Abstract
Introduction
Although consensus guidelines recommend checking serum B12 in patients with dementia, clinicians are often faced with various questions: (1) Which patients should be tested? (2) What test should be ordered? (3) How are inferences made from such testing? (4) In addition to serum B12, should other tests be ordered? (5) Is B12 deficiency compatible with dementia of the Alzheimer’s type? (6) What is to be expected from treatment? (7) How is B12 deficiency treated?
Methods
On January 31st, 2009, a Medline search was performed revealing 1,627 citations related to cobalamin deficiency, hyperhomocysteinemia, and dementia. After limiting the search terms, all abstracts and/or articles and other references were categorized into six major groups (general, biochemistry, manifestations, associations and risks, evaluation, and treatment) and then reviewed in answering the above questions.
Results
The six major groups above are described in detail. Seventy-five key studies, series, and clinical trials were identified. Evidence-based suggestions for patient management were developed.
Discussion
Evidence is convincing that hyperhomocysteinemia, with or without hypovitaminosis B12, is a risk factor for dementia. In the absence of hyperhomocysteinemia, evidence is less convincing that hypovitaminosis B12 is a risk factor for dementia. B12 deficiency manifestations are variable and include abnormal psychiatric, neurological, gastrointestinal, and hematological findings. Radiological images of individuals with hyperhomocysteinemia frequently demonstrate leukoaraiosis. Assessing serum B12 and treatment of B12 deficiency is crucial for those cases in which pernicious anemia is suspected and may be useful for mild cognitive impairment and mild to moderate dementia. The serum B12 level is the standard initial test: 200 picograms per milliliter or less is low, and 201 to 350 picograms per milliliter is borderline low. Other tests may be indicated, including plasma homocysteine, serum methylmalonic acid, antiparietal cell and anti-intrinsic factor antibodies, and serum gastrin level. In B12 deficiency dementia with versus without pernicious anemia, there appear to be different manifestations, need for further workup, and responses to treatment. Dementia of the Alzheimer’s type is a compatible diagnosis when B12 deficiency is found, unless it is caused by pernicious anemia. Patients with pernicious anemia generally respond favorably to supplemental B12 treatment, especially if pernicious anemia is diagnosed early in the course of the disease. Some patients without pernicious anemia, but with B12 deficiency and either mild cognitive impairment or mild to moderate dementia, might show some degree of cognitive improvement with supplemental B12 treatment. Evidence that supplemental B12 treatment is beneficial for patients without pernicious anemia, but with B12 deficiency and moderately-severe to severe dementia is scarce. Oral cyanocobalamin is generally favored over intramuscular cyanocobalamin.
Introduction
The notion of vitamin B12 deficiency (ie, B12 hypovitaminosis), its psychiatric and neurological manifestations, and its treatment is an age-old issue that has generated much controversy over many years. Although consensus guidelinesCitation1–Citation4 recommend checking serum B12 levels in patients with dementia, clinicians are often faced with questions regarding how to interpret and what to do with the results.Citation5 In order to help in clarifying these uncertainties, six questions were posed and then answered: 1) In which patients should vitamin B12 be routinely assessed? 2) What test or tests should be ordered, and how are inferences made from such testing? 3) Does the finding of low serum B12 or elevated homocysteine (Hcy) require evaluation for other medical conditions? 4) When vitamin B12 deficiency is found in dementia, is dementia of the Alzheimer’s type (DAT) a compatible diagnosis? 5) Based on the benefit-to-risk ratio in treatment of dementia, if vitamin B12 deficiency is determined, should supplemental B12 be initiated? 6) How is vitamin B12 deficiency treated?
By assimilating data from in vitro, in vivo animal, and in vivo human studies and epidemiological studies, this article clarifies these and other issues regarding hypovitaminosis B12, hyperhomocysteinemia (HHcy), and dementia.
Methods/results
The study design is a qualitative and quantitative review of the literature. The problem discussed is that in clinicians’ geriatric practices, patients with dementia and low vitamin B12 were not showing significant improvement with supplemental B12 therapy. Our hypothesis is that patients with dementia and low vitamin B12 improve with supplemental B12 therapy. The null hypothesis is that patients with dementia and low vitamin B12 do not improve with supplemental B12 therapy.
On January 31st, 2009 a Medline search was performed using the search terms: (Alzheimer OR Alzheimer’s OR dementia OR cognitive impairment OR cognitive dysfunction) AND (cobalamin OR cyanocobalamin OR B12 OR B-12 OR B 12 OR homocysteine OR hyperhomocysteinemia OR homocystinuria), which revealed 1,627 citations. “Title/Abstract” field limits decreased the search to 1,095 citations, which included 230 review articles. Using a Boolean operation, the review articles were removed, reducing the search to 865 citations. Subsequently, the search was limited to only citations with abstracts, so as to exclude publications such as ‘Letters to the Editor’ and case reports, which revealed 824 citations. Furthermore, in order to not miss any positive findings of individual case reports or case series, on September 6th, 2009 another Medline search was performed using the search terms: pernicious anemia AND dementia AND (case report OR case series) revealing 20 citations. Of the 844 articles, all abstracts were reviewed and, when useful, relevant articles were obtained and reviewed. Bibliographies from relevant articles were reviewed and, when applicable, review articles, ‘Letters to the Editor,’ and case reports were included in the overall review. Data from (1) other Medline searches, (2) Internet searches, (3) basic and clinical science textbooks, and (4) personal communications were added for clarification of technical issues. All abstracts, articles, and other references were categorized, allowing duplications, into six major categories: (1) general information, (2) biochemical evidence suggesting that hypovitaminosis B12 or HHcy are causal factors in dementia, (3) clinical and radiological manifestations, (4) associations between hypovitaminosis B12 or HHcy and cognitive impairment, (5) evaluation, and (6) treatment. Endnote version X.0.2 (Thomson Reuters, Philadelphia, PA) was used to maintain the reference library, which contained 839 citations. Evidenced-based medicine was used to develop suggestions for vitamin B12 workup and treatment in patients with suspected mild cognitive impairment (MCI) or dementia.
There are 511 articles and other references relevant to the six questions posed in the introduction and the six major categories listed above. Question 5 in the Introduction considers treatment benefits and risks. In terms of treatment benefits, the study objective was to determine whether or not vitamin B12 is beneficial for B12-deficient dementia. Letting the null hypothesis be, “Patients with dementia and low vitamin B12 do not improve with supplemental B12 therapy,” not rejecting the null hypothesis when it is not true would be a type 2 error (false negative). In order to avoid a type 2 error, thus concluding B12 treatment is not beneficial, when in truth it is beneficial, all published studies and reports contained in Medline (Box 1), including case series, in which supplemental B12 was an exposure and cognitive change was an outcome are included in the discussion and tables (N = 38), regardless of the quality of the study or number of subjects. Also included are all published cohort and longitudinal studies in Medline, where exposure pertains to metabolic or serum B12 deficiency and outcomes pertain to change in cognitive function or development or prevention of dementia (N = 37). Also included are the majority of the retrospective and cross-sectional studies in Medline that examine similar outcomes and exposures. Articles pertaining to genetics, biochemistry, pathophysiology, clinical manifestations, and radiological manifestations illuminating our understanding on relationships between HHcy with and without cobalamin deficiency and dementia are included in the review, as are articles relevant to evaluation, prognosis, and treatment.
Box 1 Studies and reports in which supplemental B12 is an exposure and cognitive change is an outcome
Discussion
General information
Vitamin B12 is composed of a central cobalt atom, attached to a dimethylbenzimidazole group, four nitrogen atoms, each pertaining to four pyrrole rings, and an R group (-CN, -OH, -CH3, or adenosyl group), denoting the specific type of cobalamin.Citation6–Citation8 The definition of vitamin B12 deficiency is a quantitative lack of vitamin B12 in the diet, body fluids, or cells or a qualitative lack of intracellular B12 utilization.
B12 deficiency occurs in roughly 10% of generalCitation9–Citation23 and 17% of dementedCitation10,Citation24–Citation26 elderly populations. B12 deficiency in demented individuals ranges from oneCitation10,Citation27,Citation28 to fiveCitation25,Citation29 times that of controls. Serum B12 levels and cerebral spinal fluid (CSF) folate levels decrease with advancing age,Citation9–Citation12,Citation30–Citation34 whereas serum folate levels may either increase or decrease with age.Citation32 Hcy is a nonessential thiol amino acid.Citation35,Citation36 HHcy is defined as an abnormally high level of total Hcy in the plasma. HHcy occurs in 6% to 81% of individuals, depending on the population studied.Citation37–Citation44 Causes of HHcy and B12 deficiency are listed in .Citation7,Citation18,Citation19,Citation39,Citation45–Citation62 Further details regarding differential diagnoses are available on the Internet.Citation62
Table 1 Causes of hyperhomocysteinemia and B12 deficiency
Biochemical evidence suggesting that hypovitaminosis B12 or hyperhomocysteinemia are causal factors in dementia
The means by which HHcy is involved in dementia may possibly be explained by aging and reduced vitamin B12 or folate supply versus demand ratio,Citation63,Citation64 with functional, structural, genetic, and nutritional determinants. Possible functional determinants include Hcy agonism of N-methyl-D-aspartic acid (NMDA) receptors, which causes excessive intracellular calcium influx and neuronal deathCitation65,Citation66 and HHcy creating a state of hypomethylation in the pathogenesis of Alzheimer’s disease (AD),Citation21,Citation58,Citation67 causing deoxyribonucleic acid (DNA) damage and apoptosis.Citation68–Citation71 HHcy inhibits adult mammal hippocampal neurogenesis. Citation72 Hcy may compete with gamma-amino butyric acid (GABA) at the GABA receptor and may affect its inhibitory function.Citation73,Citation74
Potential structural determinants include HHcy causing blood–brain barrier (BBB) dysfunctionCitation75–Citation78 and endothelial cell toxicity,Citation47,Citation64 whereby Hcy changes endothelial cell surface properties from anticoagulant to procoagulant.Citation47,Citation52,Citation73,Citation79 Genetic determinants include various polymorphisms and homozygous monogenic deficiencies, involving methylenetetrahydrofolate reductase (MTHFR), trans-cobalamin (TC) II, methionine synthetase (MS), and cystathione beta-synthetase (CBS). These may contribute to hypovitaminosis B12, HHcy, and dementia.Citation7,Citation47,Citation52,Citation64,Citation73,Citation80–Citation82 Meta-analysesCitation83–Citation89 of the MTHFR polymorphism show that those with 677 TT alleles compared to 677 CC alleles have elevated Hcy and increased risk for myocardial infarction (MI), transient ischemic attack, and stroke. Hyperhomocysteinemic individuals with certain butyrylcholinesterase-K (BuChE-K) alleles cognitively decline more rapidly than those with wild-type BuChE alleles.Citation90 Nutritional determinants include decreased vitamin B12 ingestionCitation9,Citation45,Citation47 and food-cobalamin malabsorption.Citation9,Citation15,Citation19,Citation45,Citation49,Citation91–Citation93
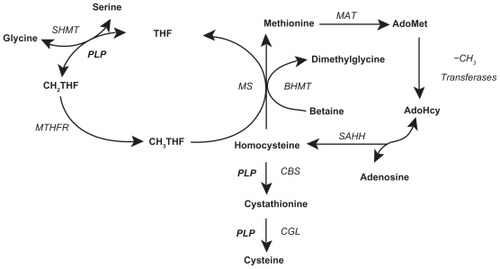
In vitro, in vivo animal, and in vivo human studies suggest AD pathophysiology conceivably involves a hypomethylation state,Citation67,Citation94–Citation97 reactive oxygen species (ROS) generation,Citation27,Citation98–Citation101 immune activation,Citation102–Citation110 and anomalous protein development.Citation111 Abnormal Hcy metabolism is involved in each of these four processes. Hcy is metabolized by the methionine cycle and trans-sulfuration pathway (),Citation112 where it has one of three possible fates: methylation to methionine (Met), transsulfuration to cystathionine, or adenosylation to S-adenosylhomocysteine (SAH).Citation113,Citation114 With normal Hcy levels, the first two reactions are maintained, the first occurring in the brain and body and the second predominately occurring in the body, promoting homeostatic methylation reactions and maintaining healthy cells. With Hcy elevation the third reaction ensues, promoting a hypomethylation state leading to disease.Citation115
Figure 1 Folate cycle, methionine cycle, and transsulfuration pathway. Copyright © 2005. Adapted with permission from Davis SR, Quinlivan EP, Shelnutt KP, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C->T polymorphism and by dietary folate restriction in young women. J Nutr. 2005;135(5):1045–1050.
Abbreviations: AdoHcy, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; BHMT, betaine-homocysteine methyltransferase; CBS, cystathionine β-synthase; CGL, cystathionine gamma-lyase; CH2THF, methylenetetrahydrofolate; -CH3, methyl group; CH3THF methyl tetrahydrofolate; DHFR, dihydrofolate reductase; MAT, methionine adenosyltransferases; MS, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; PLP, pyridoxal phosphate (the active form of vitamin B6, pyridoxine); ROS, reactive oxygen species; SAHH, S-adenosylhomocysteine hydrolase; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate.
On the one hand, without oxidative chemical reactions, as the basis for cellular respiration, we would not have life, at least as we know it. On the other hand, without these reactions, we would not have ROS.Citation116 There are thousands of publications related to ROS and aging, the greatest known risk factor for sporadic AD.Citation114,Citation116,Citation117 Oxidative metabolism generates a very small fraction of ROS,Citation117 which can be beneficial or detrimental to the central nervous system. Oxidative stress occurs when ROS generation exceeds ROS defense,Citation118 leading to potential molecular and cellular damage.Citation117 Aberrant mitochondrial enzymes may facilitate this process, thereby contributing to the pathophysiology of AD.Citation116
Hcy is rapidly auto-oxidized to homocysteine thiolactone, homocystine, and mixed disulfides,Citation7,Citation119 producing ROS,Citation18,Citation21,Citation47,Citation52,Citation68,Citation120 including singlet oxygen, superoxide anions, hydroxyl radicals, and hydrogen peroxide.Citation21,Citation42,Citation52,Citation68,Citation117,Citation118 Hcy elevation is associated with microglia activation and proliferationCitation71 and immune activation and deposition,Citation108 which is associated with choroid plexus dysfunction,Citation121–Citation123 possibly impeding vitamin B12Citation123–Citation125 and folateCitation122,Citation125 influx to, and amyloid beta (Aβ) peptideCitation123,Citation126,Citation127 clearance from, brain tissue in AD. In certain systems, Hcy elevation leads to amyloid precursorCitation128 and tauCitation128–Citation132 protein hyperphosphorylation, and Hcy oxidation produces products that crosslink with Aβ and tau proteins, causing their precipitation.Citation119,Citation128,Citation133 Hcy elevation is associated with increased Aβ peptide, in both brainCitation134 and plasma.Citation51,Citation135,Citation136
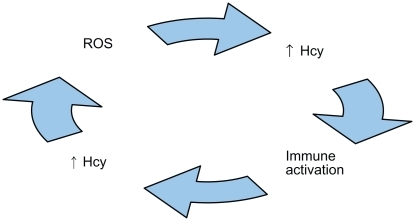
A deleterious cycle may occur between ROS generation and immune activation, where the former may cause the latterCitation137–Citation139 and vice versa.Citation63,Citation64,Citation140–Citation144
Hcy may promote a means for such a cycle,Citation64,Citation106,Citation120 because ROS generation is associated with Hcy elevation,Citation120,Citation142,Citation145–Citation147 which is associated with immune activation,Citation64,Citation71,Citation79,Citation148–Citation150 and immune activation is associated with Hcy elevation,Citation64,Citation142,Citation151,Citation152 which is associated with ROS generation ().Citation47,Citation64,Citation71,Citation153–Citation155 Additional evidence verifies ROS,Citation145,Citation151,Citation156 hypomethylation,Citation21,Citation58,Citation67,Citation94,Citation95,Citation128,Citation136,Citation157 immunological components,Citation64,Citation79,Citation106,Citation109,Citation110,Citation142 and Aβ and tau proteinsCitation58,Citation65,Citation66,Citation120,Citation136,Citation158 interact with one another, producing a neurodegeneration cascade in AD. ROS generation precedes both Aβ peptide depositionCitation159–Citation162 and tau-associated neurodegeneration.Citation163 ROS generation deregulates tau protein phosphorylation,Citation164,Citation165 and is associated with decreased S-adenosylmethionine (SAM),Citation120 increased SAH, a decreased SAM/SAH ratio,Citation115 and hyperconsumption and depletion of antioxidantsCitation98,Citation151,Citation156 and tetrahydrofolate (THF).Citation64,Citation120,Citation151,Citation156,Citation166
Figure 2 Illustration of a biologically plausible deleterious cycle of reactive oxygen species (ROS), homocysteine (Hcy), and immune activation that possibly may be involved in the pathogenesis of Alzheimer’s disease.
