Abstract
Paclitaxel is a widely used chemotherapeutic agent; however, its therapeutic index is limited by low tumor exposure and high systemic exposure. Paclitaxel poliglumex (PPX) is macromolecular drug conjugate that links paclitaxel with a biodegradable polymer, poly-L-glutamic acid. PPX enhances tumor exposure by taking advantage of the hyperpermeable vasculature and suppressed lymphatic clearance characteristic of tumor tissue. The release of paclitaxel from the polymeric backbone is, at least in part, dependent on the metabolism of PPX by the lysosomal protease cathepsin B, which is upregulated in many tumor types. Retrospective analysis of clinical data from two phase III trials in advanced lung cancer suggests that PPX activity may be modulated by estradiol: a trend toward improved survival in the PPX arm compared with the control arm was observed in female, but not in male patients. Estrogens are known to induce cathepsin B activity; cathepsin B-mediated proteolysis is a key enzymatic processing step in PPX metabolism. The association between estrogens and PPX activity is being further explored in ongoing preclinical studies. An addition phase III trial will enroll women with advanced NSCLC to prospectively evaluate the efficacy of PPX in relation to pre- and post-menopausal estrogen levels.
Introduction
Paclitaxel, one of the most widely used cytotoxic agents, induces mitotic arrest and apoptosis in proliferating cells by targeting tubulin, a component of the mitotic spindle (CitationManfredi et al 1982; CitationBhalla 2003). Like other small, hydrophobic agents, paclitaxel binds extensively to plasma proteins, and its pharmacokinetic profile is characterized by a short plasma elimination half-life with a broad tissue distribution (CitationKumar et al 1993; CitationSonnichsen and Relling 1994). These unfavorable pharmacokinetic characteristics are associated with limited tumor and high systemic tissue exposure, thus reducing the therapeutic index of paclitaxel. In addition, the intravenous administration of hydrophobic agents requires the use of solubilizing agents, such as Cremophor® EL/ethanol. Cremophor EL is a biologically and pharmacologically active compound, and its use is associated with acute hypersensitivity reactions (CitationGelderblom et al 2001).
As a chemotherapeutic agent, paclitaxel is indicated for first-line platinum-based combination treatment in advanced ovarian carcinoma and non-small cell lung carcinoma (NSCLC). Paclitaxel is also indicated for second-line treatment of ovarian and breast carcinoma. The toxicity profile of paclitaxel is characterized by bone marrow suppression, neuropathy, and alopecia (CitationRowinsky and Donehower 1995). Hematological toxicities can be managed with the prophylactic use of hematopoietic growth factors, particularly in patients at risk for myelosuppression (CitationMarkman 2003). Also patients require pretreatment corticosteroids and anti-histamines to prevent acute hypersensitivity reactions. The administration of paclitaxel typically requires a 3-hour infusion, on a 3-week schedule. Weekly taxane schedules are currently being investigated, as more frequent administration at a lower dose may reduce myelosuppression and febrile neutropenia (CitationSeidman 2005).
Biodegradable, macromolecular polymer–drug conjugates allow for a more sustained and targeted delivery of chemotherapeutic agents. The performance of these nano-sized (5–100 nm) polymer-based pharmaceuticals is influenced by morphological characteristics, surface chemistry, and molecular weight (CitationLanger 1998; CitationBala et al 2004). When well-designed, these polymer–drug conjugates preferentially deliver active drug to tumor tissue, limiting exposure of normal tissues. In addition, the slow release of drug from the polymer yields lowers peak plasma concentrations of active drug. Paclitaxel poliglumex, a polymer–drug conjugate of paclitaxel and poly-L-glutamic acid, was designed to enhance the therapeutic index of paclitaxel by improving its pharmacokinetic profile, and to provide a water-soluble alternative to the standard paclitaxel formulation.
