Abstract
Adaptive radiotherapy (ART) is a new radiotherapy technology based on image-guided radiation therapy technology, used to avoid radiation overexposure to residual tumors and the surrounding normal tissues. Tumors undergoing the same radiation doses and modes can occur unequal shrinkage due to the variation of response times to radiation doses in different patients. To perform ART effectively, eligible patients with a high probability of benefits from ART need to be identified. Confirming the precise timetable for ART in every patient is another urgent problem to be resolved. Moreover, the outcomes of ART are different depending on the various image guidance used. This review discusses ‘who, when and how’ as the three key factors involved in the most effective implementation for the management of ART.
Plain language summary
Adaptive radiotherapy (ART) is an image-guided radiotherapy technology used to treat tumors. This article explores who can gain more benefits from ART, when is the optimal time to conduct ART and how to better implement ART. Non-small-cell lung cancer patients with large tumor lesions and high radiosensitivity are the most suitable people for ART. The period of 30–50 Gy and 15–25 fractions of radiotherapy may be the most appropriate time for ART. All imaging guidances have their advantages and disadvantages. Although ART has more benefits, ART is not universally used in the clinic because of the associated labor, economic burden and need for equipment updating. This paper helps guide and popularize the application of the ART strategy in the clinic within economic benefits and feasibility.
Lung cancer, originating from the bronchial mucosa or gland, is the malignant tumor with the highest worldwide incidence and mortality rate [Citation1]. Non-small-cell lung cancer (NSCLC) comprises approximately 85% of all histological types of lung cancer, including mainly lung adenocarcinoma and lung squamous cell carcinoma [Citation2]. Most patients with lung cancer are diagnosed at an unresectable local advanced stage or distant metastasis stage [Citation3]. Radiotherapy is required for about 50% of NSCLC patients for either cure or palliative relief of symptoms [Citation4]. Unresectable stage III NSCLC accounts for about 30% of patients preliminarily diagnosed with NSCLC [Citation5]. Concurrent chemoradiotherapy (CCRT) and sequential consolidation immunotherapy (CIT) have been approved as the standard treatment for patients with unresectable stage III NSCLC based on the results of the Pacific study [Citation6]. The Pacific study (NCT02125461) is a randomized, double-blind, placebo-controlled, large-scale, multicenter phase III clinical study to evaluate the efficacy of durvalumab in the consolidation treatment of locally advanced NSCLC (stage III) patients who have no disease progression after chemotherapy combined with radiotherapy. This study showed that the median overall survival (OS) was close to 4 years, and the 5-year OS rate increased to 43% in the group with CCRT and sequential CIT [Citation6,Citation7]. However, the local control rate is still not ideal in NSCLC patients who receive conventional fractionation radiation. In the real world, tumor progression during CCRT (32.4%), treatment-related pneumonia (12.6%) and PD-L1 tumor proportion score <1% (22.3%) have disqualified over 50% of NSCLC patients using the Pacific criteria [Citation8,Citation9]. Improving the tumor control probability (TCP) can arrest the tumor progression during CCRT, but simultaneously the normal tissue compatibility probability (NTCP) should be decreased to reduce treatment-related adverse reactions. A boosted radiation dose for tumors can effectively improve the local control rate and OS [Citation10]. However, the data of RTOG0617 identified that dose escalation increased the toxicity to the surrounding organs at risk (OAR), resulting in shorter median OS [Citation11]. Radiotherapy-associated acute toxicities, especially radiation-induced pneumonitis (RP), are the major cause contributing to unsuitability for CIT after CCRT in patients with stage III unresectable NSCLC [Citation12]. Therefore, predicting and managing radiotherapy-related toxicity, especially RP, is an important improvement strategy for radiotherapy in lung cancer patients.
Intensity-modulated radiotherapy with simultaneous integrated boost has been approved to improve the prescription dose of gross tumor volume (GTV) and reduce the irradiation dose of clinical target volumes and OAR, resulting in an enhanced therapeutic effect [Citation13]. The change of surrounding anatomical structures caused by tumor retraction, changes in atelectasis, pleural effusion or tumor baseline shifts during radiotherapy will lead to significant inconsistency between the coverage area of the planned prescription dose and the actual dose [Citation14,Citation15]. Additionally, anatomical variations during radiotherapy of NSCLC induced irradiation dose changes to OARs such as the lung and heart [Citation15]. Image-guided radiation therapy technology can partly avoid the unnecessary irradiation of surrounding normal tissues caused by changed anatomical locations for radiation administration [Citation16]. Adaptive radiotherapy (ART) is another new radiotherapy technology based on image-guided radiation therapy. The concept of ART was introduced in the late 1990s as radiation replanning according to the monitoring of treatment variations to improve radiation outcomes [Citation17]. The most common rationale is to avoid overexposure to OAR and residual tumors by adjusting and optimizing the radiotherapy plan according to the anatomical location changes of surrounding normal tissues during ART [Citation17–19]. Two categories of ART – offline adaptive replanning based on scheduled images and online ART with daily replanning – have been applied in various antitumor radiotherapies, such as liver tumors, bladder cancers and cervical cancers [Citation20–22].