With absolutely or relatively low folate or vitamin B12, MS-mediated Hcy clearance is impeded,Citation52,Citation120 resulting in a hypomethylation state. Also, Hcy elevation may lead to hyperconsumption and depletion of vitamin B12 in various cases of AD and vascular dementia (VaD).Citation146 Hcy potentiates Aβ peptide-induced ROS generation and apoptosis.Citation69,Citation154,Citation155,Citation167 Aβ and tau proteins are concentrated sources for further ROS generation, Hcy elevation, and immune activation, thereby perpetuating the deleterious cycle.Citation120,Citation143,Citation168–Citation170 Also, in vivo human studies show that free cobalt is elevated in individuals with AD compared to controls.Citation8 In vitro studies show free cobalt generates oxidative stress, as measured by reduced glutathione, increases Aβ peptide secretion, and produces neuroblastoma cytotoxicity.Citation8,Citation120
Within the central nervous system, a balance may occur between endogenous neurotoxic agents on one hand and endogenous neurotrophic agents on the other hand.Citation171 Examples of potential neurotoxic agents include tumor necrosis factor-alpha (TNF-α), nerve growth factor (NGF), and the soluble CD40-soluble CD40 ligand dyad (sCD40-sCD40). Examples of potential neurotrophic agents include interleukin-6 (IL-6), epidermal growth factor (EGF), and transforming growth factor-beta1 (TGF-β1). In animals, if the balance is tilted in favor of TNF-α, NGF, and sCD40-sCD40 (eg, by the administration of exogenous TNF-α),Citation172 as opposed to IL-6, NGF, and TGF-β1, then the morphological changes of subacute combined degeneration (SCD) are observed: white matter interstitial edema, intra-myelinic edema, spongy vacuolation, and astrogliosis.Citation172–Citation175 Vitamin B12-depleted animals exhibit increased levels of TNF-α, NGF, and sCD40-sCD40Citation172,Citation176 and decreased levels of IL-6 and EGF,Citation176 thereby tilting the balance and developing the myelopathic changes of SCD. Not only does treatment with vitamin B12 reduce or reverse these changes,Citation172,Citation175 but treatment with anti-TNF-α antibodies, IL-6, EGF, and TGF-β1 does so as well.Citation172,Citation176 Interestingly, a similar observation has been observed in humans. Serum TNF-α is higher and serum EGF is lower in subjects with severe B12 deficiency compared to controls, where a direct correlation is found between plasma Hcy and serum TNF-α.Citation173 The association is translative into the CSF, where CSF B12 and EGF are lower and CSF Hcy and TNF-α are higher in subjects with SCD compared to non-B12 deficient controls.Citation177 B12-repletion lowers serum TNF-α and raises serum EGF, thereby normalizing the imbalance, which occurs concomitantly with clinical and hematological disease remission.Citation173 Hence, in addition to well-known enzymatic roles for vitamin B12, it is thought to also have nonenzymatic roles, where it is associated with downgrading synthesis and release of TNF-α and upgrading synthesis and release of EGF.Citation173,Citation177,Citation178
Clinical and radiological manifestations
Clinical manifestations of low vitamin B12 include abnormal psychiatric, neurological, and gastrointestinal findings. Psychiatric manifestations consist of psychoses, including paranoia, delusions, and hallucinations,Citation23,Citation58,Citation179–Citation184 cognitive dysfunction, including memory impairment, delirium, and dementia,Citation7,Citation21,Citation23,Citation49,Citation58,Citation93,Citation180,Citation181,Citation185–Citation188 and affective syndromes, including mania and depression,Citation7,Citation21,Citation23,Citation58,Citation93,Citation180,Citation181,Citation186,Citation189–Citation191 which also occurs with elevated HcyCitation192–Citation194 and low SAM.Citation67,Citation192 Associations exist between HHcy and cognitive dysfunction in bipolar disorderCitation195–Citation197 and perhaps schizophrenia.Citation198 Neurological manifestations include myelopathy and peripheral, autonomic, and optic neuropathies.Citation199 Paresthesia is caused by a sensory lesion anywhere between the peripheral nerve and brain and is often the initial symptom.Citation23,Citation58,Citation92,Citation180,Citation181,Citation188 SCD refers to myelopathy affecting posterior and lateral columns, characterized by a pernicious sequence of vacuolar demyelination, axonal degeneration, and neuronal death.Citation18,Citation58,Citation200–Citation202 Posterior column myelopathy affects afferent pathways, causing the most common neurological signs: ataxia, diminished proprioception and vibratory senses, and presence of Romberg’s sign.Citation18,Citation49,Citation58,Citation180,Citation185,Citation188,Citation200,Citation201,Citation203 Lateral column myelopathy affects efferent pathways, causing the second most common neurological signs: extremity muscular weakness, spasticity, hyperactive reflexes, and Babinski’s sign.Citation18,Citation49,Citation58,Citation180,Citation181,Citation200,Citation201 Peripheral and autonomic neuropathies cause hypoactive reflexes, sensory loss, orthostatic hypotension, fecal and urinary incontinence, and impotence. Citation18,Citation23,Citation49,Citation58,Citation92,Citation180,Citation181,Citation185,Citation188,Citation200,Citation203 Although optic neuropathy is uncommon,Citation23,Citation58,Citation180,Citation203 visual impairment may occasionally be the earliest or sole manifestation of the disease.Citation203 Gastrointestinal manifestations include epithelial atrophy of the tongue, referred to as atrophic glossitis, which causes the tongue to be sore and beefy red,Citation18,Citation23 and epithelial atrophy of the stomach.Citation15
Computerized axial tomographic (CAT) and magnetic resonance imaging (MRI) scans of nondemented elderly brains may show age-related cerebral atrophy and various grades of periventricular white matter disease consistent with chronic microvascular ischemia.Citation204–Citation208 Although linear and volume measurement methods, evaluating ventricle-to-brain ratios and medial temporal lobe atrophy, reveal significant differences in group means, between those with AD and controls,Citation205,Citation207,Citation209–Citation217 such strategies are not recommended for the purpose of diagnosing AD.Citation3,Citation218 Assuming normal distributions of both nondemented and demented groups, a certain degree of overlap may exist,Citation205,Citation207,Citation216 unless specified by scan angle-adjusted temporal lobe neuroimaging.Citation207,Citation214 Even so, such imaging may not distinguish non-Alzheimer’s dementia from controls.Citation217 Although CAT scans of nondemented elderly commonly show age-related cerebral atrophy, those of demented elderly often show cerebral atrophy more than expected for age,Citation210 and are read as variably judged atrophy and differently interpreted white matter changes.Citation218,Citation219 Whether or not such findings relate to dementia with low vitamin B12 and/or elevated Hcy is often unclear.
Accordingly, a literature review finds radiological manifestations of low vitamin B12 and/or elevated Hcy to include leukoaraiosis, brain atrophy, and silent brain infarcts.Citation199,Citation220–Citation237 Leukoaraiosis is a radiological term that refers to brain white matter hypodensity on CAT scans or hyperintensity on T2-weighted magnetic resonance imaging (MRI).Citation238 It is associated with aging,Citation221,Citation230,Citation239–Citation244 chronic microvascular hypo-perfusion, Citation245 BBB dysfunction,Citation246 hypertension,Citation221,Citation230,Citation240,Citation241,Citation242,Citation244 stroke,Citation230,Citation239,Citation240–Citation242,Citation244,Citation247 and death.Citation239 Leukoaraiosis is described pathologically as periventricular leukoencephalopathy or subcortical arteriosclerotic encephalopathy; it occurs in brains of individuals with ADCitation243 and dementia of the Binswanger type (DBT),Citation248 which is a relatively rarer type of dementia and is associated with other findings. HHcy increases the risk for leukoaraiosis.Citation220,Citation221,Citation223–Citation226,Citation228,Citation230,Citation232,Citation236 Independently from plasma Hcy levels, one cross-sectional studyCitation249 found an inverse association between the concentration of normal range serum B12 levels and the degree of leukoaraiosis; nonetheless, in the absence of HHcy, two studiesCitation227,Citation231 found low serum B12 does not increase the risk for leukoaraiosis. Parenthetically, one prospective studyCitation250 found an inverse association between serum folate levels and the degree of leukoaraiosis. Although leukoaraiosis is more prevalent in demented than nondemented individualsCitation225,Citation247 and it increases the risk for developing dementia,Citation247,Citation248,Citation251 results are mixed in terms of whether or not the link between HHcy and cognitive dysfunction is specifically mediated by leukoaraiosis.Citation228,Citation232,Citation240,Citation243,Citation252–Citation255 In both cross-sectional and prospective evaluations, hypovitaminosis B12 and HHcy are associated with brain atrophy.Citation199,Citation222,Citation229,Citation232,Citation234,Citation237 HHcy is associated with silent brain infarcts.Citation232
VaD,Citation61 DBT,Citation256 and AD,Citation61,Citation222,Citation243,Citation257–Citation259 represent various spectra of vascular pathology. To illustrate, cerebral amyloid angiopathy occurs in many cases of DBTCitation248 and in most cases of AD,Citation46,Citation260,Citation261 where increased vessel atrophy,Citation262 decreased microvascular density,Citation262 reduced temporal lobe blood flow,Citation263,Citation264 and spontaneous cerebral emboliCitation265,Citation266 are other significant findings. Hcy permeates through the BBB,Citation267,Citation268 causing BBB dysfunction,Citation74,Citation76–Citation78 which is observed in AD,Citation247,Citation269 DBT,Citation220,Citation223,Citation256,Citation270 and VaD,Citation75 allowing easy influx for a wide variety of proteins to cerebral interstitial fluidCitation271,Citation272 and vice versa.Citation273 With BBB dysfunction, brain parenchyma likely becomes less protected from toxic effects of systemic HHcy,Citation229 which increases risk for acute macrovascular disease, or strokes,Citation7,Citation21,Citation70,Citation84,Citation85,Citation87,Citation151,Citation223,Citation274–Citation279 causing loss of volume, and chronic microvascular disease, or ischemia,Citation135,Citation220,Citation280 causing loss of cortical-to-subcortical connections,Citation248 occasionally with findings as those in DBT.Citation220,Citation223,Citation281,Citation282
Associations between hypovitaminosis B12 or HHcy and cognitive impairment
HHcy, with hypovitaminosis B12, are common findings in the evaluation of MCI,Citation59,Citation283–Citation288 AD,Citation43,Citation60,Citation61,Citation82,Citation111,Citation222,Citation276,Citation284,Citation289–Citation291 VaD,Citation43,Citation61,Citation145,Citation276,Citation284,Citation292 and other dementia subtypes.Citation90,Citation282,Citation293,Citation294 This raises the question of whether or not these findings are the cause of, result of, or unrelated to the disease process. Although multiple retrospective and cross-sectional studies find associations between hypovitaminosis B12 and HHcy and cognitive impairment with or without dementia,Citation28,Citation39,Citation43,Citation59–Citation61,Citation82,Citation90,Citation111,Citation145,Citation186,Citation196,Citation222,Citation276,Citation283–Citation306 cohort studies are required to provide evidence that hypovitaminosis B12 or HHcy are risk factors for cognitive dysfunction. EighteenCitation106,Citation136,Citation252,Citation258,Citation301,Citation307–Citation320 of 22 cohort studies demonstrate HHcy either increases the risk for cognitive impairment or development of dementia. Depending upon whether plasma Hcy levels increase or decrease over time, the degree of change either increases the risk for developing dementiaCitation313 or decreases the risk for poorer memory performance,Citation316 respectively. The greater the baseline plasma Hcy level, the faster the rate of cognitive decline.Citation320 However, one study found that the elevated plasma Hcy risk for developing dementia diminished when controlling for low serum folic acid.Citation301 FourCitation321–Citation324 of 22 cohort studies conclude Hcy is not a factor in the development of MCI or dementia, although outcome assessment may have been a methodological weakness in one of these.Citation324 One cohort studyCitation325 concludes HHcy is a consequence of the development of cognitive impairment. Most,Citation222,Citation276,Citation316,Citation326–Citation328 but not all,Citation329 studies find HHcy is associated with intensity, rather than duration, of illness in AD. Thus, evidence that HHcy increases the risk for cognitive impairment and dementia usually is consistent and reproducible, Citation233 with HHcy predictably increasing the risk that subjects with MCI will progress to dementia.Citation233 With respect to the involvement of low vitamin B12, seven cohort studiesCitation308,Citation312,Citation313,Citation330–Citation333 show low vitamin B12 either increases the risk for cognitive impairment or development of dementia, while eightCitation13,Citation325,Citation334–Citation339 show no increased risk. Paradoxical findings in these studies include modest increases in serum B12 levels over time may increase the risk for dementiaCitation313 and individuals with normal serum B12 levels having a higher incidence of AD compared to those with low serum B12 levels. Citation13 Thus, evidence that low vitamin B12 increases the risk for cognitive impairment or dementia is inconclusive. Then again, the increased risk may occur in the opposite direction. AD may increase the risk for B12 deficiency.Citation340
Evaluation
In which patients should vitamin B12 be routinely assessed?
Guidelines for the workup of dementia are listed in .Citation4,Citation341 Although practices may range from checking serum B12 in all elderly to only particular patients with dementia, an evidence-based approach warrants checking serum B12 in all patients with MCI and mild to moderate dementia of two years or less duration. It is especially useful to assess serum B12 levels in all patients with MCI, because many will ultimately advance to dementia, where there may be a window of opportunity when vitamin B12 treatment of B12 deficiency-related cognitive dysfunction is potentially beneficial. Additionally, serum B12 should be checked in all patients with (1) history of gastric bypass surgery, partial or total gastrectomy, terminal ileum disease or resection, or pancreatic insufficiency,Citation14,Citation20,Citation33,Citation200 (2) chronic use of levodopa, histamine type-2 (H2) receptor blockers, or protein pump inhibitors (PPIs),Citation22,Citation33,Citation51,Citation54,Citation342–Citation345 or (3) findings suggestive of (a) behavioral and psychological symptoms of dementia (BPSD), (b) SCD, including paresthesias, ataxia, or loss of position or vibratory senses, or (c) pernicious anemia (PA), including low hemoglobin (Hgb), elevated mean corpuscular volume (MCV), or corpuscular changes on peripheral smear.Citation62 If such findings are absent, and patients have moderately severe to severe dementia of longer than two years duration, then universal recommendationsCitation1–Citation4 of assessing for and, when found, treating B12 deficiency may not be supported by reliable evidence.Citation13,Citation16,Citation24,Citation26,Citation92,Citation187,Citation326,Citation346–Citation364 H2 receptor blockers and PPIs impair cobalamin absorption; Citation18,Citation22,Citation33,Citation365,Citation366 their use is associated with supplemental B12 initiation.Citation367 Individuals who have had gastric surgery have a high prevalence of B12 deficiency,Citation20 and those who have had gastrectomies, who are B12-deficient, have a high prevalence of cognitive dysfunction and electroencephalographic (EEG) abnormalities.Citation332 Therefore, patients prescribed H2 receptor blockers or PPIs, and those who have had gastrectomies or gastric bypass surgery, require monitoring for hypovitaminosis B12.Citation368
Figure 3 Guidelines for the workup of dementia.
Abbreviations: AD, Alzheimer’s disease; APOE, apolipoprotein E; CAT, computerized axial tomography; CJD, Creutzfeldt-Jakob Disease; CNS, central nervous system; DAT, Dementia of the Alzheimer Type; DLB, Dementia with Lewy Bodies; DSM-IIR, Diagnostic and Statistical Manual of Mental Disorders-III-Revised; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; EEG, electroencephalogram; FTD, frontotemporal dementia; MRI, magnetic resonance imaging; NINCDS-ADRDA, National Institute of Neurologic, Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association; PET, positron emission tomography; SPECT, single photon emission computerized tomography; V aD, vascular dementia.

What test or tests should be ordered, and how are inferences made from such testing?
Common tests include the deoxyuridine suppression test (dUST), serum B12, serum TC II, plasma Hcy, serum or urinary methylmalonic acid (MMA), and the post-methionine load test. For convenience, conversion of vitamin B12 units (nanograms per liter to picograms per milliliter (pg/mL) and pg/mL to picomoles per liter) are available at http://www.cdc.gov/ncbddd/b12/index.html.
The dUST is probably the most sensitive and specific test for assessing functional folate or B12 deficiency.Citation92,Citation369 Although the test is not fully understood and probably more complex than a simple explanation,Citation369 if incubated bone marrow cells or peripheral blood lymphocytes have sufficient folate and cobalamin, nonradioactive deoxyuridine is believed to suppress the thymidylate synthetase conversion of radioactive thymidine into thymidylate, which is a normal response, but if cells have insufficient folate or cobalamin, nonradioactive deoxyuridine does not suppress the thymidylate synthetase conversion of radioactive thymidine into thymidylate, which is an abnormal response.
The most common test is the serum B12 level,Citation15 but often it is difficult to determine which serum B12 levels represent deficiency states and which do not. When using a specific serum B12 cutoff point, for example 200 pg/mL or less,Citation22,Citation181,Citation200,Citation368 in determining which individuals do and do not have B12 deficiency, problems encountered include false positives and false negatives.Citation7,Citation15,Citation19,Citation30,Citation33,Citation57,Citation188,Citation370–Citation376 One of the reasons for this is because most serum B12 assays measure total B12, including free B12 and that bound to B12 binding proteins: TC I, also called haptocorrin, TC II, simply referred to as transcobalamin, and TC III, which is produced by neutrophils.Citation7,Citation371,Citation374 TC I and III are R-proteins (R for rapid movement on electrophoresis).Citation7,Citation23,Citation200,Citation377 TC I is a storage protein and does not participate in cellular uptake.Citation200,Citation377 TC II participates in all cellular uptake.Citation7,Citation58,Citation200,Citation371,Citation372,Citation377 TC III participates in hepatocyte uptake only.Citation200 TC I and II comprise about 80% and 20% of total B12, respectively. Falsely low serum B12 occurs in congenital TC I deficiency, where TC I is low or absent, but TC II, intracellular B12, and hematopoiesis are normal.Citation7,Citation56,Citation200,Citation377 Other causes of falsely low serum B12 include folate deficiency, multiple myeloma, oral contraceptive use, and pregnancy.Citation7,Citation56 The mechanism by which low serum folate causes falsely low serum B12 is poorly understood.Citation7,Citation368 Alternatively, low serum cobalamin causes methyltetrahydrofolate (CH3-THF) trapping, elevating serum CH3-THF, which causes falsely high serum folic acid ().Citation58,Citation200,Citation372,Citation378 Falsely normal serum B12 occurs in congenital TC II deficiency, where TC I is normal, but TC II is low or absent, and intracellular B12 is insufficient, causing severe megaloblastic, macrocytic anemia.Citation7,Citation56,Citation200,Citation377,Citation379 Other causes of falsely normal serum B12 include liver disease, myelopro-liferative disorders, and intestinal bacterial overgrowth.Citation7,Citation56 Since serum TC II levels decrease with advancing age, a given serum B12 level in an elder may represent a deficiency, compared with the same level in a younger adult.Citation30,Citation380 Directly measuring serum TC II may be helpful in these cases and others.Citation17,Citation38,Citation308,Citation335,Citation351,Citation372,Citation374,Citation380–Citation383
Studies show 10%Citation56,Citation203 to 50%Citation19,Citation368 of truly vitamin B12 deficient individuals have serum B12 between 200 and 350 pg/mL, presenting clinicians with a diagnostic challenge. Based upon known biochemistry, specific tests can be ordered to help with this challenge. Cytoplasmic methylcobalamin is needed for MS-catalyzed Hcy methylation to Met, and mitochondrial adenosylcobalamin is needed for L-methylmalonyl-CoA mutase-catalyzed L-methylmalonyl-CoA conversion to succinyl-CoA.Citation7,Citation18,Citation19,Citation20,Citation52,Citation57,Citation58,Citation203 With low cellular B12, these reactions are impeded, elevating Hcy in the former and L-methylmalonyl-CoA, D-methylmalonyl-CoA, and MMA in the latter.Citation18,Citation20,Citation285 Thus, HHcy and hypermethylmalonic acidemia (HMMA) are surrogate markers for low vitamin B12 cellular levels (ie, metabolic B12 deficiency).Citation7,Citation11,Citation15,Citation19–Citation22,Citation30,Citation33,Citation44,Citation59,Citation60,Citation188,Citation203,Citation285,Citation294,Citation351,Citation353,Citation368,Citation370,Citation372,Citation373,Citation376,Citation380,Citation384,Citation385 HHcy occurs with deficiency of pyridoxine, folate, or vitamin B12;Citation19,Citation306,Citation355,Citation370,Citation386 usually, only deficiency of vitamin B12 causes HMMA.Citation19,Citation20,Citation370 Hcy may be the more sensitive and MMA the more specific surrogate marker.Citation17,Citation20,Citation38,Citation40,Citation151,Citation156,Citation285,Citation387–Citation389 Either surrogate marker is more sensitive than serum B12 in evaluating PA,Citation7,Citation19,Citation373,Citation390 and many individuals with HHcy have normal serum folate and B12 levels.Citation38,Citation39,Citation42 When used alone, B12,Citation371,Citation375 TC II,Citation371 Hcy,Citation38 or MMACitation389 may be insufficient as screening tests, but in combination, normal plasma Hcy and serum MMA rule out B12 deficiency in virtually all cases.Citation19,Citation390 Although the post-methionine load test is twice as sensitive as basal Hcy in detecting HHcy in individuals with AD,Citation391 it is not recommended as an initial test.Citation17
Most laboratory assays measure total Hcy, including reduced and oxidized forms: Hcy representing the former and homocystine and mixed disulfides (protein-bound Hcy and cysteine-Hcy) the latter.Citation52 Although the upper reference limit for plasma Hcy varies according to different conditions,Citation17,Citation33 plasma Hcy greater than 14 μmol/L in patients with vitamin B12 levels between 201 and 350 pg/mL suggests cellular B12 deficiency.Citation23,Citation41,Citation200,Citation222,Citation252,Citation258,Citation357,Citation387,Citation392 Serum B12 greater than 350 pg/mL rules out B12 deficiency in almost all individuals.Citation56,Citation200,Citation340 Since tissues store vitamin B12 for up to five yearsCitation18,Citation368,Citation393 and plasma Hcy increases only minimally after protein-rich meals,Citation17 serum B12 and plasma Hcy may be obtained fasting or nonfasting. Immediate centrifugation, or keeping samples refrigerated or ice cooled until centrifugation, prevents spuriously elevated Hcy results.Citation17,Citation394,Citation395 Suspected B12 deficiency can be confirmed when individuals having characteristic findings, including anemia, macrocytosis, corpuscular changes on peripheral smear, or signs and symptoms of SCD or peripheral neuropathy, improve with vitamin B12 treatment or when elevated Hcy or MMA are lowered with vitamin B12 treatment.Citation19,Citation20,Citation92,Citation135,Citation188,Citation347,Citation362,Citation370,Citation392,Citation396,Citation397 Although not intended to replace clinical judgment, suggestions for vitamin B12 workup and treatment in patients with suspected MCI or dementia are presented ().
