Abstract
Fungal keratitis is one of the major causes of ophthalmic mycosis and is difficult to treat. The range of common antifungal agents available for fungal keratitis remains inadequate and is generally associated with poor clinical outcomes. Voriconazole is a new generation triazole antifungal agent. Only marketed in systemic formulation and, with broad-spectrum activity and high intraocular penetration, voriconazole has demonstrated effectiveness against fungal keratitis. Systemic voriconazole, however, is not without side effects and is costly. Voriconazole eye drops have been prepared extemporaneously and used for the treatment of ophthalmic fungal keratitis. The current article sought to review the literature for evidence related to the effectiveness and safety of topical voriconazole and its corneal penetration into the aqueous humor of the eye. The voriconazole eye drops used are typically of 1% concentration, well tolerated by the eye, and are stable. Despite existing evidence to suggest that the eye drops are effective in the treatment of fungal keratitis, more studies are needed, especially in relation to using the eye drops as first-line and stand-alone treatment, preparation of higher concentrations, and optimal dosing frequency.
Introduction
Corneal disease is second only to cataracts as the most common cause of blindness worldwide, resulting in more than 1.5 million new cases of vision loss annually.Citation1 As a consequence of attention being directed towards the management of cataracts, especially in developing countries, strategies for the management of traditional infections that cause blindness have been neglected.Citation2
Ophthalmic mycosis is emerging as a major cause of vision loss and morbidity, and can be life-threatening.Citation3,Citation4 Fungal keratitis is one of the major causes of ophthalmic mycosis,Citation5 accounting for more than 50% of proven ophthalmic mycoses in some countries.Citation6 Fungal keratitis is usually characterized by a corneal epithelial defect and inflammation of the corneal stroma. If untreated, fungal keratitis can lead to corneal scarring and vision loss.Citation1
Fungal keratitis
The first description of fungal keratitis was in the late 1870s.Citation7 Fungal keratitis is most common in tropical regions and developing countries, where it constitutes over 50% of keratitis.Citation8 In South India, about 44% of corneal ulcers are caused by fungi. Although lower, the prevalence of fungal keratitis is still relatively high in other countries, being 17% in Nepal, 36% in Bangladesh, 38% in Ghana, and 35% in south Florida in the US. In China, the incidence has been increasing in the past decade.Citation9 By contrast, fungal keratitis generally accounts for only 1%–5% of the keratitis treated in developed countries and temperate regions, such as Britain and the northern US.Citation9,Citation10 This also applies to Australia, where the incidence of fungal keratitis at the Royal Victorian Eye and Ear Hospital (RVEEH) in Melbourne was reported at 5%. The RVEEH is a tertiary referral eye hospital responsible for the care of most serious corneal infections in a population of about five million across Victoria, southern New South Wales, and Tasmania.Citation10
Etiology
Filamentous fungi were long considered as a major cause of fungal keratitis.Citation11,Citation12 Ophthalmic infections from these fungi are most commonly associated with agricultural and outdoor activities.Citation13,Citation14 Of the filamentous fungi, infections from Fusarium and Aspergillus species are the most common. While Fusarium species are particularly prevalent in crop plants,Citation15 Aspergillus species are found in decaying vegetation and soil. Aspergillus is a contaminant in hospital air and has been involved in recent outbreaks of ocular infections in several hospitals.Citation16,Citation17 Keratitis caused by these filamentous fungi may involve any part of the cornea.Citation18,Citation19 Other less common keratitis-causative filamentous fungi include Paecilomyces and Acremonium species.Citation20 Paecilomyces species have been shown to be resistant to most common sterilising techniques, including those applied during surgical procedures.Citation21,Citation22 Acremonium species can be isolated from a variety of common sources, and can be associated with severe eye infections.Citation23,Citation24
Dematiaceous fungi such as Curvularia, Bipolaris, and Exserohilum species have also been reported to cause fungal keratitis. After Aspergillus and Fusarium species, Curvularia and Bipolaris species are the third most common keratitis-causative fungi worldwide.Citation20 Curvularia, Bipolaris, and Exserohilum species usually cause persistent, but low-grade ulcerations near the epithelial part of the cornea. These ulcerations, if not appropriately treated or if associated with topical steroid use, can develop into resilient infections involving the deeper layers of cornea.Citation25–Citation27 Scedosporium and Lecytophora species are also dematiaceous fungi that are known to result in very severe keratitis infections that often do not respond to medical therapies.Citation25,Citation28
Whilst filamentous and dematiaceous fungi are the common causes of fungal keratitis at a global level, yeasts are the major cause of fungal keratitis in developed countries.Citation8 Yeasts are infrequent in tropical countries, characterized by major agricultural presence, which is associated with higher prevalence of other types of fungal keratitis, such as the filamentous fungi.Citation20 Yeast infections have no geographical dominance and are most commonly caused by Candida species, especially Candida albicans.Citation8,Citation10,Citation20 Candida keratitis predominantly occurs in the stromal layer of the cornea. It is associated with epithelial defect and distinct infiltration, and is slow in development.Citation18 Cryptococcus species are another type of yeast that causes fungal keratitis, but less commonly than Candida species.Citation20
Fungal keratitis can also be caused by zygomycetes fungi such as Rhizopus and Mucorales species,Citation4,Citation29,Citation30 and other fungi such as Cladosporium, Cylindrocarpon, Penicillium, and Chrysonilia species.Citation6,Citation14,Citation31–Citation33 Keratitis due to these fungi, however, is very low in occurrence.
The incidence of different types of fungal keratitis in various areas and countries is shown in .
Table 1 Studies of the incidence of types of fungal keratitisCitation8,Citation10,Citation13,Citation20,Citation34
Risk factors
The general predisposing factors for fungal keratitis include ocular trauma, prolonged use of topical or systemic immunosuppressants, pre-existing corneal surface disease, underlying systemic disease (eg, diabetes mellitus), and contact lens wear.Citation10,Citation20,Citation35
The significance of these factors, however, varies according to geographical area. For instance, in Melbourne, ocular trauma, chronic steroid use, and ocular surface disease were the most common risk factors,Citation10 whilst the common risk factors in Philadelphia were ocular surface disease, contact lens wear, and topical steroids.Citation33 In the southern US, however, trauma was generally identified as the major risk factor for fungal keratitis. A similar trend was also observed in Singapore and Bangladesh.Citation36,Citation37 In contrast, in the northern US, ocular trauma was reported as only a secondary risk factor for fungal keratitis.Citation9
The type of predisposing risk factors relates to the type of causative fungi. For example, keratitis associated with ocular trauma is commonly caused by Aspergillus, Fusarium, and Curvularia species.Citation14 The use of lawn trimmers was found to be associated with Fusarium and Curvularia keratitis,Citation14,Citation38 while the use of topical steroids was linked to Candida, Aspergillus, Acremonium, and Curvularia keratitis. Underlying chronic diseases were frequently related to keratitis caused by Fusarium and Candida species.Citation14 Candida keratitis is common where traumatic keratitis is infrequent.Citation20 Previous corneal ulceration resulting, for example, from previous keratitis or contact lens-related trauma, is a particular risk factor for Candida keratitis.Citation18 Trauma by plant material, contaminated water, or immune suppression is a risk factor for keratitis caused by Scedosporium apiospermum.Citation20 Keratitis caused by Paecilomyces species has been reported following surgical procedures.Citation21,Citation39
Treatment
The ultimate goal in the treatment of fungal keratitis is to conserve vision. This requires timely diagnosis of the infection and administration of the appropriate antifungal therapy.Citation40 Patient with fungal keratitis can be treated with either medical or surgical therapy. Whilst surgical procedures are more effective in patients with acute corneal perforation, antifungal agents are still the major therapeutic option in fungal keratitis,Citation41 whereby success depends on the agent’s ability to penetrate into the aqueous humor and achieve therapeutic levels. Currently, the range of antifungal therapies available for fungal keratitis remains inadequate.Citation9,Citation42 The antifungal agents that can be used in fungal keratitis are broadly divided into three main groups: polyenes (amphotericin B, natamycin, and nystatin), azoles (ketoconazole, miconazole, econazole, fluconazole, itraconazole, voriconazole, and posaconazole), allylamine (terbinafine) and echinocandins (caspofungin).