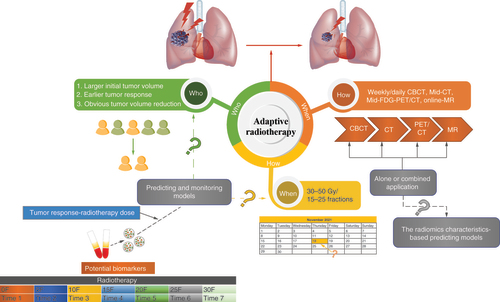
Tumor regression-induced changes are the leading cause of intrathoracic anatomical changes during the radiotherapy treatment of lung cancer patients [Citation14]. By conducting ART based on tumor shrinkage, the new target delineation and replanning of radiotherapy could result in toxicity reduction and a low rate of marginal failure in NSCLC patients treated with CCRT [Citation19]. However, the reduction of tumor volumes differs in the time frame and degrees of shrinkage in different patients under the same radiation dose and mode. Moreover, the results are different when ART is implemented based on varying imaging guidance. Three crucial elements need to be confirmed to successfully perform ART. First, the ‘who’ (selection of patients suitable for ART): due to radiosensitivity differences, GTV will change at diverse degrees during the treatment course, and ART does not need to be given to all patients. Second, the ‘when’ (selection of time frame for ART): the change in tumor volume will occur in different time frames, even if the equivalent radiation dose has been completed. Third, the ‘how’ (selection of imagery mode for ART): the image guidance methods are diverse at present. It is necessary to explore which type of image-guided technology can provide the best guidelines for the implementation of ART. This paper focuses on a comprehensive review of ART in NSCLC in terms of the above three directions () to provide ideas for how to conduct ART more effectively, contributing to improved clinical benefits.
Table 1. Studies relating to the ‘who’ of adaptive radiotherapy in patients with non-small-cell lung cancer.
Table 2. Studies relating to the ‘when’ of adaptive radiotherapy in patients with non-small-cell lung cancer.
Table 3. Studies relating to the ‘how’ of adaptive radiotherapy in patients with non-small-cell lung cancer.
Advantages of clinical application of ART in NSCLC
Dosimetric parameter benefits of ART
ART adjusts the radiotherapy plan by shrinking the GTV, leading to changes in dose coverage change for the tumor and normal tissue and vital organs around the tumor, which are reflected in the dosimetric parameter benefits of ART. According to the analysis of 158 megavoltage computed tomography images collected from seven lung patients, GTV was reduced by 60–80%, with a concomitant decrease in the volume of lung receiving 20 Gy (lung V20) of the ipsilateral lung by an average of 21% (range: 17–23%) in patients who underwent the adaptive plan. The greatest benefit of ART was observed in patients with a tumor volume that exceeded 25 cm3, which directly depends on the reduction grade of GTV during treatment [Citation44]. By ART, the mean lung dose (MLD) of lung cancer patients decreased on average from 14.6 to 12.6 Gy, and the coverage of the tumor prescription dose was significantly improved [Citation18]. Lung cancer patients enrolled in both studies included those with NSCLC and small-cell lung cancer, but the dosimetric parameter results of ART were not analyzed according to pathological type. A retrospective study by Berkovic et al. showed that the mean reductions in tumor volume, MLD and cardiac dose were 26%, 1 Gy and 0.9 Gy, respectively, in locally advanced NSCLC patients treated with ART; better progression-free survival and OS were significantly correlated with tumor reduction [Citation45]. By comparison with locally advanced NSCLC patients without ART, the reduced GTV based on ART could increase TCP by more than 40% without influencing the dose coverage of suspected microscopic disease [Citation46].
Clinical benefit of ART
The dosimetric parameter benefits for GTV and OAR brought by ART can eventually be transformed into the improvement of clinical prognosis and radiation side effects. A prospective study analyzed the local control and toxicity of ART in 50 patients with stage III NSCLC. The proportions of acute grade 3 or higher RP and radiation esophagitis were 2 and 4%, respectively; the rates for late toxicity were approximately 4 and 2%, respectively. Patients with marginal recurrence accounted for 6%, of which 20 and 4% recurred as in-field and out-of-field local failure, respectively. Therefore, ART was a great choice to reduce toxicity while keeping the low rate of marginal recurrence for NSCLC patients [Citation19]. Our previous study routinely applied ART in patients with stage III unresectable NSCLC in mid-treatment. Without a reduction in the local TCP, ART application could reduce grade 2 or higher RP and radiation esophagitis by 3 and 5%, respectively [Citation23]. A follow-up of a PET-boost trial involving 107 NSCLC patients showed that PET-guided ART could improve the radiation dose of the area with a maximum standard uptake value ≥50% to 72 Gy or above until the predefined limited dose of OAR. Compared with conventional chemoradiotherapy, PET-guided dose escalation caused a higher rate of acute and late toxicity but these were manageable. However, the outcomes of the local control rate and survival were unsatisfactory [Citation47]. To further verify the effect of PET-guided ART, Kong et al. performed a phase II randomized study. The 2-year local tumor control rate was 82% in patients treated with PET-guided dose boosting, which was significantly better than the 65% of RTOG 0617 [Citation11]. The median OS was 25 months [Citation37].