Figure 4 Suggestions for vitamin B12 workup and treatment in patients with suspected neuropathology.
Abbreviations: μmol/L, micromoles per liter; CBC, complete blood count; Hcy, homocysteine; Hgb, hemoglobin; IM, intramuscular; MCV, mean corpuscular volume; PA, pernicious anemia; pg/mL, picograms per milliliter; PO, oral; SC, subcutaneous.

Does the finding of low serum B12 or elevated Hcy require evaluation for other medical conditions?
Some authors recommend establishing the etiology of B12 deficiency as part of the diagnostic approach.Citation5 It is beyond the scope of this paper to cover all diagnostic considerations in , but levodopa treatment, folate deficiency, and PA are noteworthy.
In Parkinson’s disease (PD) it is the treatment rather than the disease that causes HHcy.Citation37,Citation54,Citation294 In such cases, HHcy mayCitation293,Citation294 or may notCitation398 be associated with cognitive impairment. Recall from , Met is converted to SAM, which is converted to SAH, which is converted to Hcy. SAM demethylation to SAH serves as a methyl group donor for the biosynthesis of nucleic acids, proteins, phospholipids, and catecholamines and the metabolism of drugs and toxins. Catechol-O-methyl transferase (COMT) catalyzes levodopa methylation to 3-O-methyldopa.Citation344 Increased methyl group demand, required for the methylation of levodopa to 3-O-methyldopa, favors the conversion of Met to SAM, demethylation to SAH, conversion to Hcy, and development of HHcy.Citation51,Citation54,Citation342,Citation344 In a cross-sectional study,Citation342 individuals on levodopa alone, compared to those on combined levodopa and COMT inhibitor therapy, had higher plasma Hcy levels. However, only oneCitation344 of threeCitation54,Citation343,Citation344 prospective studies showed addition of a COMT inhibitor to those on levodopa prevents dopamine-associated plasma Hcy elevation or serum B12 reduction.
Since the relationship between folate and vitamin B12 is biochemically and, in deficiency states, pathologically united,Citation285 there are caveats to keep in mind when working up and treating folate and B12 deficiencies. When folate and B12 deficiencies occur together, monotherapy with either folate or vitamin B12 can worsen the manifestations of the other vitamin deficiency.Citation7,Citation23,Citation180,Citation285,Citation303,Citation355,Citation371,Citation399,Citation400 Thus, it is beneficial to assess serum levels of both vitamins and treat whichever deficiency occurs. Although PA and SCD share the common etiology, type A (autoimmune) chronic atrophic gastritis, with vitamin B12 malabsorption, they have distinct pathophysiologies.Citation18,Citation201 In the methionine cycle, folate- and vitamin B12-dependent MS catalyzes Hcy methylation to Met, as CH3-THF, serving as the methyl group donor, is demethylated to THF.Citation47,Citation52,Citation58,Citation151,Citation200 Impairment of this reaction causes defective DNA synthesis, leading to megaloblastic, macrocytic anemia (ie, PA).Citation21,Citation58 Supplemental folic acid can override the impairment, restoring DNA synthesis and normalizing erythropoiesis.Citation58,Citation401 Furthermore, in the methionine cycle, Met is adenosylated to SAM, which is demethylated to SAH, which is deadenosylated to Hcy.Citation58 Impairment of SAM demethylation to SAH impedes methylation reactions, leading to vacuolar demyelination, axonal degeneration, and neuronal death (ie, SCD).Citation21,Citation58 Supplemental folic acid cannot override the methylation impairment, resulting in progressive neuropathology and neuronal death.Citation58 Treating a combined folate and B12 deficiency with folic acid alone may correct hematological abnormalities, but not neurological abnormalities, and can aggressively worsen B12-deficient neurological sequelae.Citation7,Citation18,Citation19,Citation23,Citation200,Citation203,Citation355 Thus, B12 deficiency should be ruled out before correcting folate deficiency.
When folate and vitamin B12 are in balance, increased serum folate levels are associated with decreased plasma Hcy and serum MMA levels, but when vitamin B12 is underrepresented, increased serum folate levels are associated with increased plasma Hcy and serum MMA levels.Citation285 Also, higher folate states require relatively higher vitamin B12 levels than normal folate states to protect against metabolic B12 deficiency.Citation285 Therefore, the borderline (201–350 pg/mL) serum B12 range may be higher in the presence of high folate states.
In what was described as the most severe neuropathic epidemic of modern times,Citation402 between 1991 and 1993 more than 50,000 Cubans developed peripheral neuropathy, associated with reduced nutrient intake of group B vitamins,Citation403–Citation409 in the setting of strict embargos and economic deterioration.Citation404,Citation405,Citation407,Citation408 Although widespread distribution of group B vitaminsCitation404,Citation406,Citation407,Citation409 and government-mandated folic acid fortificationCitation404,Citation410 curbed the epidemic,Citation404,Citation406 some speculate that endemic subclinical group B vitamin deficiencies, coupled with Helicobacter pylori (H. pylori) infections, are responsible for the higher prevalence of dementia in Cuba compared with other Caribbean countries.Citation404 Before and after the Cuban epidemic, in 1976 and 1995, researchersCitation411,Citation412 discovered that folate deficiency and relatively high, compared with relatively low, Hcy levels in pregnant women are associated with neural tube defects (NTDs) in infants born to such women, findings that have since been replicated.Citation7,Citation18,Citation52,Citation355,Citation393,Citation413,Citation414 Since supplemental folic acid during pregnancy decreases the risk for such abnormalities, Citation415–Citation420 in 1998 the United States Food and Drug Administration (FDA) and Health Canada required wheat and other grain products to be fortified with folic acid.Citation35,Citation58,Citation421–Citation423 To date, more than 50 countries have mandated folic acid fortification.Citation410,Citation420 It is largely accepted that high-dose folic acid may mask B12 deficiency. It is less clear whether or not low dose folic acid masks B12 deficiency.Citation285,Citation401 Although the United States FDA requires 140 μg of folic acid per 100 g of grain or flour,Citation424,Citation425 an amount chosen because it was considered high enough to prevent NTDs, but low enough to not mask B12 deficiency, some have advocated increasing this amount.Citation426 Due to insufficient sensitivity, neither Hgb nor MCV are useful in ruling out B12-deficient dementia or SCD.Citation7,Citation13,Citation33,Citation58,Citation124,Citation289,Citation300,Citation371,Citation373,Citation378,Citation385,Citation427 Although the complete blood count (CBC) is part of routine diagnostic testing for dementia,Citation368 additional vigilance is needed,Citation20,Citation58,Citation381,Citation428 especially in the era of folic acid fortification. In B12 deficiency, supplemental folic acid may protect against anemia, but not neurodegeneration;Citation19,Citation52,Citation371 thus, the CBC alone may become even less sensitive in evaluating B12-deficient psychiatric and neurological abnormalities.
Further evaluation to rule out type A chronic atrophic gastritis, or PA,Citation181,Citation429 might be warranted because of increased risk for gastric cancer, where radiographic or endoscopic screening is useful, especially in cases of MCI and early dementia.Citation15,Citation18,Citation33,Citation200,Citation201 Thus, it is useful to obtain additional testing when B12-deficient dementia occurs with anemia, macrocytosis, corpuscular changes on peripheral smear, or signs and symptoms of SCD or peripheral neuropathy.Citation7 When B12-deficient dementia occurs in the absence of such findings, the decision to rule out type A chronic atrophic gastritis may be made on a case by case basis.
Although PA, or type A chronic atrophic gastritis,Citation181,Citation429 is often cited as the most common cause of low serum B12,Citation7,Citation18,Citation19,Citation33,Citation49,Citation56,Citation58,Citation180,Citation201,Citation390 emerging evidence suggests dissociation between PA and B12-deficient dementia.Citation10,Citation13,Citation15,Citation17–Citation19,Citation21,Citation30,Citation32,Citation33,Citation45,Citation47–Citation49,Citation56,Citation58,Citation91–Citation93,Citation146,Citation180,Citation190,Citation200,Citation201,Citation203,Citation290,Citation350,Citation368,Citation371,Citation373,Citation376,Citation393,Citation427–Citation434 Thus, B12-deficient dementia may be subdivided into B12-deficient dementia with PA and B12-deficient dementia without PA. Studies support the postulate that these two disease states are different.Citation10,Citation13–Citation15,Citation17,Citation19,Citation21,Citation25,Citation30,Citation32,Citation33,Citation45,Citation47–Citation49,Citation58,Citation91–Citation93,Citation146,Citation190,Citation201,Citation203,Citation289,Citation290,Citation350,Citation368,Citation427,Citation429,Citation431–Citation435 They appear to have different etiologies, pathophysiological findings, prevalences, and responses to treatment.
B12-deficient dementia with PA and B12-deficient dementia without PA have separate etiologies, whereby the former is caused by type A chronic atrophic gastritisCitation181,Citation429 and the latter is generally caused by type B (nonautoimmune) chronic atrophic gastritis, age-associated decrease in hydrochloric acid production, or decreased vitamin B12 ingestion.Citation9,Citation10,Citation14,Citation17,Citation21,Citation22,Citation30,Citation45,Citation47,Citation49,Citation58,Citation91,Citation92,Citation203,Citation368 Type B chronic atrophic gastritis and age-associated decrease in hydrochloric acid production cause food-cobalamin malabsorption.Citation30 Less established causes of relative B12 deficiency include impairment of vitamin B12 transfer, from capillaries to CSFCitation436 and from CSF to neurons,Citation382 and vitamin B12 hyperconsumption,Citation54,Citation63,Citation340,Citation342 inactivation,Citation146 or destruction.Citation290 Type A chronic atrophic gastritis is due to antiparietal cell and anti-intrinsic factor antibodies,Citation15,Citation33,Citation58,Citation181,Citation201,Citation203,Citation429 which occur in 90% and 55% of cases, respectively,Citation7,Citation203 causing parietal cell insufficiency and intrinsic factor dysfunction, sparing the antrum.Citation33,Citation58,Citation201 Type B chronic atrophic gastritis is usually caused by H. pylori infection, involving the antrum.Citation15,Citation58,Citation201,Citation203 This is especially worth mentioning, because gastritis involving both body and antrumCitation437 and H. pylori infection, confirmed by serological,Citation437–Citation439 histological,Citation437,Citation438,Citation440 and rapid ureaseCitation438 tests, is significantly more prevalent in individuals with MCICitation437 and ADCitation438–Citation440 compared to controls. In those with MCI, there is a correlation between serum anti- H. pylori immunoglobulin G concentration and degree of cognitive impairment,Citation437 which may be mediated by elevated Hcy levels.Citation437 One studyCitation438 showed H. pylori eradication improved both cognitive and functional measures in patients with AD. Since gastrin is secreted by antral G-cells, serum gastrin is elevated with type A,Citation378 but low with type B,Citation21 chronic atrophic gastritis.
B12-deficient dementia with and without PA have separate pathophysiological findings, where myeloneuropathy is characteristic and macrocytosis or anemia are present in the former,Citation181,Citation190,Citation429 but myeloneuropathy may be rareCitation19 and macrocytosis and anemia are often absentCitation13,Citation19,Citation25,Citation30,Citation92,Citation146,Citation289,Citation290,Citation350,Citation427,Citation431–Citation433 in the latter. Neuropathy associated with PA classically involves disease progression, beginning in the cervicothoracic region of the spine,Citation202,Citation441 with upper extremity findings classically occurring before lower extremity findings, followed by peripheral nerve involvement, and lastly, if at all, brain involvement.Citation23,Citation200,Citation203 Dementia is believed to be a late and rare finding of neuropathy associated with PA.Citation19,Citation25,Citation48,Citation203,Citation290 Alternatively, dementia with B12 deficiency often occurs independently from PA.Citation13,Citation25,Citation146,Citation290,Citation427,Citation432,Citation433
B12-deficient dementia without PA is relatively common,Citation25,Citation146,Citation290,Citation427,Citation432,Citation433 but B12-deficient dementia with PA is considered rare,Citation14,Citation19,Citation25,Citation30,Citation32,Citation48,Citation93,Citation203,Citation290,Citation427,Citation432 where it causes only a small fraction of B12 deficiency in demented individuals.Citation14,Citation25,Citation30,Citation32,Citation93,Citation290,Citation427,Citation432 PA incidence peaks late in midlife,Citation181,Citation429 while dementia incidence increases with age.Citation233 Both chronic atrophic gastritis type A and B are characterized by low pepsinogen I/pepsinogen II ratios and achlorhydria;Citation7,Citation15,Citation21,Citation22,Citation33,Citation378 however, low pepsinogen I/pepsinogen II ratios occur in roughly 30% of elderly populationsCitation378 and PA occurs in only about 2%,Citation23,Citation428 suggesting the majority of cases with low ratios are caused by factors other than PA.
Meta-analyses or systematic reviews confirm that HHcy is a risk factor for AD,Citation111,Citation233 but they also confirm that vitamin B12 treatment of B12-deficient dementia is ineffective for improving cognitive function.Citation16,Citation348,Citation355 Thus, B12-deficient dementia without PA and B12-deficient dementia with PA have distinct responses to supplemental B12 treatment, where it appears that moderately severe to severe dementia does not improve in the former,Citation13,Citation15,Citation16,Citation24,Citation26,Citation33,Citation92,Citation187,Citation326,Citation349,Citation350,Citation355,Citation356,Citation358,Citation359,Citation362 but may improve in the latter.Citation185,Citation190,Citation199,Citation429,Citation434,Citation435 The apparent dissociation of hematological findings and neurocognitive impairment provides additional support to the postulate that B12-deficient dementia with or without PA represent two separate disease states. Progressive neurocognitive decline often occurs in the presence of normal Hgb and MCV, where one or both values are normal in many individuals with B12-deficient dementiaCitation13,Citation33,Citation124,Citation188,Citation200,Citation433 or neurological abnormalities.Citation18,Citation20,Citation124,Citation180,Citation188,Citation373 When anemia is absent, Hgb is indirectly proportional to cognitive function,Citation18,Citation19,Citation200 and when present, it is an inconsistent risk factor for cognitive dysfunction.Citation92,Citation430 Neurological impairment occurring concomitantly with anemia is more likely to improve with supplemental B12 therapy in those cases where the anemia is more severe and less likely to improve where the anemia is less severe.Citation180,Citation393 With supplemental B12 therapy, increased reticulocytosis occurs within one week,Citation376,Citation393 but neurological improvement may require six months or longer.Citation180,Citation393 Vitamin B12 supplementation reverses hematological abnormalities in almost all patients,Citation15,Citation33,Citation185,Citation362 may improve neurological abnormalities in roughly one-half,Citation15,Citation33,Citation185 and arrests or reverses dementia in only very few.Citation13,Citation15,Citation16,Citation24,Citation26,Citation33,Citation92,Citation187,Citation326,Citation347,Citation349,Citation350,Citation355,Citation361,Citation362
In B12 deficiency dementia with versus without PA, there appear to be different manifestations, need for further workup, and responses to treatment. Therefore, when findings include low serum B12 or elevated Hcy, it is useful to rule out PA. In the workup for type A chronic atrophic gastritis, assessment of antiparietal cell and anti-intrinsic factor antibodies is an initial option.Citation32,Citation181,Citation368,Citation429 Since the former are sensitive, occurring in up to 10% of elderly populations,Citation56 and the latter are specific, the absence of antiparietal cell antibodies suggests low likelihood, Citation7 while the presence of anti-intrinsic factor antibodies suggests high likelihood,Citation7 for type A chronic atrophic gastritis. If antiparietal cell antibodies are present and anti-intrinsic factor antibodies are absent, assessment of serum gastrin levels is a further option.Citation7,Citation33 Type A and B chronic atrophic gastritis also can be distinguished by endoscopy with mucosal surface biopsy.Citation7,Citation181,Citation429 Although Schilling’s test has been largely supplanted by the aforementioned tests,Citation203,Citation368 it may be useful if such tests are unremarkable or equivocal.Citation434 However, decreased vitamin B12 ingestionCitation9,Citation47 and food-cobalamin malabsorptionCitation9,Citation45,Citation49 are common causes of low serum B12 in elderly populations, and Schilling’s test is most often negative (ie, step one results are normal) in these conditions.Citation25,Citation33,Citation93,Citation203
When vitamin B12 deficiency is found in dementia, is dementia of the Alzheimer’s type a compatible diagnosis?
AD is diagnosed by tissue pathology. According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), DAT is essentially a diagnosis of exclusion, after other causes of dementia, including B12 deficiency, have been ruled out, thus dichotomizing dementia due to B12 deficiency and DAT. Although two studiesCitation359,Citation442 support this dichotomy, most studiesCitation13,Citation26,Citation82,Citation222,Citation333,Citation336,Citation382 suggest DAT occurs with or without B12 deficiency. When MCI and various subtypes of dementia are compared with one another, similar concentrations of serum B12 and Hcy are observed.Citation276,Citation284,Citation443 When B12-deficient dementia is compared to other subtypes of dementia, similar rates of decline are observed across almost all measured outcomes.Citation336,Citation350,Citation444 Nonetheless, there is likely a DAT subgroup, having low serum B12Citation427,Citation445 and perhaps elevated platelet monoamine oxidase (MAO) activity.Citation29 Since B12 deficiency dementia with PA may arrest or reverse with treatmentCitation190,Citation429,Citation434 and B12 deficiency dementia without PA is usually progressively neurodegenerative despite treatment, the presence of PA would suggest dementia due to B12 deficiency, while its absence would suggest DAT.Citation446
Treatment
Based on the benefit-to-risk ratio in treatment of dementia, if vitamin B12 deficiency is determined, should supplemental B12 be initiated?
Vitamin B12 treatment is considered one of the safest medical treatments available.Citation447 Although severe adverse events are very rare, those reported include anaphylactic shock and death with administration of parenteral vitamin B12, hypokalemia with sudden death in conditions of severe anemia, and severe and sudden optic atrophy in those with Leber’s hereditary optic neuropathy.Citation448 Other adverse events include polycythemia, thrombosis, pruritus, rash, skin eruptionsCitation449 congestive heart failure, diarrhea, edema, and swelling. One randomized controlled trial (RCT)Citation347 found a greater prevalence of depression in the treatment group, who received high-dose pyridoxine, folate, and vitamin B12, than in the placebo group.