Amphotericin B has poor ocular penetration after intravenous (IV) administration and, hence, the administration of higher doses may be required to ensure adequate concentration of amphotericin B in the eye;Citation20,Citation43 however, IV administration of high-dose amphotericin B is known to cause severe renal toxicity, which can occur in up to 80% of patients.Citation20,Citation43 To minimize renal toxicity, low-dose amphotericin B is often used, which in many cases, results in suboptimal doses,Citation44 especially when taking its poor ocular penetration into consideration. Amphotericin B eye drops are manufactured extemporaneously by hospital pharmacy departments. The most commonly prescribed concentration of the eye drops for fungal keratitis is 0.15%.Citation10,Citation20 Topical amphotericin B penetrates well into the stroma and can achieve sufficient concentrations against susceptible fungi;Citation20 however, its penetration through the cornea with intact epithelium is poor. Whilst amphotericin B is active against Aspergillus and Candida keratitis, it has no activity against keratitis caused by Fusarium species.Citation9,Citation45
Natamycin is the only commercially available topical antifungal preparation approved by the Food and Drug Adminstration for ophthalmic use.Citation28,Citation46 It is insoluble in water and is stable in 5% suspension.Citation20 Natamycin is the standard of care in many countries, especially developed countries,Citation9,Citation10,Citation20 and is initially administered as one drop every one or two hours.Citation47 It adheres well to the cornea surface, is well tolerated, has good activity against Candida, Aspergillus, and Fusarium, and is routinely used for keratitis caused by filamentous fungi.Citation9,Citation20 This antifungal, however, has poor penetration into deeper structures of the eye and, hence, is generally effective against superficial infections that are not severe.Citation9,Citation48 In addition, only about 2% of the drug is bioavailable after topical application.Citation49 The usefulness of topical natamycin is further complicated by the fact that it settles out on the cornea upon instillation and degrades easily.Citation28
Nystatin is another polyene that can be used topically as a suspension in fungal keratitis. However, nystatin is rarely used clinically due to the availability of more potent polyene agents.Citation50
Ketoconazole has a broad spectrum of activity, including against Aspergillus, Candida, and Fusarium species.Citation28 It is available orally and, although it has demonstrated good tissue distribution after administration,Citation46 it has not been used for fungal keratitis. Long-term administration of high-dose ketoconazole may result in impotence, gynecomastia, or alopecia, which is problematic considering the long-term nature of keratitis therapy.Citation51 Topical 1% eye drops and suspension formulations of ketoconazole have been extemporaneously prepared and used for fungal keratitis. These have been reported to inhibit the progression of corneal fungal infections and were not associated with significant corneal toxicity. Citation52,Citation53
Miconazole has been used in patients with S. apiospermum orbital infections.Citation20 It has a broad spectrum of activity, including against Aspergillus, Candida, and Scedosporium species.Citation20 Systemic miconazole, however, is associated with significant toxicity and has resulted in undetectable concentrations in the cornea.Citation20,Citation54 Topical application of extemporaneously prepared 1% miconazole eye drops achieved high concentrations in the ocular tissues.Citation54 The eye drops are generally well tolerated and are used as second-line therapy in fungal keratitis that is unresponsive to natamycin.Citation54
Econazole has a broad spectrum of activity against filamentous fungi, and is effective against Fusarium keratitis.Citation55 Topical application of 2% econazole appears to be as effective as 5% natamycin in fungal keratitis,Citation56 but has been associated with ocular irritation.Citation55
Oral and IV fluconazole are very safe, and penetrate very well into the corneal tissue.Citation20,Citation57,Citation58 Whilst oral fluconazole is a commonly used agent for the treatment of fungal keratitis,Citation10 topical application of 0.2% fluconazole solution is as effective as systemic fluconazole. Fluconazole, when applied topically, penetrates well into the cornea, is safe, and has been used successfully against fungal keratitis.Citation59–Citation61 A major limitation associated with fluconazole, however, is its narrow spectrum of antifungal activity. Fluconazole is inactive against Aspergillus and Fusarium species;Citation62 although active against Candida species, it is less active against Candida glabrata and Candida krusei than against C. albicans.Citation46
Although itraconazole is commonly associated with gastrointestinal side effects, it is considered relatively safe.Citation63 It has activity against Candida and Aspergillus species; however, is rarely used for the treatment of fungal keratitis.Citation28 Itraconazole is inactive against Fusarium speciesCitation10,Citation28 but, more importantly, it has poor penetration into the cornea after systemic administration.Citation64 Experimental use of topical itraconazole (1% solution) has been reported, but appears to demonstrate insufficient corneal penetration.Citation65
Voriconazole, a more recent azole antifungal, is available commercially for systemic administration in the form of oral and IV formulations. It has an excellent broad spectrum of antifungal activity and is active against species that are known to be resistant to the other antifungal agents commonly used in fungal keratitis.Citation28 Voriconazole is increasingly being used, orally in particular, against fungal keratitis. Oral voriconazole is highly bioavailable (96%) and has demonstrated good penetration into the different parts of the eye,Citation66,Citation67 with sufficient concentrations achieved to cover a wide range of keratitis-causative fungi.Citation28 However, oral voriconazole can be associated with side effects as well as significant drug interactions.Citation68 Topical voriconazole eye drops, manufactured extemporaneously and used in an off-label manner, have also been prescribed for the treatment of keratitis, with promising results.Citation69 With topical administration, voriconazole demonstrated good penetration through the cornea into the aqueous humour,Citation69 without compromising intraocular safety.Citation70
Posaconazole has an excellent broad spectrum of activity and is as active as voriconazole, with added activity against zygomycetes.Citation71 It is safe, with mild gastrointestinal side effects being the most common adverse events.Citation72 Posaconazole was only recently introduced worldwide and, as such, studies on its ocular penetration are lacking. In a number of recent case reports involving the use of oral posaconazole alone as salvage therapy, or in combination with topical posaconazole, this antifungal agent demonstrated success against fungal keratitis.Citation73,Citation74 The formulation used for the topical posaconazole was the same formulation used for the oral suspension (10 mg/0.1 mL).Citation28
Terbinafine is fungicidal against many molds, but only a few types of yeast.Citation75 Despite its activity, its clinical efficacy and use are limited by its pharmacokinetic characteristics after the systemic administration.Citation76,Citation77 When used as 0.25% eye drops, however, it is as effective against filamentous mycotic keratitis as 5% natamycin, especially in cases with smaller and shallower ulcers.Citation78 The eye drops are safe, but required longer durations of treatment when compared with other common topical therapies.Citation78
Caspofungin has significantly less systemic toxicity than azoles. Intravenously administered caspofungin does not penetrate well into the eye and, hence, it is not used for fungal keratitis.Citation79 Nonetheless, in one recent case report, when administered topically (0.5%) as adjunctive therapy, caspofungin demonstrated clinical success against fungal keratitis.Citation60 Caspofungin is safe,Citation79 but lacks activity against Fusarium species.Citation9,Citation80
Of the aforementioned antifungal agents, amphotericin B, natamycin, fluconazole, and miconazole have been used routinely to treat fungal keratitis for quite some time; however, poor corneal penetration after topical administration, poor ocular penetration after systemic administration, limited spectra of antifungal activity, and/or limited clinical success associated with these agents are major limitations and have rendered these therapies challenging and inadequate for fungal keratitis. The limited clinical success is particularly true with the topical use of these agents, as they often require co-administration of an additional systemic antifungal agent,Citation20 which increases the risk of toxicity and is costly. This has led to consideration of using newer antifungal agents, such as voriconazole, posaconazole, and caspofungin, and/or in-house preparations of these agents as a means to overcome the shortcomings of the current therapies.
