Abstract
The lung is an attractive target for drug delivery due to noninvasive administration via inhalation aerosols, avoidance of first-pass metabolism, direct delivery to the site of action for the treatment of respiratory diseases, and the availability of a huge surface area for local drug action and systemic absorption of drug. Colloidal carriers (ie, nanocarrier systems) in pulmonary drug delivery offer many advantages such as the potential to achieve relatively uniform distribution of drug dose among the alveoli, achievement of improved solubility of the drug from its own aqueous solubility, a sustained drug release which consequently reduces dosing frequency, improves patient compliance, decreases incidence of side effects, and the potential of drug internalization by cells. This review focuses on the current status and explores the potential of colloidal carriers (ie, nanocarrier systems) in pulmonary drug delivery with special attention to their pharmaceutical aspects. Manufacturing processes, in vitro/in vivo evaluation methods, and regulatory/toxicity issues of nanomedicines in pulmonary delivery are also discussed.
Introduction
Burgeoning interest in colloidal carriers (nanocarrier systems) has led to increasing attention for pulmonary drug delivery. The lung is an attractive target for drug delivery due to noninvasive means to provide not only local lung effects but possibly high systemic bioavailability, avoidance of first-pass metabolism, more rapid onset of therapeutic action, and the availability of a huge surface area.Citation1,Citation2 Nanocarrier systems in pulmonary drug delivery offer many advantages. These advantages include the following: 1) the potential to achieve relatively uniform distribution of drug dose among the alveoli; 2) an achievement of enhanced solubility of the drug than its own aqueous solubility; 3) the sustained-release of drug which consequently reduces the dosing frequency; 4) suitability for delivery of macromolecules; 5) decreased incidence of side effects; 6) improved patient compliance; and 7) the potential of drug internalization by cells.Citation3,Citation4
Nanotechnology has been described as the manipulation, precision placement, measurement, modeling or manufacture of matter in the sub-100 nm range,Citation5 although, depending on the context, the term sometimes identifies particles in the 1 to 200 nm range.Citation3,Citation6 However, the 100 nm limit is constraining, as in effect it would disregard many recent achievements and a plethora of basic science (interfacial and colloidal chemistry) literature and pharmaceutical research literature reports. In addition, there are a number of literature reports in both the basic science and pharmaceutical literature which scientifically define dimensions of nanoparticles ranging in size from 1 to 1000 nm.Citation3,Citation7–Citation9 In drug delivery systems, submicron size is significant because biodistribution of submicron particles are critical and some safety issues may be occurred when we use microparticles. For instance, after intravenous injection of parenteral formulation, large particles (>5 μm) can cause pulmonary embolism, which can induce fatal results,Citation10,Citation11 therefore submicron particle size is required for parenteral formulations. Although the upper limit of particle size for ophthalmic application is about 5 μm, the optimum particle size is less than 1,000 nm because a scratching feeling can occur when microparticles are applied to the eyes.Citation12 Phagocytosis is sensitive to particle size, and it is generally thought that particles of 0.5–3 μm in diameter are taken up by macrophages,Citation13 and particles of less than 0.26 μm can escape from phagocytosis by macrophages.Citation1 Phagocytosis is also the main mechanism responsible for the rapid clearance of particulate drug delivery systems from the body. Furthermore, cytosolic delivery of drugs can be achieved by endocytosis of submicron drug carriers. Therefore, in this review, the authors consider all particulates for which at least one dimension is 1–1000 nm.
There are numerous applications for nanotechnology, however, especially the treatment, diagnosis, monitoring and control of biological systems have recently been referred to as “nanomedicine” by the National Institutes of Health (Bethesda, MD, USA).Citation14 In short, nanomedicine is the application of nanotechnology to medicine, and two main types of nanomedicine products are currently in clinical trials: diagnostic tests and drug delivery devices.Citation15 Over the past decades, efforts have been focused on the development of nanomedicines such as nanoparticles, liposomes, nanoemulsions, or dendrimers for the specific delivery of drugs to the target tissues.
The modern inhalation devices can be divided into three different categories, nebulizers, pressurized metered dose inhalers (pMDI), and dry powder inhalers (DPI).Citation1 In most cases, nanocarriers can be delivered to the lungs by nebulization of colloidal dispersions or using pMDIs and DPIs in solid form.Citation1,Citation3 Due to small size and strong particle-particle interactions of nanocarriers, particle agglomeration and settlement can occur in colloidal dispersions. Moreover, chemical instability of colloidal dispersions is another issue owing to carrier hydrolysis and drug degradation. To improve physical and chemical instability of nanocarrier dispersions, freeze-drying of nanocarriers has been explored as a means to provide a storage form. However, redispersibility and the use of stabilizers for lyophilization are still problematic in nebulization. The use of a nanocarrier itself for delivery to lungs is severely limited because individual nanocarriers do not deposit efficiently in the lungs by diffusion, sedimentation or impaction, which results in the exhalation of a majority of the inhaled dose.Citation3 Therefore, micron-sized powder carriers containing nanoparticles or agglomerated nanoparticles were designed to improve the inhalation aerosol delivery of nanoparticles for deep lung delivery by using MDIs and DPIs.Citation1 A thorough discussion of incorporating nanoparticles into micron-scale structures or processing methods for agglomerated nanoparticles are beyond the scope of this review, but the topic has been recently reviewed elsewhere. Citation3,Citation4,Citation16
This review focuses on the current status and explores the potential of colloidal carriers (nanocarrier systems) in pulmonary drug delivery with special attention to and in depth presentation of their pharmaceutical aspects. Manufacturing processes, in vitro/in vivo evaluation methods, and regulatory/toxicity issues of nanomedicines in pulmonary delivery are also presented and discussed.
Drugs for inhalation
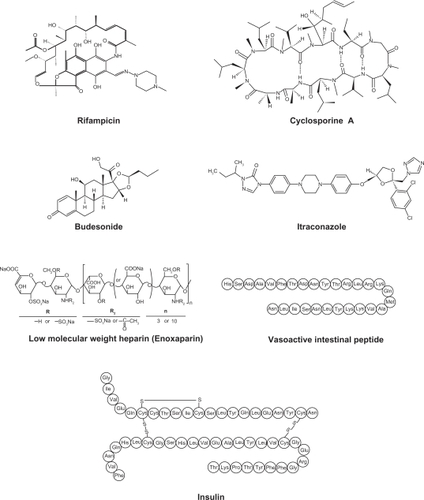
Various drugs are investigated for local or systemic pulmonary delivery.Citation2 These include small molecules, protein/peptide drug and genes (). In case of small molecule drugs, many studies were focused on local application for the treatment of chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). However, pulmonary protein/peptide delivery offers great potential for both local targeting for the treatment of respiratory diseases and systemic targeting for the treatment of diabetes mellitus or thrombosis. Gene delivery to the lungs are mainly focused on the localized delivery of drugs to the site of disease, the lungs and airways, including lung cancer, genetic disorders affecting the airways (cystic fibrosis, alpha-1-antitrypsin deficiency), obstructive lung diseases (asthma), and vaccination. Since original aerosol technology was developed for small molecule drugs, it is necessary to evolve the reengineering of nanocarrier self-assembly systems for macromolecular pulmonary delivery.Citation17 Examples of drugs for pulmonary nanocarrier systems are shown in .Citation18–Citation28,Citation29–Citation76
Table 1 Examples of drugs for pulmonary delivery using colloidal carrier self-assembly systems
Nanocarrier systems for pulmonary drug delivery
Polymeric nanoparticulate pulmonary delivery
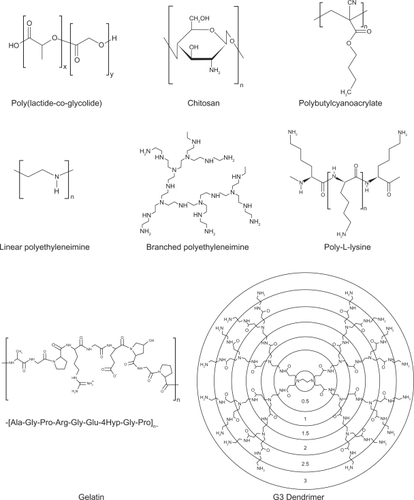
Polymeric nanoparticles are widely studied in drug delivery system for parenteral administration;Citation77,Citation78 however their application to the pulmonary routes are also widely recognized.Citation4 The main roles of polymeric nanoparticles in drug delivery system are to carry the drug molecules, to protect drugs from degradation, and to control drug release.Citation78 Therapeutically used polymeric nanoparticles are composed of biodegradable or biocompatible materials, such as poly(ɛ-caprolactone) (PCL), poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), alginic acid, gelatin and chitosan. Various polymers for pulmonary drug delivery using nanocarrier systems are shown in . The chemical structures of polymers for polymeric nanoparticles used in pulmonary delivery systems are shown .Citation26,Citation29,Citation38,Citation39,Citation54–Citation57,Citation59,Citation61,Citation69,Citation70,Citation74,Citation75,Citation79–Citation93
Table 2 Various polymers for colloidal pulmonary drug delivery systems
Due to their biocompatibility, surface modification capability, and sustained-release properties, polymeric nanoparticles are intensively studied using various important pulmonary drugs. These pulmonary drug include antiasthmatic drugs,Citation22,Citation26 antituberculosis drugs,Citation38,Citation39 pulmonary hypertension drugs,Citation29 and anticancer drugs.Citation54 However, to avoid accumulation of polymer carriers following repeated dosing, the biodegradability and toxicity of polymers over the long term should be closely examined in the formulation of polymeric nanoparticles for pulmonary delivery. Additionally, in vitro lung surfactant models and in vivo studies are required to establish the pulmonary acceptability of polymeric nanocarrier systems, as polymers and their degradation products can affect the vital surfactant properties in the alveoli which in turn will affect pulmonary immunity control and adversely affect the work of breathing.
