability (~35%) after oral administration (CitationPolinsky 1998). The assumption of greater efficacy for transdermal rivastigmine is based on lower metaboli zation into the lesser potent NAP226-90, compared with oral administration. Both oral and transdermal administration underlie a nonlinear pharmacokinetic mechanism, due to the saturation of the rivastigmine-metabolizing enzymes (CitationHossain et al 2002).
The principal metabolites of the drug are renally excreted; only <1% of the drug is eliminated through the feces, and only traces of unchanged rivastigmine are found in the urine.
After removal of the patch the elimination half-time amounts to approximately 3 h.
Interactions
The interaction potential of the rivastigmine patch has not been investigated in a clinical study. But because of the very small interaction with the cytochrome P450 system, only a marginal interaction potential is to be expected.
Adverse effects
Gastrointestinal side effects such as vomiting, nausea, diarrhea, and other cholinergic side effects are a frequent problem in treatment with AchEIs especially in the titration phase. Gastrointestinal side effects sometimes result from fast titration of the drug, particularly when rivastigmine is administrated orally, thereby circumventing the administration of the maximal admitted dosage and leading to discontinuation of the treatment. This result is especially important, because meta-analyses have shown that rivastigmine exerts a definite clinical effect only at higher doses (CitationInstitute for Quality and Efficiency in Health Care 2007). There is evidence that cholinergic side effects are the result of an abrupt rise in stimulation of the acetylcholine receptor in the brain after administration of the rivastigmine capsules (CitationLefevre et al 2008; CitationYang and Keating 2007).
This problem accelerated the development of other formulations of rivastigmine. The rivastigmine patch theoretically offers, with continuous release of the drug and constant plasma drug level, the strongest possibility of minimizing fluctuation-associated side effects (CitationCummings and Winblad 2007). And indeed, in clinical trials the patch-treated patients develop fewer adverse gastrointestinal effects than patients treated with capsules at 3 to 12 mg/day (CitationWinblad et al 2007a). shows the different profiles of adverse effects of the two formulations.
Table 2 Adverse events reported in the IDEAL-study (safety population)
Contraindictions
The rivastigmine patch is contraindicated in patients with known intolerance of rivastigmine, or other constituents of the TDS. No clinical studies have been conducted on pregnant woman and nursing mothers. Patients with low body weight should be carefully monitored due to the incidence of more adverse effects, and the possibility of more weight loss caused by rivastigmine.
Clinical efficacy of the rivastigmine patch
Effects
To evaluate the clinical efficacy, the available literature was systematically reviewed. In order to identify relevant studies, we searched keywords such as “rivastigmine”, “Alzheimer”, “patch” along with combinations of these in the following databases: Pubmed (January 1997–December 2007), the databases of the Centre for Reviews and Dissemination (until December 2007), and the database of the Deutsches Institut für medizinische Dokumentation und Information (DIMDI) (until December 2007). In addition, we screened the following journals for abstracts: Annals of Neurology, European Journal of Neurology, Neurology, Journal of Neurology. Abstracts were considered only when a) they were in English/German/Spanish/French and b) an evaluation of rivastigmine was explicitly mentioned. Four studies were identified in which the rivastigmine patch was assessed in patients suffering from AD.
The clinical efficacy of the rivastigmine patch was investigated in the IDEAL study (Investigation of Transdermal Exelon® in AD) (CitationWinblad et al 2007a; CitationWinblad et al 2007b).