This review will focus on the use of voriconazole eye drops as a treatment for fungal keratitis.
Topical voriconazole for fungal keratitis
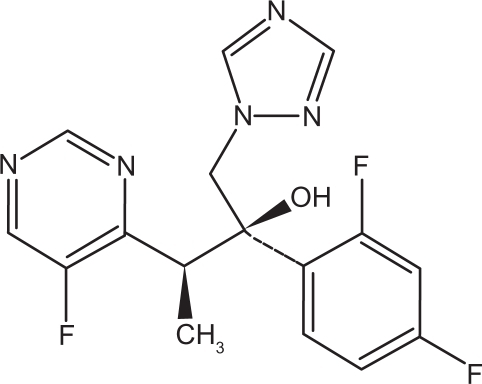
Voriconazole acts by inhibiting the synthesis of ergosterol in the fungal membranes and, ultimately, the growth of the microorganism (). Voriconazole binds to the active site of the P450-dependent enzyme lanosterol 14-demethylase (CYP51 or Erg11p) and ligates the iron heme cofactor via a nitrogen atom. This results in depletion of ergosterol and the accumulation of 14-methyl sterols such as lanosterol, affecting the integrity and function of the fungal membrane. Voriconazole is a derivative of fluconazole with the addition of a methyl group to the propyl backbone and the replacement of a triazole moiety with a fluoropyrimidine group, which significantly increased the affinity of the compound for 14-demethylase and its potency to inhibit CYP51. Voriconazole concentrations needed for a 50% decrease in ergosterol synthesis (IC50) in fungi extracts of C. albicans and C. krusei are 2 and 20 μg/L, respectively, compared with 10 and 230 μg/L, respectively, with fluconazole. With these two fungi, voriconazole is considered to be a more potent inhibitor of CYP51 than fluconazole. Similarly, the IC50 for Aspergillus fumigatus is 0.48 with voriconazole against 4.8 for fluconazole.Citation81,Citation82
Figure 1 Chemical structure of voriconazole.Citation83
Voriconazole is ideal for use in the treatment of fungal keratitis, as it has a broad spectrum of activity with low minimum inhibitory concentrations (MIC), as well as a high systemic intraocular penetration profile.Citation28,Citation81
Voriconazole is potent against a wide spectrum of keratitis-causative fungi, namely, the most common pathogens C. albicans, C. parapsilosis, C. tropicalis, A. fumigatus, Aspergillus flavus, and Fusarium solani,Citation81,Citation84,Citation85 and other less common pathogens from the Paecilomyces, Histoplasma, Scedosporium, Curvularia, and Acremonium species.Citation81,Citation84 The in vitro MICs of voriconazole against typical keratitis-causative fungi are shown in .
Table 2 In vitro minimum inhibitory concentrations (MIC90) with voriconazoleCitation69,Citation81,Citation96
Although the MIC of voriconazole against Fusarium species is higher than that for other fungi, compared with other antifungal agents, voriconazole has the best activity against Fusarium species.Citation86 In a study by Marangon et al,Citation86 in which the in vitro susceptibility of common pathogens to voriconazole was compared with that for amphotericin B, fluconazole, itraconazole, and ketoconazole, voriconazole demonstrated the lowest MIC90, as shown in . In addition, voriconazole was the only antifungal agent that demonstrated 100% antifungal activity against 541 different fungal isolates comprising Candida, Aspergillus, and Fusarium species ().
Figure 2 In vitro susceptibility of 541 fungal isolates to common antifungals.Citation86
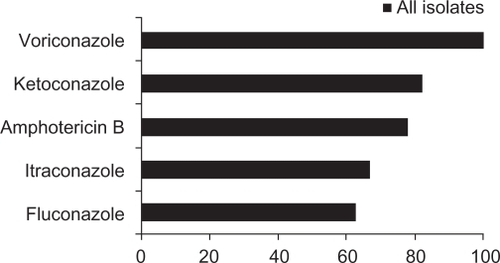
Table 3 In vitro minimum inhibitory concentrations (MIC90) of common antifungalsCitation86
Intraocular penetration of systemic voriconazole
In a prospective clinical study by Hariprasad et al,Citation67 systemically administered voriconazole was demonstrated to achieve good penetration into the aqueous and vitreous humors of the human eye. Two 12-hourly 400 mg doses of voriconazole were administered to 14 patients with noninflamed eyes and attending elective surgery. The aqueous and vitreous humor samples were collected within three hours after drug administration. The mean measured plasma, aqueous, and vitreous voriconazole concentrations were 2.13, 1.13, and 0.81 μg/mL, respectively. The voriconazole concentration in the aqueous humor was 53% of the concentration obtained in the plasma, and was sufficiently high to be effective against most common fungi associated with fungal keratitis. A similar outcome was reported by Nulens et al,Citation87 where a case of S. apiospermum keratitis was successfully treated with oral voriconazole. The voriconazole concentration in the aqueous humor (1.8 μg/mL) was measured after 12 days of drug administration and was also 53% of the voriconazole concentration observed in the patient’s plasma (3.4 μg/mL). Surprisingly, however, and despite good intraocular penetration of oral voriconazole, the reported success of oral voriconazole against fungal keratitis may not necessarily translate into success against fungal infections occurring in the posterior part of the eye (eg, endophthalmitis). Indeed, while in the case reported by Nulens et al,Citation87 oral voriconazole resulted in a voriconazole concentration in the aqueous humor that is sufficient to treat S. apiospermum keratitis, in a case report by Nochez et al,Citation88 the voriconazole concentration in the vitreous humor, resulting from oral voriconazole, was not sufficiently high to manage successfully an endophthalmitis infection that is also caused by S. apiospermum. Concomitant administration of intravitreal voriconazole was required to achieve a successful outcome.Citation88
Although it has good intraocular penetration, systemic voriconazole may result in side effects (including ocular events), complications, and interactions with concomitant medications.Citation68 Whilst mostly reversible, these side effects may lead to the discontinuation of therapy.Citation81 In addition, systemic voriconazole is very costly. The cheapest of its formulations (ie, oral voriconazole tablets) costs about AU$3,000 (US$2,600) per month of therapy for fungal keratitis.Citation61 When administered intravenously, it can cost up to AU$11,400 (US$9,600) per month.Citation89 Therefore, an efficient and economical strategy of using voriconazole for the treatment of fungal keratitis is highly desirable and would be invaluable in clinical practice.
Eye drops and ophthalmic drug delivery
The topical administration of medications to the eye is a typical strategy for treating disorders of anterior eye structures, such as the cornea.Citation90,Citation91 Eye drops are the most common dosage form used,Citation91 because they are an economical and efficient method of delivering drugs into the eye, and have four main advantages.Citation90 Firstly, the drug effect is localized where it is needed and a minimal amount of the drug reaches the systemic circulation. Secondly, drug concentrations that are hard to achieve at the site via systemic administration, can be achieved via topical administration. Thirdly, topically administered drugs avoid hepatic metabolism. Lastly, topical administration is convenient, simple, and painless.