Although no overdosage has been reported, in treating hypovitaminosis B12 with supplemental B12, there is a fine line between help and harm. The serum cobalamin-plasma Hcy concentration–response curve appears curvilinear.Citation17 As serum cobalamin increases from the lowest detectable level to 950 pg/mL, plasma Hcy decreases, but as serum cobalamin further increases from 950 pg/mL to 1,350 pg/mL, plasma Hcy begins to rise. One cohort study,Citation386 found a dose-dependent association between increasing serum B12 levels and incidence of coronary artery disease (CAD) and mortality, where each 100 pg/mL increase in serum cobalamin was associated with a 10% increased incidence for such events. One RCTCitation450 found that low-dose pyridoxine, folic acid, and vitamin B12 treatment decreased the risk for stroke, CAD events, and death, while moderately high dose pyridoxine, folic acid, and vitamin B12 treatment did not. Although the earliest twoCitation451,Citation452 of eight other RCTs showed combined pyridoxine, folic acid, and vitamin B12 treatment decreased morbidity in individuals with or at risk for CAD, the later sixCitation453–Citation458 failed to validate these findings, and fourCitation454,Citation456–Citation458 of the six showed treatment potentially increased the risk for harm. One of these RCTsCitation455 was prematurely terminated due to potential risk for harm demonstrated by group B vitamin treatment.Citation454 Therefore, some authorsCitation386,Citation454 suggest supplemental B12 should not be administered unless B12 deficiency is present, and when present, only enough supplemental B12 should be administered to correct the deficiency. Parenthetically, meta-analyses of folic acid treatment, aimed at lowering HHcy, show mixed results in terms of whether or not treatment decreases the risk for stroke.Citation459,Citation460 Since most prospective studies show supplemental B12 does not improve cognitive function or prevent dementia in cognitively intact individuals,Citation306,Citation354,Citation360 including those with hypovitaminosis B12Citation351,Citation353 or HHcy,Citation357 it is not recommendedCitation401 for these purposes.
HHcy increases the risks for birth defects, cognitive dysfunction, dementia, cerebrovascular disease, stroke, CAD, MI, peripheral vascular disease, venous thrombosis, osteoporosis, hip fractures, and death.Citation21,Citation39,Citation279,Citation386,Citation461–Citation468 Benefits of treating B12 deficiency include lowering Hcy levels,Citation19,Citation21,Citation47,Citation135,Citation198,Citation326,Citation347,Citation351,Citation357,Citation360–Citation362,Citation397,Citation469 lowering Aβ peptide levels,Citation470 decreasing tau hyperphosphorylation,Citation132 anti-inflammation, Citation471 neuroprotection,Citation471 reduction of MAO activity,Citation29 and causing the BBB to be less leaky.Citation469
It may not be feasible to perform a meta-analysis on studies that have ascertained whether or not vitamin B12 treatment is associated with improved cognitive function or prevention of dementia due to the heterogeneity of these studies (). Published studies include multiple designs (eg, case-control, other retrospective studies, RCTs, and other prospective studies), subjects (eg, those who are healthy, ill, communitydwelling, residents of tertiary care facilities, with and without cognitive dysfunction, and with and without vitamin B12 deficiency), interventions, including vitamin B12 alone or in combination with other drugs or dietary supplements, type of vitamin B12 administered, and route of administration.
Table 2 Examples of multiple binary variables in studies examining efficacy of B12 treatment on cognition
Traditional recommendations of assessing for and, when found, treating B12-deficient dementia are supported by case-control studies,Citation350,Citation359 retrospective series,Citation49,Citation180,Citation188,Citation435,Citation472 and case reports,Citation190,Citation429,Citation434 where B12 deficiency was specifically caused by PA. Evidence supporting these recommendations appears to diminish with many RCTs,Citation16,Citation185,Citation198,Citation298,Citation306,Citation326,Citation347,Citation348,Citation351–Citation353,Citation355,Citation357,Citation360,Citation361,Citation364,Citation473,Citation474 clinical trials (CTs),Citation13,Citation24,Citation185,Citation187,Citation199,Citation346,Citation356,Citation362,Citation397,Citation469 and other prospective studiesCitation26,Citation92,Citation349,Citation358,Citation475 ( and ). TwelveCitation16,Citation306,Citation326,Citation347,Citation351–Citation353,Citation355,Citation357,Citation360,Citation361,Citation364 of 16 RCTs found supplemental B12 therapy was of no benefit for improving cognitive function. FourCitation185,Citation198,Citation473,Citation474 of 16 RCTs found supplemental B12 therapy was beneficial for improving cognitive function; however, subjects in these studies included nondemented, healthy adult women,Citation473 individuals with schizophrenia and HHcy,Citation198 and individuals with dementia, irrespective of serum B12 levels, treated with multiple vitamins and other dietary supplements.Citation474 The design of one of these studiesCitation185 did not include a separate control group. In all other RCTs of individuals with hypovitaminosis B12,Citation16,Citation351,Citation353,Citation355 HHcy,Citation357 HMMA,Citation352 dementia,Citation16,Citation326,Citation347,Citation355,Citation361 or at risk for dementia,Citation326,Citation348,Citation352,Citation360,Citation364 vitamin B12 treatment group results were no better than placebo group results. Nonetheless, the results from the RCTs may not generalize to all patients. Five of theseCitation306,Citation351,Citation353,Citation357,Citation360 did not examine subjects with MCI or dementia, threeCitation16,Citation326,Citation355 may have included subjects in various dementia stages, and six appear to have enrolled subjects, with either normalCitation347,Citation361 or irrespectiveCitation306,Citation326,Citation360,Citation364 of baseline folate and vitamin B12 statuses.
Table 3a Studies showing vitamin B12 treatment is not associated with improved cognitive function or prevention of dementia
Table 3b Studies showing vitamin B12 treatment is associated with improved cognitive function or prevention of dementia
Although few included control groups,Citation198,Citation199,Citation473,Citation474 CTs and other prospective studies with positive resultsCitation24,Citation185,Citation187,Citation198,Citation199,Citation346,Citation356,Citation358,Citation397,Citation469,Citation473–Citation475 show cognitive improvement may be associated with five factors: Hcy state,Citation37,Citation57,Citation200,Citation358,Citation397 disease duration,Citation18,Citation24,Citation30,Citation180,Citation346,Citation356 disease intensity,Citation24,Citation180,Citation350,Citation358,Citation359 and treatment type,Citation474,Citation475 as shown in .Citation17,Citation24,Citation37,Citation58,Citation146,Citation180,Citation290,Citation306,Citation346,Citation350,Citation351,Citation353,Citation356–Citation359,Citation361,Citation473–Citation495 The fifth factor, and perhaps the most striking, is whether or not the B12 deficiency-associated cognitive dysfunction is due to PA. If so, then these individuals may respond remarkably well to supplemental B12 treatment, regardless of the severity of the dementia.Citation185,Citation190,Citation429,Citation434 Parenthetically, all RCTs show supplemental folic acid is no better than placebo at improving cognitive function in individuals with dementia.Citation348,Citation355,Citation361,Citation496
Table 4 Factors associated with cognitive improvement in B12 supplementation of B12-deficient dementia
BPSD include symptoms and signs of disturbed behavior, mood, psychomotor activity, and thought content (eg, delusions and hallucinations) in patients with dementia. Exogenous SAM improves depression in patients with PDCitation481 and other conditions.Citation494,Citation495 In individuals with AD, those with HHcy have increased EEG slow wave activity (ie, increased theta and delta waves) compared to those without HHcy.Citation184,Citation257 In nondemented individuals, lowering plasma Hcy levels with vitamin B12 treatment reverses such EEG findings.Citation184,Citation397 Accordingly, prospective studies of individuals with B12-deficient dementia show patients with deliriumCitation187 or psychosesCitation359 improve with vitamin B12 supplementation. In demented individuals, prospective studies show vitamin B12 supplementation enhances vigilance when combined with bright light therapy,Citation497 improves mood disturbances,Citation475 but may worsen motor performance.Citation353 Although not a consistent finding, Citation187,Citation362 supplemental B12 in B12-deficient dementia may be beneficial for some patients with BPSD.Citation359,Citation475 Independent from serum folic acid and B12 levels, an association was found between HHcy and schizophrenia;Citation498 in spite of the HHcy association being independent from group B vitamins in that study, another studyCitation198 showed combined pyridoxine, folic acid and vitamin B12 treatment in individuals with HHcy and schizophrenia improved positive symptoms, negative symptoms, and cognitive function. Depression, mania, psychoses, and delirium associated with PA or hypovitaminosis B12 may improve with supplemental B12 treatment.Citation181–Citation184,Citation190,Citation191
Considering the potential risks for adverse events, added cost, and added administration, and the potential benefit for arresting or reversing dementia, if B12 deficiency is determined, it should be treated. Vitamin B12 treatment should be initiated in those cases where serum B12 levels are 200 pg/mL or less and those where serum B12 levels are 201–350 pg/mL with plasma Hcy greater than 14 μmol/L.Citation320 Duration of therapy is based on etiology and other factors, with an optimum serum B12 not being more than 950 pg/mL. In cases of PA, treatment is continued for life. In cases of dietary deficiency, low vitamin B12 in dementia normalizes with dietary correction.Citation14 Possible explanations on why dementia does not reverse or arrest with supplemental B12 include treatment being an ineffective form,Citation146,Citation290 initiation outside of a time course window,Citation30,Citation346,Citation356 or dementia not solely being caused by PA or low serum B12.Citation13,Citation26,Citation82,Citation222,Citation333,Citation336,Citation359,Citation442
How is vitamin B12 deficiency treated?
Since bacteria produce natural forms of vitamin B12, which humans ingest by consuming animal products,Citation19,Citation58,Citation201 strict vegetarians may require oral (PO) vitamin B12 supplementation.Citation58 The recommended daily allowance (RDA) is 2.4 μg daily. Citation499 Supplemental forms of vitamin B12 include cyanocobalamin, used in the United States, hydroxocobalamin, used in Europe, and mecobalamin, used in Asia.Citation7,Citation361,Citation475 Mecobalamin is a synthetic form of methylcobalamin, which is the type of cobalamin utilized intracellularly. Although it may be advantageous in cognitive improvement or protection,Citation475 it has not been compared with cyanocobalamin or hydroxocobalamin in clinical trials, and it may not be readily available in many western countries. Routes for cyanocobalamin administration include PO, sublingual (SL), intranasal (IN), intramuscular (IM), and subcutaneous (SC). Although the intravenous (IV) route has been used in patients with renal failure,Citation500,Citation501 this route is not recommended for general use.Citation448 Compared to PO cyanocobalamin, IM and IV cyanocobalamin potentially pose greater risks for anaphylaxis. Up to 98% of an IM dose is lost in the urine, and even more is lost with an IV dose.Citation387,Citation448 Alternatively, 1% of PO cyanocobalamin is absorbed, throughout the gastrointestinal tract, by passive diffusion, independent of intrinsic factor.Citation18,Citation52,Citation201,Citation368,Citation387 Thus, 10 μg will be absorbed from a 1,000 μg dose, well above the RDA;Citation18,Citation201,Citation502 this effect is seen even in those with PA, prior gastrectomies, or diseases of the terminal ileum.Citation52,Citation368,Citation387,Citation503 Most physicians favor IM over PO routes,Citation20,Citation23,Citation31,Citation203,Citation504 while most patients favor PO over IM routes.Citation503,Citation505 Patient preference is supported by a CTCitation91 and RCTsCitation31,Citation34,Citation185,Citation392 showing PO cyanocobalamin provides equal, if not greater, serum B12 therapeutic levels. Since PO cyanocobalamin is as effective as IM cyanocobalamin in treating B12 deficiency, and its complications, it may be the preferred route of administration, unless there is concern regarding PO cyanocobalamin adherence, such as dysphagia.Citation19,Citation200,Citation376,Citation505 Variance exists in recommendations for dose and administration frequency: common practices include cyanocobalamin 1,000 μg PO dailyCitation203,Citation503,Citation505 or cyanocobalamin 1,000 μg IM daily for one week, then weekly for one month, then monthly thereafter.Citation23,Citation185,Citation200,Citation203,Citation346,Citation356,Citation387,Citation393 Alternative regimens are recommended for those with manifestations of severe PA.Citation448
Conclusions
Although this paper represents a synopsis of our current understanding between HHcy and hypovitaminosis B12 and MCI or dementia, some investigations and reviews reveal contrary results or recommendations.Citation27,Citation41,Citation62,Citation188,Citation340,Citation398,Citation401,Citation506–Citation508 Possible errors from this review and its conclusions are that Medline was the primary database, searches may not have captured all relevant studies and case reports, and it is presumed that there are unpublished vitamin B12 treatment responses. In order to attempt to minimize erroneous conclusions, results were crosschecked with Internet searches and basic and clinical science textbooks, other Medline searches were performed using various search terms, and communication took place with experts who care for demented patients. Owing to the latter, it is crucial that PA be ruled out in all demented patients. Also, the trials that have been reported to date may have been of insufficient size or duration to determine a beneficial effect.Citation233
Biological data support the notion that ROS generation may lead to elevated Hcy, but this may be, at least in part, an adaptive mechanism to generate cysteine, which is used in the biosynthesis of glutathione,Citation120,Citation509 an antioxidant that protects cells from oxidative stress. Biochemical and epidemiological evidence is convincing that HHcy, with or without hypovitaminosis B12, is a risk factor for dementia. Evidence is less convincing that hypovitaminosis B12 is a risk factor for dementia in the absence of HHcy. B12 deficiency manifestations are variable and include abnormal psychiatric, neurological, gastrointestinal, and hematological findings. Radiological images of nondemented and demented individuals with HHcy frequently demonstrate leukoaraiosis, potentially related to BBB dysfunction. Thus, the finding of leukoaraiosis on CAT or MRI scan is an indication for checking plasma Hcy and serum B12 levels.
Although historically neuropathy related to PA was described according to a classical disease progression, patient samples now show that symptoms and signs secondary to the neuropathy can be variable.Citation180,Citation188 Nonetheless, dementia secondary to PA, occurring separately from other manifestations of SCD or peripheral neuropathy, and in the absence of macrocystosis and anemia, may still be considered sufficiently rare to be the basis of case reports.Citation190,Citation199,Citation434
On the one hand, literature suggests assessing serum B12 and treatment of B12 deficiency is useful early in the course of cognitive dysfunction, for instance, MCICitation199,Citation350,Citation469 and mild to moderate dementia of less than two years duration.Citation24,Citation180,Citation346,Citation356,Citation358,Citation359 If low serum B12 is determined, it may be worthwhile to check for antiparietal cell and anti-intrinsic factor antibodies and obtain testing for H. pylori, for management and prognostic purposes. On the other hand, literature suggests assessing serum B12 and treatment of B12 deficiency may not be useful late in the course of cognitive dysfunction, for instance, moderately severe to severe dementia, if symptoms and signs of SCD and peripheral neuropathy, hematological manifestations of PA, and other factors listed on are absent. However, if one has not inquired about neurological symptoms, examined the mouth and tongue, performed a neurological examination, and obtained a CBC on a moderately-severe to severely demented patient, then it could be a ‘medical-legal pitfall’ not to check the serum B12 level.Citation62 The suggestions for vitamin B12 workup and treatment in patients with suspected MCI or dementia are evidence-based. However, since the rate of dementia progression is poorly measured and variable and there are no specific criteria for vitamin B12 testing, the suggestions are not intended to replace care based upon clinical decisions. The serum B12 level remains the standard initial test.Citation181 DAT is a compatible diagnosis when B12 deficiency is found, unless it is caused by PA. Cyanocobalamin PO is favorable over IM, in terms of both therapeutic efficacy and patient preference, unless there is concern regarding PO adherence.
Since the original observations by Biermer in 1872, to the more recent epidemic neuropathy in Cuba, it is evident that adequate folate and vitamin B12 function is required for physical and mental health. Issues surrounding folate, vitamin B12, Hcy, and MMA as they relate to health, especially microvascular disease and cognitive dysfunction, are high interest among researchers and clinicians. Puzzling questions remain. Since B12 deficiency can lead to depression, mania, and psychoses, should clinicians routinely check serum B12 levels in patients with such findings? Among those individuals who are B12 deficient, what are the prevalences of antiparietal, anti-intrinsic factor, and anti-H. pylori anti-bodies? Is HHcy a risk factor for other types of dementia, such as DBT, dementia due to PD, or dementia with Lewy bodies?Citation193,Citation510,Citation511 Since HHcy has been prospectively shown to be an ‘independent’ risk factor for dementia, then why are Hcy-lowering therapies ineffective, or at best modestly effective, at preventing or treating dementia? Answers to questions such as these may one day unleash knowledge necessary for more effective treatment. The notions of age-related, and perhaps disease-related, degradation of the BBB and oxidative chemical reactions, generating ROS and elevating Hcy, coupled with immune activation, both without and within the brain, remain novel areas for further work.Citation120
Acknowledgments
The author wishes to thank the reviewers of this manuscript, for their thoughtful work and kind suggestions, which were highly valuable in the final writing of this publication. The author reports no conflicts of interest in this work.