Although cationic lipid-based gene carriers are currently being clinically evaluated further than polymer-based gene carriers,Citation94 cationic polymers are one of the popular carriers for gene delivery to the lungs.Citation95,Citation96 Although polyethyleneimine (PEI) and polyamino acids, such as poly-l-lysine, have been shown to be effective agents for DNA delivery both in vitro and in vivo,Citation97,Citation98 cytotoxicityCitation99 and low transfection efficacy problems when delivered via inhalation have to be overcome.Citation100 To solve these problems, various modifications of PEI with liposomes/PEGs or conjugations of PEI with ligands such as transferrin have been investigated extensively.Citation101–Citation107 Noninvasive pulmonary gene delivery using cationic polymers has been reviewed in detail elsewhere.Citation95
Liposomal pulmonary delivery
Liposomes are one of the most extensively investigated systems for controlled delivery of drug to the lung.Citation108 Liposomes seem particularly appropriate for therapeutic agent delivery to lung, since these vesicles can be prepared from compounds endogenous to the lungs, such as the components of lung surfactant, and these properties make liposomes attractive candidates as drug delivery vehicles.Citation37 The first pharmaceutical liposomal products in market include the synthetic lung surfactant Alveofact® (Dr Karl Thomae GmbH, Biberach, Germany) for pulmonary instillation for the treatment of respiratory distress syndrome (RDS).Citation109 Typically, liposomal formulations have been delivered to the lung in the liquid state, and nebulizers have been used extensively for the aerosol delivery of liposomes in the liquid state.Citation110 However, concerns arise from drug stability in the liquid state and leakage when nebulizers are used to deliver a liposomal encapsulated agent.Citation111 Recently, liposomal dry powder formulations have been intensively examined in order to successfully circumvent these issues.Citation72,Citation112–Citation114 Liposomal dry powder formulations have been shown to be very promising in the delivery of various types of pulmonary drugs and some of these formulations are currently in clinical trials.
Much interest has focused on cationic liposomes for pulmonary gene delivery because cationic liposomes offer the advantage of self-assembly with DNA material through favorable cationic–anionic electrostatic interactions. Additional advantages include evasion from complement inactivation after in vivo administration, the low cost and relative ease in producing nucleic acid–liposome complexes in large scale.Citation95 After first report of inhalation gene delivery success,Citation115 many reports have been published on gene delivery using cationic liposomes by pulmonary administration.Citation104,Citation116–Citation123 Many recent reviewsCitation95,Citation124,Citation125 present gene delivery system using cationic liposomes. Moreover, liposomes conjugated with cell-penetrating peptides are recognized as potential nanocarrier systems for intracellular delivery of macromolecules to the lung. Liposomes modified with cell-penetrating peptides, antennapedia, the HIV-1 transcriptional activator, and octaarginine have been reported to enhance the cellular uptake of liposomes to airway cells.Citation126
Solid lipid nanoparticles in pulmonary delivery
Solid lipid nanoparticles (SLNs) are made from solid lipids (ie, lipids solid at room temperature), surfactant(s) and water.Citation127 Since the beginning of 1990s, the SLNs have been focused on an alternative to polymeric nanoparticles. Citation109 The advantages of drug release from SLNs in the lung are control of the release profile, achievement of a prolonged release and having a faster in vivo degradation compared to particles made from PLA or PLGA. In addition, SLNs proved to possess a higher tolerability in the lungs compared to particles made from some polymeric materials.Citation109,Citation128,Citation129 Although SLNs for the pulmonary delivery is not fully appreciated, toxicological profile of SLNs, when using physiological lipids, is expected to be better than that of polymer-based systems, because physiological lipids have little or no cytotoxicicity.Citation130,Citation131 It is feasible that aqueous suspensions and perhaps dry powder formulations of SLN can be used for pulmonary inhalation aerosol administration of drugs using nebulizers and dry powder inhalers.Citation109
Several studies have been published on the pulmonary applications of SLNs as local delivery carriers for small moleculesCitation42 or as systemic delivery carriers for macromolecules.Citation73,Citation132 Pandey and KhullerCitation42 studied the chemotherapeutic potential of SLNs incorporating rifampicin, isoniazid and pyrazinamide against experimental tuberculosis, and observed the slow and sustained-release of drugs from the SLNs in vitro and in vivo. Gene delivery of SLN-based gene vectors was introduced by Rudolph and colleagues.Citation132 They reported that SLN gene vectors mediate gene expression in the mouse lungs upon aerosol application, which was increased by the TAT peptide, and aerosol application of fragile gene delivery systems can be achieved by a mild nebulization technology based on a novel perforated membrane technology. Liu and colleaguesCitation73 studied novel nebulizer-compatible SLNs containing insulin for pulmonary delivery. They concluded that SLNs could be successfully applied as a pulmonary carrier system for insulin, which might provide novel solutions to the currently unmet medical needs for systemic delivery of proteins.
Deposition and clearance of SLNs were assessed by Videira and colleaguesCitation133,Citation134 after inhalation of aerosolized insoluble particles using gamma-scintigraphy imaging analysis. It was observed that a few minutes after deposition, inhaled material began to translocate to regional lymph nodes indicating that inhalation can be an effective route to deliver drug-containing lipid partices to the lymphatic systems and lipid particles can be used as potential drug carriers for lung cancer therapy, as well as for vaccine delivery.Citation133,Citation134
Submicron emulsions in pulmonary delivery
Until now, the submicron emulsion system has not yet been fully exploited for pulmonary drug delivery and very little has been published in this area.Citation65 Cationic submicron emulsion loaded with Mycobacterium tuberculosis Ag85B DNA vaccine was explored vaccine for the purpose of pulmonary mucosal vaccination.Citation65 Emulsion systems have been introduced as alternative gene transfer vectors to liposomes.Citation135 Other emulsion studies for gene delivery (nonpulmonary route) have shown that binding of the emulsion/DNA complex was stronger than liposomal carriers.Citation136 This stable emulsion system delivered genes to endothelial cells in the mouse nasal cavity more efficiently than commercially available liposomes.Citation137 Bivas-Benita and colleaguesCitation65 have reported that cationic submicron emulsions are promising carriers for DNA vaccines to the lung since they are able to transfect pulmonary epithelial cells, which possibly induce cross priming of antigen-presenting cells and directly activate dendritic cells, resulting in stimulation of antigen-specific T-cells. Therefore the nebulization of submicron emulsions will be a new and upcoming research area. However, extensive studies are required for the successful formulation of inhalable submicron emulsions due to possible adverse effects of surfactants and oils on lung alveoli function (adverse interactions with lung surfactant).
Dendrimer-based nanoparticles for lung delivery
Dendrimers are polymers, which have hyperbranched structures, with layered architectures.Citation138 The research in dendrimer-mediated drug delivery has mainly been focused on the delivery of DNA drugs into the cell nucleus for gene or antisense therapy, and many studies have been reported on the possible use of dendrimers as nonviral gene transfer agents.Citation138 Several studies have been published regarding pulmonary applications of dendrimers as systemic delivery carriers for macromolecules.Citation74,Citation75,Citation93,Citation139
Kukowska-Latallo and colleaguesCitation93 investigated the ability of polyamido amine (PAMAM) dendritic polymers (dendrimers) to augment plasmid DNA gene transfer in vivo and evaluates the targeting of the lung by alternative routes of administration. They suggested that vascular administration seemed to achieve expression in the lung parenchyma, mainly within the alveoli, while endobronchial administration primarily targeted bronchial epithelium, indicating that each delivery route requires different vectors to achieve optimal transgene expression, that each approach appears to target different cells within the lung.
Rudolph and colleaguesCitation139 compared the properties of branched polyethylenimine (PEI) 25 kDa and fractured PAMAM dendrimers for topical gene transfer to the airways in vivo. Their results demonstrated that gene transfer mediated by PEI under optimal conditions was two orders of magnitude higher compared to fractured dendrimers. Therefore, branched PEI 25 kDa was superior to fractured dendrimers for gene delivery to the airways.
Bai and colleaguesCitation74 produced low molecular weight heparin (LMWH)–dendrimer complex through electrostatic interactions using various PAMAM dendrimers, then evaluated both the safety and the efficacy of the drug–dendrimer formulations in preventing deep vein thrombosis in vivo and in situ. They concluded that cationic dendrimers can be used as pulmonary delivery carriers for a relatively large molecular weight anionic drug. These carriers bind anionic drug molecules most likely via electrostatic interactions and increase drug absorption through charge neutralization. In addition, preliminary safety studies using the frog palate model and bronchoalveolar lavage analysis suggest that dendrimers could be viable carriers for pulmonary delivery of LMWH. In another study the same groupCitation75 also investigated pegylated dendrimers (mPEG–dendrimer) in order to increase pulmonary absorption and circulation time of the drug. Briefly, half-life and absorption of LMWH administered via the pulmonary route can be increased by encapsulating the drug in dendrimeric micelles, and their study suggests that LMWH loaded in the mPEG–dendrimer could potentially be used as noninvasive delivery system for the treatment of thromboembolic disorder. However, the potential of dendrimers in the pulmonary drug administration route still remains as a challenge that needs further research to achieve lower cytotoxicity and higher biocompatibility.
Manufacture of nanoparticulate inhalation aerosols
In formulation preparation, several processing technologies have been used to obtain nanoparticles for use as pulmonary inhalation aerosols with desirable attributes, such as narrow particle size distribution, enhanced stability, controlled and targeted release, and improved bioavailability. Jet-milling of the drug under nitrogen gas is the traditional way of creating respirable aerosol particles in the solid-state.Citation140,Citation141 The basic procedure of jet-milling is to grind a bulk crystallized particles into small particles by one of the following mechanical forces: pressure, friction, attrition, impact, or shear. However, up until very recently, this technology had a limitation to generate particles in the nanosized range, although a few cases have been reported to produce nanoparticles, such as insulin nanoparticlesCitation142 and budesonide nanoparticles,Citation143 by wet milling process. The recent availability of new nanojet milling instruments which produce nanoparticles by jet milling may increase the routine use of creating nanoparticles by this method.