The corneal tissues comprise three major layers of cells, ie, a lipophilic outermost layer called the epithelium, a hydrophilic middle layer called the stroma, and an innermost layer of single cells called the endothelium.Citation92,Citation93
Given that the cornea comprises both lipophilic and hydrophilic substances, it represents an effective barrier against delivering both lipophilic and hydrophilic drugs into the eye.Citation90 A lipophilic compound encounters minimal resistance in penetrating the outer epithelium of the cornea, but more resistance in infiltrating the stroma. The converse applies to hydrophilic compounds, which encounter more resistance to absorption from the epithelium and less by the stroma. As the corneal epithelium is the main and first barrier to drug absorption into the eye,Citation94 it is not surprising that lipophilic compounds are more favorable for corneal absorption.Citation90
Formulation of voriconazole eye drops
Whilst lipophilic compounds (or drugs) have higher corneal permeability, they usually have limited aqueous solubility. As such, formulating eye drops for drugs with low aqueous solubility can be challenging.Citation90 Voriconazole is a lipophilic compound with low solubility (0.061% at pH 7), and is unstable in aqueous environments.Citation90,Citation95 For the IV formulation of voriconazole to be feasible, the manufacturer encapsulated voriconazole with a β-cyclodextrin derivative in the form of lyophilized powder of cyclodextrin-voriconazole complex.Citation68 This increases the solubility and stability of voriconazole in aqueous solutions, while maintaining its lipophilicity and high corneal permeability.Citation90,Citation94 Cyclodextrins are a group of homologous cyclic oligosaccharides that, in complex formation with a drug, increase dissolution rate (solubility), aqueous stability, and/or bioavailability of the drug.Citation94
Currently, voriconazole eye drops are not commercially available, and are aseptically manufacturedCitation61,Citation96 by diluting the IV formulation of voriconazole. IV voriconazole (Vfend®, Pfizer) is available as a glass vial that contains a white lyophilized powder containing 200 mg voriconazole and 3,200 mg sulfobutyl ether β-cyclodextrin sodium. As per the voriconazole package insert, the powder is reconstituted with 19 mL of water for injection to produce a 20 mL aqueous voriconazole solution with a concentration of 10 mg/mL (1%).Citation68 This voriconazole solution is what is typically being used as eye drops.Citation96,Citation97
Pharmacokinetic data on the corneal penetration of topically administered voriconazole are lacking; however, a number of studies have suggested good penetration of voriconazole through the cornea into the aqueous humor.
Animal studies
Several studies have been performed to assess the penetration and tolerability of voriconazole eye drops in animals. In a study by Sponsel et al,Citation98 topical voriconazole (5 or 10 μg/mL) was evaluated for efficacy against Paecilomyces lilacinus keratitis in 10 rabbits (10 infected eyes). Voriconazole demonstrated good and deep penetration into the rabbits’ eyes. The measured tissue concentrations in the cornea were sufficiently high (24.3 and 51 ng/mL with 5 and 10 μg/mL voriconazole, respectively), and the experimental keratitis was treated successfully.
Topical application of voriconazole eye drops was also investigated in a horse model by Clode et al,Citation99 where voriconazole eye drops (0.5%, 1.0%, and 3% solutions) were administered to seven healthy horses (four eyes for each concentration). With the measured aqueous humor concentrations being 1.43, 2.35, and 2.4 μg/mL, respectively, topical voriconazole was shown to penetrate effectively through the cornea and achieve detectable levels.
It is important to recognize, however, that extrapolating penetration data from animal models to humans may not be reliable. Rabbits, for instance, have a very low blink rate and a large epithelial eye surface, which enhances the penetration of lipophilic and nonirritating drugs, such as voriconazole, into the cornea.Citation100 In addition, while the drainage rate of eye drops from the ocular surface in rabbits is about 4 μL per minute, it is over twice as much in humans.Citation94
Studies in nonkeratitis patients
Three studies have investigated voriconazole penetration through the human cornea into the aqueous humor. Two of the studies investigated 1% voriconazole eye drops,Citation61,Citation96 and one study investigated 2% voriconazole eye drops.Citation101
In a prospective study by Vemulakonda et al,Citation96 13 patients scheduled for vitrectomy surgery were recruited to receive a two-hourly 1% voriconazole eye drop for 24 hours. Samples were taken 24 ± 14 minutes after the last dose. Topical voriconazole was well tolerated by the eye, and the mean measured voriconazole concentration in the aqueous humor was 6.49 ± 3.04 μg/mL, which is sufficiently high to be effective against common pathogens. The concentration, however, was a peak level concentration, because it was taken 24 minutes after a two-hourly dosing regimen (peak concentration is reached 20 to 30 minutes after eye drop administration).Citation91 While this study did not demonstrate that the two-hourly dosing regimen results in sustained and adequate therapeutic trough voriconazole concentrations in the eye, the ability of topically administered voriconazole eye drops to achieve high aqueous humor concentrations was demonstrated.
In another prospective study by Lau et al,Citation61 10 patients scheduled for anterior segment surgery were recruited to receive either a 1% voriconazole eye drop every six hours for three days, or every hour for four doses. The eye drops were well tolerated, but the aqueous humor concentrations achieved were not sufficiently high to be effective against all common pathogenic fungi. After the six-hourly and hourly dosing, the voriconazole concentrations in the aqueous humor were 0.94 ± 1.21 and 1.9 ± 1.12 μg/mL, with average sampling times of 2.1 ± 0.6 and 1.1 ± 0.5 hours after the last dose, respectively. If samples from the six-hourly regimen were to be taken six hours after the last dose (ie, at trough level), the concentrations measured would be even less than 0.94 μg/mL, suggesting that six-hourly dosing of 1% voriconazole eye drops may be ineffective. Samples taken after hourly dosing were collected approximately one hour after the last dose, representing trough level concentrations. Although the measured 1.9 μg/mL concentration is effective against Aspergillus and Candida keratitis, it is ineffective against other common types, such as Fusarium keratitis.
Al-Badriyeh et al investigated the penetration of 2% voriconazole eye drops,Citation101 with the hypothesis that increasing the concentration of voriconazole in the eye drops will increase the amount of voriconazole penetrating into the eye. Hourly 2% voriconazole eye drops were given to 13 human subjects four hours prior to elective anterior segment eye surgery. The mean voriconazole concentration in the aqueous humor was 1.67 ± 0.97 μg/mL, while the mean sampling time after the last eye drop administration was at 1.3 ± 0.3 hours. No side effects or toxicities were reported. The design of this study was similar to that of Lau et al,Citation61 in that both studies had identical numbers and frequencies of doses administered; trough voriconazole levels were measured; the same volumes (∼0.05 mL) of eye drops were administered at each dose,Citation102 and 0.01% benzalkonium chloride solution (a preservative) was used as a diluent for the preparation of the eye drops. It should be noted that the clinical studies by Lau et al,Citation61 and Al-Badriyeh et al,Citation101 are the only studies in the literature that involved the use of benzalkonium chloride solution. Benzalkonium chloride is a quaternary ammonium compound with a broad range of antimicrobial activity.Citation103 It is the most frequently used preservative in ophthalmic solutions, and its concentration ranges from 0.004 to 0.02%.Citation103 In addition to preventing microbial contamination, benzalkonium chloride is known to act as a corneal penetrating enhancer, promoting drug penetration through the strong corneal barrier.Citation104 The study by Al-Badriyeh et al found that the concentration of voriconazole in the aqueous humor resulting from the 2% voriconazole eye drops was not significantly different from that reported for the 1% solution,Citation61,Citation101 suggesting that the penetration of voriconazole through an intact infection-free cornea is not concentration-dependent, at least for the concentration range studied. This appears to be counterintuitive to the hypothesis of the study but is consistent with data from the horse model by Clode et al,Citation99 in which the voriconazole level in the corneas of horses with fungal keratitis did not change when the concentration of the administered voriconazole eye drops was changed from 1% to 3%.
