Abstract
Aim: This study investigated the clinical factors associated with functional ambulatory outcomes and gender differences in prestroke depressive patients. Materials & methods: Clinical risk factors associated with improved functional outcomes with recombinant tissue plasminogen activator-treated cohorts were determined using binary logistic regression. Results: In the adjusted analysis, improvement in ambulation was associated with male patients that had higher National Institute of Health stroke scale score (p = 0.04), a stroke history (p = 0.026), lower serum creatinine levels (p = 0.049) and were taking cholesterol reducing medication (p = 0.014). Improvement in females was associated with taking antidepressants (p = 0.027) and having lower National Institute of Health stroke scale score (p = 0.002). Conclusion: Our findings indicate gender disparities between male and female prestroke depressive patients.
It has long been known that, in the USA, the number one cause for long-term disability is stroke [Citation1]. Stroke is currently the third leading cause of death in females and the fourth leading cause of death among males [Citation2]. There is an evidence that, for women, recovering from a stroke is more difficult than for men, and women may have worse outcomes [Citation3,Citation4], in comparison with a similar cohort of males [Citation5,Citation6]. Many studies provide evidence for differences in gender within the stroke system [Citation7,Citation8]. Evidence shows that women with a history of stroke are more likely to have a higher incidence of comorbidities, such as atrial fibrillation [Citation9] and hypertension [Citation10], while men are more likely to have a history of diabetes and heart disease [Citation10].
Depression is a major risk factor for stroke [Citation11], and there is also an association with increasing morbidity and mortality in ischemic stroke patients [Citation12–14]. Women of all ages tend to be more likely to have greater symptoms of depression than males of the same age [Citation15]. Prestroke depression is also associated with a greater likelihood of depression [Citation11,Citation16,Citation17], which also has an association with an increased risk of stroke [Citation18], low cognitive function [Citation19], higher mortality rates [Citation20] and an increase in hospitalizations [Citation21,Citation22]. Moreover, women tend to have higher rates of depression [Citation15] and worse functional outcomes after ischemic events [Citation23]. Therefore, if women present with higher rates of depression and worse outcomes after stroke, it then suggests that functional outcomes following treatment with thrombolytic therapy may not be the same in the population of male and female prestroke depressive ischemic stroke patients. One possibility is that there may be more demographic or clinical risk factors that have an association with improved functional ambulation in comparison with no-improvement in functional ambulation following thrombolytic therapy.
No precise or generally accepted motor assessment tool is currently available [Citation24–26]; therefore, existing studies on poststroke motor recovery are based on different evaluation tools, including the Barthel Index score and the functional independence measure motor score, among others [Citation27,Citation28], and they are very good in providing cumulative assessment. Since the heterogeneity of stroke manifestations provides a source of variability that should be amenable to clinical and motor parsing, the accurate prediction of motor recovery following an ischemic stroke and treatment with thrombolytic therapy represents a tool to evaluate ambulatory outcome. The prediction of motor recovery would represent a suitable cut-off assessment based on direct evaluation of ambulation status before and even after treatment. Thus, an accurate prediction of motor recovery for stroke patients who are most likely to benefit from thrombolytic therapy will help clinicians to plan rehabilitation programs and provide realistic goals for the patient.
Our objective is to identify the clinical and demographic factors that may contribute to gender differences in functional ambulation among prestroke depressive ischemic stroke patients. We determine whether treatment with recombinant tissue plasminogen activator (rtPA) is more likely to have better functional improvement in ambulation in male prestroke depressive patients with ischemic strokes than in females of a similar cohort. The goal of this study is to determine clinical risk factors that have an association with functional ambulatory outcome in male and female prestroke depressive ischemic stroke patients. Knowledge of the specific clinical risk factors that contribute to gender differences in prestroke depressive ischemic stroke patients will be helpful in providing information about the measurable and qualitative risks of prestroke depression that can effectively improve the eligibility of thrombolytic therapy for prestroke depressive ischemic strokes.
Material & methods
Retrospective data of acute ischemic stroke patients treated with thrombolytic therapy were collected from the PRISMA Health stroke registry between 2010 and 2016. The PRISMA Health stroke registry used in this study contributes to the Get with The Guidelines Stroke registry. This is a national registry, launched by the American Heart Association and American Stroke Association (both TX, USA), that facilitates quality improvement of hospital systems in the care of ischemic stroke patients [Citation29]. Previous studies [Citation24–28] describe the PRISMA Health stroke registry. The PRISMA stroke center, located in (SC, USA), is well known for its pioneering work in stroke treatment, diagnostic technologies and improved outcomes in stroke care in South Carolina. Eligibility for rtPA was determined based upon the guidelines outlined by the American Heart Association for the treatment and management of patients with acute ischemic strokes [Citation15]. Within this cohort, 352 patients had been identified as having a prestroke depression prior to presentation with an acute ischemic stroke. Out of this dataset, 171 were administered rtPA, while 181 were excluded from receiving rtPA treatment. These contraindications included mild ischemic stroke events and improvements demonstrated clinically involved in the symptoms of the event, whether they progressed too mildly or rapidly [Citation14].