Paclitaxel poliglumex
A macromolecular polymer-drug conjugate
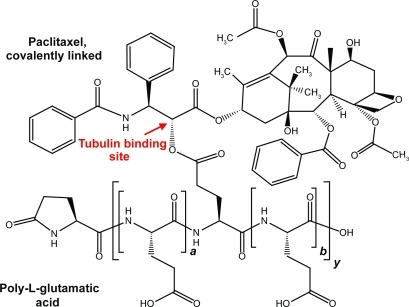
Paclitaxel poliglumex (PPX) is a polymer–drug conjugate that links paclitaxel to a biodegradable polymeric backbone consisting of L-glutamic acid residues (CitationSinger et al 2005). Paclitaxel is conjugated by ester linkage to the γ-carboxylic acid side chains of poly-L-glutamic acid. Because the conjugation site is through the 2′ hydroxyl of paclitaxel, a site crucial for tubulin binding, conjugated paclitaxel does not interact with β-tubulin and is biologically inactive (CitationGueritte-Voegelein et al 1991). The median molecular weight of PPX is 38.5 kDa. Conjugated paclitaxel represents approximately 36% by weight of PPX, equivalent to about one paclitaxel ester linkage per 11 glutamic acid units ().
Figure 1 Schematic representation of PPX. The structure shown is illustrative of a fragment of the molecule, but specific conjugation sites are not implied. On average there are approximately 10.4 non-conjugated monomer glutamic acid units (a + b) for every molecule conjugated to a paclitaxel molecule (y). The a-poly-L-glutamic degree of polymerization and the number of conjugation sites with paclitaxel are variable within the drug substance’s specifications.
The rate of release of paclitaxel from PPX by hydrolysis in buffered saline solution and in mouse or human plasma was evaluated. Incubation of PPX in buffered saline or plasma for 24 hours at 37°C showed that less than 14% of the bound paclitaxel had been hydrolyzed. This indicates that PPX is relatively resistant to plasma esterases and is unlikely to release substantial amounts of paclitaxel in the circulation, even with prolonged clearance times.
The characteristics of PPX were evaluated using gel-permeation chromatography in an organic mobile phase. When combined with multiple angle laser-light scattering and reflective index detection, the elution profile provides information on the apparent average molecular weight and polydispersity of the polymer. Nuclear magnetic spectroscopy confirmed the conjugation of paclitaxel to the γ and α positions of polyglutamate. Spectral changes were consistent with conjugation through the paclitaxel 2′- position with additional minor amounts of conjugation in the 7′-position. The distribution of paclitaxel on the polyglutamate backbone was studied through limited proteolysis of PPX with pronase. The resulting peptide mixtures were analyzed by reverse-phase HPLC and mass spectrometry. A wide range of peptide species were observed in the enzyme digest; they can be described as (Glu)n-(paclitaxel)x, where n = 1–20 and x = 1–6. This is in good agreement with a distribution of paclitaxel that would be expected if the conjugation occurred in a non-directed fashion (CitationSinger et al 2005).
Tumor accumulation
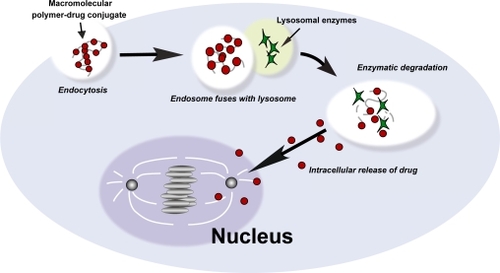
Polymer–drug conjugates passively accumulate in tumor tissue by taking advantage of the hyperpermeable tumor vasculature and reduced lymphatic clearance, a phenomenon known as the enhanced permeation and retention (EPR) effect. Tumor vasculature is more permeable to macromolecules than normal vasculature because of structural differences between the neovasculature in tumors and the mature vasculature in normal organs (CitationGerlowski and Jain 1986; CitationRoberts and Palade 1997). The paucity of lymphatic vessels in tumor tissue allows the retention of these macromolecules in the interstitial space, resulting in a 10- to 100-fold increase in intra-tumoral drug concentrations when compared with an equivalent dose of the drug given conventionally (CitationMatsumura and Maeda 1986; CitationGreish et al 2003). Macromolecules, characterized by high molecular weight, cannot be internalized into cells by simple diffusion; instead, macromolecules enter cells through endocytosis. Following internalization through endocytosis, polymer–drug conjugates are transported via the endosomal compartment to the lysosomes. Active drug is released through degradation by lysosomal enzymes, followed by diffusion of the active agent into the cytoplasm or nucleus () (CitationDuncan 1992, Citation2003).