The studies by Vemulakonda et al, Lau et al, and Al-Badriyeh et al explored the penetration of voriconazole eye drops into the human aqueous humor using different dosage regimens and concentrations.Citation61,Citation96, Citation101 However, it is important to recognize the major limitation of these studies, ie, that the eye drops were applied to noninfected eyes. It has been widely observed that corneal drug penetration will generally be enhanced with the destruction of the corneal epithelium.Citation105 For instance, the removal of the surface of the corneal epithelium is recommended to improve the penetration of topical amphotericin B.Citation100 On the other hand, in the rabbit model by Sponsel et al,Citation98 when the penetration of topical voriconazole into the infected eyes was compared with penetration into the noninfected eyes of the rabbits, it was found that the corneal concentration of voriconazole in the noninfected eyes (after topical administration) was higher than that in the infected corneas. However, in a recent case series by Thiel et al (where voriconazole concentrations in the aqueous humor, following the administration of voriconazole eye drops, were compared among patients with different degrees of corneal injuries) voriconazole concentrations in the infected eyes depended neither on the size of the epithelial defect nor on epithelial removal.Citation100 These, however, are preliminary findings, and the effect of corneal damage on the penetration of voriconazole into the human eye remains to be fully elucidated.
Studies in keratitis patients
To date, the penetration of topical voriconazole eye drops through the infected cornea in humans has only been reported twice, and in the form of case reports. In the case reported by Klont et al,Citation69 the aqueous humor voriconazole concentration was measured after 13 days of topical 1% voriconazole, co-administered with oral voriconazole, for the treatment of a patient with Fusarium keratitis. The advantage of topical voriconazole was demonstrated by an aqueous humor concentration of 3.2 μg/mL, which was 160% of the voriconazole concentration in plasma (2 μg/mL). In the previously mentioned case series by Thiel et al,Citation100 six patients, including five patients with fungal keratitis, received IV and topical voriconazole for the treatment of Aspergillus and Candida infections. The aqueous humor samples were collected at stages of therapy where voriconazole eye drops were used alone, yielding voriconazole concentrations ranging from 0.61 to 3.3 μg/mL. The results were highly variable, but provided support for the benefit of using voriconazole eye drops.
Efficacy
Although voriconazole concentrations were detected in the aqueous humor after topical administration of voriconazole eye drops, this may not necessarily correlate with efficacy in the clinical setting of fungal keratitis.Citation20 Well-designed clinical studies of voriconazole eye drops in patients with active fungal keratitis are difficult to perform and, therefore, lacking. The difficulties in conducting such studies relate to the low incidence of fungal keratitis as well as the need for long treatment duration. In addition, in clinical settings, patients will mostly be receiving other antifungal therapies that will interfere with the outcomes measured.
Currently, evidence of the clinical efficacy of voriconazole eye drops in fungal keratitis is based solely on case reports. A review of the published literature identified nine reports on the use of voriconazole eye drops for the treatment of fungal keratitis.Citation69,Citation74,Citation106–Citation111 The case reports are summarized in .
Table 4 Case reports of the use of topical voriconazole in fungal keratitisCitation69,Citation74,Citation106–Citation111
In most of the reported cases, voriconazole eye drops were used in combination with systemic voriconazole, except for the case reports by Al-Badriyeh et al,Citation110,Citation111 where voriconazole eye drops were used as monotherapy. Voriconazole 1% eye drops were used in all cases, except in the case reported by Polizzi et al,Citation106 where 2% voriconazole was used. Brief summaries of these cases are given below.
The first of these cases, reported by Reis et al,Citation108 involved a 16-year-old girl diagnosed with keratitis caused by F. solani after swimming in a lake. After months of antifungal therapy, the fungal keratitis failed to respond. The patient was initially prescribed topical amphotericin B and fluconazole, followed by itraconazole at a later stage. These, however, had no effect on the infection and, hence, IV voriconazole followed by oral voriconazole was administered. A significant improvement was noticed, followed by resolution upon the addition of topical voriconazole to therapy, which was discontinued after eight weeks.
The case report by Klont et al also reported the use of 1% voriconazole eye drops in the treatment of Fusarium keratitis.Citation69 A 23-year-old man with F. solani keratitis failed to respond to treatment despite initial topical amphotericin B and itraconazole. The patient was then prescribed, as salvage therapy, concomitant IV and topical voriconazole followed by oral and topical voriconazole. The treatment was ceased at week 6, with a successful outcome.
In the case report by Prats et al,Citation107 a 19-year-old man was admitted with an incisive eye wound, with the cornea totally sectioned upon trauma. S. apiospermum keratitis was diagnosed. Upon failure of initial empirical antifungal therapy, systemic (IV and oral) and topical voriconazole were commenced. The infection resolved, and the eye did not have to be enucleated. This was the first case report where voriconazole was used for the treatment of S. apiospermum keratitis. Five months after the incident, a penetrating keratoplasty and chamber intraocular lens implantation was performed with a favorable visual outcome.
Jones et al demonstrated that voriconazole was effective in a 52-year-old woman diagnosed with Aspergillus niger keratitis.Citation109 The patient was initially treated with topical amphotericin B, which was not effective. When the patient was switched to a combination of oral and topical voriconazole, the infection improved rapidly and resolved after five weeks.
In the first of the two cases reported by Tu et al,Citation74 a 29-year-old man received oral and topical voriconazole for the treatment of trauma-induced Fusarium keratitis. In the second case, a 43-year-old woman received a combination of IV, topical, and intravitreal voriconazole for keratitis caused by F. solani that was associated with contact lens wear. In both of these cases, voriconazole was initially effective until it had to be discontinued because of severe hepatotoxicity. Patients were then switched to posaconazole as salvage therapy.
In the two case reports by Al-Badriyeh et al,Citation110,Citation111 1% voriconazole was used as a stand-alone therapy. In one case report,Citation110 a 54-year-old woman presented with a painful injected eye. Despite empirical therapy, symptoms persisted. Keratitis was later diagnosed and identified as a rare S. apiospermum keratitis. Primary antifungal therapy with natamycin 5% was not successful and was switched to 1% voriconazole eye drops. Vision improved in five days, and the corneal defect completely re-epithelialized in a week. In the other report by Al-Badriyeh et al,Citation111 a 48-year-old presented with keratitis following exposure to dust, the cause of which was later identified as C. albicans. Despite empirical antibacterial therapy, the epithelial defect persisted. Primary antifungal therapy with 1% voriconazole eye drops was initiated. The corneal infiltrate resolved in two days, the epithelial defect was completely healed, and visual acuity was restored in seven days.
Polizzi et al reported the only case where 2% voriconazole eye drops were used.Citation106 A 56-year-old man developed corneal ulceration caused by F. solani upon accidental contact with vegetation. Corneal ulceration developed and a perforating keratoplasty was performed. Systemic and topical amphotericin B and fluconazole were administered initially, but the patient did not improve. A new abscess formed on the transplanted graft and a wound leak developed. Therapy was then switched to IV and oral voriconazole in combination with topical 2% voriconazole eye drops. The patient completely recovered after 20 days of treatment with voriconazole.
A number of important considerations are associated with the above cases. One is that the use of voriconazole eye drops was associated with successful outcomes in most cases of fungal keratitis. Whilst the voriconazole therapy appeared to fail in the cases reported by Tu et al,Citation74 the failure was not due to lack of efficacy, but to severe side effects from systemically administered voriconazole, which required discontinuation of treatment. A second issue is that voriconazole eye drops were used as an adjunct to systemic voriconazole in most reported cases. Only Al-Badriyeh et al have demonstrated the clinical benefit of topical voriconazole when used alone as primary and salvage therapies.Citation110,Citation111 Thirdly, whilst the 2% voriconazole eye drops were effective,Citation106 the advantage of using 2% compared with 1% voriconazole eye drops remains unknown. A fourth issue is that, in the reports by Al-Badriyeh et al,Citation110,Citation111 the voriconazole eye drops were prepared as a solution containing 0.01% benzalkonium chloride as preservative, whereas, in all other case reports, the eye drops were prepared in sterile water for injection. In these case reports, voriconazole eye drops were typically administered with a dosing frequency of one drop every 0.5, 1.0, or 2.0 hours,Citation69,Citation74,Citation106–Citation111 and the average duration of administration of the voriconazole eye drops as adjunct therapy (ie, one to two months) was similar to the average duration of administration of the voriconazole eye drops given as monotherapy (ie, one month). However, it is common for the administration of the eye drops to extend beyond the resolution of fungal keratitis, with a duration that is more related to local clinical practices rather than time to resolution.