Data for this study were collected from patients that presented with signs and symptoms indicative of acute ischemic stroke. Charts were reviewed manually, and data obtained from the registry for identified ischemic stroke patients who were diagnosed by radiological interpretation via CT scan. Baseline data collected included demographic characteristics such as race, ethnicity, sex and age. Clinical variables and history of comorbid risk factors such as atrial fibrillation, substance abuse, smoking, alcohol, diabetes mellitus, hypertension and a history of transient ischemic attacks (TIAs) or prior strokes was also collected. Ambulatory outcomes were documented for prestroke depressive individuals who were administered rtPA after the diagnosis of an acute ischemic stroke, as well as those that were not administered rtPA after the diagnosis of an acute ischemic event. These ambulatory outcomes were recorded for each of the gender specific, male and female, prestroke depressed patient populations.
Data analysis
For all statistical analyses, the SPSS package version 24 (IL, USA) was utilized, and p < 0.05 was used to establish statistical significance for group comparisons. The dataset of prestroke depressed patients was divided by gender (‘males’ vs ‘females’) and grouped based upon treatment with thrombolytic therapy (‘rtPA group’ vs no ‘rtPA group’). These data were then further subdivided based on ambulatory status (‘improvement or no improvement in ambulatory outcome’) at discharge and compared with the status upon the time of presentation. Additionally, an improvement of ambulation from the time of presentation to the time of discharge was assigned a value of 1, while if no improvement during this time was noted, a value of 0 was given. Ambulatory status as a reliable and valid metric has been well documented and discussed previously [Citation24]. Therefore, ambulation was utilized in this study as a metric to measure the functional outcomes in male and female rtPA-treated stroke patients. The method to compute this metric of measure has been validated in a previous study [Citation24]. To determine ambulatory outcomes, first the ability to ambulate prior to the current event, at admission and at discharge was determined. For example, on admission, the ambulatory status of all patients was evaluated by the nurse and a score of 0–3 was given based on the possible respective outcomes; patients with undocumented ambulation were assigned a score of 0, while those who were unable to ambulate were assigned a score of 1. Patients that were able to ambulate with assistance were scored 2, while those that ambulated independently without any assistance were scored 3. Changes in ambulation were tracked from admission to discharge to determine those that improved and those that did not improve after discharge. Second, to compute ambulatory outcome (improvement or no improvement), a new variable was determined from the existing data (i.e., improvement or nonimprovement in ambulation from the time of admission to discharge). Finally, a value of ‘1’ was associated with an improvement in ambulation from the time of admission to the time of discharge, while ‘0’ was associated with no improvement. This was used to build a model for ambulatory outcomes for male and female ischemic stroke patients who received rtPA.
Clinical and demographic characteristics for the patients in all groups were calculated with descriptive statistics. The normal distribution of our data was confirmed using the Kolomogorov–Smirnov test, following the determination of mean and standard deviation. We validated the normal distribution of our data using the Lilliefors test. The student’s t-test was used for continuous variables, while Mann–Whitney U or Pearson’s χ2 test was used as appropriate to analyze discrete variables and determine comparisons between the two groups. For all continuous variables, the mean, standard deviation and range were calculated and for all discrete variables, the number of patients and percentage of patients in that category were determined. To further explore the numerous associable variables linked to improved functional outcomes relative to gender (male vs female) in the prestroke-depressed ischemic patient population, a multivariable logistic regression was performed to determine the association of rtPA with clinical and demographic factors. The logistic regression identified demographic and clinical factors that predicted improved functional ambulatory outcomes stratified by gender. The primary outcomes were demographic and clinical risk factors that predicted gender differences in functional ambulation in the prestroke-depressed ischemic patient population. A backward selection method was considered, which allowed for the use of the least squares model that contained all variables as predictors, regardless of the factor’s associated univariate analysis p-value. The resulting odds ratios (OR) was used to predict the likelihood of improvement in functional ambulation. A 95% CI was considered for the OR while the significance level was set at the probability level of 0.05. The receiver-operating characteristic (ROC) curve was then used to determine the model discrimination, while variance inflation factors were utilized to determine multicollinearity, with values >5 suggestive of multicollinearity.