References
- ArnoldSEKumarAReversible dementiasMed Clin North Am19937712152308419719
- American Psychiatric AssociationPractice guideline for psychiatric evaluation of adultsAm J Psychiatry199515211 Suppl63809565542
- KnopmanDSDeKoskySTCummingsJLPractice parameter: diagnosis of dementia (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology20015691143115311342678
- FeldmanHHJacovaCRobillardADiagnosis and treatment of dementia: 2. DiagnosisCMAJ2008178782583618362376
- SchneedeJUelandPMNovel and estabslished markers of cobalamin deficiency: complementary or exclusive diagnostic strategiesSemin Vasc Med20055214015516047266
- VanderjagtDJMcCarthyDMGlewRHNinomiyaYPernicious AnemiaClinical Studies in Medical Biochemistry2nd EdNew York, NYOxford University Press2006122133
- KleeGGCobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folateClin Chem2000468 Pt 21277128310926922
- OlivieriGHessCSavaskanEMelatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased beta-amyloid secretionJ Pineal Res200131432032511703561
- AllenLHHow common is vitamin B-12 deficiency?Am J Clin Nutr2009892693S696S19116323
- BasunHFratiglioniLWinbladBCobalamin levels are not reduced in Alzheimer’s disease: results from a population-based studyJ Am Geriatr Soc19944221321368126323
- ClarkeRRefsumHBirksJScreening for vitamin B-12 and folate deficiency in older personsAm J Clin Nutr20037751241124712716678
- ClarkeRGrimley EvansJSchneedeJVitamin B12 and folate deficiency in later lifeAge Ageing2004331344114695861
- CrystalHAOrtofEFrishmanWHGruberAHershmanDAronsonMSerum vitamin B12 levels and incidence of dementia in a healthy elderly population: a report from the Bronx Longitudinal Aging StudyJ Am Geriatr Soc19944299339368064100
- ElsborgLHansenTRafaelsenOJVitamin B12 concentrations in psychiatric patientsActa Psychiatr Scand1979592145152420034
- LechnerKFodingerMGrisoldWPuspokASillaberCVitamin B12 deficiency. New data on an old themeWien Klin Wochenschr20051171757959116395986
- MaloufRAreosa SastreAVitamin B12 for cognitionCochrane Database Syst Rev20033CD00432612918012
- RefsumHSmithADUelandPMFacts and recommendations about total homocysteine determinations: an expert opinionClin Chem200450133214709635
- SinghNNThomasFPDiamondALDiamondRVitamin B-12 associated neurological diseases2006 Available from: http://emedicine.medscape.com/article/1152670-overviewAccessed on Nov 3, 2009
- StablerSPLindenbaumJAllenRHVitamin B-12 deficiency in the elderly: current dilemmasAm J Clin Nutr19976647417499322547
- SumnerAEChinMMAbrahmJLElevated methylmalonic acid and total homocysteine levels show high prevalence of vitamin B12 deficiency after gastric surgeryAnn Intern Med199612454694768602704
- WoltersMStrohleAHahnAAge-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequencesZ Gerontol Geriatr200437210913515103481
- WoltersMStrohleAHahnACobalamin: a critical vitamin in the elderlyPrev Med20043961256126615539065
- WorrallBRowlandLRowlandLNutritional disorders: Malnutrition, malabsorption, and B12 and other vitamin deficienciesMerritt’s Neurology11th edPhiladelphia, PALippincott Williams & Wilkins200510911098
- CunhaUGRochaFLPeixotoJMMottaMFBarbosaMTVitamin B12 deficiency and dementiaInt Psychogeriatr19957185887579024
- KarnazeDSCarmelRLow serum cobalamin levels in primary degenerative dementia. Do some patients harbor atypical cobalamin deficiency states?Arch Intern Med198714734294313827417
- TeunisseSBollenAEvan GoolWAWalstraGJDementia and subnormal levels of vitamin B12: effects of replacement therapy on dementiaJ Neurol199624375225298836942
- GreilbergerJKoidlCGreilbergerMMalondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s diseaseFree Radic Res200842763363818654878
- WhyteEMMulsantBHButtersMACognitive and behavioral correlates of low vitamin B12 levels in elderly patients with progressive dementiaAm J Geriatr Psychiatry200210332132711994220
- ReglandBGottfriesCGOrelandLVitamin B12-induced reduction of platelet monoamine oxidase activity in patients with dementia and pernicious anaemiaEur Arch Psychiatry Clin Neurosci199124045288291
- Bopp-KistlerIRuegger-FreyBGrobDSixPVitamin B12 deficiency in geriatricsSchweiz Rundsch Med Prax1999884518671875
- ButlerCCVidal-AlaballJCannings-JohnROral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trialsFam Pract200623327928516585128
- NaggaKRajaniRMardhEBorchKMardhSMarcussonJCobalamin, folate, methylmalonic acid, homocysteine, and gastritis markers in dementiaDement Geriatr Cogn Disord200316426927514512723
- Nilsson-EhleHAge-related changes in cobalamin (vitamin B12) handling. Implications for therapyDrugs Aging19981242772929571392
- Vidal-AlaballJButlerCCCannings-JohnROral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiencyCochrane Database Syst Rev20053CD00465516034940
- Diaz-ArrastiaRHomocysteine and neurologic diseaseArch Neurol200057101422142711030793
- BrosnanJTBrosnanMEThe sulfur-containing amino acids: an overviewJ Nutr20061366 Suppl1636S1640S16702333
- BaronePBurnDJvan LaarTHsuCPoeweWLaneRMRivastigmine versus placebo in hyperhomocysteinemic Parkinson’s disease dementia patientsMov Disord200823111532154018581467
- Gonzalez-GrossMSolaRAlbersUB-vitamins and homocysteine in Spanish institutionalized elderlyInt J Vitam Nutr Res2007771223317685092
- MarengoniACossiSDe MartinisMCalabresePAOriniSGrassiVHomocysteine and disability in hospitalized geriatric patientsMetabolism20045381016102015281011
- NilssonKGustafsonLHultbergBThe plasma homocysteine concentration is better than that of serum methylmalonic acid as a marker for sociopsychological performance in a psychogeriatric populationClin Chem200046569169610794752
- RavagliaGFortiPMaioliFBlood homocysteine and vitamin B levels are not associated with cognitive skills in healthy normally ageing subjectsJ Nutr Health Aging20004421822211115804
- WelchGNLoscalzoJHomocysteine and atherothrombosisN Engl J Med199833815104210509535670
- BrianiCCagninAGalloLAnti-heparan sulphate antibodies and homocysteine in dementia: markers of vascular pathologyJ Neurol Sci2005229230215218
- NilssonKGustafsonLHultbergBPlasma homocysteine is a sensitive marker for tissue deficiency of both cobalamines and folates in a psychogeriatric populationDement Geriatr Cogn Disord199910647648210559563
- AndresELoukiliNHNoelEVitamin B12 (cobalamin) deficiency in elderly patientsCMAJ2004171325125915289425
- BergeronCRanalliPJMiceliPNAmyloid angiopathy in Alzheimer’s diseaseCan J Neurol Sci19871445645693690426
- CookSHessOMHomocysteine and B vitaminsHandb Exp Pharmacol200517032533816596805
- El OtmaniHEl MoutawakilBMoutaouakilFGamIRafaiMASlassiIPostoperative dementia: toxicity of nitrous oxideEncephale2007331959717457299
- El OtmaniHMoutaouakilFMidafiNCobalamin deficiency: Neurological aspects in 27 casesRev Neurol (Paris)2009165326326719056098
- HadithiMMulderCJStamFEffect of B vitamin supplementation on plasma homocysteine levels in celiac diseaseWorld J Gastroenterol200915895596019248194
- IrizarryMCGurolMERajuSAssociation of homocysteine with plasma amyloid beta protein in aging and neurodegenerative diseaseNeurology20056591402140816275827
- JacobsenDWGatautisVJGreenRRapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjectsClin Chem19944068738818087981
- MylesPSChanMTLeslieKPeytonPPaechMForbesAEffect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgeryBr J Anaesth2008100678078618400808
- O’SuilleabhainPEBottiglieriTDeweyRBJrSharmaSDiaz-ArrastiaRModest increase in plasma homocysteine follows levodopa initiation in Parkinson’s diseaseMov Disord200419121403140815390053
- SaibeniSLecchiAMeucciGPrevalence of hyperhomocysteinemia in adult gluten-sensitive enteropathy at diagnosis: role of B12, folate, and geneticsClin Gastroenterol Hepatol20053657458015952099
- SnowCFLaboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physicianArch Intern Med1999159121289129810386505
- StablerSPLindenbaumJAllenRHThe use of homocysteine and other metabolites in the specific diagnosis of vitamin B-12 deficiencyJ Nutr19961264 Suppl1266S1272S8642468
- WeirDGScottJMBrain function in the elderly: role of vitamin B12 and folateBr Med Bull199955366968210746355
- KoikeTKuzuyaMKandaSRaised homocysteine and low folate and vitamin B-12 concentrations predict cognitive decline in community-dwelling older Japanese adultsClin Nutr200827686587118835069
- LiLCaoDDesmondRCognitive performance and plasma levels of homocysteine, vitamin B12, folate and lipids in patients with Alzheimer diseaseDement Geriatr Cogn Disord200826438439018931498
- NilssonKGustafsonLHultbergBPlasma homocysteine levels and different forms of vascular disease in patients with dementia and other psychogeriatric diseasesDement Geriatr Cogn Disord2009271889519155623
- ConradMEPernicious anemia Available from: http://emedicine.medscape.com/article/204930-overviewAccessed September 5, 2009
- SchroecksnadelKFrickBWinklerCLeblhuberFWirleitnerBFuchsDHyperhomocysteinemia and immune activationClin Chem Lab Med200341111438144314656023
- SchroecksnadelKFrickBWirleitnerBWinklerCSchennachHFuchsDModerate hyperhomocysteinemia and immune activationCurr Pharm Biotechnol20045110711814965213
- LehmannJTsaiCWoodPLHomocysteic acid as a putative excitatory amino acid neurotransmitter: I. Postsynaptic characteristics at N-methyl-D-aspartate-type receptors on striatal cholinergic interneuronsJ Neurochem1988516176517702846784
- LiptonSAKimWKChoiYBNeurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptorProc Natl Acad Sci U S A19979411592359289159176
- BottiglieriTGodfreyPFlynnTCarneyMWTooneBKReynoldsEHCerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionineJ Neurol Neurosurg Psychiatry19905312109610982292704
- HuangRFHuangSMLinBSWeiJSLiuTZHomocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL-60 cellsLife Sci200168252799281111432446
- KimHJChoHKKwonYHSynergistic induction of ER stress by homocysteine and beta-amyloid in SH-SY5Y cellsJ Nutr Biochem2008191175476118430556
- KrumanIICulmseeCChanSLHomocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicityJ Neurosci200020186920692610995836
- ZouCGZhaoYSGaoSYHomocysteine promotes proliferation and activation of microgliaNeurobiol Aging200916 [Epub ahead of print]
- RabanedaLGCarrascoMLopez-ToledanoMAHomocysteine inhibits proliferation of neuronal precursors in the mouse adult brain by impairing the basic fibroblast growth factor signaling cascade and reducing extracellular regulated kinase 1/2-dependent cyclin E expressionFASEB J200822113823383518703672
- KumarMTyagiNMoshalKSHomocysteine decreases blood flow to the brain due to vascular resistance in carotid arteryNeurochem Int20085368214219
- KumarMTyagiNMoshalKSGABAA receptor agonist mitigates homocysteine-induced cerebrovascular remodeling in knockout miceBrain Res2008122114715318547546
- HerrmannWObeidRBiomarkers of folate and vitamin B(12) status in cerebrospinal fluidClin Chem Lab Med200745121614162017892439
- KamathAFChauhanAKKisuckaJElevated levels of homocysteine compromise blood-brain barrier integrity in miceBlood2006107259159316189268
- LominadzeDRobertsAMTyagiNMoshalKSTyagiSCHomocysteine causes cerebrovascular leakage in miceAm J Physiol Heart Circ Physiol20062903H1206121316258031
- TyagiSCLominadzeDRobertsAMHomocysteine in microvascular endothelial cell barrier permeabilityCell Biochem Biophys2005431374416043881
- PoddarRSivasubramanianNDiBelloPMRobinsonKJacobsenDWHomocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular diseaseCirculation2001103222717272311390343
- BiXHZhaoHLZhangZXZhangJWAssociation of RFC1 A80G and MTHFR C677T polymorphisms with Alzheimer’s diseaseNeurobiol Aging200930101601160718258338
- DeshmukhARodrigueKMKennedyKMLandSJacobsBSRazNSynergistic effects of the MTHFR C677T polymorphism and hypertension on spatial navigationBiol Psychol200880224024519013496
- KimJMStewartRKimSWMethylenetetrahydrofolate reductase gene and risk of Alzheimer’s disease in KoreansInt J Geriatr Psychiatry200823545445917932993
- WeihMJunge-HulsingJMehraeinSZiemerSEinhauplKMHereditary thrombophilia with ischemiC stroke and sinus thrombosis. Diagnosis, therapy and meta-analysisNervenarzt2000711293694511139989
- KellyPJRosandJKistlerJPHomocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysisNeurology200259452953612196644
- ClarkeRLewingtonSLandrayMHomocysteine, renal function, and risk of cardiovascular diseaseKidney Int Suppl200384S13113312694328
- KimRJBeckerRCAssociation between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studiesAm Heart J2003146694895714660985
- CasasJPBautistaLESmeethLSharmaPHingoraniADHomocysteine and stroke: evidence on a causal link from mendelian randomisationLancet2005365945522423215652605
- HaywoodSLiesnerRPindoraSGanesanVThrombophilia and first arterial ischaemic stroke: a systematic reviewArch Dis Child200590440240515781933
- CroninSFurieKLKellyPJDose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysisStroke20053671581158715947278
- LaneRHeYMorrisCLeverenzJBEmreMBallardCBuChE-K and APOE epsilon4 allele frequencies in Lewy body dementias, and influence of genotype and hyperhomocysteinemia on cognitive declineMov Disord200924339240019006190
- AndresEKaltenbachGNoblet-DickMHematological response to short-term oral cyanocobalamin therapy for the treatment of cobalamin deficiencies in elderly patientsJ Nutr Health Aging20061013616453051
- CarmelRGottPSWatersCHThe frequently low cobalamin levels in dementia usually signify treatable metabolic, neurologic and electrophysiologic abnormalitiesEur J Haematol19955442452537789470
- KarnazeDSCarmelRNeurologic and evoked potential abnormalities in subtle cobalamin deficiency states, including deficiency without anemia and with normal absorption of free cobalaminArch Neurol1990479100810122396929
- FusoACavallaroRAZampelliAgamma-Secretase is differentially modulated by alterations of homocysteine cycle in neuroblastoma and glioblastoma cellsJ Alzheimers Dis200711327529017851177
- FusoANicoliaVCavallaroRAB-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in miceMol Cell Neurosci200837473174618243734
- HerrmannWSignificance of hyperhomocysteinemiaClin Lab20065278367374
- WestRLLeeJMMarounLEHypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patientJ Mol Neurosci1995621411468746452
- FoyCJPassmoreAPVahidassrMDYoungISLawsonJTPlasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s diseaseQJM1999921394510209671
- HarrisMEHensleyKButterfieldDALeedleRACarneyJMDirect evidence of oxidative injury produced by the Alzheimer’s beta-amyloid peptide (1–40) in cultured hippocampal neuronsExp Neurol199513121932027895820
- NourhashemiFGillette-GuyonnetSAndrieuSAlzheimer disease: protective factorsAm J Clin Nutr2000712643S649S10681273
- PraticoDClarkCMLiunFRokachJLeeVYTrojanowskiJQIncrease of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer diseaseArch Neurol200259697297612056933
- BlaskoIStampfer-KountchevMRobatscherPVeerhuisREikelenboomPGrubeck-LoebensteinBHow chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytesAging Cell20043416917615268750
- DavisGKBaboolalNSSealesDRamchandaniJMcKellSMcRaeAPotential biomarkers for dementia in Trinidad and TobagoNeurosci Lett20074241273017703882
- MrakREGriffinWSPotential inflammatory biomarkers in Alzheimer’s diseaseJ Alzheimers Dis20058436937516556968
- StreitWJMicroglia and Alzheimer’s disease pathogenesisJ Neurosci Res20047711815197750
- van den KommerTNDikMGComijsHCJonkerCDeegDJHomocysteine and inflammation: Predictors of cognitive decline in older personsNeurobiol Aging20081110 [Epub ahead of print]
- VielTALima CaetanoANaselloAGIncreases of kinin B1 and B2 receptors binding sites after brain infusion of amyloid-beta 1–40 peptide in ratsNeurobiol Aging200829121805181417570564
- FrickBGruberBSchroecksnadelKLeblhuberFFuchsDHomocysteine but not neopterin declines in demented patients on B vitaminsJ Neural Transm2006113111815181916988797
- TeunissenCELutjohannDvon BergmannKCombination of serum markers related to several mechanisms in Alzheimer’s diseaseNeurobiol Aging200324789390212928047
- TeunissenCEvan BoxtelMPBosmaHSerum markers in relation to cognitive functioning in an aging population: results of the Maastricht Aging Study (MAAS)Tijdschr Gerontol Geriatr200334161212629905
- Van DamFVan GoolWAHyperhomocysteinemia and Alzheimer’s disease: A systematic reviewArch Gerontol Geriatr200948342543018479766
- DavisSRQuinlivanEPShelnuttKPHomocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C -->T polymorphism and by dietary folate restriction in young womenJ Nutr200513551045105015867279
- De La HabaGCantoniGThe enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteineJ Biol Chem1959234360360813641268
- GarciaAZanibbiKHomocysteine and cognitive function in elderly peopleCMAJ2004171889790415477631
- TchantchouFGravesMFalconeDSheaTBS-adenosylmethionine mediates glutathione efficacy by increasing glutathione S-transferase activity: implications for S-adenosyl methionine as a neuroprotective dietary supplementJ Alzheimers Dis200814332332818599958
- GibsonGESheuKFBlassJPAbnormalities of mitochondrial enzymes in Alzheimer diseaseJ Neural Transm199810589855870
- MarkesberyWRThe role of oxidative stress in Alzheimer diseaseArch Neurol199956121449145210593298
- TurrensJFMitochondrial formation of reactive oxygen speciesJ Physiol2003552Pt 233534414561818
- TooheyJIMercaptopropionaldehyde from homocysteine: implications for Alzheimer’s diseaseJ Alzheimers Dis200712324124318057557
- McCaddonAHudsonPRAlzheimer’s disease, oxidative stress and B-vitamin depletionFuture Neurology200725537547
- SerotJMBeneMCFaureGCComparative immunohistochemical characteristics of human choroid plexus in vascular and Alzheimer’s dementiaHum Pathol19942511118511907959663
- SerotJMChristmannDDubostTBeneMCFaureGCCSF-folate levels are decreased in late-onset AD patientsJ Neural Transm20011081939911261750
- SerotJMBeneMCFaureGCChoroid plexus, aging of the brain, and Alzheimer’s diseaseFront Biosci20038s515s52112700093
- NijstTQWeversRASchoonderwaldtHCHommesORde HaanAFVitamin B12 and folate concentrations in serum and cerebrospinal fluid of neurological patients with special reference to multiple sclerosis and dementiaJ Neurol Neurosurg Psychiatry199053119519542283525
- SpectorRCancillaPDamasioAIs idiopathic dementia a regional vitamin deficiency state?Med Hypotheses197957763767514118
- CrossgroveJSLiGJZhengWThe choroid plexus removes betaamyloid from brain cerebrospinal fluidExp Biol Med (Maywood)20052301077177616246905
- JohansonCEDuncanJA3rdKlingePMBrinkerTStopaEGSilverbergGDMultiplicity of cerebrospinal fluid functions: new challenges in health and diseaseCerebrospinal Fluid Res2008511018479516
- SontagENunbhakdi-CraigVSontagJMProtein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulationJ Neurosci200727112751275917360897
- ChanAYAlsarabyASheaTBFolate deprivation increases tau phosphorylation by homocysteine-induced calcium influx and by inhibition of phosphatase activity: Alleviation by S-adenosyl methionineBrain Res2008119913313718279842
- LuoYZhouXYangXWangJHomocysteine induces tau hyperphosphorylation in ratsNeuroreport200718182005200818007203
- ObeidRKasohaMKnappJPFolate and methylation status in relation to phosphorylated tau protein(181P) and beta-amyloid(1–42) in cerebrospinal fluidClin Chem20075361129113617384003
- ZhangCETianQWeiWHomocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampusNeurobiol Aging200829111654166517537547
- AgnatiLFGenedaniSLeoGAbeta peptides as one of the crucial volume transmission signals in the trophic units and their interactions with homocysteine. Physiological implications and relevance for Alzheimer’s diseaseJ Neural Transm20071141213116969627
- Pacheco-QuintoJRodriguez de TurcoEBDeRosaSHyperhomocysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid beta peptide levelsNeurobiol Dis200622365165616516482
- ViswanathanARajSGreenbergSMPlasma Abeta, homocysteine, and cognition: the Vitamin Intervention for Stroke Prevention (VISP) trialNeurology200972326827219153374
- BlaskoIJellingerKKemmlerGConversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteineNeurobiol Aging200829111117055615
- KangJParkEJJouIKimJHJoeEHReactive oxygen species mediate A beta(25–35)-induced activation of BV-2 microgliaNeuroreport20011271449145211388427
- WangJYWenLLHuangYNChenYTKuMCDual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammationCurr Pharm Des200612273521353317017945
- HarmanDAlzheimer’s disease: role of aging in pathogenesisAnn N Y Acad Sci2002959384395 discussion 463–38511976212
- HwangJZhengLTOckJLeeMGSukKAnti-inflammatory effects of m-chlorophenylpiperazine in brain glia cellsInt Immunopharmacol20088121686169418771755
- LiuBGaoHMWangJYJeohnGHCooperCLHongJSRole of nitric oxide in inflammation-mediated neurodegenerationAnn N Y Acad Sci200296231833112076984
- SchroecksnadelKLeblhuberFFrickBWirleitnerBFuchsDAssociation of hyperhomocysteinemia in Alzheimer disease with elevated neopterin levelsAlzheimer Dis Assoc Disord200418312913315494618
- WilkinsonBLLandrethGEThe microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s diseaseJ Neuroinflammation200633017094809
- YamasakiRZhangJKoshiishiIInvolvement of lysosomal storage-induced p38 MAP kinase activation in the overproduction of nitric oxide by microglia in cathepsin D-deficient miceMol Cell Neurosci200735457358417570679
- KoseogluEKaramanYRelations between homocysteine, folate and vitamin B12 in vascular dementia and in Alzheimer diseaseClin Biochem2007401285986317532313
- McCaddonAReglandBHudsonPDaviesGFunctional vitamin B(12) deficiency and Alzheimer diseaseNeurology20025891395139912011287
- SheaTBEffects of dietary supplementation with N-acetyl cysteine, acetyl-L-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4Neuromolecular Med20079326426917914184
- DawsonHCollinsGPyleRDeep-DixitVTaubDDThe immunoregulatory effects of homocysteine and its intermediates on T-lymphocyte functionMech Ageing Dev2004125210711015037011
- SiowYLAu-YeungKKWooCWOKHomocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activationBiochem J20063981738216626305
- WangGOKHomocysteine stimulates the expression of monocyte chemoattractant protein-1 receptor (CCR2) in human monocytes: possible involvement of oxygen free radicalsBiochem J2001357Pt 123324011415454
- WidnerBEnzingerCLaichAWirleitnerBFuchsDHyperhomocysteinemia, pteridines and oxidative stressCurr Drug Metab20023222523212003353
- WidnerBLeblhuberFFrickBLaichAArtner-DworzakEFuchsDModerate hyperhomocysteinaemia and immune activation in Parkinson’s diseaseJ Neural Transm2002109121445145212486485
- GuidiIGalimbertiDLonatiSOxidative imbalance in patients with mild cognitive impairment and Alzheimer’s diseaseNeurobiol Aging200627226226916399211
- HoPICollinsSCDhitavatSHomocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stressJ Neurochem200178224925311461960
- WhiteARHuangXJoblingMFHomocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: possible risk factors in the Alzheimer’s-type neurodegenerative pathwaysJ Neurochem20017651509152011238735
- FuchsDJaegerMWidnerBWirleitnerBArtner-DworzakELeblhuberFIs hyperhomocysteinemia due to the oxidative depletion of folate rather than to insufficient dietary intake?Clin Chem Lab Med200139869169411592434
- FusoASeminaraLCavallaroRAD’AnselmiFScarpaSS-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid productionMol Cell Neurosci200528119520415607954
- JinYBrennanLEffects of homocysteine on metabolic pathways in cultured astrocytesNeurochem Int20085281410141518417255
- PerryGCashADSmithMAAlzheimer Disease and Oxidative StressJ Biomed Biotechnol20022312012312488575
- PraticoDUryuKLeightSTrojanoswkiJQLeeVMIncreased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosisJ Neurosci200121124183418711404403
- PraticoDSungSLipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s diseaseJ Alzheimers Dis20046217117515096701
- SwerdlowRHKhanSMA “mitochondrial cascade hypothesis” for sporadic Alzheimer’s diseaseMed Hypotheses200463182015193340
- Dias-SantagataDFulgaTADuttaroyAFeanyMBOxidative stress mediates tau-induced neurodegeneration in DrosophilaJ Clin Invest2007117123624517173140
- GamblinTCKingMEKuretJBerryRWBinderLIOxidative regulation of fatty acid-induced tau polymerizationBiochemistry20003946142031421011087369
- LiuQSmithMAAvilaJAlzheimer-specific epitopes of tau represent lipid peroxidation-induced conformationsFree Radic Biol Med200538674675415721985
- PercyMMoalemSGarciaAInvolvement of ApoE E4 and H63D in sporadic Alzheimer’s disease in a folate-supplemented Ontario populationJ Alzheimers Dis2008141698418525129
- LeoGGenedaniSFilaferroMHyper-homocysteinemia alters amyloid peptide-clusterin interactions and neuroglial network morphology and function in the caudate after intrastriatal injection of amyloid peptidesCurr Alzheimer Res20074330531317627488
- AkamaKTAlbaneseCPestellRGVan EldikLJAmyloid betapeptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanismProc Natl Acad Sci U S A19989510579558009576964
- ButterfieldDALauderbackCMLipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stressFree Radic Biol Med200232111050106012031889
- CenteMFilipcikPPevalovaMNovakMExpression of a truncated tau protein induces oxidative stress in a rodent model of tauopathyEur J Neurosci20062441085109016930434
- ScalabrinoGVeberDMuttiEExperimental and clinical evidence of the role of cytokines and growth factors in the pathogenesis of acquired cobalamin-deficient leukoneuropathyBrain Res Rev2008591425418538413
- BuccellatoFRMilosoMBragaMMyelinolytic lesions in spinal cord of cobalamin-deficient rats are TNF-alpha-mediatedFASEB J19991322973049973317
- PeracchiMBamonti CatenaFPomatiMDe FranceschiMScalabrinoGHuman cobalamin deficiency: alterations in serum tumour necrosis factor-alpha and epidermal growth factorEur J Haematol200167212312711722601
- ScalabrinoGCobalamin (vitamin B(12)) in subacute combined degeneration and beyond: traditional interpretations and novel theoriesExp Neurol2005192246347915755562
- ScalabrinoGVeberDMuttiENew pathogenesis of the cobalamin-deficient neuropathyMed Secoli200719191818447164
- ScalabrinoGThe multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiencyProg Neurobiol200988320322019394404
- ScalabrinoGCarpoMBamontiFHigh tumor necrosis factor-alpha [corrected] levels in cerebrospinal fluid of cobalamin-deficient patientsAnn Neurol200456688689015562428
- ScalabrinoGPeracchiMNew insights into the pathophysiology of cobalamin deficiencyTrends Mol Med200612624725416690356
- DoganMOzdemirOSalEAPsychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiencyJ Trop Pediatr200955320520719095695
- HealtonEBSavageDGBrustJCGarrettTJLindenbaumJNeurologic aspects of cobalamin deficiencyMedicine (Baltimore)19917042292451648656
- DurandCMarySBrazoPDollfusSPsychiatric manifestations of vitamin B12 deficiency: a case reportEncephale200329656056515029091
- KoSMLiuTCPsychiatric syndromes in pernicious anaemia – a case reportSingapore Med J199233192941598618
- LassenEEwaldHAcute organic psychosis caused by thyrotoxicosis and vitamin B12 def iciency: case reportJ Clin Psychiatry19854631061073972777
- EvansDLEdelsohnGAGoldenRNOrganic psychosis without anemia or spinal cord symptoms in patients with vitamin B12 deficiencyAm J Psychiatry198314022182216849439
- BolamanZKadikoyluGYukselenVYavasogluIBarutcaSSenturkTOral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label studyClin Ther200325123124313414749150
- CuberoLMayorJAlmirallPValdesMMemory disorders in the epidemic neuropathyRev Neurol199928434334810714309
- KwokTLeeJLamLWooJVitamin B(12) supplementation did not improve cognition but reduced delirium in demented patients with vitamin B(12) deficiencyArch Gerontol Geriatr200846327328217561285
- LindenbaumJHealtonEBSavageDGNeuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosisN Engl J Med198831826172017283374544
- HeokKEHoRThe many faces of geriatric depressionCurr Opin Psychiatry200821654054518852559
- SaracaceanuETramoniAVHenryJMAn association between subcortical dementia and pernicious anemia – a psychiatric maskCompr Psychiatry19973863493519406742
- GoggansFCA case of mania secondary to vitamin B12 deficiencyAm J Psychiatry198414123003016691503
- BottiglieriTLaundyMCrellinRTooneBKCarneyMWReynoldsEHHomocysteine, folate, methylation, and monoamine metabolism in depressionJ Neurol Neurosurg Psychiatry200069222823210896698
- MeeksTWRopackiSAJesteDVThe neurobiology of neuropsychiatric syndromes in dementiaCurr Opin Psychiatry200619658158617012935
- ReifAPfuhlmannBLeschKPHomocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychosesProg Neuropsychopharmacol Biol Psychiatry20052971162116816055253
- DittmannSSeemullerFSchwarzMJAssociation of cognitive deficits with elevated homocysteine levels in euthymic bipolar patients and its impact on psychosocial functioning: preliminary resultsBipolar Disord20079126370
- DittmannSSeemullerFGrunzeHCThe impact of homocysteine levels on cognition in euthymic bipolar patients: A cross-sectional studyJ Clin Psychiatry2008e1e8
- OsherYBersudskyYSilverHSelaBABelmakerRHNeuropsychological correlates of homocysteine levels in euthymic bipolar patientsJ Affect Disord20081051322923317659352
- LevineJStahlZSelaBAHomocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemiaBiol Psychiatry200660326526916412989
- KalitaJMisraUKVitamin B12 deficiency neurological syndromes: correlation of clinical, MRI and cognitive evoked potentialJ Neurol2008255335335918350363
- GoldmanLAACecil Textbook of Medicine22nd edPhiladelphia, PASaunders, Elsevier Inc2004
- TohBHvan DrielIRGleesonPAPernicious anemiaN Engl J Med199733720144114489358143
- PuntambekarPBashaMMZakITMadhavanRRare sensory and autonomic disturbances associated with vitamin B12 deficiencyJ Neurol Sci20092871228528719800081
- RopperABrownRRopperABrownRDiseases of the nervous system due to nutritional deficiencyAdams and Victor’s Principles of Neurology8th edNew York, NYMcGraw-Hill20059831003
- HuckmanMSFoxJTopelJThe validity of criteria for the evaluation of cerebral atrophy by computed tomographyRadiology1975116185921079610
- de LeonMJGeorgeAEReisbergBAlzheimer’s disease: longitudinal CT studies of ventricular changeAJR Am J Roentgenol19891526125712622785749
- TakedaSMatsuzawaTAge-related brain atrophy: a study with computed tomographyJ Gerontol19854021591633973356
- LeMayMRadiologic changes of the aging brain and skullAJR Am J Roentgenol198414323833896377860
- ItoMHatazawaJYamauraHMatsuzawaTAge-related brain atrophy and mental deterioration – a study with computed tomographyBr J Radiol1981546413843907237008
- CreaseyHSchwartzMFredericksonHHaxbyJVRapoportSIQuantitative computed tomography in dementia of the Alzheimer typeNeurology19863612156315683785670
- DeCarliCKayeJAHorwitzBRapoportSICritical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer typeNeurology19904068728832189080
- DeCarliCMurphyDGMcIntoshARTeichbergDSchapiroMBHorwitzBDiscriminant analysis of MRI measures as a method to determine the presence of dementia of the Alzheimer typePsychiatry Res19955721191307480379
- FrisoniGBBeltramelloAWeissCGeroldiCBianchettiATrabucchiMLinear measures of atrophy in mild Alzheimer diseaseAJNR Am J Neuroradiol19961759139238733967
- HornROstertunBFricMSolymosiLSteudelAMollerHJAtrophy of hippocampus in patients with Alzheimer’s disease and other diseases with memory impairmentDementia1996741821868835880
- JobstKASmithADSzatmariMDetection in life of confirmed Alzheimer’s disease using a simple measurement of medial temporal lobe atrophy by computed tomographyLancet19923408829117911831359259
- KawamuraJMeyerJSIchijoMKobariMTerayamaYWeathersSCorrelations of leuko-araiosis with cerebral atrophy and perfusion in elderly normal subjects and demented patientsJ Neurol Neurosurg Psychiatry19935621821878437007
- LuxenbergJSFriedlandRPRapoportSIQuantitative X-ray computed tomography (CT) in dementia of the Alzheimer type (DAT)Can J Neurol Sci1986134 Suppl5705723491665
- PhilpotMBurnsAJobstDetection in life of confirmed Alzheimer’s disease using a simple measurement of medial temporal lobe atrophy by computed tomographyBr J Psychiatry19931638098128306125
- WhitehousePJClinical Practice Guidelines: Guidelines Abstracted from the American Academy of Neurology’s Dementia Guidelines for Early Detection, Diagnosis, and Management of DementiaThe American Geriatrics Society2008 Available from: http://www.americangeriatrics.org/products/positionpapers/aan_dementia.shtmlAccessed on August 21, 2009
- BurnsAJacobyRLevyRComputed tomography in Alzheimer’s disease: a longitudinal studyBiol Psychiatry19912943833902036479
- BertschTMielkeOHolySHomocysteine in cerebrovascular disease: an independent risk factor for subcortical vascular encephalopathyClin Chem Lab Med200139872172411592441
- CensoriBPartziguianTManaraOPoloniMPlasma homocysteine and severe white matter diseaseNeurol Sci200728525926317972040
- ClarkeRSmithADJobstKARefsumHSuttonLUelandPMFolate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer diseaseArch Neurol19985511144914559823829
- EversSKochHGGrotemeyerKHLangeBDeufelTRingelsteinEBFeatures, symptoms, and neurophysiological findings in stroke associated with hyperhomocysteinemiaArch Neurol19975410127612829341574
- HassanAHuntBJO’SullivanMHomocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunctionBrain2004127Pt 121221914607791
- HogervorstERibeiroHMMolyneuxABudgeMSmithADPlasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leukoaraiosis) in patients with Alzheimer diseaseArch Neurol200259578779312020261
- NakaHNomuraETakahashiTPlasma total homocysteine levels are associated with advanced leukoaraiosis but not with asymptomatic microbleeds on T2*-weighted MRI in patients with strokeEur J Neurol200613326126516618343
- QuadriPFragiacomoCPezzatiRHomocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementiaAm J Clin Nutr200480111412215213037
- SachdevPParslowRSalonikasCHomocysteine and the brain in midadult life: evidence for an increased risk of leukoaraiosis in menArch Neurol20046191369137615364682
- SachdevPSHomocysteine and brain atrophyProg Neuropsychopharmacol Biol Psychiatry20052971152116116102882
- SchwartzGLBaileyKRMosleyTAssociation of ambulatory blood pressure with ischemic brain injuryHypertension20074961228123417404188
- ScottTMTuckerKLBhadeliaAHomocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disordersAm J Geriatr Psychiatry200412663163815545331
- SeshadriSWolfPABeiserASAssociation of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring StudyArch Neurol200865564264918474741
- SmithADThe worldwide challenge of the dementias: a role for B vitamins and homocysteine?Food Nutr Bull2008292 SupplS14317218709889
- VogiatzoglouARefsumHJohnstonCVitamin B12 status and rate of brain volume loss in community-dwelling elderlyNeurology2008711182683218779510
- WilliamsJHPereiraEABudgeMMBradleyKMMinimal hippocampal width relates to plasma homocysteine in community-dwelling older peopleAge Ageing200231644044412446289
- WrightCBPaikMCBrownTRTotal homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan StudyStroke20053661207121115879345
- YangLKWongKCWuMYLiaoSLKuoCSHuangRFCorrelations between folate, B12, homocysteine levels, and radiological markers of neuropathology in elderly post-stroke patientsJ Am Coll Nutr200726327227817634173
- JimenezIAgullaJPousoMCognitive impairment associated to leukoaraiosis: its pathophysiology, clinical manifestations and treatmentRev Neurol2008471053654419012258
- FuJHLuCZHongZDongQLuoYWongKSExtent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic strokeJ Neurol Neurosurg Psychiatry200576679379615897500
- LongstrethWTJrManolioTAArnoldAClinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health StudyStroke1996278127412828711786
- StreiflerJYEliasziwMBenaventeORPrognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosisStroke20023361651165512053006
- StreiflerJYEliasziwMBenaventeORDevelopment and progression of leukoaraiosis in patients with brain ischemia and carotid artery diseaseStroke20033481913191612869713
- GeorgeAEde LeonMJGentesCILeukoencephalopathy in normal and pathologic aging: 1. CT of brain lucenciesAJNR Am J Neuroradiol1986745615663088933
- BasileAMPantoniLPracucciGAge, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) StudyCerebrovasc Dis20062156315322
- Bastos-LeiteAJKuijerJPRomboutsSACerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensitiesAJNR Am J Neuroradiol20082971296130118451090
- FarrallAJWardlawJMBlood-brain barrier: Ageing and microvascular disease – systematic review and meta-analysisNeurobiol Aging200930333735217869382
- SchwartzGLFornageMMosleyTTurnerSTTreatment of leukoaraiosisCurr Treat Options Cardiovasc Med20057317317716004848
- RomanGCSenile dementia of the Binswanger type. A vascular form of dementia in the elderlyJAMA198725813178217883625988
- de LauLMRefsumHSmithADJohnstonCBretelerMMPlasma folate concentration and cognitive performance: Rotterdam Scan StudyAm J Clin Nutr200786372873417823439
- de LauLMSmithADRefsumHJohnstonCBretelerMMPlasma vitamin B12 status and cerebral white-matter lesionsJ Neurol Neurosurg Psychiatry200980214915718977824
- InzitariDSimoniMPracucciGRisk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS studyArch Intern Med20071671818817210882
- DufouilCAlperovitchADucrosVTzourioCHomocysteine, white matter hyperintensities, and cognition in healthy elderly peopleAnn Neurol200353221422112557288
- PolyakZSternFBernerYNHyperhomocysteinemia and vitamin score: correlations with silent brain ischemic lesions and brain atrophyDement Geriatr Cogn Disord2003161394512714799
- StensetVHofossDJohnsenLWhite matter lesion severity is associated with reduced cognitive performances in patients with normal CSF Abeta42 levelsActa Neurol Scand2008118637337818510598
- WongAMokVFanYHLamWWLiangKSWongKSHyperhomocysteinemia is associated with volumetric white matter change in patients with small vessel diseaseJ Neurol2006253444144716267639
- WallinASjogrenMCerebrospinal fluid cytoskeleton proteins in patients with subcortical white-matter dementiaMech Ageing Dev2001122161937194911589912
- BabiloniCBoscoPGhidoniRHomocysteine and electroencephalographic rhythms in Alzheimer disease: a multicentric studyNeuroscience2007145394295417321055
- SeshadriSBeiserASelhubJPlasma homocysteine as a risk factor for dementia and Alzheimer’s diseaseN Engl J Med2002346747648311844848
- MillerJWGreenRMungasDMReedBRJagustWJHomocysteine, vitamin B6, and vascular disease in AD patientsNeurology200258101471147512034781
- AndoSNakayamaHCerebral amyloid angiopathy in Alzheimer’s disease: with special reference to its incidence and distributionNo To Shinkei19843611110911166525324