More sophisticated and advanced manufacturing technologies are utilized to produce respirable aerosol nanoparticles. These respirable particles may be encapsulated in microparticles in the respirable aerodynamice size range of 1–5 microns or the nanoparticles may be designed to aggregate to a favorable aerodynamic size range. These manufacturing nanotechnologies include spray drying (sometimes referred to as advanced spray drying or nanospray drying), spray-freeze drying, supercritical fluid technology, double emulsion/solvent evaporation technology, antisolvent precipitation, particle replication in nonwetting templates (PRINT), and thermal condensation using capillary aerosol generator.
Spray-drying
Spray-drying is an advanced pharmaceutical manufacturing process used to efficiently produce respirable colloidal particles in the solid-state.Citation144,Citation145 In the process, the feed solution is supplied at room temperature and pumped to the nozzle where it is atomized by the nozzle gas. The atomized solution is then dried by preheated drying gas in a special chamber to remove water moisture from the system, thus forming dry particles. These prepared particles are collected with a cyclone separation device. A schematic representation of a spray drying process is shown in .
The spray-drying process is suitable for thermolabile materials such as proteins and peptides, because mechanical high energy input is avoided in this process.Citation146–Citation148 Moreover, the spray-drying system can be modified to meet specific needs. For example, Maa and colleaguesCitation149 replaced the bag-filter unit of a spray-drying system with a vacuum to reduce the drying airflow resistance. It allows the protein (recombinant humanized anti-lgE antibody) to be dried at a much lower temperature than usual and the production scale to be increased.Citation146 In another study, poly(lactic-co-glycolic acid) particles containing proteins were successfully dried by ultrasonic atomization of feed solution into an atmosphere under reduced pressure.Citation147 Solution spray-drying ensures compositional homogeneity of the drug powder, since the drug and the excipients are dissolved prior to the process. More importantly, spray-drying can result in uniform particle morphology.Citation114,Citation149,Citation150 In industry, spray-drying is a continuous production method, scalable for commercial production volumes.
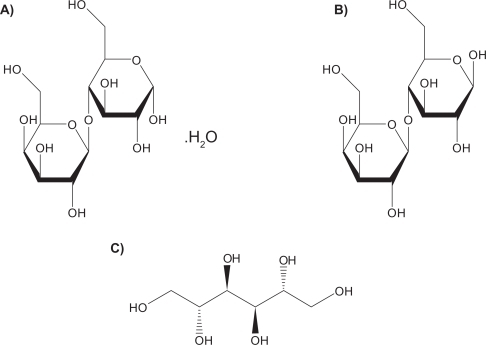
Lactose monohydrate is the only US Food and Drug Administration (FDA)-approved sugar carrier for dry powder aerosol formulations and one of a few FDA approved excipients.Citation151 For example, lactose with either gelatin or poly(butylcyanoacrylate) nanoparticles were spray dried to produce particles for pulmonary delivery.Citation84 Lactose in solid form can be either crystalline as lactose monohydrate or amorphous as lactose. Crystalline lactose can exist in one of two distinct forms: α-lactose monohydrate () and anhydrous β-lactose (). Amorphous lactose may contain both α- and β-lactose molecules that are arranged in a more or less random state. α-lactose monohydrate and anhydrous β-lactose are superior in offering better physical stability than amorphous lactose as the later has a tendency to recrystallize spontaneously.Citation152,Citation153 A disadvantage of lactose monohydrate, a reducing sugar, as a sugar carrier and/or excipient in the solid-state is that it participates in Maillard decomposition reactions with certain small molecular weight pulmonary drugs (eg, budesonide, formoterol), proteins and peptides.Citation154 Recently, D-mannitol, a nonreducing sugar alcohol, whose molecular structure is shown in , has been used as an alternative sugar carrier of pulmonary drugs in dry powder inhalation aerosols, since it does not participate in the solid-state decomposition Maillard reaction.Citation155
Spray-freeze-drying (SFD)
Spray-freeze-drying (SFD) is an advanced particle engineering method which combines spray-drying and freeze-drying processing steps. This technique involves the atomization of an aqueous drug solution into a spray chamber filled with a cryogenic liquid (liquid nitrogen) or halocarbon refrigerant such as chlorofluorocarbon or fluorocarbon.Citation156 The water is removed by sublimation after the liquid droplets solidify.Citation157 SFD is capable of producing porous particles with high fine particle fraction (FPF) at subambient temperatures.Citation157 They are usually low-density composite amorphous particles with high specific surface area.Citation158 Thermolabile protein and peptide substances, such as insulinCitation159 and plasmid DNA,Citation160 can also be formulated into dry powder inhalation products by SFD.
Supercritical fluid technology (SCF)
The basic feature of the supercritical fluid process is the controlled crystallization of drugs from dispersion in supercritical fluids, carbon dioxide. This method has demonstrated a wide range of application in producing pulmonary inhalable formulations.Citation23,Citation161,Citation162 Supercritical fluid technology can be divided into several classes. The most important two are supercritical antisolvent precipitation (SAS)Citation163,Citation164 and supercritical fluid extraction of emulsions (SFEE).Citation165,Citation166 The fundamental mechanism of SAS is based on rapid precipitation when a drug solution is brought into contact with a supercritical CO2. SFEE is based on extraction of the organic phase in oil-in-water or multiple emulsions using supercritical CO2.Citation165,Citation166 The schematic representation of SAS and SFEE processes is shown in . Because most of the drugs (eg, asthma drugs) are not soluble in CO2, SAS processes provide an easy and excellent way to produce dry powder inhalation formulations.Citation163,Citation164 SFEE can provide uniform crystalline drug nanoparticles, composite nanoparticles containing polymeric materials and the drugs, and nanosuspensions.Citation165,Citation166 For instance, Chattopadhyay and colleaguesCitation23 used a continuous SFEE method to produce nanoparticle suspensions containing one of three lipids (tripalmitin, tristearin, or gelucire 50/13), and one of two model drugs (indomethacin or ketoprofen). The first step of this process was to produce nanoemulsions by mixing organic phase containing lipids, a selected drug and chloroform with aqueous phase containing sodium glycocholate, under high pressure homogenization. Then the nanoemulsions were introduced to an extraction chamber countercurrently to a stream of supercritical CO2. The CO2 extracted the organic solvent from the dispersed droplets, forming nanoparticles with a volume mean diameter between 10–30 nm with a high drug loading efficiency for the gelucire particles (∼80%–90%). In another example, nanoparticles containing cholesterol acetate (CA), griseofulvin (GF), and megestrol acetate (MA) were produced by extraction of the internal phase of oil-in-water emulsions using supercritical carbon dioxide.Citation165 This method offered advantages such as the control of particle size, crystallinity, and surface properties. Meanwhile, it shortened the processing time, improved the product purity, and reduced the large waste streams.
Double emulsion/solvent evaporation technique
Respiratory nanoparticle formation from double emulsion/solvent evaporation system involves preparation of oil/water (o/w) emulsions with subsequent removal of the oil phase (ie, typically a volatile organic solvent) through evaporation. The emulsions are usually prepared by emulsifying the organic phase containing the drug, polymer and organic solvent in an aqueous solution containing emulsifier. The organic solvent diffuses out of the polymer phase and into the aqueous phase, and is then evaporated, forming drug-loaded polymeric nanoparticles. By this method, biodegradable polymers, including poly(l-lactic acid) (PLA), poly(glycolic) acid (PGA), and poly(lactide-co-glycolide) acid (PLGA), have been intensively investigated as carriers for solid drug nanoparticles.Citation167
Antisolvent precipitation
Crystalline drug particles with narrow size distribution could be prepared by direct controlled crystallization.Citation168 This process involves antisolvent precipitation of drug solution in a water-miscible organic solvent, followed by addition of a bridging solvent, which is immiscible or partially miscible with water. Growth-retarding stabilizing additives, such as hydroxylpropylmethylcellulose (HPMC), is usually added in the medium to yield particles with small size. The precipitated drug crystals exhibit a high FPF and low amorphous content.Citation169
Particle replication in nonwetting templates (PRINT)
PRINT is a top-down particle fabrication technique developed by Dr. Joseph DeSimone and his group. This technique is able to generate uniform populations of organic micro- and nanoparticles with complete control of size, shape and surface functionality, and permits the loading of delicate cargos such as small organic therapeutics, peptides, proteins, oligonucleotides, siRNA, contrast agents, radiotracers, and fluorophores.Citation170–Citation172 All these features are essential for controlling in vivo transport, biodistribution, and drug-release mechanisms of nanoparticles. The principle of PRINT is to utilize a low surface energy fluoropolymeric mold that enables high-resolution imprint lithography, an emerging technique from the microelectronics industry, to fabricate a variety of organic particles. PRINT is therefore an adaptation of lithographic techniques found in the microelectronics industry to fabricate carriers of precise size for use in nanomedicine. Through the use of an appropriately designed master template, PRINT can precisely manipulate particle size ranging from 20 nm to more than 100 μm. The shape of particles can be sphere, cylinder, discs, and toroid with defined aspect ratios. PRINT is a promising and novel technology in nanoparticulate design and manufacture for use in pulmonary delivery.