Results
Out of 352 patients who presented with a diagnosis of acute ischemic stroke with a previous diagnosis of depression, 171 received rtPA while 181 were excluded from rt-PA (). The characterization of prestroke-depressed ischemic stroke patients who received rtPA, respective of improvement status and gender, is presented in . This showed that 66 female stroke patients and 42 male stroke patients did not present with improved ambulation after being treated with rtPA. Females in the group that did not demonstrate improvement upon rtPA treatment were more likely to have presented with a previous TIA (18.2 vs 2.2%), have a family history of stroke (21.2 vs 6.7%) and a higher serum creatinine (1.072 ± 0.4211 vs 0.918 ± 0.2733). Females with no improvement also presented with a lower National Institute of Health stroke scale (NIHSS) score (8.53 ± 7.923 vs 13.27 ± 7.644). In the group that did show improvement, females had higher rates of peripheral vascular disease (13.3 vs 3%). Males who showed improvement were more likely to have carotid stenosis (27.8 vs 7.1%), less likely to have hypertension (77.8 vs. 95.2%) and less likely to be taking antihypertensives (66.7 vs 88.1%). Males in the improvement group also presented with a higher calculated NIHSS score than those who did not improve (11.22 ± 8.135 vs 7.33 ± 5.859). In summary, females presented with four clinical characteristics that were significantly associated with no improvement: previous TIA (p = 0.01), family history of stroke (p = 0.037), higher serum creatinine (p = 0.023) and lower-calculated NIHSS score (p = 0.002). Taking antihypertensives (p = 0.049), lower NIHSS score (p = 0.041) and hypertension (P = 0.039) were significant clinical variables associated with males who showed no improvement after rtPA was administered.
Table 1. Demographic and clinical characteristics of improvement and nonimprovement in ischemic stroke patients with recombinant tissue plasminogen activator and who had a diagnosis of depression prior to the event.
Table 2. The characterization of prestroke depressive ischemic stroke patients who received recombinant tissue plasminogen activator respective of improvement status and gender.
To determine clinical factors associated with ambulation in males () who presented with acute ischemic stroke and a history of depression, an adjusted analysis was performed and the following was determined as significant: history of a previous stroke (OR = 7.177; 95% CI: 1.271–40.533; p = 0.026), NIHSS score (OR = 1.093; 95% CI: 1.004–1.191; p = 0.04), cholesterol reducing medication (OR = 8.421; 95% CI: 1.546–45.862; p = 0.014) and lower serum creatinine level (OR = 1.818; 95% CI: 1.002–3.298; p = 0.049) were associated with improved ambulation. The use of antihypertensive medication (OR = 0.028; 95% CI: 0.003–0.308; p = 0.003) and diabetic medication (OR = 0.027; 95% CI: 0.002–0.35; p = 0.006) were linked to reduced improvement in ambulation following thrombolytic therapy. As shown in , the model had a strong indication demonstrated by the ROC curve, with area under the curve = 0.817 (95% CI: 0.646–0.971; p < 0.001). For the female patients (), congestive heart failure (OR = 0.083; 95% CI: 0.018–0.383; p = 0.001) and previous TIA (OR = 0.064; 95% CI: 0.01–0.405; p = 0.003) were associated with reduced improvement, while the use of antidepressants prior to hospital admission (OR = 4.549; 95% CI: 1.188–17.424; p = 0.027) was associated with improved ambulatory outcomes. The higher area under the curve = 0.822 (95% CI: 0.751–0.897; p < 0.001) in the ROC analysis indicated a better discrimination of the score for the measured outcome ().
Table 3. Clinical factors that were associated with improvement in ambulation for male ischemic stroke male population with prestroke depression and who received recombinant tissue plasminogen activator.
(A): area under the curve = 0.817; 95% CI: 0.646–0.971; p < 0.001. (B): area under the curve = 0.822; 95% CI: 0.751–0.897; p < 0.001.
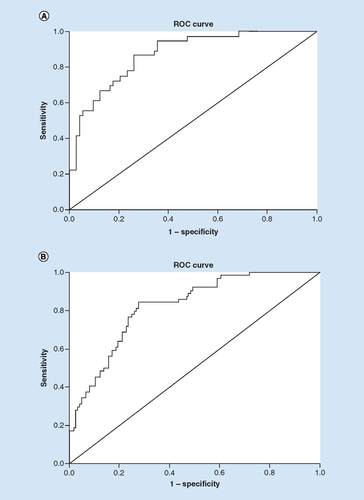
Table 4. Clinical factors that were associated with improvement for the ischemic stroke female population with prestroke depression and who received recombinant tissue plasminogen activator.