Figure 2 Internalization of macromolecules by endocytosis and release of active drug. Macromolecules are internalized through endocytosis. Enzymatic degradation of the polymeric backbone by lysosomal enzymes mediates the release of active drug.
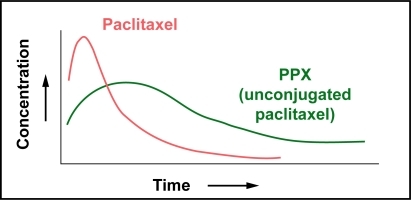
To take advantage of the EPR effect, macromolecules have to remain in circulation for at least 6 hours (CitationMatsumura and Maeda 1986). Clinical plasma pharmacokinetics of PPX show a biphasic decline with a prolonged distribution phase, and an elimination phase with a long terminal half-life. Following a 10- to 30-minute infusion of single-agent PPX, plasma concentrations of conjugated taxanes decline biphasically (). The distribution phase is prolonged and the elimination phase, which appears approximately 48 hours after drug administration, is characterized by a long terminal half-life, t1/2,z, of 108–261.5 hours (CitationBernareggi 2005). PPX is relatively stable in circulation; the area under the curve (AUC) of unconjugated paclitaxel is 1%–2% of the AUC of conjugated paclitaxel. The total systemic exposure to unconjugated paclitaxel is similar after administration of equivalent doses of PPX and standard paclitaxel; however, the Cmax values for paclitaxel are significantly lower in patients treated with PPX ().
Figure 3A Clinical plasma pharmacokinetics. Plasma samples were collected from patients receiving PPX as a 10- to 30-minute infusion once every 3 weeks. Conjugated and unconjugated paclitaxel were measured by validated HPLC/MS methods. The plasma concentration of conjugated paclitaxel declines biphasically and is characterized by a prolonged distribution phase and an elimination phase with a long terminal half-life.
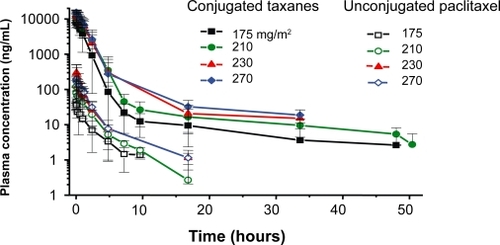
Figure 3B Schematic representation of the plasma pharmacokinetics of paclitaxel vs PPX (unconjugated paclitaxel). Total systemic exposure to PPX (unconjugated of PPX (unconjugated paclitaxel) and standard paclitaxel are similar; however, Cmax paclitaxel) is lower than equivalent doses of standard paclitaxel.
The prolonged circulation time of PPX facilitates tumor accumulation through the EPR effect, as was demonstrated in model animals (CitationSinger et al 2005). To determine the pharmacokinetic profile of PPX and its tissue distribution, female mice with subcutaneous B16 murine melanomas were given equivalent doses of tritium-labeled paclitaxel 40 mg/ kg iv, either as [3H]paclitaxel in Cremophor EL/ethanol or as [3H]paclitaxel poliglumex in phosphate buffer. Tumor samples were collected at regular intervals up to 144 hours after the injection, and the concentrations of PPX and paclitaxel were determined by LC/MS analysis. Tumor exposure of B16 melanomas to total taxanes was increased by a factor of 3 (Cmax) or factor of 12 (AUC) in mice treated with [3H]PPX compared with animals treated with [3H]paclitaxel (; ). Distribution of paclitaxel to the tumor was faster with [3H]paclitaxel, but overall tumor exposure to paclitaxel, with steady concentrations between 24 and 120 hours, was higher after administration of [3H]PPX. In addition to tumor tissue, PPX also accumulates in tissues with abundant reticular endothelial systems through active phagocytosis (eg, liver, spleen).