The IDEAL study was a 24-week, randomized, double-blind, double-dummy, placebo- and active controlled, multicenter trial of the rivastigmine patch on 1195 AD patients. The patients were randomized in one of four groups with target doses of: 9.5 mg/24 h (10 cm2) rivastigmine patch (n = 293), 17.4 mg/24 h (20 cm2) rivastigmine patch (n = 303), 3 mg/day to a maximum of 12 mg/day rivastigmine capsules (n = 297) or placebo (n = 302). The patches were placed by the caregivers on the upper back every morning, with normal daily activities like bathing being permitted. The patients were investigated at baseline, week 16 and week 24. The primary outcomes were a change from baseline to week 24 in the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) (CitationRosen et al 1984) and, to assess the global impression of the patient, a change from baseline to week 24 in the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGIC) (CitationSchneider and Olin 1996). Compared to placebo, all rivastigmine-treated patients (patches and capsules) at week 24 showed a significant improvement in the ADAS-Cog score and a significant reduction in ADCS-CGIC scores, indicating an improvement of cognitive functions and global impression. Secondary outcomes comprise the Mini Mental State Examination (MMSE) (CitationFolstein et al 1975), Neuropsychiatric Inventory (NPI-12, with the NPI-Distress), psychiatric disturbance and behaviour (Cummings et al 1994), Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL) (CitationGalasko et al 1997), Trail Making Test Part A (TMTA) (motor processing speed, attention, and visual tracking) (CitationCorrigan and Hinkeldey 1987), and the Ten-Point Clock-Drawing Test (TCD) (executive and visuospatial functions) (CitationWatson et al 1993). Compared with placebo, the 10 cm2 and the 20 cm2 rivastigmine patches and the rivastigmine capsules led to a significant improvement in results for the ADCS-ADL, MMSE, and the TMTA at week 24. No significant difference was observed in the NPI-12, NPI-Distress score, and TCD in the 3 rivastigmine-treated groups compared with the placebo group at week 24. There was no statistical difference between the efficacy of the rivastigmine capsules and the 10 cm2 patch. Compared with the patients treated with the 10 cm2 patch, the 20 cm2 patch group showed numerically better scores on the ADAS-Cog at week 24.
Adverse effects
The most reported side effects in AchEI treatment are cholinergic side effects such as nausea, vomiting, and diarrhea. In the IDEAL study of 1195 patients, adverse events in the four groups: 10 cm2 rivastigmine patch, 20 cm2 rivastigmine patch, capsule (3 to 12 mg rivastigmine per day), and placebo, were analyzed (CitationWinblad et al 2007a; CitationWinblad et al 2007b) (). Patients in the 10 cm2 patch group developed nearly two-thirds fewer nausea (7%) and vomiting (6%) reactions than the 20 cm2 patch group (21% nausea, 19% vomiting), and the patients receiving capsules (23% nausea, 17% vomiting). After administration of the 20 cm2 patch and the capsules, significantly more adverse effects were noted than in the placebo group. The incidence of adverse events in the 10 cm2 patch-treated group did not differ statistically from the placebo group. The reported side effects were mainly mild to moderate.
Because of adverse events, 10% of the patients in the 20 cm2 group, 11% in the 10 cm2 group, and 9% of patients in the placebo group, have discontinued the trial. Severe adverse events occurred in 8% of patients receiving the 10 cm2 patch, 12% receiving the 20 cm2 patch, 7% receiving the capsules, and 9% receiving placebo.
Weight loss equal to or more than 7% of the baseline weight was observed in 8% of the patients of the 10 cm2 patch group, 12% of the patients receiving the 20 cm2 patch, 11% of the patients treated with the capsules, and 6% of the patients receiving placebo.
In conclusion, in the IDEAL study, the 10 cm2 patch was well tolerated. In contrast to the 20 cm2 patch and the capsules, the incidence of side effects was not significantly different compared with the placebo. Moreover, in a study including 40 healthy volunteers, the 10 cm2 patch was also well tolerated (CitationLefevre et al 2007). In the IDEAL study, skin irritation was rarely reported. Skin irritation was assessed by the investigators and caregivers: 8% of patients receiving a patch (all sizes) and 4% receiving the placebo patch developed an erythema; 7% of the patch-treated group and 3% of the placebo patch group reported pruritus; 2% of patients treated with a patch, and no patients from the placebo group, discontinued the treatment because of skin irritation (CitationWinblad et al 2007a). Another study investigated the tolerance of the patch on different regions of the body (CitationLefevre et al 2007). More incidents of erythema occurred when the patch was applied to the thigh and abdomen (compared with the upper back), whereas patch adherence to the upper arm and the chest led to fewer such incidents. Erythemic instances were marginal, visible, or mild, and self-resolved within 24 h of patch displacement. No other skin reactions, such as papules or edema, were observed (CitationLefevre et al 2007).