Discussion
The neurological impacts and ramifications of depression on cognitive function have been well studied [Citation30–33]; however, the effect of prestroke depression in ischemic stroke and related gender differences with regards to rtPA administration and ambulatory outcome is not very clear. In the univariate analysis, females that received rtPA who did not show functional improvement were more likely to present with a previous TIA, a family history of stroke, a higher serum creatinine level and a lower NIHSS score. Females who did show improvement after the administration of rtPA had higher rates of peripheral vascular disease. Meanwhile, males who showed improvement were more likely to have carotid stenosis but less likely to have hypertension or be taking antihypertensives than those who did not improve. Baseline NIHSS score was a strong predictor of functional outcome and represented a major evaluation tool to assess the efficacy of rtPA treatment [Citation34]. In this study, stroke severity was between 0 and 11 for most male patients and 0 and 7.6 for female patients that showed an improvement in ambulation following thrombolytic therapy. This suggests that the severity of the stroke itself does not provide an explanation for the observed results [Citation34]. Instead, it was likely due to the differences observed in clinical risk factors where socio-demographic factors were involved [Citation35–37].
In the adjusted analysis for the male patients, factors such as previous strokes within the past 3 months, higher NIHSS score, serum creatinine levels and cholesterol reducing medication prior to admission were associated with improved ambulation, while the use of antihypertensive and diabetic medication were associated with nonimprovement in ambulation in patients that received rtPA. We found that previous strokes within the past 3 months were associated with improved ambulation. Similar finding were reported by other studies, where the administration of intravenous thrombolysis to patients with a stroke within the past 3 months was associated with an improved outcome [Citation38,Citation39]. Although thrombolytic therapy remained substantially underutilized, because of many contraindications, our findings indicated that administration of intravenous thrombolysis to prestroke-depressed ischemic stroke patients with a prior stroke in the past 3 months was associated with improved ambulation recovery. We found that low creatinine was associated with improved ambulation, which indicated that a low serum level was associated with improvement in ambulatory recovery in prestroke-depressed ischemic stroke patients that received rtPA. Low serum creatinine levels have been associated with early neurological improvement [Citation40], which suggests that ambulatory recovery and effectiveness of thrombolytic therapy may be dependent on renal function in prestroke-depressed ischemic stroke patients. Several studies have also reported the benefits in the use of cholesterol reducing medication in the event of acute ischemic strokes [Citation41–44]. Other reports have indicated good outcomes in ischemic cerebrovascular events in patients using cholesterol reducing medication when compared with patients given placebo treatment [Citation44]. These findings support our results that the use of a cholesterol reducing medication was possibly associated with improvement in ambulatory recovery in prestroke-depressed ischemic stroke patients that received rtPA. On the other hand, uncontrolled diabetes in ischemic stroke patients has been known to result in poor functional outcomes, including limb weakness, when compared with those without diabetes [Citation45]. Therefore, the nonimprovement in ambulation observed in the group associated with diabetic medication was possibly associated with the effects of prestroke depression.
We also observed that antihypertensive medication was associated with a nonimprovement in ambulation following thrombolytic therapy. Poorer treatment outcomes in the event of acute ischemic strokes have been linked to the cases of untreated hypertension [Citation46]. The American Heart Association guidelines on hypertension [Citation47] recommend the control of hypertension, especially throughout the acute stages of a stroke. However, there have been concerns that some baseline clinical risk factors were able to alter the benefits of thrombolysis resulting in poor outcomes, even in stroke patients with treated hypertension [Citation46]. Our finding indicate that antihypertensive medication is an independent predictor of decreased ambulatory improvement and this may be associated with the pharmacological interactions that occurred between rtPA treatment and antihypertensive medication [Citation46].
In the adjusted analysis for the female patients, heart failure and previous TIA were associated with a poor outcome, while lower NIHSS and the use of antidepressants prior to admission were associated with improved outcomes. Heart failure has been directly linked with a poorer prognosis in ischemic stroke patients [Citation48–50]. Moreover, heart dysfunction was found to increase the risk for acute ischemic strokes and was predicted to ultimately impact poststroke prognosis [Citation51,Citation52]. An estimated 20% of patients with a stroke are predicted to present with a history of heart failure [Citation53], while, on average, more than 8% of patients with a history of heart failure may have an ischemic stroke [Citation54]. Patients with ischemic strokes and heart failure present with higher mortality rates, more severe neurologic deficits, longer hospital stays and more unfavorable long-term outcomes when compared with ischemic stroke patients without heart failure [Citation54,Citation55]. Heart failure is an important cause of morbidity and mortality in women, and women develop heart failure at an older age compared with men [Citation50]. Findings from existing studies supported our result, that heart failure may be an independent risk factor that can impact the efficacy of thrombolytic therapy, resulting in no improvement in ambulation in female prestroke-depressed ischemic stroke patients.