Figure 4 Preclinical tumor pharmacokinetics. To determine the tissue pharmacokinetic profile of PPX, female mice with subcutaneous B16 murine melanomas were given tritium-labeled paclitaxel 40 mg/kg intravenously, either as [3H]paclitaxel in polyoxyethylated castor oil/ethanol or [3H]PPX in phasophate buffer. Samples were collected from 0 to 144 hours; the concentration of extractable taxanes was determined by HPLC/MS.
Table 1 Preclinical tumor pharmacokinetics
![Figure 4 Preclinical tumor pharmacokinetics. To determine the tissue pharmacokinetic profile of PPX, female mice with subcutaneous B16 murine melanomas were given tritium-labeled paclitaxel 40 mg/kg intravenously, either as [3H]paclitaxel in polyoxyethylated castor oil/ethanol or [3H]PPX in phasophate buffer. Samples were collected from 0 to 144 hours; the concentration of extractable taxanes was determined by HPLC/MS. Table 1 Preclinical tumor pharmacokineticsDownload CSVDisplay Table](/cms/asset/223d7942-673e-437d-ac14-ee83e7f923b6/dijn_a_14375_f0005_c.jpg)
Metabolism
Anti-tumor activity requires the release of paclitaxel from the conjugate through the proteolysis of the polymeric backbone. Initially, two PPX metabolites, monoglutamyl-paclitaxel isomers Glu-2′-TXL (2′-[L-γ-glutamyl]-paclitaxel) and Glu-7′-TXL (7′-[L-γ-glutamyl]-paclitaxel), were identified in tumor tissue from B16 tumor-bearing mice exposed to [3H]-labeled PPX. Subsequent pharmacokinetic analysis indicated a gradual increase of [3H]-labeled PPX metabolites in tumor tissue, reaching Tmax at 72 hours post-administration. In comparison, administration of [3H]-paclitaxel resulted in a Tmax at 1.5 hours. The gradual accumulation of PPX in tumor tissue was associated with prolonged tumor exposure to PPX metabolites Glu-2′-TXL and paclitaxel. These observations demonstrate the intra-tumoral degradation of the poliglutamate backbone of PPX in association with the release of paclitaxel (CitationSinger et al 2005).
PPX biodegradation was further characterized in vitro and in vivo using qualitative and quantitative LC/MS analysis (CitationShaffer et al 2007). The intracellular, time-dependent generation of 5 PPX metabolites was observed in RAW 264.7 monocytes as well as the human HT-29 colon adenocarcinoma and NCI-H460 non-small lung carcinoma cell lines. In cell-free assays, mainly the carboxy exopeptidases, cathepsin B and X, could mediate PPX metabolism. In vitro, the release of conjugated paclitaxel was dependent on the activity of cathepsin B. Data from a cathepsin B deficient animal model confirmed that cathepsin B is an important in vivo mediator of PPX metabolism and the subsequent release of paclitaxel; however, other proteolytic pathways may contribute as well.
In normal physiological conditions, cathepsin B expression and activity is tightly regulated (CitationYan and Sloane 2003). In malignant tumors and premalignant lesions, cathepsin B expression is increased, which may also be associated with secretion and localization at the cell membrane (CitationPoole et al 1978; CitationSpiess et al 1994; CitationLinebaugh et al 1999). Both intra-cellular and membrane-bound cathepsin B contributes to the degradation of the extracellular matrix, a crucial step in the process of tumor cell invasion (CitationSzpaderska and Frankfater 2001; CitationPremzl et al 2003).