- CoriaFCastanoEPrelliFIsolation and characterization of amyloid P component from Alzheimer’s disease and other types of cerebral amyloidosisLab Invest19885844544582965774
- BueeLHofPRBourasCPathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disordersActa Neuropathol (Berl)19948754694808059599
- EberlingJLJagustWJReedBRBakerMGReduced temporal lobe blood flow in Alzheimer’s diseaseNeurobiol Aging19921344834911508299
- JobstKASmithADBarkerCSAssociation of atrophy of the medial temporal lobe with reduced blood flow in the posterior parietotemporal cortex in patients with a clinical and pathological diagnosis of Alzheimer’s diseaseJ Neurol Neurosurg Psychiatry19925531901941564478
- PurandareNBurnsADalyKJCerebral emboli as a potential cause of Alzheimer’s disease and vascular dementia: case-control studyBMJ200633275501119112416648133
- PurandareNVoshaarRCMorrisJAsymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementiaBiol Psychiatry200762433934417531959
- AgnatiLFGenedaniSRasioGStudies on homocysteine plasma levels in Alzheimer’s patients. Relevance for neurodegenerationJ Neural Transm2005112116316915599614
- LeeESChenHSolimanKFCharltonCGEffects of homocysteine on the dopaminergic system and behavior in rodentsNeurotoxicology200526336137115935208
- ChoiBHKimRCVaughanPJDecreases in protease nexins in Alzheimer’s disease brainNeurobiol Aging19951645575628544905
- HanyuHAsanoTTanakaYIwamotoTTakasakiMAbeKIncreased blood-brain barrier permeability in white matter lesions of Binswanger’s disease evaluated by contrast-enhanced MRIDement Geriatr Cogn Disord20021411612053125
- ArshavskyYIAlzheimer’s disease, brain immune privilege and memory: a hypothesisJ Neural Transm2006113111697170716932992
- D’AndreaMRAdd Alzheimer’s disease to the list of autoimmune diseasesMed Hypotheses200564345846315617848
- GroppaSAChekhoninVPSpecific brain antigens as the indicators of the blood-brain barrier permeability in Alzheimer’s diseaseZh Nevropatol Psikhiatr Im S S Korsakova199191950521723824
- Homocysteine and risk of ischemic heart disease and stroke: a meta-analysisJAMA2002288162015202212387654
- BautistaLEArenasIAPenuelaAMartinezLXTotal plasma homocysteine level and risk of cardiovascular disease: a meta-analysis of prospective cohort studiesJ Clin Epidemiol200255988288712393075
- LiuXQJiangPHuangHYanYPlasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereofZhonghua Yi Xue Za Zhi200888322246224919087670
- MollerJNielsenGMTvedegaardKCAndersenNTJorgensenPEA meta-analysis of cerebrovascular disease and hyperhomocysteinaemiaScand J Clin Lab Invest200060649149911129065
- WaldDSLawMMorrisJKHomocysteine and cardiovascular disease: evidence on causality from a meta-analysisBMJ20023257374120212446535
- BousheyCJBeresfordSAOmennGSMotulskyAGA quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakesJAMA199527413104910577563456
- TroenAMShea-BudgellMShukitt-HaleBSmithDESelhubJRosenbergIHB-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in miceProc Natl Acad Sci U S A200810534124741247918711131
- YaoHSadoshimaSKuwabaraYIchiyaYFujishimaMCerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger typeStroke19902112169416992264076
- PicardiANavajasFSpotoSPalma ModoniADe GalassoLCostantinoSA 74-year-old woman with macrocytic anemiaClin Ter20021531656711963638
- ChinAVRobinsonDJO’ConnellHVascular biomarkers of cognitive performance in a community-based elderly population: the Dublin Healthy Ageing studyAge Ageing200837555956418667454
- SalaIBelen Sanchez-SaudinosMMolina-PorcelLHomocysteine and cognitive impairment. Relation with diagnosis and neuropsychological performanceDement Geriatr Cogn Disord200826650651219023204
- SelhubJMorrisMSJacquesPFRosenbergIHFolate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiencyAm J Clin Nutr2009892702S706S19141696
- StewartRAsonganyiBSherwoodRPlasma homocysteine and cognitive impairment in an older British African-Caribbean populationJ Am Geriatr Soc20025071227123212133017
- RavagliaGFortiPMaioliFHomocysteine and cognitive function in healthy elderly community dwellers in ItalyAm J Clin Nutr200377366867312600859
- PrinsNDDen HeijerTHofmanAHomocysteine and cognitive function in the elderly: the Rotterdam Scan StudyNeurology20025991375138012427887
- ColeMGPrchalJFLow serum vitamin B12 in Alzheimer-type dementiaAge Ageing19841321011056731164
- MalaguarneraMFerriRBellaRAlagonaGCarnemollaAPennisiGHomocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer diseaseClin Chem Lab Med20044291032103515497469
- McCaddonADaviesGHudsonPTandySCattellHTotal serum homocysteine in senile dementia of Alzheimer typeInt J Geriatr Psychiatry19981342352399646150
- HagneliusNOWahlundLONilssonTKCSF/serum folate gradient: physiology and determinants with special reference to dementiaDement Geriatr Cogn Disord200825651652318463447
- ZoccolellaSLambertiPIlicetoGPlasma homocysteine levels in L-dopa-treated Parkinson’s disease patients with cognitive dysfunctionsClin Chem Lab Med200543101107111016197306
- OzerFMeralHHanogluLPlasma homocysteine levels in patients treated with levodopa: motor and cognitive associationsNeurol Res200628885385817288745
- BudgeMMde JagerCHogervorstESmithADTotal plasma homocysteine, age, systolic blood pressure, and cognitive performance in older peopleJ Am Geriatr Soc200250122014201812473014
- DuthieSJWhalleyLJCollinsARLeaperSBergerKDearyIJHomocysteine, B vitamin status, and cognitive function in the elderlyAm J Clin Nutr200275590891311976166
- EliasMFRobbinsMABudgeMMHomocysteine and cognitive performance: modification by the ApoE genotypeNeurosci Lett20084301646918023533
- EussenSJUelandPMClarkeRThe association of betaine, homocysteine and related metabolites with cognitive function in Dutch elderly peopleBr J Nutr200798596096817537289
- GarciaAAHaronYEvansLRSmithMGFreedmanMRomanGCMetabolic markers of cobalamin deficiency and cognitive function in normal older adultsJ Am Geriatr Soc2004521667114687317
- HinHClarkeRSherlikerPClinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 studyAge Ageing200635441642216709605
- KadoDMKarlamanglaASHuangMHHomocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful AgingAm J Med2005118216116715694902
- MillerJWGreenRRamosMIHomocysteine and cognitive function in the Sacramento Area Latino Study on AgingAm J Clin Nutr200378344144712936927
- MorrisMSJacquesPFRosenbergIHSelhubJFolate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortificationAm J Clin Nutr200785119320017209196
- RiggsKMSpiroA3rdTuckerKRushDRelations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging StudyAm J Clin Nutr19966333063148602585
- StyczynskaMStrosznajderJBReligaDAssociation between genetic and environmental factors and the risk of Alzheimer’s diseaseFolia Neuropathol200846424925419169966
- WoltersMHicksteinMFlintermannATewesUHahnACognitive performance in relation to vitamin status in healthy elderly German women-the effect of 6-month multivitamin supplementationPrev Med200541125325915917019
- AnnerboSWahlundLOLokkJThe relation between homocysteine levels and development of Alzheimer’s disease in mild cognitive impairment patientsDement Geriatr Cogn Disord200520420921416088136
- ClarkeRBirksJNexoELow vitamin B-12 status and risk of cognitive decline in older adultsAm J Clin Nutr20078651384139117991650
- EliasMFSullivanLMD’AgostinoRBHomocysteine and cognitive performance in the Framingham offspring study: age is importantAm J Epidemiol2005162764465316107567
- GabryelewiczTStyczynskaMLuczywekEThe rate of conversion of mild cognitive impairment to dementia: predictive role of depressionInt J Geriatr Psychiatry200722656356717136705
- GarciaAHaronYPulmanKHuaLFreedmanMIncreases in homocysteine are related to worsening of stroop scores in healthy elderly persons: a prospective follow-up studyJ Gerontol A Biol Sci Med Sci200459121323132715699533
- HaanMNMillerJWAielloAEHomocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on AgingAm J Clin Nutr200785251151717284751
- KimJMStewartRKimSWChanges in folate, vitamin B12, and homocysteine associated with incident dementiaJ Neurol Neurosurg Psychiatry200879886486818252751
- RavagliaGFortiPMaioliFBlood inflammatory markers and risk of dementia: The Conselice Study of Brain AgingNeurobiol Aging200728121810182017011077
- WrightCBLeeHSPaikMCStablerSPAllenRHSaccoRLTotal homocysteine and cognition in a tri-ethnic cohort: the Northern Manhattan StudyNeurology200463225426015277617
- NurkERefsumHTellGSPlasma total homocysteine and memory in the elderly: the Hordaland Homocysteine StudyAnn Neurol200558684785716254972
- NurkEDrevonCARefsumHCognitive performance among the elderly and dietary fish intake: the Hordaland Health StudyAm J Clin Nutr20078651470147817991661
- McCaddonAHudsonPDaviesGHughesAWilliamsJHWilkinsonCHomocysteine and cognitive decline in healthy elderlyDement Geriatr Cogn Disord200112530931311455131
- TeunissenCEBlomAHVan BoxtelMPHomocysteine: a marker for cognitive performance? A longitudinal follow-up studyJ Nutr Health Aging20037315315912766792
- OulhajARefsumHBeaumontHHomocysteine as a predictor of cognitive decline in Alzheimer’s diseaseInt J Geriatr Psychiatry2009529 [Epub ahead of print]
- CampbellAKJagustWJMungasDMLow erythrocyte folate, but not plasma vitamin B-12 or homocysteine, is associated with dementia in elderly LatinosJ Nutr Health Aging200591394315750664
- LuchsingerJATangMXSheaSMillerJGreenRMayeuxRPlasma homocysteine levels and risk of Alzheimer diseaseNeurology200462111972197615184599
- ReitzCTangMXMillerJGreenRLuchsingerJAPlasma homocysteine and risk of mild cognitive impairmentDement Geriatr Cogn Disord2008271111719088473
- KalmijnSLaunerLJLindemansJBotsMLHofmanABretelerMMTotal homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam StudyAm J Epidemiol1999150328328910430233
- MooijaartSPGusseklooJFrolichMHomocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus studyAm J Clin Nutr200582486687116210718
- ClarkeRHarrisonGRichardsSEffect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementiaJ Intern Med20032541677512823643
- NilssonKGustafsonLHultbergBPlasma homocysteine concentration relates to the severity but not to the duration of Alzheimer’s diseaseInt J Geriatr Psychiatry200419766667215254923
- PostiglioneAMilanGRuoccoAGallottaGGuiottoGDi MinnoGPlasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer’s dementia. A case-control studyGerontology200147632432911721146
- LocascioJJFukumotoHYapLPlasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer diseaseArch Neurol200865677678518541797
- CorderEHBeaumontHSusceptibility groups for Alzheimer’s disease (OPTIMA cohort): integration of gene variants and biochemical factorsMech Ageing Dev20071281768217116317
- EngelborghsSVloeberghsEMaertensKCorrelations between cognitive, behavioural and psychological findings and levels of vitamin B12 and folate in patients with dementiaInt J Geriatr Psychiatry200419436537015065230
- RoosDWillangerRVarious degrees of dementia in a selected group of gastrectomized patients with low serum B12Acta Neurol Scand1977555363376855644
- WangHXWahlinABasunHFastbomJWinbladBFratiglioniLVitamin B(12) and folate in relation to the development of Alzheimer’s diseaseNeurology20015691188119411342684
- EussenSJFerryMHiningerIHallerJMatthysCDirrenHFive year changes in mental health and associations with vitamin B12/folate status of elderly EuropeansJ Nutr Health Aging200261435011813081
- GarrodMGGreenRAllenLHFraction of total plasma vitamin B12 bound to transcobalamin correlates with cognitive function in elderly Latinos with depressive symptomsClin Chem20085471210121718451312
- LopezOLBeckerJTKlunkWResearch evaluation and diagnosis of possible Alzheimer’s disease over the last two decades: IINeurology200055121863186911134386
- LuchsingerJATangMXMillerJGreenRMayeuxRRelation of higher folate intake to lower risk of Alzheimer disease in the elderlyArch Neurol2007641869217210813
- SmallBJViitanenMWinbladBBackmanLCognitive changes in very old persons with dementia: the influence of demographic, psychometric, and biological variablesJ Clin Exp Neuropsychol19971922452609240484
- SmallBJBackmanLPredictors of longitudinal changes in memory, visuospatial, and verbal functioning in very old demented adultsDement Geriatr Cogn Disord1998952582669701677
- ProdanCICowanLDStonerJARossEDCumulative incidence of vitamin B12 deficiency in patients with Alzheimer diseaseJ Neurol Sci20092841214414819380153
- American Academy of NeurologyPractice Parameter: Diagnosis of Dementia. American Academy of Neurology Guideline Summary for CliniciansDetection, Diagnosis, and Management of Dementia: American Academy of Neurology Current guideline – reaffirmed February 13, 2004. Available from: http://www.aan.com/professionals/practice/pdfs/dementia_guideline.pdfAccessed on September 7, 2009
- ValkovicPBenetinJBlazicekPValkovicovaLGmitterovaKKukumbergPReduced plasma homocysteine levels in levodopa/entacapone treated Parkinson patientsParkinsonism Relat Disord200511425325615878587
- PostumaRBEspayAJZadikoffCVitamins and entacapone in levodopa-induced hyperhomocysteinemia: a randomized controlled studyNeurology200666121941194316801668
- LambertiPZoccolellaSIlicetoGEffects of levodopa and COMT inhibitors on plasma homocysteine in Parkinson’s disease patientsMov Disord2005201697215390046
- LambertiPZoccolellaSArmeniseEHyperhomocysteinemia in L-dopa treated Parkinson’s disease patients: effect of cobalamin and folate administrationEur J Neurol200512536536815804266
- AbyadAPrevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacementJ Nutr Health Aging20026425426012486445
- AisenPSSchneiderLSSanoMHigh-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trialJAMA2008300151774178318854539
- BalkEMRamanGTatsioniAChungMLauJRosenbergIHVitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trialsArch Intern Med20071671213017210874
- CunhaUGAn investigation of dementia among elderly outpatientsActa Psychiatr Scand19908232612632248054
- EastleyRWilcockGKBucksRSVitamin B12 deficiency in dementia and cognitive impairment: the effects of treatment on neuropsychological functionInt J Geriatr Psychiatry200015322623310713580
- EussenSJde GrootLCJoostenLWEffect of oral vitamin B12 with or without folic acid on cognitive function in older people with mild vitamin B12 deficiency: a randomized, placebo-controlled trialAm J Clin Nutr200684236137016895884
- HvasAMJuulSLauritzenLNexoEEllegaardJNo effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled studyJ Affect Disord200481326927315337331
- KwokTTangCWooJLaiWKLawLKPangCPRandomized trial of the effect of supplementation on the cognitive function of older people with subnormal cobalamin levelsInt J Geriatr Psychiatry19981396116169777425
- LinYTLinMHLaiHYChenLKHwangSJLanCFRegular vitamin B(12) supplementation among older Chinese men in a veterans care home in TaiwanArch Gerontol Geriatr200949118618918775574
- MaloufMGrimleyEJAreosaSAFolic acid with or without vitamin B12 for cognition and dementiaCochrane Database Syst Rev20034CD00451414584018
- MartinDCFrancisJProtetchJHuffFJTime dependency of cognitive recovery with cobalamin replacement: report of a pilot studyJ Am Geriatr Soc19924021681721740602
- McMahonJAGreenTJSkeaffCMKnightRGMannJIWilliamsSMA controlled trial of homocysteine lowering and cognitive performanceN Engl J Med2006354262764277216807413
- NilssonKGustafsonLHultbergBImprovement of cognitive functions after cobalamin/folate supplementation in elderly patients with dementia and elevated plasma homocysteineInt J Geriatr Psychiatry200116660961411424170
- OsimaniABergerAFriedmanJPorat-KatzBSAbarbanelJMNeuropsychology of vitamin B12 deficiency in elderly dementia patients and control subjectsJ Geriatr Psychiatry Neurol2005181333815681626
- StottDJMacIntoshGLoweGDRandomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular diseaseAm J Clin Nutr20058261320132616332666
- SunYLuCJChienKLChenSTChenRCEfficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patientsClin Ther200729102204221418042476
- van DyckCHLynessJMRohrbaughRMSiegalAPCognitive and psychiatric effects of vitamin B12 replacement in dementia with low serum B12 levels: a nursing home studyInt Psychogeriatr2008110
- van UffelenJGChinAPMJHopman-RockMvan MechelenWThe effect of walking and vitamin B supplementation on quality of life in community-dwelling adults with mild cognitive impairment: a randomized, controlled trialQual Life Res20071671137114617616840
- van UffelenJGChinapawMJvan MechelenWHopman-RockMWalking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trialBr J Sports Med200842534435118308888
- RuscinJMPageRL2ndValuckRJVitamin B(12) deficiency associated with histamine(2)-receptor antagonists and a proton-pump inhibitorAnn Pharmacother200236581281611978157
- ValuckRJRuscinJMA case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adultsJ Clin Epidemiol200457442242815135846
- MitchellSLRockwoodKThe association between antiulcer medication and initiation of cobalamin replacement in older personsJ Clin Epidemiol200154553153411337218
- OhRBrownDLVitamin B12 deficiencyAm Fam Physician200367597998612643357
- MetzJThe deoxyuridine suppression testCrit Rev Clin Lab Sci19842032052416426860
- AllenRHStablerSPSavageDGLindenbaumJDiagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrationsAm J Hematol199034290982339683
- ClarkeRSherlikerPHinHDetection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalaminClin Chem200753596397017363419
- HerrmannWObeidRSchorrHGeiselJFunctional vitamin B12 deficiency and determination of holotranscobalamin in populations at riskClin Chem Lab Med200341111478148814656029
- LindenbaumJSavageDGStablerSPAllenRHDiagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrationsAm J Hematol1990342991072339684
- MillerJWGarrodMGRockwoodALMeasurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiencyClin Chem200652227828516384886
- PennypackerLCAllenRHKellyJPHigh prevalence of cobalamin deficiency in elderly outpatientsJ Am Geriatr Soc19924012119712041447433
- SmithDLAnemia in the elderlyAm Fam Physician20006271565157211037074
- ObeidRMorkbakALMunzWNexoEHerrmannWThe cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birthClin Chem200652226326916384893
- KrasinskiSDRussellRMSamloffIMFundic atrophic gastritis in an elderly population. Effect on hemoglobin and several serum nutritional indicatorsJ Am Geriatr Soc198634118008063771980
- CarmelRMild transcobalamin I (haptocorrin) deficiency and low serum cobalamin concentrationsClin Chem20034981367137412881454
- MetzJBellAHFlickerLThe significance of subnormal serum vitamin B12 concentration in older people: a case control studyJ Am Geriatr Soc19964411135513618909352
- BorMVCetinMAytacSAltayCNexoENonradioactive vitamin B12 absorption test evaluated in controls and in patients with inherited malabsorption of vitamin B12Clin Chem200551112151215516166166
- McCaddonAHudsonPAbrahamssonLOlofssonHReglandBAnalogues, ageing and aberrant assimilation of vitamin B12 in Alzheimer’s diseaseDement Geriatr Cogn Disord200112213313711173886
- RefsumHSmithADLow vitamin B12 status in confirmed Alzheimer’s disease as revealed by serum holotranscobalaminJ Neurol Neurosurg Psychiatry200374795996112810791