While the history of an established TIA within 3 months of the stoke is not a contraindication for thrombolytic therapy [Citation56], current TIA may affect the overall outcomes after thrombolytic therapy administration, especially in patients that have recent TIA, invisible on CT scans and that are presented with ischemic lesions and intracerebral microbleeds [Citation57,Citation58]. Our data suggest that thrombolytic therapy in prestroke-depressed patients with recent TIAs may result in decreased ambulatory outcomes. This finding further supports the necessity for the assessment of the efficacy in treatment with rtPA in prestroke-depressed ischemic stroke patients with a history of recent TIA.
Although depression has been known to occur in up to two-thirds of patients with stroke, there is evidence suggesting improvement in poststroke depressive symptoms with antidepressant drug treatment [Citation59]. Moreover, while antidepressants have been known to improve treatment outcomes in ischemic stroke patients [Citation60] during poststroke recovery in patients who suffered severe strokes [Citation61], our findings indicate that antidepressant use in prestroke-depressed ischemic stroke patients may also be associated with improvement in ambulation after thrombolytic therapy.
In the adjusted analysis, the effect of a previous stroke was attenuated in the female patient population but not in the male population. While the effect of a previous TIA was significant in the univariate analysis, it disappeared in the adjusted analysis. Lower NIHSS score and serum creatinine levels were significant for the female patients in the univariate analysis, but the effect of both disappeared in the adjusted analysis. While antihypertensive medication which was significant in the univariate for the male patients, this effect was sustained in the adjusted analysis when predicting a lack of improvement.
In this study, we identified baseline clinical factors that were associated with either an increase or decrease in odds of improvement of ambulation and demonstrated that these factors differed for male and female stroke patients who had experienced prestroke depression.
There were many limitations that should be taken into consideration prior to the interpretation of the results of this study. This was a retrospective study and there is the potential for measurement bias in NIHSS score as it was not documented who performed the initial assessment or whether this assessment was performed in person or via an electronic resource. Depression was a clinical diagnosis and as a result, it was often difficult to make any assessment addressing the clinical severity of prestroke depression and variations in our findings based upon this. A strength of this study was the large and diverse population made available for analysis from a designated stroke center designed to monitor quality treatment. Therefore, this study was well equipped to determine rtPA treatment and exclusive criteria in a population with comorbid depression. A major contribution of this study to existing literature was the stratification of clinical characteristics and an assessment of functional ambulatory outcomes on the specific population of acute ischemic patients with prestroke depression and the variations of this population with regards to gender.
Conclusion
In prestroke depressive patients, gender seemed to play a role in the determined functional outcomes and associated clinical factors. We observed that antihypertensive and diabetic medications were associated with no significant improvement in ambulation following thrombolytic therapy while previous strokes, a higher NIHSS score, cholesterol reducing medications prior to admission and serum creatinine levels were associated with improved ambulation in male patients. In the female patients, heart failure and previous TIAs were associated with poorer outcomes while lower NIHSS scores and the use of antidepressants prior to admission were associated with improved outcomes. An early and reliable prediction of poor or improved ambulatory outcomes can have potential implications for the clinical management and posthospitalization recovery management for acute ischemic stroke patients.
Future perspective
It is important to identify clinical risk factors that are associated with an increase or decrease in the odds of improvement or no improvement in ambulation among prestroke-depressed ischemic stroke patients. This will help in the development of management strategies to improve the eligibility of thrombolytic therapy for prestroke-depressed ischemic stroke patients. Moreover, it may provide clues for future studies and give detailed insight into how these clinical risk factors interact with thrombolytic therapy to impact treatment outcomes in ischemic stroke patients with prestroke depression.
Aim
This study investigated the clinical factors associated with functional ambulatory outcomes and gender differences in prestroke-depressed patients.
Methods
Clinical risk factors associated with improved functional outcomes with recombinant tissue plasminogen activator-treated cohorts were determined using binary logistic regression.
Results
In the adjusted analysis, the odds of improvement in ambulation among male patients was associated with higher National Institute of Health stroke scale (NIHSS) scores (odds ratio [OR] = 0.089; 95% 95% CI: 1.004–1.191; p = 0.04), a previous stroke history (OR = 1.27; 95% CI: 1–40.533; p = 0.026), lower serum creatinine levels (OR = 1.00; 95% CI: 2–3.298; p = 0.049) and history of cholesterol reducing medication (OR = 2.131; 95% CI: 1.546–45.862; p = 0.014).
Improvement in females was associated with a history of taking antidepressants prior to hospitalization (OR = 1.515; 95% CI: 1.188–17.424; p = 0.027) and lower NIHSS scores (OR = 1.088; 95% CI: 1.031–1.148; p = 0.002).
Antihypertensive and diabetic medications were associated with the reduced odds of no significant improvement in ambulation following thrombolytic therapy.