Safety
The safety of voriconazole in the eye was first evaluated in a rat model by Gao et al,Citation85 who demonstrated that intravitreal voriconazole concentration as high as 25 mg/mL did not result in any electroretinographic or histologic abnormalities in the rat retina. This, however, cannot be extrapolated as an evidence of the safety of voriconazole when topically applied to the human cornea.
In a stability study by Al-Badriyeh et al,Citation112 changes in the pH of 1% and 2% voriconazole eye drops were followed for over three months, and were found to range between 6.02 and 6.27. This was consistent with the findings of the only other stability study of voriconazole eye drops, performed by Dupuis et al,Citation97 where a pH of 7 was maintained for 1% voriconazole eye drops with a storage duration of 30 days. These pH values are usually well tolerated by the eye.Citation112 It is unlikely, therefore, that any eye irritation resulting from the use of the voriconazole eye drops would be a consequence of low pH.
Indeed, in the above studies that evaluated the application of voriconazole eye drops to the eye of animals and humans, the drops were well tolerated, and associated with mild or no side effects. In the animal model by Sponsel et al,Citation98 all rabbits responded well to the voriconazole eye drops with no apparent side effects. In the horse model by Clode et al,Citation99 0.5% and 1% voriconazole eye drops resulted in no side effects, and only the higher 3% concentration was associated with ocular toxicity. In the clinical study by Vemulakonda et al,Citation96 only two patients reported a mild transient stinging sensation on instillation of the 1% voriconazole eye drops. Visualization was excellent in all 13 patients. Of the 10 patients in the study by Lau et al,Citation61 four patients reported one or two instances of mild stinging, and one patient reported sneezing after the initial dose. None of the patients in the clinical study by Al-Badriyeh et al reported any side effects with the 2% voriconazole eye drops.Citation101 These outcomes were consistent with the outcome in another study by Lau et al that evaluated voriconazole penetration into the vitreous humor of the eye after topical application of the eye drops.Citation113 Here also, no side effects were reported. Similarly, in all of the case reports of using topical voriconazole in fungal keratitis, the eye drops were well tolerated with no side effects reported.Citation69, Citation74,Citation106–Citation111
Systemic side effects resulting from the topical administration of the voriconazole solution should be negligible. In the case of the 2% eye drops (the highest concentration that has been reported in humans), each drop contains only 0.001 mg of voriconazole which, when compared with the standard systemic dose of 200 mg oral or IV voriconazole twice daily,Citation68 is unlikely to result in systemic concentration that is high enough to induce side effects.
Stability of voriconazole eye drops
According to the stability study by Al-Badriyeh et al,Citation112 1% voriconazole eye drops, prepared in sterile benzalkonium chloride 0.01% solution, were stable for at least 14 weeks when stored at 2–8°C, while 2% voriconazole eye drops, also prepared in sterile benzalkonium chloride solution, were stable for 16 weeks at 2–8, 25, and 40°C. This was consistent with the stability study by Dupuis et al,Citation97 where 1% voriconazole eye drops, prepared in sterile water for injection, were stable for at least four weeks when stored at 4°C. Such long-term stability data will help minimize wastage and is pivotal to facilitate the use of the eye drops in the outpatient setting.
Patient adherence and satisfaction
Given the observed efficacy, safety, and stability, voriconazole eye drops appear to be a good option in the treatment of fungal keratitis. However, non-adherence to the prescribed dosing regimen may pose a challenge to achieving the desired treatment outcome and, consequently, lead to progression of the infection. The benefits of using voriconazole eye drops, especially in the outpatient setting, could be diminished due to poor patient adherence if the dosing regimen is intense (eg, hourly to two-hourly drops) and is for an extended duration. The outpatient setting is different from the situation in clinical studies, in which maximal compliance can be achieved because the eye drops are often administered by nursing staff in hospitals.Citation100 To date, there are no published data available about patient adherence to voriconazole eye drops.
The appropriate dosing frequency of voriconazole eye drops is not clear. The voriconazole aqueous humor concentration measured by Vemulakonda et al (6.49 ± 3.04 mg/L),Citation96 where 1% eye drops were administered every two hours for 24 hours, is more than three times the concentration reported by Lau et al (1.90 ± 1.12 mg/L),Citation61 where one drop was administered every hour for four hours. Based on these data, it is tempting to assume that the higher concentration measured by Vemulakonda et al was a result of drug accumulation in the eye; however, the significant difference in the post-dose sampling time (0.4 hours versus 1.1 hours in the Vemulakonda et al and Lau et al studies, respectively) could have been the key factor behind the differences in the measured voriconazole level in the aqueous humor.
Conclusion
Voriconazole eye drops appear to be effective when used for the treatment of fungal keratitis caused by a variety of fungi, including F. solani, C. albicans, S. apiospermum, and A. niger. The eye drops are well tolerated in the reported clinical trials and case studies. In addition, stability data for the extemporaneously prepared eye drops are available, minimizing the manufacturing cost and wastage associated with the eye drops.
Case reports have shown that voriconazole eye drops are effective when used as adjunctive therapy or monotherapy, and as primary or salvage therapy for the management of ophthalmic fungal keratitis. However, the number of these case reports and, hence, their significance remains limited. Whilst large-scale, randomized, controlled studies are needed, such studies are difficult to conduct and, consequently, audits or large case series on the use of voriconazole eye drops as monotherapy are necessary to confirm current findings and help establish the extent of their effectiveness.
Increasing the concentration of voriconazole eye drops may lead to increased efficacy and/or reduced dosing frequency; however, the benefit of using concentrations greater than 1% has not been evaluated in patients with fungal keratitis beyond a single case report. Studies using intact corneas have suggested concentration-independent penetration of voriconazole through the cornea and, consequently, it appears that administering 2% over 1% voriconazole eye drops in fungal keratitis is unlikely to give any additional benefit. Although some studies have suggested that epithelial damage is not necessary for voriconazole penetration, future studies that evaluate the penetration of 2% versus 1% voriconazole eye drops in patients with fungal keratitis, despite being difficult to perform, will be important. Given that the optimal dosing of the voriconazole eye drops remains unknown, studies to investigate the extent of voriconazole clearance from the human eye after topical administration should be conducted to guide the dosing frequency.
Current literature has provided some evidence on the effectiveness of voriconazole eye drops for the treatment of fungal keratitis; however, more data are required before a definite conclusion regarding their utility is drawn.
Disclosures
The authors report no financial or other conflicts of interest in this work.