Caregiver
In a sub-study of the IDEAL trial, the caregiver preference for the rivastigmine patch, compared with capsule treatment, was investigated (CitationWinblad et al 2007c). At baseline, week 8 and week 24, the AD Caregiver Preference Questionnaire (ADCPQ) (CitationWinblad et al 2007c) was used to evaluate the satisfaction, preferences, and expectations of the caregivers. The double-dummy nature of the study ensured that this assessment was not influenced by perceptions of efficacy and tolerability (CitationWinblad et al 2007c). At week 8, 68% of the caregivers preferred the patch over the capsules. At week 24, 72% of the caregivers decided in favor of the patch. At week 24, the most frequently assigned reasons in favor of the patch were: simplicity of the schedule and ease of use. The more specific reasons given for patch preference included greater self-sufficiency, fewer or no adverse events, and more convenient handling. Those caregivers preferring the capsules declared most frequently, ease of use.
Health-related quality of life
No clinical trial has investigated the effect of the rivastigmine patch on health-related quality of life (HRQOL). Nor has the effect on HRQOL of treatment with rivastigmine capsules been investigated (CitationInstitute for Quality and Efficiency in Health Care 2007).
Cost
In Germany, the daily therapy cost of rivastigmine capsules was €5.50 in 2006. In comparison, the daily therapy cost of donezepil amounted to €3.72 and galantamine to €4.31 (CitationSchwabe and Paffrath 2007). In a recent meta-analysis of comparative health economic evaluations a cost saving effect was found using rivastigmine capsules. In patients with mild to moderate AD these models estimated a cost per quality-adjusted life year gain, ranging from £16.000–£46.000 (CitationNICE 2007). Unfortunately, no evaluations for rivastigmine patch treatment are currently available. In Germany, the cost of the small and large patch is approximately €4 per patch.
Conclusion
In recent years, TDS have become an increasingly important treatment option for the application of certain drugs (CitationPriano et al 2006). This trend is due especially to continuous drug delivery, leading to consistent drug plasma level, improved tolerance, greater systemic bioavailability, and is also due to avoiding the first-pass effect, simple usage, easy scheduling, and strong adhesiveness. Most available patches result in only rare disadvantages, such as skin irritations.
The rivastigmine patch, the first patch for treating mild to moderate AD, was approved in July 2007 in the US. In the IDEAL study the 10 cm2 patch significantly improved cognition, global impression, activities of daily living, motor processing speed, and attention and visual tracking, compared with placebo (CitationPriano et al 2006; CitationWinblad et al 2007a; CitationWinblad et al 2007b). Concomitantly, patients treated with the 10 cm2 patch developed approximately two-thirds fewer gastrointestinal side effects, such as vomiting and nausea, compared with patients treated with rivastigmine capsules (3–12 mg/day). In contrast to the capsules, the incidence of adverse events is not significantly different compared with placebo (CitationWinblad et al 2007a; CitationWinblad et al 2007b). The lower incidence of adverse events may be the result of reduced fluctuation of the drug plasma level due to continuous drug delivery, because the acute, centrally-mediated gastrointestinal effects correlated with the maximum plasma level and the time it took to reach this peak after oral administration (CitationJann et al 2002; CitationDarvesh et al 2003). The infrequent gastrointestinal side effects after administration of the patch might compensate for the higher incidence of nausea and vomiting in the therapy with rivastigmine capsules compared with donezepil and galantamine, which is probably associated with inhibition of the AchE and the BchE of rivastigmine (CitationInglis 2002). Well designed clinical trials should investigate the incidence of adverse events with the rivastigmine patch, compared with the oral formulation of donezepil and galantamine.
In the IDEAL study, due to gastrointestinal effects approximately 75% fewer patients treated with the 10 cm2 patch discontinued the therapy compared with patients receiving capsules (CitationWinblad et al 2007b). This should contribute to a better compliance of patients suffering from AD (CitationCummings and Winblad 2007). This result has proved to be very important, because consistent therapy is essential, in order to reduce the risks of rapid cognitive deterioration, increasing medical and healthcare costs, and the resignation of caregivers (CitationBlesa et al 2007; CitationSmall and Dubois 2007). Compared with the capsules, another advantage of the patch is the easier dosing schedule with one increase of the dosage after 4 weeks only.