Thermal condensation aerosols
Thermal condensation is a cutting edge technology which uses capillary aerosol generator (CAG) to produce high concentration condensation submicron to micron sized aerosols from drug solutions. The drug solution is pumped through a heated capillary tube. Precisely controlled heating of the capillary causes the solution to evaporate. Formulation vapor exiting the tip of the capillary tube mixes with the cooler surrounding air, and then becomes supersaturated and initiates nucleation. The condensation of surrounding vapor onto the generated nuclei results in an aerosol. Various drugs, such as perphenazine,Citation173 prochlorperazine,Citation174 rizatriptanCitation175 and benzyl,Citation176 have been aerosolized using this technique. Propylene glycol (PG) is a popular solvent chosen to dissolve the drugs.Citation177 However, in some cases, rapid heating of thin films of pharmaceutical compounds can also vaporize the molecules, leading to formation of aerosol particles of optimal size for pulmonary drug delivery.Citation178 By controlling the film thickness, the purity of aerosols can be enhanced by reducing the amount of aerosol decomposition.Citation179
In vitro evaluation methods for pulmonary drug delivery systems
In vitro characterization of nanoparticulate aerosol systems
Nanoparticles for pulmonary drug delivery can be evaluated by comprehensive characterization methods. lists several important techniques as well as their functions in the in vitro characterization study of the behavior of nanoparticulate pulmonary inhalation aerosols.Citation180
Table 3 Methods to characterize the physicochemical and aerodynamic properties of particles (microparticles and nanoparticles) for pulmonary inhalation delivery
Inertial impaction is the standard method to measure the particle or droplet aerodynamic size from pharmaceutical aerosol delivery systems.Citation181,Citation182 It describes the phenomenon of the deposition of aerosol particles on the walls of an airway conduct. The impaction (obstruction) tends to occur where the airway direction changes. The big particles have high momentum (inertia) and are more likely to travel in the initial direction of airflow, while those with low momentum adjust to the new direction of flow and pass around the obstruction. Inertial impaction employs Stokes’ law to determine the aerodynamic diameter of particles being evaluated. This has the advantage of incorporating shape and density effects into a single term.Citation141
In addition to the conventional commercially available cascade impactors, MSP Corporation (Minneapolis, MN, USA) has recently developed a new commercially available nanomicroorifice uniform deposition impactor (Nano-MOUDI™) device with high accuracy in sampling and collecting size-fractionated airborne particle samples and pharmaceutical aerosol particle samples for gravimetric and/or chemical analysis. The Nano-MOUDI™ differs from conventional cascade impactors by using microorifice nozzles to reduce the jet velocity, pressure drop, particle bounce, and evaporative loss. These impactors also have a uniform deposit feature by rotating the impaction plates with respect to the nozzles to spread out the particle deposit uniformly on the collection substrates. The uniform deposit prevents heavy particles build-up under each nozzle to reduce particle bounce and blow-off that may otherwise occur. Nano-MOUDI™ is also superior to conventional cascade impactors in its aerodynamic design features. It is designed to prevent cross-flow interference between adjacent nozzles, which results in sharp cut-size characteristics not available with other cascade impactors. Because of its superior aerodynamic design and outstanding performance characteristics, Nano-MOUDI™ has an increasing application in environmental air quality and air pollution studies.Citation183–Citation190 We believe its application in pharmaceutical nanoaerosol drug delivery characterization and modeling is very promising, as well.
X-ray powder diffraction (XRPD) is one of the most important characterization tools used in solid state chemistry and materials science, since it directly measures the degree of molecular long-range vs short-range order. Molecular long-range order is indicative of crystallinity, while short-range order is indicative of noncrystallinity such as liquid crystallinity and amorphicity. It gives information about the extent and nature of crystallinity and molecular order for solid-state materials, ie, how the atoms pack together in the crystalline state and what the interatomic distance and angle are.Citation191–Citation194
Differential scanning calorimetry (DSC) is a powerful and routinely used pharmaceutical thermal analytical method for phase behavior study on polymorphs, hydrates, binding interactions, amorphocity, thermotropic and lyotropic phase transitions of pharmaceutical materials, including nanoparticles. DSC directly measures the gain and loss of enthalpy, that is, order-to-disorder (eg, melting) and disorder-to-order (eg, crystallization) phase transitions.Citation195
Scanning electron microscopy (SEM) is used to visualize the surface morphology of particles with a high magnification. The resolution allows identification of specific surface features and asperities that lead to mechanical interlocking (ie, structural cohesion) and that contain high-energy “active” sites on the surface which influence surface energetic properties and interparticulate interactions and ultimately influence aerosol dispersion performance.Citation180 Surface and interfacial/interparticulate forces are of great importance in the properties of nanoparticles and in the properties of aerosols. Atomic force microscopy (AFM) is used in surface nanotopographical imaging, measurement of surface energy, and measurement of interparticulate forces. This microscopy works in mesoscopic scale resolution (10−6–10−9 m).Citation196 Inverse gas chromatography (IGC) measures the surface free energy of bulk powders such as polymers, fibers, and composite materials.Citation197
Karl Fischer titration is used to analytically quantify small amounts of water present in the inhalation powder which has important consequences on capillary condensation (ie, capillary force is an important interparticulate force in inhalation aerosol particles), solid-state phase behavior, solid-state properties, and solid-state stability of pharmaceutical particles in the solid-state.Citation198
In vitro pulmonary cell culture models
The lung can be anatomically divided into several parts: the trachea, the main bronchi, the conducting bronchioles, the terminal bronchioles and the alveoli.Citation199,Citation200 Drug delivery via inhalation may be absorbed throughout the conducting airway from the trachea down to the terminal bronchioles and ultimately the distal alveoli.Citation201 The airway and alveolar epithelium of the lung, which have different cell types, provide barrier capability to drug absorption.
In order to better understand the drug absorption process in different regions (eg, tracheal, bronchial and alveolar) of the respirable barriers, in vitro pulmonary cell culture models have been developed.Citation201–Citation205 The cell lines used include A427, A549, HBE14o, and the Calu line (-1, -2, and -3).Citation205–Citation210 These are immortal (continuous) cell lines and hence have different membrane structural features compared with mortal cell lines which influence drug absorption and efflux. For example, A549 cell line represents the alveolar type II pulmonary epithelial cell, and has been reported to be an ideal model to study the metabolic and macromolecule mechanisms of drug delivery at the alveolar pulmonary epithelium because the endocytic ability of the pulmonary epithelium and localization of cytochrome P450 systems is largely a function of type II pneumocytes.Citation209 Calu-3 and HBE14o model the upper airways (bronchi) and many studies have been conducted on them. For example, the permeability data of small lipophilic molecule (eg, testosterone) and high molecular weight substance (eg, fluorescein isothiocyanate-transferrin) across Calu-3 cell line has been examined by Foster and colleagues.Citation208 The authors demonstrated this cell line is useful for studying the contributions of bronchial epithelial cells to the mechanisms of drug delivery at the respiratory epithelium. Similar conclusions were drawn when the apparent permeability of the glucocorticosteroid budesonide was investigated.Citation206
Primary cultured cells have also been used in pulmonary absorption and transport studies. These cell cultures have advantages over continuous cells by exhibiting tight junctions. It is important for cell cultures to form a sufficient and appropriately tight polarized monolayer, so that the transport kinetics of test molecules can be properly assessed.Citation211
In vivo evaluation methods for pulmonary drug delivery systems
Pharmacokinetic study after nebulization of colloidal dispersion of drugs
Colloidal drug dispersions have been delivered to male ICR mice via nebulization using a restraint-free small animal inhalation dosing chamber. Then, the mice were sacrificed at pre-determined time points post-dosing. Blood samples were taken by cardiac puncture, and the lungs were harvested for analysis by analytical instrument. From these experiments, lung and serum (blood) pharmacokinetics of colloidal drug dispersions can be determined as reported previously.Citation212–Citation214
Biodistribution study using radiolabeled SLNs
To assess the inhaled radiolabelled SLNs biodistribution, nanoparticles (200 nm) were radiolabeled with 99mTc and biodistribution studies were carried out following aerosolisation and administration of a 99mTc-HMPAO-SLNs suspension to a group of adult male Wistar rats. After administration of radiolabeled SLNs, dynamic image acquisition was performed in a gamma-camera, followed by static image collection. Then radiation counting was performed in organ samples, collected after the animals were sacrificed. From these experiments, biodistribution of SLNs can be traced and evaluated.Citation133,Citation134
Fluorescence/bioluminescence imaging systems for pulmonary gene delivery
Visualization and evaluation of both the pulmonary delivery and the gene expression properties after dry powder inhalation in mice were recently reported by Mizuno and colleagues.Citation215 It was shown that the pulmonary delivery and the gene expression can be evaluated using fluorescence of indocyanine green (ICG) as a fluorescent label and the detection of luciferase activity, respectively, by using a non-destructive real-time in vivo imaging system. They concluded that the dry powder containing both ICG and pCMV-Luc was useful as a dual imaging system to visualize pulmonary delivery and gene expression in mice.
Regulatory and toxicity issues
Inhaled microparticles and nanoparticles of different sizes can target into different regions of respiratory tract, including nasopharyngeal, tracheal, bronchial, and alveolar regions, with several mechanisms. Meanwhile, the surface chemistry, charge, shape and aggregation status of nanoparticles also have influences on their disposition efficacy.Citation216 For example, inhaled large particles and nanoparticles deposit in the respiratory tract by distinctive mechanisms. Large particles deposit via inertial impaction, gravitation settling and interception mechanisms, while nanoparticles deposits via diffusion due to displacement when they collide with air molecules. The presence of albuminCitation217 and phospholipids (eg, lecithin)Citation218 in alveolar epithelial lining fluid is important to facilitate epithelial cell uptake of nanoparticles after deposition in the alveolar space. Nanoparticles coated with them may translocate across the alveolo–capilliary barrier, whereas uncoated particles do not. Therefore, when evaluating the efficacy and fate of nanoparticles by inhalation delivery, all the variables should be taken into consideration.Citation1
After delivery into the lung, some nanoparticles may be translocated to extrapulmonary sites and reach other target organs by cellular endocytosis, transcytosis, neuronal,Citation219 epithelialCitation220 and circulatoryCitation221 translocation and distribution, which makes them desirable for medical therapeutic or diagnostic application. However, these features can also pose potential toxicity. Transcytosis absorbs nanoparticles and translocate them into the interstitial sites, where they gain access to the blood circulation via lymphatics, resulting in distribution throughout the body. Neuronal translocation involves uptake of nanoparticles by sensory nerve endings embedded in airway epithelia, followed by axonal translocation to ganglionic and central nerve systems (CNS) structures. For example, nanoparticles facilitating drug delivery to the CNS in the brain raises the question of fate of nanoparticles after their translocation to the specific cell types or to subcellular structures. This kind of questions includes whether mitochondrial localization induces oxidative stress and how persistent the coating or the core of nanoparticles is, which is essential in nanoparticle toxicology and safety evaluation.Citation222
The clearance of nanoparticles in the alveolar region is predominantly mediated by alveolar macrophages, through phagocytosis of deposited nanoparticles. After macrophages recognize the deposited nanoparticles and phagocytize them, macrophages with internalized nanoparticles gradually move toward the mucociliary escalator, and then the clearance process is started. The retention half-time of solid particles in the alveolar region based on this mechanism is very slow, up to 700 days in humans. Moreover, unlike larger particles, results from several studies show the apparent inefficiency of alveolar macrophage phagocytosis of nanosized particles.Citation223–Citation225 The ultrafine nanoparticles are not easily phagocytized by macrophages and, consequently, are not readily cleared in the alveolar region.Citation226 These nanoparticles are either in epithelium cells or are further translocated to the interstitium, which may cause a long-term accumulation in the lung and subsequent toxicity issues.