References
- WhitcherJSrinivasanMUpadhyayMCorneal blindness: A global perspectiveBull World Health Organ200179321422111285665
- DandonaLDandonaRNaduvilathTIs current eye-care-policy focus almost exclusively on cataract adequate to deal with blindness in India?Lancet19983519112131213169643793
- LevinLAveryRShoreJWoogJBakerAThe spectrum of orbital aspergillosis: A clinicopathological reviewSurv Ophthalmol19964121421548890440
- YohaiRBullockJAzizAMarkertRSurvival factors in rhino-orbital-cerebral mucormycosisSurv Ophthalmol19943913227974189
- SeeJWongTYeoKTrends in the pattern of blindness and major ocular diseases in Singapore and AsiaAnn Acad Med Singapore19982745405469791663
- HaganMWrightENewmanMDolinPJohnsonGCauses of suppurative keratitis in GhanaBr J Ophthalmol19957911102410288534648
- PuttannaSPrimary keratomycosisJ All India Ophthalmol Soc19691751712004914371
- GalarretaDTuftSRamsayADartJFungal keratitis in London: Microbiological and clinical evaluationCornea20072691082108617893539
- SrinivasanMFungal keratitisCurr Opin Ophthalmol200415432132715232472
- BhartiyaPDaniellMConstantinouMIslamFTaylorHFungal keratitis in MelbourneClin Experiment Ophthalmol200735212413017362452
- NelsonPEDignaniMCAnaissieEJTaxonomy, biology, and clinical aspects of Fusarium speciesClin Microbiol Rev1994744795047834602
- ThomasPMycotic keratitis – an underestimated mycosisJ Med Vet Mycol19943242352567983569
- GopinathanUGargPFernandesMSharmaSAthmanathanSRaoGThe epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in South IndiaCornea200221655555912131029
- RosaRMillerDAlfonsoEThe changing spectrum of fungal keratitis in south FloridaOphthalmology19941016100510138008340
- CueroRGEcological distribution of Fusarium solani and its opportunistic action related to mycotic keratitis in Cali, ColombiaJ Clin Microbiol19801234554617217337
- StevensDKanVJudsonMPractice guidelines for diseases caused by aspergillus. Infectious Diseases Society of AmericaClin Infect Dis200030469670910770732
- TabbaraKal JabartiAHospital construction-associated outbreak of ocular aspergillosis after cataract surgeryOphthalmology199810535225269499785
- SmolinGFosterCSAzarDTDohlmanCHThe Cornea: Scientific Foundations and Clinical Practice4th edPhiladelphia, PALippincott Williams & Wilkins Inc.2005
- KlintworthGThe cornea – structure and macromolecules in health and disease. A reviewAm J Pathol1977893718808339743
- ThomasPACurrent perspectives on ophthalmic mycosesClin Microbiol Rev200316473079714557297
- GordonMNortonSCorneal transplant infection by Paecilomyces lilacinusSabouraudia19852342953013901331
- KozarskyAStultingRWaringGCornellFWilsonLCavanaghHPenetrating keratoplasty for exogenous Paecilomyces keratitis followed by postoperative endophthalmitisAm J Ophthalmol19849855525576541878
- FincherRFisherJLovellRNewmanCEspinel-IngroffAShadomyHInfection due to the fungus Acremonium (cephalosporium)Medicine (Baltimore)19917063984091956281
- GuarroJGamsWPujolIGeneJAcremonium species: New emerging fungal opportunists – in vitro antifungal susceptibilities and reviewClin Infect Dis1997255122212299402385
- GargPGopinathanUChoudharyKRaoGKeratomycosis: Clinical and microbiologic experience with dematiaceous fungiOphthalmology2000107357458010711898
- JonesBPrinciples in the management of oculomycosis. XXXI Edward Jackson memorial lectureAm J Ophthalmol19757957197511096622
- SternGButtrossMUse of corticosteroids in combination with antimicrobial drugs in the treatment of infectious corneal diseaseOphthalmology19919868478531866135
- HariprasadSMMielerWFLinTKSponselWEGraybillJRVoriconazole in the treatment of fungal eye infections: A review of current literatureBr J Ophthalmol200892787187818577634
- FairleyCSullivanTBartleyPAllworthTLewandowskiRSurvival after rhino-orbital-cerebral mucormycosis in an immunocompetent patientOphthalmology2000107355555810711895
- MarshallDBrownsteinSJacksonWMintsioulisGGilbergSal-ZeerahBPost-traumatic corneal mucormycosis caused by Absidia corymbiferaOphthalmology19971047110710119224461
- Mino de KasparHZoulekGParedesMMycotic keratitis in ParaguayMycoses1991345–62512541795722
- PandaASharmaNDasGKumarNSatpathyGMycotic keratitis in children: Epidemiologic and microbiologic evaluationCornea19971632952999143801
- TanureMCohenESudeshSRapuanoCLaibsonPSpectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, PennsylvaniaCornea200019330731210832689
- SrinivasanMGonzalesCAGeorgeCEpidemiology and aetiological diagnosis of corneal ulceration in Madurai, south IndiaBr J Ophthalmol199781119659719505820
- SonyPSharmaNVajpayeeRRayMTherapeutic keratoplasty for infectious keratitis: A review of the literatureCLAO J200228311111812144228
- DunlopAWrightEHowladerSSuppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitisAust N Z J Ophthalmol19942221051107917262
- WongTNgTFongKTanDRisk factors and clinical outcomes between fungal and bacterial keratitis: A comparative studyCLAO J19972342752819348453
- ClinchTRobinsonMBarronBInslerMLiangKKaufmanHFungal keratitis from nylon line lawn trimmersAm J Ophthalmol199211444374401415454
- OkhraviNDartJKTowlerHMLightmanSPaecilomyces lilacinus endophthalmitis with secondary keratitis: A case report and literature reviewArch Ophthalmol199711510132013249338682
- ManzouriBVafidisGWyseRPharmacotherapy of fungal eye infectionsExpert Opin Pharmacother20012111849185711825321
- FlorCruzNVPeczonIJrMedical interventions for fungal keratitisCochrane Database Syst Rev2008CD00424118254043
- O’DayDSelection of appropriate antifungal therapyCornea1987642382453319407
- KhooSHBondJDenningDWAdministering amphotericin B – a practical approachJ Antimicrob Chemother19943322032138182001
- BatesDSuLYuDMortality and costs of acute renal failure associated with amphotericin B therapyClin Infect Dis200132568669311229835
- O’DayDHeadWRobinsonRClantonJCorneal penetration of topical amphotericin B and natamycinCurr Eye Res19865118778823490954
- O’BrienTPTherapy of ocular fungal infectionsOphthalmol Clin North Am1999123350
- Natacyn [Package insert]Alcon Laboratories Inc.Texas52008
- PandaASharmaNAngraSTopical fluconazole therapy of Candida keratitisCornea19961543733758776563
- SealDPleyerUOcular Infection and Immunity2nd edNew York, NYInforma Healthcare Inc.2007
- GanegodaNRaoSKAntifungal therapy for keratomycosesExpert Opin Pharmacother20045486587415102569
- O’DayDHeadWRobinsonRWilliamsTGeddeSAnomalous effect of subconjunctival miconazole on Candida albicans keratitis in rabbitsAm J Ophthalmol199111255625661951595
- TorresMAMohamedJCavazos-AdameHMartinezLATopical ketoconazole for fungal keratitisAm J Ophthalmol198510022932984025470
- FosterCSLassJHMoran-WallaceKGiovanoniROcular toxicity of topical antifungal agentsArch Ophthalmol1981996108110846263236
- FosterCSStefanyszynMIntraocular penetration of miconazole in rabbitsArch Ophthalmol197997917031706475641
- ThomasPAFungal infections of the corneaEye (Lond)200317885286214631389
- PrajnaNVJohnRKNirmalanPKLalithaPSrinivasanMA randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitisBr J Ophthalmol200387101235123714507756
- AvundukABeuermanRWarnelEKaufmanHGreerDComparison of efficacy of topical and oral fluconazole treatment in experimental Aspergillus keratitisCurr Eye Res200326211311712815530
- NuneryWWelshMSaylorRPseudallescheria boydii (Petriellidium boydii) infection of the orbitOphthalmic Surg19851652963004011116
- Sonego-KroneSSanchez-Di MartinDAyala-LugoRClinical results of topical fluconazole for the treatment of filamentous fungal keratitisGraefes Arch Clin Exp Ophthalmol2006244778278716133016
- TuEAlternaria keratitis: Clinical presentation and resolution with topical fluconazole or intrastromal voriconazole and topical caspofunginCornea200928111611919092423