A disadvantage of the rivastigmine patch is higher therapy costs compared to capsules. In Germany the annual cost for the 9.5 mg/24 h patch accounts for €1,424 compared with €1,318 for treatment with 12 mg capsules daily (based on Red List price, 2008: €352.25 per 90 9.5 mg/24 h patch package vs €202.79 per 112 6 mg capsules package).
Furthermore, there is less clinical experience compared with treatment with oral AchEI, and the evidence of clinical efficacy is based on only a few trials. However, the good efficacy, rare systemic adverse events, dynamic skin tolerance, handling ease, and the satisfaction of caregivers, means that the new rivastigmine patch is an important step in the treatment of AD.
Disclosures
AW has no conflict of interest. RD and WO have received honoraria for scientific presentations at different meetings including Novartis, Pfizer and Eisai.
References
- BlesaRBallardCOrgogozoJM2007Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer diseaseNeurology694 Suppl 1S23817646620
- Corey-BloomJAnandRVeachJA1998A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartate), a new acetylcholinesterase inhibitor in patients with mild to moderate severe Alzheimer`s diseaseInt J Geriatr Psychopharmacol15565
- CorriganJDHinkeldeyNS1987Relationships between parts A and B of the Trail Making TestJ Clin Psychol4340293611374
- CrisbyMCarlsonLAWinbladB2002Statins in the prevention and treatment of Alzheimer diseaseAlzheimer Dis Assoc Disord16131612218642
- CummingsJWinbladB2007A rivastigmine patch for the treatment of Alzheimer’s disease and Parkinson’s disease dementiaExpert Rev Neurother714576317997695
- CummingsJL2004Alzheimer’s diseaseN Engl J Med351566715229308
- DarveshSWalshRKumarR2003Inhibition of human cholines-terases by drugs used to treat Alzheimer diseaseAlzheimer Dis Assoc Disord171172612794390
- DewachterIVan LeuvenF2002Secretases as targets for the treatment of Alzheimer’s disease: the prospectsLancet Neurol14091612849363
- DodartJCBalesKRGannonKS2002Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease modelNat Neurosci5452711941374
- DodelRCHampelHDuY2003Immunotherapy for Alzheimer’s diseaseLancet Neurol22152012849209
- FabbriniGBrotchieJMGrandasF2007Levodopa-induced dyskinesiasMov Disord22137989 quiz 52317427940
- FarlowMAnandRMessinaJJr2000A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s diseaseEur Neurol442364111096224
- FarlowMRCummingsJL2007Effective pharmacologic management of Alzheimer’s diseaseAm J Med1203889717466645
- FolsteinMFFolsteinSEMcHughPR1975“Minimental state”. A practical method for grading the cognitive state of patients for the clinician”J Psychiatr Res12189981202204
- GalaskoDBennettDSanoM1997An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative StudyAlzheimer Dis Assoc Disord11Suppl 2S3399236950
- HardyJSelkoeDJ2002The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeuticsScience297353612130773
- HossainMJheeSSShiovitzT2002Estimation of the absolute bio-availability of rivastigmine in patients with mild to moderate dementia of the Alzheimer’s typeClin Pharmacokinet412253411929322
- in t’ VeldBARuitenbergAHofmanA2001Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s diseaseN Engl J Med34515152111794217
- InglisF2002The tolerability and safety of cholinesterase inhibitors in the treatment of dementiaInt J Clin Pract Suppl127456312139367
- Institute for Quality and Efficiency in Health Care2007Cholinesterase inhibitors in Alzheimer’s disease
- JannMWShirleyKLSmallGW2002Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitorsClin Pharmacokinet417193912162759
- LefevreGSedekGHuangHL2007Pharmacokinetics of a rivastigmine transdermal patch formulation in healthy volunteers: relative effects of body site applicationJ Clin Pharmacol47471817389556
- LefevreGSedekGJheeSS2008Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patientsClin Pharmacol Ther831061417522596
- McGeerPLSchulzerMMcGeerEG1996Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studiesNeurology47425328757015