- McCrackenCHudsonPEllisRMcCaddonAMethylmalonic acid and cognitive function in the Medical Research Council Cognitive Function and Ageing StudyAm J Clin Nutr20068461406141117158424
- StablerSPAllenRHSavageDGLindenbaumJClinical spectrum and diagnosis of cobalamin deficiencyBlood19907658718812393714
- ZeitlinAFrishmanWHChangCJThe association of vitamin b 12 and folate blood levels with mortality and cardiovascular morbidity incidence in the old old: the Bronx aging studyAm J Ther1997478275281
- DharmarajanTSNorkusEPApproaches to vitamin B12 deficiency. Early treatment may prevent devastating complicationsPostgrad Med2001110199105 quiz 10611467046
- HultbergBIsakssonANilssonKGustafsonLHomocysteine and methylmalonic acid as markers of cobalamin/folate status. The association to neuropsychiatric symptoms in the elderly is exploredLakartidningen2000973841314134413611068379
- NilssonKGustafsonLHultbergBOptimal use of markers for cobalamin and folate status in a psychogeriatric populationInt J Geriatr Psychiatry2002171091992512325051
- SavageDGLindenbaumJStablerSPAllenRHSensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficienciesAm J Med19949632392468154512
- GalimbertiGContiEZiniMPost-methionine load test: A more sensitive tool to reveal hyperhomocysteinemia in Alzheimer patientsClin Biochem200841101191491617988658
- KuzminskiAMDel GiaccoEJAllenRHStablerSPLindenbaumJEffective treatment of cobalamin deficiency with oral cobalaminBlood1998924119111989694707
- LittleDRAmbulatory management of common forms of anemiaAm Fam Physician19995961598160410193599
- HillDMJohnsonLJBurnsPJNealeAMHarmeningDMKenneyACEffects of temperature on stability of blood homocysteine in collection tubes containing 3-deazaadenosineClin Chem200248112017202212406988
- HansraniMStansbyGExtended storage of whole blood with 3-deazaadenosine for homocysteine assayAnn Clin Biochem200744Pt 438839017594787
- JoostenEPelemansWDevosPCobalamin absorption and serum homocysteine and methylmalonic acid in elderly subjects with low serum cobalaminEur J Haematol199351125308348941
- van AsseltDZPasmanJWvan LierHJCobalamin supplementation improves cognitive and cerebral function in older, cobalamin-deficient personsJ Gerontol A Biol Sci Med Sci20015612M77577911723153
- AnnanmakiTPessala-DriverAHokkanenLMurrosKUric acid associates with cognition in Parkinson’s diseaseParkinsonism Relat Disord200814757657818321759
- MorettiRTorrePAntonelloRMCazzatoGCattaruzzaTScapicchioPLVitamin B12 and folate depletion: clinical evidence in a neurological populationNeurologist200410633834315518600
- SelhubJMorrisMSJacquesPFIn vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrationsProc Natl Acad Sci U S A200710450199952000018056804
- ClarkeRSherlikerPHinHFolate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UKBr J Nutr200810051054105918341758
- Santiesteban-FreixasRSerrano-VerduraCGutierrez-GilJThe neuropathy epidemic in Cuba: eight years of investigation and follow-upRev Neurol200031654956611055062
- The Cuba Neuropathy Field Investigation TeamEpidemic optic neuropathy in Cuba – clinical characterization and risk factorsN Engl J Med199533318117611827565972
- DominguezYLHernandezMMatosCMZhouDIs B vitamins deficiency associated with prevalence of Alzheimer’s disease in Cuban elderly?Nutr Health200618210311816859173
- HedgesTR3rdHiranoMTuckerKCaballeroBEpidemic optic and peripheral neuropathy in Cuba: a unique geopolitical public health problemSurv Ophthalmol19974143413539104771
- RomanGCAn epidemic in Cuba of optic neuropathy, sensorineural deafness, peripheral sensory neuropathy and dorsolateral myeloneuropathyJ Neurol Sci1994127111287699385
- RomanGCOn politics and health: an epidemic of neurologic disease in CubaAnn Intern Med199512275305337872589
- RomanGCEpidemic neuropathy in Cuba: a public health problem related to the Cuban Democracy Act of the United StatesNeuroepidemiology19981731111159648115
- TuckerKHedgesTRFood shortages and an epidemic of optic and peripheral neuropathy in CubaNutr Rev199351123493578108040
- The Flour Fortification InitiativeCountries with Mandatory Fortification of Flour with Iron and/or Folic Acid (Total = 57) Available from: http://www.sph.emory.edu/wheatflour/COUNTRYDATA/Mandatory_countries.pdfAccessed on August 11, 2009
- SmithellsRWSheppardSSchorahCJVitamin dificiencies and neural tube defectsArch Dis Child197651129449501015847
- MillsJLMcPartlinJMKirkePNHomocysteine metabolism in pregnancies complicated by neural-tube defectsLancet199534589431491517741859
- Bjorke-MonsenALUelandPMSchneedeJVollsetSERefsumHElevated plasma total homocysteine and C677T mutation of the methylenetetrahydrofolate reductase gene in patients with spina bifidaQJM19979095935969349452
- VollsetSERefsumHIrgensLMPlasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine studyAm J Clin Nutr200071496296810731504
- CzeizelAEDudasIPrevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementationN Engl J Med199232726183218351307234
- MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin StudyLancet199133887601311371677062
- SmithellsRWSheppardSSchorahCJPossible prevention of neural-tube defects by periconceptional vitamin supplementationLancet1980181643393406101792
- LaurenceKMJamesNMillerMHTennantGBCampbellHDouble-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defectsBr Med J (Clin Res Ed)1981282627515091511
- CzeizelAEPrevention of congenital abnormalities by periconceptional multivitamin supplementationBMJ19933066893164516488324432
- Trends in wheat-flour fortification with folic acid and iron – worldwide, 2004 and 2007MMWR Morb Mortal Wkly Rep200857181018185494
- JacobsenDWHomocysteine and vitamins in cardiovascular diseaseClin Chem1998448 Pt 2183318439702993
- PitkinRMFolate and neural tube defectsAm J Clin Nutr2007851285S288S17209211
- LiuSWestRRandellEA comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defectsBMC Pregnancy Childbirth2004412015450123
- LewisDPVan DykeDCStumboPJBergMJDrug and environmental factors associated with adverse pregnancy outcomes. Part III: Folic acid: pharmacology, therapeutic recommendations, and economicsAnn Pharmacother19983210108710959793602
- LeyvaMFolic acid intake and its effects on the prevention of neural tube defects, the masking of vitamin B12 deficiency and the reduction of homocysteineJ Okla State Med Assoc200295533934212043109
- MalinowMRDuellPBHessDLReduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart diseaseN Engl J Med199833815100910159535664
- KristensenMOGulmannNCChristensenJEOstergaardKRasmussenKSerum cobalamin and methylmalonic acid in Alzheimer dementiaActa Neurol Scand19938764754818356878
- CarmelRPrevalence of undiagnosed pernicious anemia in the elderlyArch Intern Med199615610109711008638997
- KurodaSMoritaSA case of pernicious anemia with type A gastritis in an extremely elderly patient with dementia and heart failureNippon Ronen Igakkai Zasshi200845333533718622120
- ArgyriadouSVlachonikolisIMelisopoulouHKatachanakisKLionisCIn what extent anemia coexists with cognitive impairment in elderly: a cross-sectional study in GreeceBMC Fam Pract20012511707152
- McCaddonAKellyCLFamilial Alzheimer’s disease and vitamin B12 deficiencyAge Ageing19942343343377976784
- NilssonKGustafsonLFaldtRAnderssonAHultbergBPlasma homocysteine in relation to serum cobalamin and blood folate in a psychogeriatric populationEur J Clin Invest19942496006067828631
- McCaddonATandySHudsonPAbsence of macrocytic anaemia in Alzheimer’s diseaseClin Lab Haematol200426425926315279662
- GrossJSWeintraubNTNeufeldRRLibowLSPernicious anemia in the demented patient without anemia or macrocytosis. A case for early recognitionJ Am Geriatr Soc19863486126143722679
- FoxJHTopelJLHuckmanMSDementia in the elderly – a search for treatable illnessesJ Gerontol19753055575641181360
- IkedaTFurukawaYMashimotoSTakahashiKYamadaMVitamin B12 levels in serum and cerebrospinal fluid of people with Alzheimer’s diseaseActa Psychiatr Scand19908243273292260490
- KountourasJTsolakiMBozikiMAssociation between Helicobacter pylori infection and mild cognitive impairmentEur J Neurol200714997698217718688
- KountourasJBozikiMGavalasEEradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s diseaseJ Neurol2009256575876719240960
- MalaguarneraMBellaRAlagonaGFerriRCarnemollaAPennisiGHelicobacter pylori and Alzheimer’s disease: a possible linkEur J Intern Med200415638138615522573
- KountourasJTsolakiMGavalasERelationship between Helicobacter pylori infection and Alzheimer diseaseNeurology200666693894016567719
- VasconcellosLFCorreaRBChimelliLMyelopathy due to vitamin B12 deficiency presenting as transverse myelitisArq Neuropsiquiatr200260115015411965427
- GottfriesJBlennowKLehmannMWReglandBGottfriesCGOne-carbon metabolism and other biochemical correlates of cognitive impairment as visualized by principal component analysisJ Geriatr Psychiatry Neurol200114310911411563432
- Faxen-IrvingGBasunHCederholmTNutritional and cognitive relationships and long-term mortality in patients with various dementia disordersAge Ageing200534213614115644407
- JonesSSmallBJFratiglioniLBackmanLPredictors of cognitive change from preclinical to clinical Alzheimer’s diseaseBrain Cogn200249221021315259392
- McCaddonAKellyCLAlzheimer’s disease: a ‘cobalaminergic’ hypothesisMed Hypotheses19923731611651350050
- OpalaGJasinska-MygaBOchudloSJaniecKDiagnostic problems in the case of dementia with coexisting hematological disordersWiad Lek20015478456461
- CarmelREllenbogenLHerbertVDDietary Supplement Fact Sheet Vitamin B12Bethesda, MDOffice of Dietary Supplements, National Institutes of Health2006 Available from: From http://ods.od.nih.gov/factsheets/VitaminB12_pf.aspAccessed on January 18, 2009
- American Regent ICyanocobalamin Injection, USP. Package InsertShirley, NYAmerican Regent2003
- Braun-FalcoOLinckeHThe problem of vitamin B6/B12 acne. A contribution on acne medicamentosa (author’s transl)MMW Munch Med Wochenschr19761186155160130553
- TooleJFMalinowMRChamblessLELowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trialJAMA2004291556557514762035
- SchnyderGRoffiMPinRDecreased rate of coronary restenosis after lowering of plasma homocysteine levelsN Engl J Med2001345221593160011757505
- SchnyderGRoffiMFlammerYPinRHessOMEffect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trialJAMA2002288897397912190367
- AlbertCMCookNRGazianoJMEffect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trialJAMA2008299172027203618460663
- BonaaKHNjolstadIUelandPMHomocysteine lowering and cardiovascular events after acute myocardial infarctionN Engl J Med2006354151578158816531614
- EbbingMBleieOUelandPMMortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trialJAMA2008300779580418714059
- LangeHSuryapranataHDe LucaGFolate therapy and in-stent restenosis after coronary stentingN Engl J Med2004350262673268115215483
- LonnEYusufSArnoldMJHomocysteine lowering with folic acid and B vitamins in vascular diseaseN Engl J Med2006354151567157716531613
- MannJFSheridanPMcQueenMJHomocysteine lowering with folic acid and B vitamins in people with chronic kidney disease – results of the renal Hope-2 studyNephrol Dial Transplant200823264565318003666
- BazzanoLAReynoldsKHolderKNHeJEffect of folic acid supplementation on risk of cardiovascular diseases: a metaanalysis of randomized controlled trialsJAMA2006296222720272617164458
- WangXQinXDemirtasHEfficacy of folic acid supplementation in stroke prevention: a meta-analysisLancet200736995761876188217544768
- BrattstromLWilckenDEOhrvikJBrudinLCommon methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysisCirculation19989823252025269843457
- DangourADBreezeEClarkeRShettyPSUauyRFletcherAEPlasma homocysteine, but not folate or vitamin B-12, predicts mortality in older people in the United KingdomJ Nutr200813861121112818492844
- den HeijerMRosendaalFRBlomHJGerritsWBBosGMHyperhomocysteinemia and venous thrombosis: a meta-analysisThromb Haemost19988068748779869152
- Den HeijerMLewingtonSClarkeRHomocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studiesJ Thromb Haemost20053229229915670035
- Dhonukshe-RuttenRAPluijmSMde GrootLCLipsPSmitJHvan StaverenWAHomocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly peopleJ Bone Miner Res200520692192915883631
- KuoHKSorondFAChenJHHashmiAMilbergWPLipsitzLAThe role of homocysteine in multisystem age-related problems: a systematic reviewJ Gerontol A Biol Sci Med Sci20056091190120116183962
- SatoYHondaYIwamotoJKanokoTSatohKHomocysteine as a predictive factor for hip fracture in stroke patientsBone200536472172615781007
- SatoYIwamotoJKanokoTSatohKHomocysteine as a predictive factor for hip fracture in elderly women with Parkinson’s diseaseAm J Med2005118111250125516271909
- LehmannMReglandBBlennowKGottfriesCGVitamin B12-B6- folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairmentDement Geriatr Cogn Disord200316314515012826740
- FlickerLMartinsRNThomasJB-vitamins reduce plasma levels of beta amyloidNeurobiol Aging200829230330517113685
- StuerenburgHJMueller-ThomsenTMethnerAVitamin B12 plasma concentrations in Alzheimer diseaseNeuro Endocrinol Lett200425317617715349081
- La RueAKoehlerKMWayneSJChiulliSJHaalandKYGarryPJNutritional status and cognitive functioning in a normally aging sample: a 6-y reassessmentAm J Clin Nutr199765120298988908
- BryanJCalvaresiEHughesDShort-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various agesJ Nutr200213261345135612042457
- RemingtonRChanAPaskavitzJSheaTBEfficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer’s disease: a placebo-controlled pilot studyAm J Alzheimers Dis Other Demen2009241273319056706
- IkedaTYamamotoKTakahashiKTreatment of Alzheimer-type dementia with intravenous mecobalaminClin Ther19921434264371638584
- AlfthanGTapaniKNissinenKSaarelaJAroAThe effect of low doses of betaine on plasma homocysteine in healthy volunteersBr J Nutr200492466566915522136
- BellKMPlonLBunneyWEJrPotkinSGS-adenosylmethionine treatment of depression: a controlled clinical trialAm J Psychiatry19881459111011143046382
- BottiglieriTAdemetionine (S-adenosylmethionine) neuropharmacology: implications for drug therapies in psychiatric and neurological disordersExpert Opin Investig Drugs199764417426
- BressaGMS-adenosyl-l-methionine (SAMe) as antidepressant: meta-analysis of clinical studiesActa Neurol Scand Suppl19941547147941964
- D’AngeloASelhubJHomocysteine and thrombotic diseaseBlood19979011119207431
- Di RoccoARogersJDBrownRWernerPBottiglieriTS-Adenosyl- Methionine improves depression in patients with Parkinson’s disease in an open-label clinical trialMov Disord20001561225122911104210
- DudmanNPGuoXWGordonRBDawsonPAWilckenDEHuman homocysteine catabolism: three major pathways and their relevance to development of arterial occlusive diseaseJ Nutr19961264 Suppl1295S1300S8642474
- HoPIAshlineDDhitavatSFolate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteineNeurobiol Dis2003141324213678664
- JacobRAJendenDJAllman-FarinelliMASwendseidMEFolate nutriture alters choline status of women and men fed low choline dietsJ Nutr1999129371271710082779
- KaganBLSultzerDLRosenlichtNGernerRHOral S-adenosylmethionine in depression: a randomized, double-blind, placebo-controlled trialAm J Psychiatry199014755915952183633
- KnopmanDPattersonMAn open-label, 24-week pilot study of the methyl donor betaine in Alzheimer disease patientsAlzheimer Dis Assoc Disord200115316216511522934
- LipinskiJFCohenBMFrankenburgFOpen trial of S-adenosylmethionine for treatment of depressionAm J Psychiatry198414134484506367496
- McCaddonAHomocysteine and cognitive impairment; a case series in a General Practice settingNutr J20065616480506
- Melse-BoonstraAHolmPIUelandPMOlthofMClarkeRVerhoefPBetaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrationsAm J Clin Nutr20058161378138215941890
- MitsuyamaYKogohHSerum and cerebrospinal fluid vitamin B12 levels in demented patients with CH3-B12 treatment – preliminary studyJpn J Psychiatry Neurol198842165713398357
- Montero BrensCDalmau SerraJCabello TomasMLGarcia GomezAMRodes MonegalMVilaseca BuscaAHomocystinuria: effectiveness of the treatment with pyridoxine, folic acid, and betaineAn Esp Pediatr199339137418363149
- SchwabUTorronenAToppinenLBetaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjectsAm J Clin Nutr200276596196712399266
- SelhubJBagleyLCMillerJRosenbergIHB vitamins, homocysteine, and neurocognitive function in the elderlyAm J Clin Nutr2000712614S620S10681269
- ShippyRAMendezDJonesKCergnulIKarpiakSES-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDSBMC Psychiatry200443815538952
- TavoniAJeracitanoGCiriglianoGEvaluation of S-adenosylmethionine in secondary fibromyalgia: a double-blind studyClin Exp Rheumatol19981611061079543578
- ConnellyPJPrenticeNPCouslandGBonhamJA randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s diseaseInt J Geriatr Psychiatry200823215516017600848
- ItoTYamaderaHItoRSuzukiHAsayamaKEndoSEffects of vitamin B12 on bright light on cognitive and sleep-wake rhythm in Alzheimer-type dementiaPsychiatry Clin Neurosci200155328128211422876
- HaidemenosAKontisDGaziAKallaiEAllinMLuciaBPlasma homocysteine, folate and B12 in chronic schizophreniaProg Neuropsychopharmacol Biol Psychiatry20073161289129617597277
- KingJCDietary Guidelines for Americans 2005US Department of Health and Human Services and US Department of Agriculture2005 Available from: http://www.cnpp.usda.gov/Publications/DietaryGuidelines/2005/2005DGPolicyDocument.pdfAccessed on January 18, 2009
- DierkesJDomroseUAmbroschASupplementation with vitamin B12 decreases homocysteine and methylmalonic acid but also serum folate in patients with end-stage renal diseaseMetabolism199948563163510337865
- HofferLJSaboohiFGoldenMBarrePECobalamin dose regimen for maximum homocysteine reduction in end-stage renal diseaseMetabolism200554683584015931623
- EussenSJde GrootLCClarkeROral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trialArch Intern Med2005165101167117215911731
- NyholmETurpinPSwainDOral vitamin B12 can change our practicePostgrad Med J20037993021822012743340
- LederleFAOral cobalamin for pernicious anemia: back from the verge of extinctionJ Am Geriatr Soc1998469112511279736106
- KwongJCCarrDDhallaIATom-KunDUpshurREOral vitamin B12 therapy in the primary care setting: a qualitative and quantitative study of patient perspectivesBMC Fam Pract200561815723708
- HengstermannSHanemannANieczajRDoes an association between increased homocystein levels and cognitive dysfunction also exist in multimorbid geriatric patients?Z Gerontol Geriatr200942213113618535757
- HengstermannSLaemmlerGHanemannATotal serum homocysteine levels do not identify cognitive dysfunction in multimorbid elderly patientsJ Nutr Health Aging200812641141618548180
- HuangYCKuoYWLeeTHHypoalbuminemia and not hyperhomocysteinemia as a risk factor for dementia in hemodialysis patientsJ Ren Nutr200818434735418558299
- StipanukMHDominyJEJrLeeJIColosoRMMammalian cysteine metabolism: new insights into regulation of cysteine metabolismJ Nutr20061366 Suppl1652S1659S16702335
- FernandezHHWuCKOttBRPharmacotherapy of dementia with Lewy bodiesExpert Opin Pharmacother20034112027203714596656
- McKeithIDel SerTSpanoPEfficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international studyLancet200035692472031203611145488