Previous strokes, NIHSS scores, cholesterol reducing medication and serum creatinine levels were associated with improved ambulation in male prestroke-depressed ischemic stroke patients.
Congestive heart failure and a history of previous transient ischemic attack were associated with the odds of poor outcome or no improvement in ambulation.
NIHSS scores and the use of antidepressants prior to admission were associated with the odds of improvement in ambulation among female prestroke-depressed ischemic stroke patients.
Conclusion
Gender disparities in ambulatory outcomes exist between male and female prestroke-depressed ischemic patients treated with thrombolytic therapy.
An early and reliable prediction of poor or improved ambulatory outcomes can help in the clinical management and posthospitalization recovery management for prestroke-depressed ischemic stroke patients.
Author contributions
MJ Tate, RM Shugar, RA Moraney, LE Brechtel, B Blum and T Nathaniel designed the concept, experimental design and data analysis, critically revised the drafts, read and approved the last version of this manuscript.
Ethical conduct of research
This study was performed with the approval of the Institutional Review Board of Greenville Health System and the Institutional Committee for Ethics. Being a retrospective data analysis with blinded data, no consent was needed.
Acknowledgments
The authors thank the stroke unit of PRISMA Health system for helping in the data collection.
Availability of data & materials
All materials are available for use from the corresponding author.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- AronAW, StaffI, FortunatoG, McculloughLD. Prestroke living situation and depression contribute to initial stroke severity and stroke recovery. J. Stroke Cerebrovasc. Dis.24(2), 492–499 (2015).
- GarganoJW, WehnerS, ReevesM. Sex differences in acute stroke care in a Statewide stroke registry. Stroke39(1), 24–29 (2008).
- LiuMS, LiGQ, TangJet al.The influence of sex in stroke thrombolysis: a systematic review and meta-analysis. J. Clin. Neurol.14(2), 141–152 (2018).
- WiszniewskaM, NiewadaM, CzlonkowskaA. Sex differences in risk factor distribution, severity, and outcome of ischemic stroke. Acta Clin. Croat.50(1), 21–28 (2011).
- AhnstedtH, McculloughLD, CipollaMJ. The importance of considering sex differences in translational stroke research. Transl. Stroke Res.7(4), 261–273 (2016).
- GaineyJ, BrecthtelL, BlumBet al.Functional outcome measures of recombinant tissue plasminogen activator–treated stroke patients in the telestroke technology. J. Exp. Neurosci.12, 1–11 (2018).
- BerglundA, Schenck-GustafssonK, Von EulerM. Sex differences in the presentation of stroke. Maturitas99, 47–50 (2017).
- LiOL, SilverFL, LichtmanJet al.Sex differences in the presentation, care, and outcomes of transient ischemic attack results from the Ontario Stroke Registry. Stroke47(1), 255–257 (2016).
- FukudaM, KandaT, KamideN, AkutsuT, SakaiF. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern. Med.48(12), 967–973 (2009).
- DentiL, ArtoniA, ScodittiUet al.Impact of gender-age interaction on the outcome of ischemic stroke in an Italian cohort of patients treated according to a standardized clinical pathway. Eur. J. Intern. Med.24(8), 807–812 (2013).
- BarlinnK, KepplingerJ, PuetzV, IlligensBM, BodechtelU, SiepmannT. Exploring the risk-factor association between depression and incident stroke: a systematic review and meta-analysis. Neuropsychiatr. Dis. Treat.11, 1–14 (2015).
- DongJY, ZhangYH, TongJ, QinLQ. Depression and risk of stroke a meta-analysis of prospective studies. Stroke43(1), 32–U108 (2012).
- WuQE, ZhouAM, HanYPet al.Poststroke depression and risk of recurrent stroke: a meta-analysis of prospective studies. Medicine98(42), e17235 (2019).
- PanA, SunQ, OkerekeOI, RexrodeKM, HuFB. Depression and risk of stroke morbidity and mortality a meta-analysis and systematic review. JAMA306(11), 1241–1249 (2011).
- GirgusJS, YangKT, FerriCV. The gender difference in depression: are elderly women at greater risk for depression than elderly men?Geriatrics2(4), 35 (2017).
- Taylor-RowanM, MomohO, AyerbeL, EvansJJ, StottDJ, QuinnTJ. Prevalence of pre-stroke depression and its association with post-stroke depression: a systematic review and meta-analysis. Psychol. Med.49(4), 685–696 (2019).
- Taylor-RowanM, StottD, EvansJ, QuinnT. Prevalence of pre-stroke depression and its association with post-stroke depression: a systematic review and meta-analysis. Int. J. Stroke12, 41–41 (2017).
- YuS, ArimaH, BertmarCet al.Depression but not anxiety predicts recurrent cerebrovascular events. Acta Neurol. Scand.134(1), 29–34 (2016).