Clinical development
Performance status (PS) measures the impact of tumor-related symptoms and co-morbidities, such as co-existing illnesses and older age, on a patient’s functional status; poor PS is defined by a score of 2 or more on the Eastern Cooperative Oncology Group ECOG performance scale. In NSCLC, poor PS is associated with a poor prognosis and an increased vulnerability to chemotherapy-related toxicities (CitationSweeney et al 2001; CitationCrinò et al 2002). In the absence of clinical trials enrolling significant numbers of poor PS patients, no standard of care has been established for the treatment of patients with advanced NSCLC and poor PS. When considering treatment options for patients with advanced NSCLC and poor PS, meaningful clinical benefit is not only determined by improved survival, but also symptom relief and tolerability (CitationBlackhall et al 2005). Two phase III studies evaluated the efficacy and safety of PPX in chemotherapy-naïve patients with advanced NSCLC and a poor performance status. In STELLAR 3, PPX in combination with carboplatin was compared with paclitaxel in combination with carboplatin, and in STELLAR 4, PPX as a single agent was compared with physician’s choice of either gemcitabine or vinorelbine. A third phase III study, STELLAR 2, compared PPX with docetaxel in patients with relapsed/refractory NSCLC. The primary efficacy endpoint for all three trials was survival, and PPX was shown to be as effective as current treatment options in NSCLC ().
Table 2 PPX phase III efficacy summary (no statistically significant differences between the treatment arms were observed in STELLAR 2, 3, and 4)
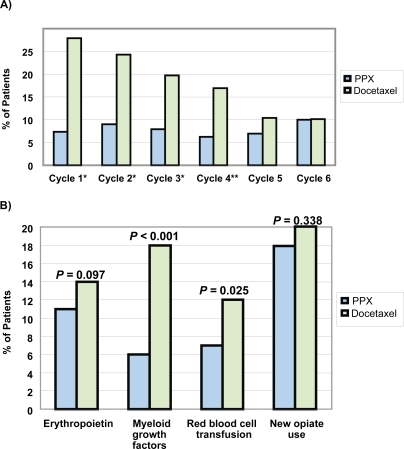
Compared with docetaxel, PPX was associated with a favorable hematological toxicity profile, reducing the incidence of severe neutropenia and infection (). The reduced hematologic toxicity was clinically relevant as patients in the PPX arm showed a significant decrease in the requirement for supportive care (). The non-hematologic toxicity profile of PPX is characterized by minimal hair loss, a distressing side-effect of chemotherapy associated with a lowered self-esteem and a negative body image. In general, PPX-related non-hematologic severe (grade 3 or 4) adverse events are similar to those of other taxanes, with dose-related neuropathy as the most clinically important issue (CitationLanger et al 2005; CitationO’Byrne 2005a, Citationb).
Figure 5 Clinical benefits of reduced myelosupression. A: Percentage of patients with grade 3/4 neutropenia (by treatment cycle) in STELLAR 2. B: Reduced need for supportive measures to manage effects of myelosuppression. *p<0.0001; **p<0.01.
Table 3 Hematologic toxicity profile: PPX vs docetaxel (STELLAR 2)
Future directions
In the phase III studies, chemo-naïve patients receiving PPX had similar overall survival compared with patients in the control arms. However, in STELLAR 4, a trend towards improved survival was noted for female patients receiving PPX compared with female patients in the control arm: 49 women received PPX and 56 received gemcitabine or vinorelbine; the median survival for females in the PPX arm was 312 days compared with 209 days for females in the control arm (log rank p = 0.069; hazard ratio = 0.65). In contrast, survival was similar for male patients, regardless of treatment, indicating a potential gender-specific benefit associated with PPX for the treatment of lung cancer (CitationRoss 2006).