According to the FDA guidance for dry powder inhaler (DPI) drug products, α-lactose monohydrate is the only approved sugar that can be used as a large carrier particle in dry powder inhalation aerosol products to fluidize and disperse the respiratory drug while itself not being delivered to the lung. Other novel materials, including phospholipids, specifically lecithin and amino acids (lysine, polylysine) have also been developed for use in pulmonary formulations as excipients that are delivered to the lung. A thorough assessment of these alternatives associated with any inhalable substance is required, from a variety of sources: human, animal, and/or in vitro test models.Citation227
Conclusions
Inhalable colloidal carriers (nanocarrier systems) offer numerous advantages. The decrease in particle size leads to an increase in surface area leading to enhanced dissolution rate, as well as relatively uniform distribution of drug dose among the alveoli. In addition, by suspending the drugs in nanoparticles, one can achieve a dose that is higher than that offered by a pure aqueous solution, which is thermodynamically limited by the aqueous solubility of the drug. Nanocarrier systems can provide the advantage of sustained-release in the lung tissue, resulting in reduced dosing frequency and improved patient compliance. Local delivery of inhalable nanocarriers may be a promising alternative to oral or intravenous administration, thus decreasing the incidence of side effects associated with a high drug serum concentration. As with all formulations designed for pulmonary drug delivery, the potential long-term risk of excipient toxicity and nanoscale carrier itself are issues that need to be considered in the successful product development of pulmonary drug delivery systems. Nevertheless, their inherently small size and surface modification properties enable further opportunities for innovative controlled drug release and pulmonary cell targeting therapeutic platforms. The integration of nanotechnology and pulmonary delivery has the potential to improve the targeting, release, and therapeutic effects of drugs and needle-free inhalation vaccines with significant potential capability of overcoming the physicochemical and biological hurdles.
Disclosure
The authors report no conflicts of interest in this work.
References
- YangWPetersJIWilliamsRO3rdInhaled nanoparticles–a current reviewInt J Pharm200835623924718358652
- PattonJSByronPRInhaling medicines: delivering drugs to the body through the lungsNat Rev Drug Discov20076677417195033
- SungJCPulliamBLEdwardsDANanoparticles for drug delivery to the lungsTrends Biotechnol20072556357017997181
- BaileyMMBerklandCJNanoparticle formulations in pulmonary drug deliveryMed Res Rev20092919621218958847
- ShekunovBNanoparticle technology for drug delivery – From nanoparticles to cutting-edge delivery strategies – Part I – 21–22 March 2005, Philadelphia, PA, USAIdrugs2005839940115883921
- KreuterJNanoparticle-based drug delivery systemsJ Control Release199116169176
- BriggerIDubernetCCouvreurPNanoparticles in cancer therapy and diagnosisAdv Drug Deliv Rev20025463165112204596
- TiwariSBAmijiMMA review of nanocarrier-based CNS delivery systemsCurr Drug Deliv2006321923216611008
- KaurIPBhandariRBhandariSKakkarVPotential of solid lipid nanoparticles in brain targetingJ Control Release20081279710918313785
- DriscollDBhargavaHLiLZaimRBabayanVBistrianBPhysicochemical stability of total nutrient admixturesAm J Health Syst Pharm1995526236347606577
- KosterVSKuksPFMLangeRTalsmaHParticle size in parenteral fat emulsions, what are the true limitations?Int J Pharm1996134235238
- ZimmerAKreuterJMicrospheres and nanoparticles used in ocular delivery systemsAdv Drug Deliv Rev1995166173
- ChonoSTaninoTSekiTMorimotoKInfluence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomesJ Drug Target20061455756617043040
- ParkJHLeeSKimJHParkKKimKKwonICPolymeric nanomedicine for cancer therapyProg Polym Sci200833113137
- ResnikDBTinkleSSEthical issues in clinical trials involving nanomedicineContemp Clin Trials20072843344117166777
- AzarmiSRoaWHLobenbergRTargeted delivery of nanoparticles for the treatment of lung diseasesAdv Drug Deliv Rev20086086387518308418
- CryanSACarrier-based strategies for targeting protein and peptide drugs to the lungsAAPS J20057E20E4116146340
- JoshiMRMisraALiposomal budesonide for dry powder inhaler: preparation and stabilizationAAPS Pharm Sci Tech2001225
- KonduriKSNandedkarSDuzgunesNEfficacy of liposomal budesonide in experimental asthmaJ Allergy Clin Immunol200311132132712589352
- StentonGRUlanovaMDeryREInhibition of allergic inflammation in the airways using aerosolized antisense to Syk kinaseJ Immunol20021691028103612097411
- JoshiMMisraADisposition kinetics of ketotifen from liposomal dry powder for inhalation in rat lungClin Exp Pharmacol Physiol20033015315612603343
- SeongJHLeeKMKimSTJinSEKimCKPolyethylenimine-based antisense oligodeoxynucleotides of IL-4 suppress the production of IL-4 in a murine model of airway inflammationJ Gene Med2006831432316292779
- ChattopadhyayPShekunovBYYimDCipollaDBoydBFarrSProduction of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx systemAdv Drug Deliv Rev20075944445317582648
- HajosFStarkBHenslerSPrasslRMosgoellerWInhalable liposomal formulation for vasoactive intestinal peptideInt J Pharm200835728629418328650
- StarkBDebbagePAndreaeFMosgoellerWPrasslRAssociation of vasoactive intestinal peptide with polymer-grafted liposomes: structural aspects for pulmonary deliveryBiochim Biophys Acta2007176870571417204237
- WernigKGriesbacherMAndreaeFDepot formulation of vasoactive intestinal peptide by protamine-based biodegradable nanoparticlesJ Control Release200813019219818601963
- WijagkanalanWKawakamiSTakenagaMIgarashiRYamashitaFHashidaMEfficient targeting to alveolar macrophages by intratracheal administration of mannosylated liposomes in ratsJ Control Release200812512113018037185
- GongFTangHLinYGuWWangWKangMGene transfer of vascular endothelial growth factor reduces bleomycin-induced pulmonary hypertension in immature rabbitsPediatr Int20054724224715910444
- KimuraSEgashiraKChenLNanoparticle-mediated delivery of nuclear factor {kappa}B decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertensionHypertension2009
- ChouguleMBPadhiBKMisraANano-liposomal dry powder inhaler of Amiloride HydrochlorideJ Nanosci Nanotechnol200663001300917048511
- GibbonsAMMcElvaneyNGTaggartCCCryanSADelivery of rSLPI in a liposomal carrier for inhalation provides protection against cathepsin L degradationJ Microencapsul2008110
- BeaulacCSachetelliSLagaceJAerosolization of low phase transition temperature liposomal tobramycin as a dry powder in an animal model of chronic pulmonary infection caused by Pseudomonas aeruginosaJ Drug Target19997334110614813
- MarierJFBrazierJLLavigneJDucharmeMPLiposomal tobramycin against pulmonary infections of Pseudomonas aeruginosa: a pharmacokinetic and efficacy study following single and multiple intratracheal administrations in ratsJ Antimicrob Chemother20035224725212837733
- LabanaSPandeyRSharmaSKhullerGKChemotherapeutic activity against murine tuberculosis of once weekly administered drugs (isoniazid and rifampicin) encapsulated in liposomesInt J Antimicrob Agents20022030130412385689
- VyasSPKannanMEJainSMishraVSinghPDesign of liposomal aerosols for improved delivery of rifampicin to alveolar macrophagesInt J Pharm2004269374914698575
- ChangsanNChanHKSeparovicFSrichanaTPhysicochemical characterization and stability of rifampicin liposome dry powder formulations for inhalationJ Pharm Sci20099862863918484099
- JustoORMoraesAMIncorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalationDrug Deliv20031020120712944141
- PandeyRSharmaAZahoorASharmaSKhullerGKPrasadBPoly(DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosisJ Antimicrob Chemother20035298198614613962
- ZahoorASharmaSKhullerGKInhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosisInt J Antimicrob Agents20052629830316154726
- EsmaeiliFHosseini-NasrMRad-MalekshahiMSamadiNAtyabiFDinarvandRPreparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticlesNanomedicine2007316116717468055
- OhashiKKabasawaTOzekiTOkadaHOne-step preparation of rifampicin/poly(lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosisJ Control Release2009135192419121349
- PandeyRKhullerGKSolid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosisTuberculosis (Edinb)20058522723415922668
- WongJPYangHBlasettiKLSchnellGConleyJSchofieldLNLiposome delivery of ciprofloxacin against intracellular Francisella tularensis infectionJ Control Release20039226527314568408
- SweeneyLGWangZLoebenbergRWongJPLangeCFFinlayWHSpray-freeze-dried liposomal ciprofloxacin powder for inhaled aerosol drug deliveryInt J Pharm200530518018516242277
- ShahSPMisraADevelopment of liposomal amphotericin B dry powder inhaler formulationDrug Deliv20041124725315371106
- VyasSPQuraishiSGuptaSJaganathanKSAerosolized liposome-based delivery of amphotericin B to alveolar macrophagesInt J Pharm2005296122515885451
- KhannaCAndersonPMHaszDEKatsanisENevilleMKlausnerJSInterleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastasesCancer199779140914219083164