- SyedMJ, FarooqS, SiddiquiS, AwanS, WasayM. Depression and the use of selective serotonin reuptake inhibitors in patients with acute intracerebral hemorrhage. Cureus11(10), e5975 (2019).
- AyerbeL, AyisS, CrichtonSL, RuddAG, WolfeCDA. Explanatory factors for the increased mortality of stroke patients with depression. Neurology83(22), 2007–2012 (2014).
- LuXR, DuanJF, ChengQ, LuJL. The association between serum growth differentiation factor-15 and 3-month depression after acute ischemic stroke. J. Affect. Disord.260, 695–702 (2020).
- Van RijsbergenMWA, MarkRE, KopWJ, DeKort PLM, SitskoornMM. Psychological factors and subjective cognitive complaints after stroke: beyond depression and anxiety. Neuropsychol. Rehabil.29(10), 1671–1684 (2019).
- AndrewNE, SrikanthV. Sex differences in stroke outcomes a case for better health care for older women. Neurology90(22), 995–996 (2018).
- LawsonTR, BrownIE, WesterkamDLet al.Tissue plasminogen activator (rt-PA) in acute ischemic stroke: outcomes associated with ambulation. Restor. Neurol. Neurosci.33(3), 301–308 (2015).
- BrecthelL, GaineyJ, PenwellA, NathanielTI. Predictors of thrombolysis in the telestroke and non telestroke settings for hypertensive acute ischemic stroke patients. BMC Neurol.18, 215 (2018).
- FazzoneB, MorrisG, BlackLAet al.Exclusion and inclusion criteria for thrombolytic therapy in an ischemic stroke population. J. Neurol. Disord. Stroke4(2), 1112 (2016).
- NathanielTI, CochranT, ChavesJet al.Co-morbid conditions in use of recombinant tissue plasminogen activator (rt-PA) for the treatment of acute ischaemic stroke. Brain Inj.30(10), 1261–1265 (2016).
- NathanielTI, GaineyJ, BlumB, MontgomeryC, ErvinL, MadelineL. Clinical risk factors in thrombolysis therapy: telestroke versus nontelestroke. J. Stroke Cerebrovasc. Dis.27(9), 2524–2533 (2018).
- SchwammL, FonarowG, ReevesMet al.Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation119(1), 101–115 (2009).
- FowlerNR, PerkinsAJ, GaoSJ, SachsGA, BoustaniMA. Risks and benefits of screening for dementia in primary care: The Indiana University Cognitive Health Outcomes Investigation of the Comparative Effectiveness of Dementia Screening (IU CHOICE) Trial. J. Am. Geriatr. Soc.68(3), 535–543 (2020).
- KuehlLK, DeuterCE, Hellmann-RegenJ, KaczmarczykM, OtteC, WingenfeldK. Enhanced noradrenergic activity by yohimbine and differential fear conditioning in patients with major depression with and without adverse childhood experiences. Prog. Neuropsychopharmacol. Biol. Psychiatry96, 109751 (2020).
- LiuJ, DongQL, LuXWet al.Influence of comorbid anxiety symptoms on cognitive deficits in patients with major depressive disorder. J. Affect. Disord.260, 91–96 (2020).
- ZuoB, ZhangX, WenFF, ZhaoY. The influence of stressful life events on depression among Chinese university students: multiple mediating roles of fatalism and core self-evaluations. J. Affect. Disord.260, 84–90 (2020).
- FredwallM, SternbergS, BlackhurstD, LeeA, LeacockR, NathanielTI. Gender differences in exclusion criteria for recombinant tissue-type plasminogen activator. J. Stroke Cerebrovasc. Dis.25(11), 2569–2574 (2016).
- NathanielIT, GaineyJ, BlumB, MontgomeryC. Clinical risk factors in thrombolysis therapy: telestroke versus nontelestroke. J. Stroke Cerebrovasc. Dis.27(9), 2524–2533 (2018).
- NathanielIT, WilliamsJ, FazzoneFet al.Abstract P245: contraindications and exclusion criteria in guidelines for rt-pa in acute ischemic stroke: can the new aha/asa guideline expand the use of rt-PA?Hypertension68(Suppl. 1), AP245 (2016).
- NathanielIT, WormackL, GaineyJ. Development of a new predictive model for thrombolysis in the telestroke for hypertensive acute ischemic stroke patients. Presented at: 11th World Stroke Congress, Montreal, QC, Canada (2018).
- WuCJ, WuD, ChenJ, LiCH, JiXM. Why not intravenous thrombolysis in patients with recurrent stroke within 3 months?Aging Dis.9(2), 309–316 (2018).