The comparison of lung tumors from male and female patients has identified various tumor characteristics that may affect tumor etiology in a gender-specific manner (CitationPatel 2005; CitationThomas et al 2005). Particularly, lung tumorigenesis may be modulated depending on exposure to estrogen. An epidemiologic link between estrogen exposure and incidence of NSCLC has been suggested in Japanese non-smoking women (CitationLiu et al 2005). Younger, presumably pre-menopausal, women appear to have shorter survival than older women: in an analysis of patients with advanced NSCLC enrolled in Southwest Oncology Group (SWOG) trials, women over 70 had a 34% 1-year survival compared with 11% for those under 45 (CitationAlbain et al 1991).
Estrogen-induced cell proliferation is a critical step in the etiology and progression of a variety of tumor types (CitationDeroo and Korach 2006). The cellular response to estrogen is mediated by estrogen receptor alpha (ER-alpha) and ER-beta. These receptor proteins function as ligand-dependent transcription factors and regulate the expression of genes implicated in cell cycle control, signal transduction, and cell survival (CitationFrasor et al 2003; CitationEdwards 2005). In animal models, estrogen plays a role in normal pulmonary physiology (CitationMassaro and Massaro 2004, Citation2006); adult females have a larger number of alveoli that are smaller in size, than males. This difference develops as animals reach sexual maturity and seems to be mediated mainly by estrogens (CitationMassaro et al 1996). In vivo studies show that ER-beta is abundantly expressed in lung tissue and controls the transcription of platelet-derived growth factor A (PDGF-A), which plays a pivotal role in alveolar formation, and granulocyte macrophage colony-stimulating factor (GM-CSF), a key regulator of surfactant homeostasis (CitationPatrone et al 2003). ER-beta, and to a lesser extend ER-alpha, are expressed in lung tumors from both men and women; in a large series of surgically resected NSCLC tumors, ER-beta was detected in 45.8% of cases. Overexpression of ER-beta was significantly more common in tumors from non-smokers (53.5%) than smokers (36.6%, p<0.004). Among non-smokers, higher ER-beta expression was observed significantly more frequently in female patients (58.3%) than in male patients (40.9%) (CitationWu et al 2005). The complex biological effects mediated by ERs involve communication between many proteins and signaling pathways, including lysosomal vesicle trafficking.
Under the control of estrogen, intracellular trafficking of cathepsin B may be altered in malignant tumors, resulting in the increased secretion of precursor and active forms of the enzyme (CitationPoole et al 1978; CitationAchkar et al 1990; CitationLinebaugh et al 1999), its redistribution from perinuclear lysosomes to peripheral vesicles (CitationSloane et al 1994), and its association with the cell membrane (CitationSpiess et al 1994; CitationSameni et al 1995; CitationCavallo-Medved et al 2005). Cathepsin B localizes with other proteases at the tumor cell surface in caveolae, mediating cell-surface proteolytic events associated with invasion (CitationRoshy et al 2003; CitationCavallo-Medved et al 2005). The abundance of cathepsin B in tumor tissue is relevant for the efficient degradation of PPX. The interaction of lung cancer cells and infiltrating immune cells, particularly phagocytic monocytes, can further promote tumor development and metastasis. Homeostasis of human monocytes is regulated by estrogen, and monocytes are known to express both ER-alpha and ER-beta (CitationPhiel et al 2005). Interestingly, cathepsin X is upregulated in tumor-infiltrating immune cells, particularly in phagocytic monocytes (CitationKos et al 2005).
Summary
Factors limiting the therapeutic index of paclitaxel include low solubility, high systemic exposure, poor pharmacokinetic characteristics, and a lack of selective tumor uptake. The rationale for developing paclitaxel poliglumex (PPX), a macromolecular polymer–drug conjugate, was to improve standard chemotherapy with paclitaxel by overcoming some of these limitations (). Tumor pharmacokinetics show that tumor distribution is faster with paclitaxel, but overall tumor exposure is higher with PPX. The available data support a model in which PPX accumulates in tumor tissue through the EPR effect, followed by the cathepsin B-mediated release of paclitaxel.
Table 4 Paclitaxel vs PPX
The cathepsin-mediated release of paclitaxel may have therapeutic implications as cathepsin B is upregulated in malignant cells, particularly during tumor progression. Estrogen may play an important role in lung tumor biology and is a modulator of cathepsin B activity. A possible association between estrogen levels and PPX activity will be further explored in both preclinical and clinical studies.