- DensmoreCLKleinermanESGautamAGrowth suppression of established human osteosarcoma lung metastases in mice by aerosol gene therapy with PEI-p53 complexesCancer Gene Ther2001861962711593330
- GautamADensmoreCLWaldrepJCInhibition of experimental lung metastasis by aerosol delivery of PEI-p53 complexesMol Ther2000231832311020346
- KoshkinaNVAgoulnikIYMeltonSLDensmoreCLKnightVBiodistribution and pharmacokinetics of aerosol and intravenously administered DNA-polyethyleneimine complexes: optimization of pulmonary delivery and retentionMol Ther2003824925412907147
- GautamADensmoreCLMeltonSGolunskiEWaldrepJCAerosol delivery of PEI-p53 complexes inhibits B16-F10 lung metastases through regulation of angiogenesisCancer Gene Ther20029283611916242
- VerschraegenCFGilbertBELoyerEClinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignanciesClin Cancer Res2004102319232615073107
- ShahiwalaAMisraAA preliminary pharmacokinetic study of liposomal leuprolide dry powder inhaler: a technical noteAAPS Pharm Sci Tech20056E482E486
- AzarmiSTaoXChenHFormulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particlesInt J Pharm200631915516116713150
- HwangSKJinHKwonJTAerosol-delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP-1 activity in the lungs of AP-1 luciferase reporter miceGene Ther2007141353136117611588
- JinHKimTHHwangSKAerosol delivery of urocanic acid-modified chitosan/programmed cell death 4 complex regulated apoptosis, cell cycle, and angiogenesis in lungs of K-ras null miceMol Cancer Ther200651041104916648576
- TaetzSNafeeNBeisnerJThe influence of chitosan content in cationic chitosan/PLGA nanoparticles on the delivery efficiency of antisense 2’-O-methyl-RNA directed against telomerase in lung cancer cellsEur J Pharm Biopharm20097235836918703137
- JereDXuCXAroteRYunCHChoMHChoCSPoly(betaamino ester) as a carrier for si/shRNA delivery in lung cancer cellsBiomaterials2008292535254718316120
- XuCXJereDJinHPoly(ester amine)-mediated, aerosol-delivered Akt1 small interfering RNA suppresses lung tumorigenesisAm J Respir Crit Care Med2008178607318310482
- TomodaKOhkoshiTHirotaKPreparation and properties of inhalable nanocomposite particles for treatment of lung cancerColloids Surf B Biointerfaces20097117718219264458
- de SemirDPetrizJAvinyoANon-viral vector-mediated uptake, distribution, and stability of chimeraplasts in human airway epithelial cellsJ Gene Med2002430832212112648
- GilbertBEKnightCAlvarezFGTolerance of volunteers to cyclosporine A-dilauroylphosphatidylcholine liposome aerosolAm J Respir Crit Care Med1997156178917939412556
- ChouguleMPadhiBMisraANano-liposomal dry powder inhaler of tacrolimus: preparation, characterization, and pulmonary pharmacokineticsInt J Nanomedicine2007267568818203434
- Bivas-BenitaMvan MeijgaardenKEFrankenKLPulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosisVaccine2004221609161515068842
- Bivas-BenitaMOudshoornMRomeijnSCationic submicron emulsions for pulmonary DNA immunizationJ Control Release200410014515515491818
- KaipelMWagnerAWassermannEIncreased biological half-life of aerosolized liposomal recombinant human Cu/Zn superoxide dismutase in pigsJ Aerosol Med Pulm Drug Deliv20082128129018578594
- CarpenterMEpperlyMWAgarwalAInhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damageGene Ther20051268569315750616
- EpperlyMWGuoHLJeffersonMCell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) during lung radioprotective gene therapyGene Ther20031016317112571645
- YamamotoHKunoYSugimotoSTakeuchiHKawashimaYSurfacemodified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctionsJ Control Release200510237338115653158
- ZhangQShenZNagaiTProlonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal ratsInt J Pharm2001218758011337151
- HuangYYWangCHPulmonary delivery of insulin by liposomal carriersJ Control Release200611391416730838
- BiRShaoWWangQZhangNSpray-freeze-dried dry powder inhalation of insulin-loaded liposomes for enhanced pulmonary deliveryJ Drug Target20081663964818982512
- LiuJGongTFuHSolid lipid nanoparticles for pulmonary delivery of insulinInt J Pharm200835633334418281169
- BaiSThomasCAhsanFDendrimers as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparinJ Pharm Sci2007962090210617286291
- BaiSAhsanFSynthesis and evaluation of pegylated dendrimeric nanocarrier for pulmonary delivery of low molecular weight heparinPharm Res20092653954819034631
- PirasAMChielliniFFiumiCA new biocompatible nanoparticle delivery system for the release of fibrinolytic drugsInt J Pharm200835726027118313868
- PlumleyCGormanEMEl-GendyNBybeeCRMunsonEJBerklandCNifedipine nanoparticle agglomeration as a dry powder aerosol formulation strategyInt J Pharm200936913614319015016
- AlmeidaAJSoutoESolid lipid nanoparticles as a drug delivery system for peptides and proteinsAdv Drug Deliv Rev20075947849017543416
- Koping-HoggardMVarumKMIssaMImproved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomersGene Ther2004111441145215269712
- HowardKARahbekULLiuXRNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle systemMol Ther20061447648416829204
- GrenhaASeijoBRemunan-LopezCMicroencapsulated chitosan nanoparticles for lung protein deliveryEur J Pharm Sci20052542743715893461
- IssaMMKoping-HoggardMTommeraasKTargeted gene delivery with trisaccharide-substituted chitosan oligomers in vitro and after lung administration in vivoJ Control Release200611510311216901570
- BrzoskaMLangerKCoesterCLoitschSWagnerTOMallinckrodtCIncorporation of biodegradable nanoparticles into human airway epithelium cells-in vitro study of the suitability as a vehicle for drug or gene delivery in pulmonary diseasesBiochem Biophys Res Commun200431856257015120637
- ShamJOZhangYFinlayWHRoaWHLobenbergRFormulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lungInt J Pharm200426945746714706257
- KaulGAmijiMTumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studiesPharm Res20052295196115948039
- ElyLRoaWFinlayWHLobenbergREffervescent dry powder for respiratory drug deliveryEur J Pharm Biopharm20076534635317156987
- Beck-BroichsitterMGaussJPackhaeuserCBPulmonary drug delivery with aerosolizable nanoparticles in an ex vivo lung modelInt J Pharm200936716917818848609
- LynchJBehanNBirkinshawCFactors controlling particle size during nebulization of DNA-polycation complexesJ Aerosol Med20072025726817894533
- ChenJGaoXHuKGalactose-poly(ethylene glycol)-poly-ethylenimine for improved lung gene transferBiochem Biophys Res Commun200837537838318694731
- NguyenJXieXNeuMEffects of cell-penetrating peptides and pegylation on transfection efficiency of polyethylenimine in mouse lungsJ Gene Med2008101236124618780309
- ZiadyAGGedeonCRMuhammadOMinimal toxicity of stabilized compacted DNA nanoparticles in the murine lungMol Ther2003894895614664797
- ZiadyAGGedeonCRMillerTTransfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivoMol Ther2003893694714664796
- Kukowska-LatalloJFRaczkaEQuintanaAChenCRymaszewskiMBakerJRJrIntravascular and endobronchial DNA delivery to murine lung tissue using a novel, nonviral vectorHum Gene Ther2000111385139510910136
- de FougerollesARDelivery vehicles for small interfering RNA in vivoHum Gene Ther20081912513218257677
- DensmoreCLAdvances in noninvasive pulmonary gene therapyCurr Drug Deliv20063556316472094
- De SmedtSCDemeesterJHenninkWECationic polymer based gene delivery systemsPharm Res20001711312610751024
- WightmanLKircheisRRosslerVDifferent behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivoJ Gene Med2001336237211529666
- KichlerALeborgneCCoeytauxEDanosOPolyethylenimine-mediated gene delivery: a mechanistic studyJ Gene Med2001313514411318112
- CholletPFavrotMCHurbinACollJLSide-effects of a systemic injection of linear polyethylenimine-DNA complexesJ Gene Med20024849111828391
- TangMXSzokaFCThe influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexesGene Ther199748238329338011
- OgrisMWagnerETumor-targeted gene transfer with DNA polyplexesSomat Cell Mol Genet200227859512774943
- RudolphCSchillingerUPlankCNonviral gene delivery to the lung with copolymer-protected and transferrin-modified polyethylenimineBiochim Biophys Acta20021573758312383945
- LiSDHuangLSurface-modified LPD nanoparticles for tumor targetingAnn N Y Acad Sci200610821817145918
- EliyahuHJosephASchillemansJPAzzamTDombAJBarenholzYCharacterization and in vivo performance of dextranspermine polyplexes and DOTAP/cholesterol lipoplexes administered locally and systemicallyBiomaterials2007282339234917298842
- ChonoSLiSDConwellCCHuangLAn efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumorJ Control Release2008131646918674578
- ParkMRKimHWHwangCSHighly efficient gene transfer with degradable poly(ester amine) based on poly(ethylene glycol) diacrylate and polyethylenimine in vitro and in vivoJ Gene Med20081019820718064729
- KoYTKaleAHartnerWCPapahadjopoulos-SternbergBTorchilinVPSelf-assembling micelle-like nanoparticles based on phospholipid-polyethyleneimine conjugates for systemic gene deliveryJ Control Release200913313213818929605
- ZengXMMartinGPMarriottCThe controlled delivery of drugs to the lungInt J Pharm1995124149164