- BlumB, BrechtelL, NathanielT. Thrombolysis therapy in specialized and non-specialized stroke units. Arch. Med. Res.49(8), 588–597 (2018).
- GuettierS, ApolM, BonnetALet al.Abstract T P63: low creatinine level is associated with very early neurological improvement after thrombolysis for ischemic stroke. Stroke46(Suppl. 1), ATP63 (2015).
- CanaveroI, CavalliniA, PerronePet al.Clinical factors associated with statins prescription in acute ischemic stroke patients: findings from the Lombardia Stroke Registry. BMC Neurol.14, 53 (2014).
- BrookJG, GordonD, RifkindB. Prevention of stroke by cholesterol-lowering: a meta-analysis. Atherosclerosis134(1–2), 122 (1997).
- HackamDG, HegeleRA. Cholesterol lowering and prevention of stroke an overview. Stroke50(2), 537–541 (2019).
- NtaiosG, MilionisH. Low-density lipoprotein cholesterol lowering for the prevention of cardiovascular outcomes in patients with ischemic stroke. Int. J. Stroke14(5), 476–482 (2019).
- ChenR, OvbiageleB, FengWW. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am. J. Med. Sci.351(4), 380–386 (2016).
- FlemingT, BlumB, AverkampB, SullivanJ, NathanielT. Effect of antihypertensive medications on thrombolysis therapy and outcomes in acute ischemic stroke patients. J. Clin. Hypertens.21(2), 271–279 (2019).
- McevoyJW, DayaN, RahmanFet al.Association of isolated diastolic hypertension as defined by the 2017 ACC/AHA blood pressure guideline with incident cardiovascular outcomes. JAMA323(4), 329–338 (2020).
- HamataniY, NagaiT, HondaYet al.P6385 Impact of admission plasma D-dimer level on short-term risk of ischemic stroke in hospitalized patients with acute heart failure. Eur. Heart J.39(Suppl. 1), ehy566–P6385 (2018).
- HuWS, LinCL. Prediction of acute coronary syndrome, ischemic stroke, and mortality in patients with heart failure: a comparison of CHA(2)DS(2)-VASc and AHEAD scores. J. Interv. Card. Electr.55(2), 225–231 (2019).
- SiedlerG, SommerK, MachaKet al.Heart failure in ischemic stroke relevance for acute care and outcome. Stroke50(11), 3051–3056 (2019).
- SamaiA, AlbrightK, AntzoulatosE, SchluterL, Martin-SchildS. Abstract TP440: heart failure is associated with elevated von Willebrand factor (vWF) antigen but not factor VIII (FVIII) in acute ischemic stroke (AIS). Stroke47(Suppl. 1), ATP440 (2016).
- TakasugiJ, YamagamiH, ToyodaK, InvestigatorsS. The association between congestive heart failure and occlusion of internal carotid artery in acute ischemic stroke with atrial fibrillation: The SAMURAI-NVAF study. Int. J. Stroke9, 145 (2014).
- DivaniAA, VazquezG, QureshiAI, PullicinoP. Nationwide frequency and association of coexisting heart failure on stroke outcomes in the United States: 1995–2005. Stroke40(4), E238–E239 (2009).
- HaeuslerKG, LaufsU, EndresM. Chronic heart failure and ischemic stroke. Stroke42(10), 2977–U2483 (2011).
- WittBJ, GamiAS, BallmanKVet al.The incidence of ischemic stroke in chronic heart failure: a meta-analysis. J. Card. Fail.13(6), 489–496 (2007).
- SobolewskiP, BrolaW, WiszniewskaMet al.Intravenous thrombolysis with rt-PA for acute ischemic stroke within 24 h of a transient ischemic attack. J. Neurol. Sci.340(1–2), 44–49 (2014).
- DeLecinana MA, FuentesB, MasjuanJet al.Thrombolytic therapy for acute ischemic stroke after recent transient ischemic attack. Int. J. Stroke7(3), 213–218 (2012).
- PouporeN, StratD, MackeyT, NathanielIT. The association between an antecedent of transient ischemic attack prior to onset of stroke and functional ambulatory outcome. Clin. Appl. Thromb. Hemost.26, 1–11 (2020).
- StarksteinSE, MizrahiR, PowerBD. Antidepressant therapy in a post-stroke depression. Expert Opin. Pharmacother.9(8), 1291–1298 (2008).
- HeibergerCJ, BuschC, RanceKet al.Antidepressant use for improving functional ischemic stroke outcomes. Cureus11(10), e5908 (2019).
- LiJM, DaiJQ, TanYY, GuJW. P112: Effect of antidepressant therapy on neurological rehabilitation and neuron-specific enolase in elderly patients with post-stroke depression. J. Am. Geriatr. Soc.67(Suppl. 4), S636 (2019).