- LauDFedinandsMLeungLPenetration of voriconazole, 1%, eyedrops into human aqueous humor: A prospective open-label studyArch Ophthalmol2008126334334618332313
- RaoSMadhavanHRaoGPadmanabhanPFluconazole in filamentous fungal keratitisCornea19971667009395885
- RajasekaranJThomasPKalavathyCJosephPAbrahamDItraconazole therapy for fungal keratitisIndian J Ophthalmol1987355–61571602854820
- SavaniDVPerfectJRCoboLMDurackDTPenetration of new azole compounds into the eye and efficacy in experimental Candida endophthalmitisAntimicrob Agents Chemother19873116103032091
- Vanden BosscheHMackenzieDCauwenberghGAspergillus and AspergillosisNew York, NYPlenum Press Inc.1988
- JohnsonLKauffmanCVoriconazole: A new triazole antifungal agentClin Infect Dis200336563063712594645
- HariprasadSMMielerWFHolzERDetermination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humansArch Ophthalmol20041221424714718293
- Vfend [Package insert]New York, NYPfizer Inc.32008
- KlontREgginkCRijsAWesselingPVerweijPSuccessful treatment of Fusarium keratitis with cornea transplantation and topical and systemic voriconazoleClin Infect Dis20054012e110e11215909252
- GaoHPennesiMEShahKIntravitreal voriconazole: An electroretinographic and histopathologic studyArch Ophthalmol2004122111687169215534131
- TorresHHachemRChemalyRKontoyiannisDRaadIPosaconazole: A broad-spectrum triazole antifungalLancet Infect Dis200551277578516310149
- UllmannAJCornelyOABurchardtAPharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infectionAntimicrob Agents Chemother200650265866616436724
- SponselWEGraybillJRNevarezHLDangDOcular and systemic posaconazole (SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitisBr J Ophthalmol200286782983012084760
- TuEMcCartneyDBeattyRSpringerKLevyJEdwardDSuccessful treatment of resistant ocular fusariosis with posaconazole (SCH-56592)Am J Ophthalmol2007143222222717258521
- Abdel-RahmanSNahataMOral terbinafine: A new antifungal agentAnn Pharmacother19973144454569101008
- JensenJClinical pharmacokinetics of terbinafine (Lamisil)Clin Exp Dermatol19891421101132689012
- NedelmanJRGibianskyERobbinsBPharmacokinetics and pharmacodynamics of multiple-dose terbinafineJ Clin Pharmacol19963654524618739024
- LiangQFJinXYWangXLSunXGEffect of topical application of terbinafine on fungal keratitisChin Med J (Engl)2009122161884188819781365
- GoldblumDFruehBESarraGMKatsoulisKZimmerliSTopical caspofungin for treatment of keratitis caused by Candida albicans in a rabbit modelAntimicrob Agents Chemother20054941359136315793112
- LalithaPShapiroBLSrinivasanMAntimicrobial susceptibility of fusarium, aspergillus, and other filamentous fungi isolated from keratitisArch Ophthalmol2007125678979317562990
- JurkunasULangstonDColbyKUse of voriconazole in the treatment of fungal keratitisInt Ophthalmol Clin2007472475917450006
- LevequeDNivoixYJehlFHerbrechtRClinical pharmacokinetics of voriconazoleInt J Antimicrob Agents200627427428416563707
- Carrillo-MunozAQuindosGJLLRCurrent development in anti-fungal agentsCurr Med Chem200434297323
- BreitSHariprasadSMielerWShahGMillsMGrandMManagement of endogenous fungal endophthalmitis with voriconazole and caspofunginAm J Ophthalmol2005139113514015652837
- GaoHPennesiMShahKSafety of intravitreal voriconazole: Electroretinographic and histopathologic studiesTrans Am Ophthalmol Soc200310118318914971576
- MarangonFMillerDGiaconiJAlfonsoEIn vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogensAm J Ophthalmol2004137582082515126145
- NulensEEgginkCRijsAJMMWesselingPVerweijPEKeratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazoleJ Clin Microbiol20034152261226412734297
- NochezYArseneSLe GuellecCUnusual pharmacokinetics of intravitreal and systemic voriconazole in a patient with Scedosporium apiospermum endophthalmitisJ Ocul Pharmacol Ther2008241879018370878
- StewartAPowlesRHewetsonMAntrumJRichardsonCMehtaJCosts of antifungal prophylaxis after bone marrow transplantation. A model comparing oral fluconazole, liposomal amphotericin and oral polyenes as prophylaxis against oropharyngeal infectionsPharmacoeconomics19958435036110155676
- DaviesNBiopharmaceutical considerations in topical ocular drug deliveryClin Exp Pharmacol Physiol200027755856210874518
- UrttiAChallenges and obstacles of ocular pharmacokinetics and drug deliveryAdv Drug Deliv Rev200658111131113517097758
- ForresterJVDickADRobertsFThe Eye: Basic Sciences in Practice: Basic Science in Practice2nd edLondon, UKSaunders Ltd2002
- KlotzSPennCNegveskyGButrusSFungal and parasitic infections of the eyeClin Microbiol Rev200013466268511023963
- JtirvinenKUrttiAOcular absorption following topical deliveryAdv Drug Del Rev1995161319
- SilveiraFPHusainSFungal infections in solid organ transplantationMed Mycol200745430532017510855
- VemulakondaGAHariprasadSMMielerWFPrinceRAShahGKVan GelderRNAqueous and vitreous concentrations following topical administration of 1% voriconazole in humansArch Ophthalmol20081261182218195213
- DupuisATournierNLe MoalGVenisseNPreparation and stability of voriconazole eye drop solutionAntimicrob Agents Chemother200953279879919001119
- SponselWChenNDangDTopical voriconazole as a novel treatment for fungal keratitisAntimicrob Agents Chemother200650126226816377696
- ClodeADavisJSalmonJMichauTGilgerBEvaluation of concentration of voriconazole in aqueous humor after topical and oral administration in horsesAm J Vet Res200667229630116454636
- ThielMAZinkernagelASBurhenneJKaufmannCHaefeliWEVoriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitisAntimicrob Agents Chemother200751123924417060517
- Al-BadriyehDLeungLRoydhouseTProspective open-label study of the administration of two-percent voriconazole eye dropsAntimicrob Agents Chemother20095373153315519433565
- LauDPenetration of 1% voriconazole eye drops into human aqueous and vitreous humor – a prospective, open-label study (MClinPharm thesis)Melbourne, AustraliaMonash University2006
- FreemanPDKahookMYPreservatives in topical ophthalmic medications: Historical and practical review of benzalkonium chlorideExpert Rev Ophthalmol2009415964
- van der BijlPvan EykAMeyerDEffects of three penetration enhancers on transcorneal permeation of cyclosporineCornea200120550555811413407
- SenguptaKKMikherjiREssentials Ocular Pharmacology and Therapeutics1st edKent, UKAnshan Pub.2007
- PolizziASiniscalchiCMastromarinoASaccaSEffect of voriconazole on a corneal abscess caused by fusariumActa Ophthalmol Scand200482676276415606478
- PratsCHTelloFLJoseABSOtaolaurruchiJSBainesJPOVoriconazole in fungal keratitis caused by Scedosporium apiospermumAnn Pharmacother200438341441714755065
- ReisASundmacherRTintelnotKAgostiniHJensenHEAlthmausCSuccessful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazoleBr J Ophthalmol200084893293310979655
- JonesAMuhtasebMUse of voriconazole in fungal keratitisJ Cataract Refract Surg200834218318418242428
- Al-BadriyehDLeungLDaviesGEStewartKKongDSuccessful salvage treatment of Scedosporium apiospermum keratitis with topical voriconazole after failure of vatamycinAnn Pharmacother20094361139114219435962
- Al-BadriyehDLeungLDaviesGEStewartKKongDSuccessful use of topical voriconazole 1% alone as first-line antifungal therapy against Candida albicans keratitisAnn Pharmacother200943122103210719861430
- Al-BadriyehDLiJStewartKStability of extemporaneously prepared voriconazole ophthalmic solutionAm J Health Syst Pharm200966161478148319667005
- LauDLeungLFedinandsMPenetration of 1% voriconazole eye drops into human vitreous humour – a prospective, open-label studyClin Experiment Ophthalmol200937219720019723128