References
- AchkarCGongQMFrankfaterA1990Differences in targeting and secretion of cathepsins B and L by BALB/3T3 fibroblasts and Moloney murine sarcoma virus-transformed BALB/3T3 fibroblastsJ Biol Chem2651365042166039
- AlbainKSCrowleyJJLeblancM1991Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experienceJ Clin Oncol91618261651993
- BalaIHariharanSKumarMN2004PLGA nanoparticles in drug delivery: the state of the artCrit Rev Ther Drug Carrier Syst2138742215719481
- BernareggiAOldhamFBakerB2005XYOTAX™ (paclitaxel poliglumex, PPX): tumor accumulation and prolonged exposure to paclitaxel11th World Congress on Lung CancerBarcelona, Spain
- BhallaKN2003Microtubule-targeted anticancer agents and apoptosisOncogene2290758614663486
- BlackhallFHBhosleJThatcherN2005Chemotherapy for advanced non-small cell lung cancer patients with performance status 2Curr Opin Oncol17135915725918
- Cavallo-MedvedDMaiJDosescuJ2005Caveolin-1 mediates the expression and localization of cathepsin B, prourokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cellsJ Cell Sci118149350315769846
- CrinòLMigliorinoM2002A phase III randomized trial comparing three platinum-based doublets in advanced non-small cell lung cancer (NSCLC): impact of PS = 2 vs 0 or 1 and age >70 vs <70 on chemotherapy outcomeProc Am Soc Clin Oncol21315a
- DerooBJKorachKS2006Estrogen receptors and human diseaseJ Clin Invest1165617016511588
- DuncanR1992Drug-polymer conjugates: potential for improved chemotherapyAnticancer Drugs31752101525399
- DuncanR2003The dawning era of polymer therapeuticsNat Rev Drug Discov23476012750738
- EdwardsDP2005Regulation of signal transduction pathways by estrogen and progesteroneAnnu Rev Physiol673357615709962
- FrasorJDanesJMKommB2003Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotypeEndocrinology14445627412959972
- GelderblomHVerweijJNooterK2001Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulationEur J Cancer371590811527683
- GerlowskiLEJainRK1986Microvascular permeability of normal and neoplastic tissuesMicrovasc Res312883052423854
- GreishKFangJInutsukaT2003Macromolecular therapeutics: advantages and prospects with special emphasis on solid tumour targetingClin Pharmacokinet42108910514531722
- Gueritte-VoegeleinFGuenardDLavelleF1991Relationships between the structure of taxol analogues and their antimitotic activityJ Med Chem3499281672159
- KosJSekirnikAPremzlA2005Carboxypeptidases cathepsins X and B display distinct protein profile in human cells and tissuesExp Cell Res3061031315878337
- KumarGNWalleUKBhallaKN1993Binding of taxol to human plasma, albumin and alpha 1-acid glycoproteinRes Commun Chem Pathol Pharmacol80337448102493
- LangerCJSocinskiMARossH2005Paclitaxel poliglumex (PPX)/carboplatin vs paclitaxel/carboplatin for the treatment of PS2 patients with chemotherapy-naïve advanced non-small cell lung cancer (NSCLC): A phase III studyJ Clin Oncol, ASCO Annual Meeting Proceedings237011
- LangerR1998Drug delivery and targetingNature3925109579855
- LinebaughBESameniMDayNA1999Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular massEur J Biochem264100910447678
- LiuYInoueMSobueT2005Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort studyInt J Cancer117662615929081
- ManfrediJJParnessJHorwitzSB1982Taxol binds to cellular microtubulesJ Cell Biol94688966127342
- MarkmanM2003Managing taxane toxicitiesSupport Care Cancer11144712618923
- MassaroDMassaroGD2004Estrogen regulates pulmonary alveolar formation, loss, and regeneration in miceAm J Physiol Lung Cell Mol Physiol287L1154915298854