- MercierFLefevreGHuangHL2007Rivastigmine exposure provided by a transdermal patch versus capsulesCurr Med Res Opin23319920418001519
- MorganDDiamondDMGottschallPE2000A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s diseaseNature408982511140686
- NICE2007Donezepil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer’s disease (amended)NICE technology appraisal guidance 111 (amended)
- Novartis2007 http://wwwpharmausnovartiscom/product/pi/pdf/exelonpatchpdf
- OertelWRossJSEggertK2007Rationale for transdermal drug administration in Alzheimer diseaseNeurology694 Suppl 1S4917646621
- OrgogozoJMGilmanSDartiguesJF2003Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunizationNeurology611465412847155
- PermanneBAdessiCFragaS2002Are beta-sheet breaker peptides dissolving the therapeutic problem of Alzheimer’s disease?J Neural Transm Suppl6229330112456072
- PerryEKTomlinsonBEBlessedG1978Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementiaBr Med J214579719462
- PolinskyRJ1998Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s diseaseClin Ther20634479737824
- PrianoLGascoMRMauroA2006Transdermal treatment options for neurological disorders: impact on the elderlyDrugs Aging233577516823990
- PuglielliLTanziREKovacsDM2003Alzheimer’s disease: the cholesterol connectionNat Neurosci63455112658281
- RaskindMAPeskindERTruyenL2004The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trialArch Neurol61252614967774
- RosenWGMohsRCDavisKL1984A new rating scale for Alzheimer’s diseaseAm J Psychiatry1411356646496779
- RoslerMAnandRCicin-SainA1999Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trialBmj318633810066203
- ScarpiniEScheltensPFeldmanH2003Treatment of Alzheimer’s disease: current status and new perspectivesLancet Neurol25394712941576
- SchenkDBarbourRDunnW1999Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouseNature400173710408445
- SchneiderLSOlinJT1996Clinical global impressions in Alzheimer’s clinical trialsInt Psychogeriatr827788 discussion 88–908994897
- SchwabeUPaffrathD2007Arzneiverordnungs-Report 2007HeidelbergSpringer
- SmallGDuboisB2007A review of compliance to treatment in Alzheimer’s disease: potential benefits of a transdermal patchCurr Med Res Opin2327051317892635
- SmallGWKauferDMendiondoMS2005Cognitive performance in Alzheimer’s disease patients receiving rivastigmine for up to 5 yearsInt J Clin Pract59473715853867
- SotoCSaborioGPPermanneB2000Inhibiting the conversion of soluble amyloid-beta peptide into abnormally folded amyloidogenic intermediates: relevance for Alzheimer’s disease therapyActa Neurol Scand Suppl17690511261811
- SummersWKMajovskiLVMarshGM1986Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer typeN Engl J Med315124152430180
- US Food and Drug Administration2008 http://wwwfdagov/consumer/updates/exelonpatch080307html
- van GoolWAAisenPSEikelenboomP2003Anti-inflammatory therapy in Alzheimer’s disease: is hope still alive?J Neurol2507889212883918
- WatsonYIArfkenCLBirgeSJ1993Clock completion: an objective screening test for dementiaJ Am Geriatr Soc411235408227899
- WattsRLJankovicJWatersC2007Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson diseaseNeurology68272617202432
- WeggenSEriksenJLDasP2001A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activityNature414212611700559
- WinbladBCummingsJAndreasenN2007aA six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease – rivastigmine patch versus capsuleInt J Geriatr Psychiatry224566717380489
- WinbladBGrossbergGFrolichL2007bIDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer diseaseNeurology694 Suppl 1S142217646619
- WinbladBKawataAKBeusterienKM2007cCaregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer’s diseaseInt J Geriatr Psychiatry224859117407176
- XieWStribleyJAChatonnetA2000Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholinesteraseJ Pharmacol Exp Ther29389690210869390
- YangLPKeatingGM2007Rivastigmine transdermal patch: in the treatment of dementia of the Alzheimer’s typeCNS Drugs219576517927299