- MullerRHMaderKGohlaSSolid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the artEur J Pharm Biopharm20005016117710840199
- SchreierHGonzalez-RothiRJStecenkoAAPulmonary delivery of liposomesJ Control Release199324209223
- TaylorKMGTaylorGKellawayIWStevensJThe stability of liposomes to nebulisationInt J Pharm1990585761
- JoshiMMisraANPulmonary disposition of budesonide from liposomal dry powder inhalerMethods Find Exp Clin Pharmacol20012353153611957743
- ShahSPMisraALiposomal amphotericin B dry powder inhaler: effect of fines on in vitro performancePharmazie20045981281315544064
- WhiteSBennettDBCheuSEXUBERA: pharmaceutical development of a novel product for pulmonary delivery of insulinDiabetes Technol Ther2005789690616386095
- StriblingRBrunetteELiggittDGaenslerKDebsRAerosol gene delivery in vivoProc Natl Acad Sci U S A19928911277112811454808
- AltonEWMiddletonPGCaplenNJNon-invasive liposomemediated gene delivery can correct the ion transport defect in cystic fibrosis mutant miceNat Genet199351351427504552
- ChadwickSLKingstonHDSternMSafety of a single aerosol administration of escalating doses of the cationic lipid GL-67/DOPE/DMPE-PEG5000 formulation to the lungs of normal volunteersGene Ther199749379429349430
- OudrhiriNVigneronJPPeuchmaurMLeclercTLehnJMLehnPGene transfer by guanidinium-cholesterol cationic lipids into airway epithelial cells in vitro and in vivoProc Natl Acad Sci U S A199794165116569050833
- PillaiRPetrakKBlezingerPUltrasonic nebulization of cationic lipid-based gene delivery systems for airway administrationPharm Res199815174317479833997
- DensmoreCLGiddingsTHWaldrepJCKinseyBMKnightVGene transfer by guanidinium-cholesterol: dioleoylphosphatidyl-ethanolamine liposome-DNA complexes in aerosolJ Gene Med1999125126410738558
- GautamADensmoreCLWaldrepJCPulmonary cytokine responses associated with PEI-DNA aerosol gene therapyGene Ther2001825425711313798
- PitardBOudrhiriNLambertOSterically stabilized BGTC-based lipoplexes: structural features and gene transfection into the mouse airways in vivoJ Gene Med2001347848711601761
- DeshpandeDSBlanchardJDSchusterJGamma scintigraphic evaluation of a miniaturized AERx pulmonary delivery system for aerosol delivery to anesthetized animals using a positive pressure ventilation systemJ Aerosol Med200518344415741772
- TempletonNSNonviral delivery for genomic therapy of cancerWorld J Surg20093368569719023615
- MorilleMPassiraniCVonarbourgAClavreulABenoitJPProgress in developing cationic vectors for non-viral systemic gene therapy against cancerBiomaterials2008293477349618499247
- CryanSADevocelleMMoranPJHickeyAJKellyJGIncreased intracellular targeting to airway cells using octaarginine-coated liposomes: In vitro assessment of their suitability for inhalationMol Pharm2006310411216579639
- MehnertWMaderKSolid lipid nanoparticles: production, characterization and applicationsAdv Drug Deliv Rev20014716519611311991
- SchwarzCMehnertWLucksJSM?lerRHSolid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilizationJ Control Release1994308396
- WestesenKNovel lipid-based colloidal dispersions as potential drug administration systems – expectations and realityColloid Polym Sci2000278608618
- MullerRHRuhlDRungeSSchulze-ForsterKMehnertWCytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactantPharm Res1997144584629144731
- HeydenreichAVWestmeierRPedersenNPoulsenHSKristensenHGPreparation and purification of cationic solid lipid nanospheres–effects on particle size, physical stability and cell toxicityInt J Pharm2003254838712615415
- RudolphCSchillingerUOrtizAApplication of novel solid lipid nanoparticle (SLN)-gene vector formulations based on a dimeric HIV-1 TAT-peptide in vitro and in vivoPharm Res2004211662166915497694
- VideiraMAGanoLSantosCNevesMAlmeidaAJLymphatic uptake of lipid nanoparticles following endotracheal administrationJ Microencapsul20062385586217390627
- VideiraMABotelhoMFSantosACGouveiaLFde LimaJJAlmeidaAJLymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticlesJ Drug Target20021060761312683665
- LiuFYangJHuangLLiuDEffect of non-ionic surfactants on the formation of DNA/emulsion complexes and emulsion-mediated gene transferPharm Res199613164216468956328
- YiSWYuneTYKimTWA cationic lipid emulsion/DNA complex as a physically stable and serum-resistant gene delivery systemPharm Res20001731432010801220
- KimTWChungHKwonICSungHCJeongSYIn vivo gene transfer to the mouse nasal cavity mucosa using a stable cationic lipid emulsionMol Cells20001014214710850654
- BoasUHeegaardPMDendrimers in drug researchChem Soc Rev200433436314737508
- RudolphCLausierJNaundorfSMullerRHRoseneckerJIn vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimersJ Gene Med2000226927810953918
- HickeyAJLung deposition and clearance of pharmaceutical aerosols: what can be learned from inhalation toxicology and industrial hygieneAerosol Sci Technol199318290304
- HickeyAJMansourHMDelivery of drugs by the pulmonary routeFlorenceATSiepmannJModern PharmaceuticsNew York, NYTaylor and Francis, Inc2008191219
- Merisko-LiversidgeEMcGurkSLLiversidgeGGInsulin nanoparticles: a novel formulation approach for poorly water soluble ZninsulinPharm Res2004211545155315497677
- RabinowBENanosuspensions in drug deliveryNat Rev Drug Discov2004378579615340388
- MosénKBáckstromKThalbergKParticle formation and capture during spray drying of inhalable particlesPharm Dev Technol2004940941815581077
- DudduSPSiskSAWalterYHImproved lung delivery from a passive dry powder inhaler using an Engineered PulmoSphere powderPharm Res20021968969512069174
- MaaYFNguyenPASitKHsuCCSpray-drying performance of a bench-top spray dryer for protein aerosol powder preparationBiotechnol Bioeng19986030130910099432
- FreitasSMerkleHPGanderBUltrasonic atomisation into reduced pressure atmosphere – envisaging aseptic spray-drying for microencapsulationJ Control Release20049518519514980767
- StahlKClaessonMLilliehornPLindenHBackstromKThe effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalationInt J Pharm200223322723711897427
- GilaniKNajafabadiARBarghiMRafiee-TehraniMThe effect of water to ethanol feed ratio on physical properties and aerosolization behavior of spray dried cromolyn sodium particlesJ Pharm Sci2005941048105915793812
- VidgrenMTVidgrenPAParonenTPComparison of physical and inhalation properties of spray-dried and mechanically micronized disodium cromoglycateInt J Pharm198735139144
- US Food and Drug AdministrationGuidance for industry: metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products –chemistry, manufacturing, and controls documentationRockville, INUS Department of Health and Human Services1998
- KawashimaYSeriganoTHinoTYamamotoHTakeuchiHEffect of surface morphology of carrier lactose on dry powder inhalation property of pranlukast hydrateInt J Pharm1998172179188
- VanderbistFWeryBPavonIMMoesAJOptimization of a dry powder inhaler formulation of nacystelyn, a new mucoactive agentJ Pharm Pharmacol1999511229123410632079
- SteckelHBolzenNAlternative sugars as potential carriers for dry powder inhalationsInt J Pharm200427029730614726144
- HarjunenPLankinenTSalonenHLehtoVPJarvinenKEffects of carriers and storage of formulation on the lung deposition of a hydrophobic and hydrophilic drug from a DPIInt J Pharm200326315116312954190
- RogersTJohnstonKWilliamsRSolution-based particle formation of pharmaceutical powders by supercritical or compressed Fluid CO2 and cryogenic spray-freezing technologiesDrug Dev Ind Pharm2001271003101611794803
- MaaYFPrestrelskiSJBiopharmaceutical powders: particle formation and formulation considerationsCurr Pharm Biotechnol2000128330211469385
- MaaYFNguyenPASweeneyTShireSJHsuCCProtein inhalation powders: spray drying vs spray freeze dryingPharm Res19991624925410100310
- YuZGarciaASJohnstonKPWilliamsRO3rdSpray freezing into liquid nitrogen for highly stable protein nanostructured microparticlesEur J Pharm Biopharm20045852953715451527
- KuoJHHwangRPreparation of DNA dry powder for non-viral gene delivery by spray-freeze drying: effect of protective agents (polyethyleneimine and sugars) on the stability of DNAJ Pharm Pharmacol200456273314979998
- TomJWDebenedettiPGParticle formation with supercritical fluids-a reviewJ Aerosol Sci199122555584
- RehmanMShekunovBYYorkPOptimisation of powders for pulmonary delivery using supercritical fluid technologyEur J Pharm Sci20042211715113578
- SteckelHThiesJMullerBWMicronizing of steroids for pulmonary delivery by supercritical carbon dioxideInt J Pharm199715299110
- SteckelHMullerBWMetered-dose inhaler formulation of fluticasone-17-propionate micronized with supercritical carbon dioxide using the alternative propellant HFA-227Int J Pharm19981732533
- ShekunovBYChattopadhyayPSeitzingerJHuffRNanoparticles of poorly water-soluble drugs prepared by supercritical fluid extraction of emulsionsPharm Res20062319620416307386
- ChattopadhyayPHuffRShekunovBYDrug encapsulation using supercritical fluid extraction of emulsionsJ Pharm Sci20069566767916447174
- El-BaseirMMPhippsMAKellawayIWPreparation and subsequent degradation of poly(l-lactic acid) microspheres suitable for aerosolisation: a physico-chemical studyInt J Pharm1997151145153