- MassaroDMassaroGD2006Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in miceAm J Physiol Lung Cell Mol Physiol290L8667016361355
- MassaroGDMortolaJPMassaroD1996Estrogen modulates the dimensions of the lung’s gas-exchange surface area and alveoli in female ratsAm J Physiol270L11048772533
- MatsumuraYMaedaH1986A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancsCancer Res466387922946403
- O’ByrneKJ2005aPaclitaxel poliglumex vs. gemcitabine or vinorelbine for the treatment of performance status (PS) 2 patients with chemotherapy-naïve advanced non-small cell lung cancer (NSCLC): the STELLAR 4 phase III studyEur J Cancer Suppl3324
- O’ByrneKJ2005Paclitaxel poliglumex vs. docetaxel for the second-line treatment of non-small cell lung cancer (NSCLC): the STELLAR 2 phase III studyEur J Cancer Suppl35
- PatelJD2005Lung cancer in womenJ Clin Oncol233212815886308
- PatroneCCasselTNPetterssonK2003Regulation of postnatal lung development and homeostasis by estrogen receptor betaMol Cell Biol2385425214612399
- PhielKLHendersonRAAdelmanSJ2005Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populationsImmunol Lett971071315626482
- PooleARTiltmanKJReckliesAD1978Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomasNature2735457661963
- PremzlAZavasnik-BergantVTurkV2003Intracellular and extracellular cathepsin B facilitate invasion of MCF-10A neoT cells through reconstituted extracellular matrix in vitroExp Cell Res2832061412581740
- RobertsWGPaladeGE1997Neovasculature induced by vascular endothelial growth factor is fenestratedCancer Res57765729044858
- RoshySSloaneBFMoinK2003Pericellular cathepsin B and malignant progressionCancer Metastasis Rev222718612785001
- RossHBonomiPLangerC2006Effect of gender on outcome in two randomized phase III trials of paclitaxel poliglumex (PPX) in chemonaïve pts with advanced NSCLC and poor performance status (PS2)J Clin Oncol, ASCO Annual Meeting Proceedings247039
- RowinskyEKDonehowerRC1995Paclitaxel (taxol)N Engl J Med3321004147885406
- SameniMElliottEZieglerG1995Cathepsin B and D are localized at the surface of human breast cancer cellsPathol Oncol Res1435311173567
- SeidmanAD2005“Will weekly work”? Seems to be so”J Clin Oncol235873416087954
- ShafferSALee-BakerCKennedyK2007In vitro and in vivo metabolism of paclitaxel poliglumex: Identification of metabolites and active proteasesCancer Chemother PharmacolIn press.
- SingerJWShafferSBakerB2005Paclitaxel poliglumex (XYOTAX; CT-2103): an intracellularly targeted taxaneAnticancer Drugs162435415711176
- SloaneBFMoinKSameniM1994Membrane association of cathepsin B can be induced by transfection of human breast epithelial cells with c-Haras oncogeneJ Cell Sci107373848207069
- SonnichsenDSRellingMV1994Clinical pharmacokinetics of paclitaxelClin Pharmacokinet27256697834963
- SpiessEBruningAGackS1994Cathepsin B activity in human lung tumor cell lines: ultrastructural localization, pH sensitivity, and inhibitor status at the cellular levelJ Histochem Cytochem42917298014475
- SweeneyCJZhuJSandler2001Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinomaCancer9226394711745199
- SzpaderskaAMFrankfaterA2001An intracellular form of cathepsin B contributes to invasiveness in cancerCancer Res61349350011309313
- ThomasLDoyleLAEdelmanMJ2005Lung cancer in women: emerging differences in epidemiology, biology, and therapyChest1283708116002959
- WuCTChangYLShihJY2005The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancersJ Thorac Cardiovasc Surg1309798616214508
- YanSSloaneBF2003Molecular regulation of human cathepsin B: implication in pathologiesBiol Chem3848455412887051