- RasenackNSteckelHMullerBWMicronization of anti-inflammatory drugs for pulmonary delivery by a controlled crystallization processJ Pharm Sci200392354412486680
- ChowAHLTongHHYChattopadhyayPShekunovBYParticle engineering for pulmonary drug deliveryPharm Res20072441143717245651
- GrattonSEAPohlhausPDLeeJGuoJChoMJDeSimoneJMNanofabricated particles for engineered drug therapies: A preliminary biodistribution study of PRINT™ nanoparticlesJ Control Release2007121101817643544
- GrattonSERoppPAPohlhausPDThe effect of particle design on cellular internalization pathwaysProc Natl Acad Sci U S A2008105116131161818697944
- GrattonSENapierMERoppPATianSDeSimoneJMMicrofabricated particles for engineered drug therapies: elucidation into the mechanisms of cellular internalization of PRINT particlesPharm Res2008252845285218592353
- LiXBlondinoFEHindleMSoineWHByronPRStability and characterization of perphenazine aerosols generated using the capillary aerosol generatorInt J Pharm200530311312416139453
- AvramMJHenthornTKSpykerDARecirculatory pharmacokinetic model of the uptake, distribution, and bioavailability of prochlorperazine administered as a thermally generated aerosol in a single breath to dogsDrug Metab Dispos20073526226717079359
- RabinowitzJDWensleyMLloydPFast onset medications through thermally generated aerosolsJ Pharmacol Exp Ther200430976977514752061
- HongJNHindleMByronPRControl of particle size by coagulation of novel condensation aerosols in reservoir chambersJ Aerosol Med20021535936812581502
- GuptaRHindleMByronPRCoxKAMcRaeDDInvestigation of a novel condensation aerosol generator: solute and solvent effectsAerosol Sci Technol200337672681
- RabinowitzJDLloydPMMunzarPUltra-fast absorption of amorphous pure drug aerosols via deep lung inhalationJ Pharm Sci2006952438245116886198
- MyersDJTimmonsRDLuATThe effect of film thickness on thermal aerosol generationPharm Res20072433634217180726
- HickeyAJMansourHMTelkoMJPhysical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristicsJ Pharm Sci2007961282130117455324
- WilliamsRO3rdBrownJLiuJInfluence of micronization method on the performance of a suspension triamcinolone acetonide pressurized metered-dose inhaler formulationPharm Dev Technol1999416717910231878
- BarryPWO’CallaghanCAn in vitro analysis of the output of budesonide from different nebulizersJ Allergy Clin Immunol19991041168117310588997
- SchauerJJChristensenCGKittelsonDBJohnsonJPWattsWFImpact of ambient temperatures and driving conditions on the chemical composition of particulate matter emissions from nonsmoking gasoline-powered motor vehiclesAerosol Sci Technol200842210223
- MiguelAEiguren-FernandezASioutasCFinePGellerMMayoPObservations of twelve USEPA priority polycyclic aromatic hydrocarbons in the aitken size range (10–32 nm Dp)Aerosol Sci Technol200539415418
- ParkKCaoFKittelsonDBMcMurryPHRelationship between particle mass and mobility for diesel exhaust particlesEnviron Sci Technol20033757758312630475
- SardarSBFinePMMayoPRSioutasCSize-fractionated measurements of ambient ultrafine particle chemical composition in Los Angeles using the NanoMOUDIEnviron Sci Technol20053993294415773464
- LinCCChenSJHuangKLCharacteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked roadEnviron Sci Technol2005398113812216294844
- GellerMDKimSMisraCSioutasCOlsonBAMarpleVAA methodology for measuring size-dependent chemical composition of ultrafine particlesAerosol Sci Technol200236748762
- VenkatachariPHopkePKBruneWHCharacterization of wintertime reactive oxygen species concentrations in Flushing, New YorkAerosol Sci Technol20074197111
- FujitaniYHasegawaSFushimiACollection characteristics of low-pressure impactors with various impaction substrate materialsAtmos Environ20064032213229
- BatesSZografiGEngersDMorrisKCrowleyKNewmanAAnalysis of amorphous and nanocrystalline solids from their X-ray diffraction patternsPharm Res2006232333234917021963
- LarhribHZengXMMartinGPMarriottCPritchardJThe use of different grades of lactose as a carrier for aerosolised salbutamol sulphateInt J Pharm199919111410556735
- TrainiDYoungPMThielmannFAcharyaMThe influence of lactose pseudopolymorphic form on salbutamol sulfate-lactose interactions in DPI formulationsDrug Dev Ind Pharm200834992100118800259
- NewmanAWByrnSRSolid-state analysis of the active pharmaceutical ingredient in drug productsDrug Discov Today2003889890514554018
- Saleki-GerhardtAAhlneckCZografiGAssessment of disorder in crystalline solidsInt J Pharm1994101237247
- BunkerMDaviesMRobertsCTowards screening of inhalation formulations: measuring interactions with atomic force microscopyExpert Opin Drug Deliv2005261362416296789
- SethuramanVVHickeyAJPowder properties and their influence on dry powder inhaler delivery of an antitubercular drugAAPS Pharm Sci Tech20023E28
- MansourHMZografiGThe relationship between water vapor absorption and desorption by phospholipids and bilayer phase transitionsJ Pharm Sci20079637739617080427
- AguRUUgwokeMIArmandMKingetRVerbekeNThe lung as a route for systemic delivery of therapeutic proteins and peptidesRespir Res2001219820911686885
- GronebergDAWittCWagnerUChungKFFischerAFundamentals of pulmonary drug deliveryRespir Med20039738238712693798
- MathiasNRYamashitaFLeeVHLRespiratory epithelial cell culture models for evaluation of ion and drug transportAdv Drug Deliv Rev199622215249
- ForbesBEhrhardtCHuman respiratory epithelial cell culture for drug delivery applicationsEur J Pharm Biopharm20056019320515939233
- SakagamiMIn vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic deliveryAdv Drug Deliv Rev2006581030106017010473
- MobleyCHochhausGMethods used to assess pulmonary deposition and absorption of drugsDrug Discov Today2001636737511267923
- SteimerAHaltnerELehrCMCell culture models of the respiratory tract relevant to pulmonary drug deliveryJ Aerosol Med20051813718215966771
- BorchardGCassaráMLRoemeléPEHFloreaBIJungingerHETransport and local metabolism of budesonide and fluticasone propionate in a human bronchial epithelial cell line (Calu-3)J Pharm Sci2002911561156712115854
- EhrhardtCFiegelJFuchsSDrug absorption by the respiratory mucosa: cell culture models and particulate drug carriersJ Aerosol Med20021513113912184863
- FosterKAAveryMLYazdanianMAudusKLCharacterization of the Calu-3 cell line as a tool to screen pulmonary drug deliveryInt J Pharm200020811111064206
- FosterKAOsterCGMayerMMAveryMLAudusKLCharacterization of the A549 cell line as a type ii pulmonary epithelial cell model for drug metabolismExp Cell Res19982433593669743595
- ManfordFTrondeAJeppssonABPatelNJohanssonFForbesBDrug permeability in 16HBE14o-airway cell layers correlates with absorption from the isolated perfused rat lungEur J Pharm Sci20052641442016153810
- LinHLiHChoHJAir-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studiesJ Pharm Sci20079634135017080426
- VaughnJMMcConvilleJTBurgessDSingle dose and multiple dose studies of itraconazole nanoparticlesEur J Pharm Biopharm2006639510216516450
- YangWTamJMillerDAHigh bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizersInt J Pharm200836117718818556158
- McConvilleJTOverhoffKASinswatPTargeted high lung concentrations of itraconazole using nebulized dispersions in a murine modelPharm Res20062390191116715380
- MizunoTMohriKNasuSDanjoKOkamotoHDual imaging of pulmonary delivery and gene expression of dry powder inhalant by fluorescence and bioluminescenceJ Control Release200913414915419100298
- OberdorsterGSharpZAtudoreiVExtrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of ratsJ Toxicol Environ Health A2002651531154312396867
- HeckelKKiefmannRDorgerMStoeckelhuberMGoetzAEColloidal gold particles as a new in vivo marker of early acute lung injuryAm J Physiol Lung Cell Mol Physiol2004287L867L87815194564
- KatoTYashiroTMurataYEvidence that exogenous substances can be phagocytized by alveolar epithelial cells and transported into blood capillariesCell Tissue Res2003311475112483283
- OberdorsterGSharpZAtudoreiVTranslocation of inhaled ultrafine particles to the brainInhal Toxicol20041643744515204759
- OberdorsterGToxicology of ultrafine particles: in vivo studiesPhilos Trans R Soc Lond A200035827192740
- BallouBLagerholmBCErnstLABruchezMPWaggonerASNoninvasive imaging of quantum dots in miceBioconjug Chem200415798614733586
- OberdorsterGOberdorsterEOberdorsterJNanotoxicology: an emerging discipline evolving from studies of ultrafine particlesEnviron Health Perspect200511382383916002369
- KreylingWGSemmlerMErbeFTranslocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very lowJ Toxicol Environ Health A2002651513153012396866
- OberdorsterGFerinJMorrowPEVolumetric loading of alveolar macrophages (AM): a possible basis for diminished AM-mediated particle clearanceExp Lung Res199218871041572327
- OberdorsterGFinkelsteinJNJohnstonCAcute pulmonary effects of ultrafine particles in rats and miceRes Rep Health Eff Inst2000574; disc 75–86.11205815
- FerinJOberdorsterGSoderholmSCGeleinRPulmonary tissue access of ultrafine particlesJ Aerosol Med199145768
- HickeyAJMansourHMFormulation challenges of powders for the delivery of small molecular weight molecules as aerosolsRathboneMJHadgraftJRobertsMSLaneMEModifiedrelease Drug Delivery TechnologyNew York, NYInforma Healthcare2008573601