Three essential factors of ART for NSCLC
Selection of patients suitable for ART: ‘who’
The dosimetry of conventional radiotherapy plans is evaluated based on the assumption that computed tomography (CT) images will not change during radiotherapy. However, the actual doses of GTV and key OAR are different from the planned prescription doses, which are caused by the tumor retraction-induced changes in surrounding anatomical locations [Citation15,Citation25,Citation32]. The risk and seriousness of radiation toxicity, especially RP, will be reduced when the dose–volume parameters of key OARs are lower [Citation48]. In our previous study, we retrospectively analyzed 64 patients with unresectable stage III NSCLC who underwent intensity-modulated radiotherapy with simultaneous integrated boost. The contrast enhancement chest CT simulation was arranged again at 20 fractions of radiotherapy in all patients. It was found that the change in GTV ranged from +10.9% to -91.4% after 42–44 Gy in 20 fractions of radiotherapy [Citation23]. Interestingly, the dose to key OAR was also decreased significantly after ART, even in the top quartile of patients with the smallest tumor shrinkage. Our data showed that the adaptation plan could reduce the MLD by 74.8 cGy, mean esophageal dose by 183.1 cGy, grade 2 or higher RP probability by about 3% (20% relatively) and grade 2 or higher radiation esophagitis probability by 5% (12% relatively) [Citation23]. Moreover, 41 NSCLC patients who received CCRT (n = 21) or sequential chemoradiotherapy were enrolled to analyze the dosimetry and tumor volume. GTVs were decreased by 4.0–69.3%, and NSCLC patients with an initial large GTV who received CCRT were found to benefit most from ART after 15 fractions of radiotherapy [Citation24]. This suggested that NSCLC patients receiving radical radiotherapy were suitable for ART, especially those who received CCRT and had large tumor volumes. Chen et al. analyzed weekly 4D CT sets of NSCLC patients with/without ART and proposed that NSCLC patients who need ART might be identified by mean target volume variation from the planning position [Citation25].
These retrospective studies indicated that the key OAR (e.g., lung, esophagus and heart) of NSCLC patients could benefit from ART, resulting in a reduced incidence of radiotherapy-related toxicity and side effects. However, there are few reports directly involving how to predict the tumor response of NSCLC patients during radiotherapy and screen the patients suitable for ART at the appropriate time point. Recently, Amugongo et al. proposed and developed an automatic algorithm to predict the tumor volume and shape of NSCLC patients at weeks 3 and 4 of the radiotherapy course. Cone-beam CT (CBCT) images on days 1, 2, 3, 7 and 14 of radiotherapy were collected, followed by the extraction of intensity values from each voxel of plan target volumes (PTVs), and the construction of four regression models fitted to voxel intensity by linear, Gaussian, quadratic and cubic methods [Citation26]. Overall, the linear model was best in the early prediction of patients who would benefit from ART, with a sensitivity of 84% and a specificity of 99%. In addition, 21 and 23% of patients with more than 30% tumor volume reduction could benefit from ART in weeks 3 and 4, respectively [Citation26]. In another study, a mathematical model based on tumor volume change during radiotherapy was constructed to predict individual patients’ responses to radiotherapy, which provides useful guiding information for ART arrangement and dosage adjustment [Citation27]. The radiomics characteristics extracted from fluorodeoxyglucose (FDG) PET have been certified to predict tumor response to radiotherapy doses in patients with locally advanced NSCLC, which could be applied to screen the appropriate patients for ART [Citation28]. Sunassee et al. explored proliferation saturation index-related mathematical modeling in NSCLC patients by using daily CT scanning, which can accurately forecast the tumor response to subsequent radiation doses after 2 weeks and can be used as an actionable image-derived biomarker for personalization and ART [Citation29]. These studies suggest that predictive models based on the feature parameters extracted from different images could distinguish NSCLC patients who can benefit from ART. In addition, biomarkers can also be used in the prediction of suitable patients for ART due to the radiotherapy dose-dependent tumor volume change. A prospective trial including 92 consecutive stage III NSCLC patients treated with radical radiotherapy established a survival model that involved radiosensitivity-associated single-nucleotide polymorphisms, which could help to predict radiation-dose response and individualized guiding of ART [Citation30].
These results suggest that NSCLC patients with a large initial tumor volume, earlier tumor response to radiotherapy dose and obvious tumor volume reduction can receive the most benefit from ART implementation; that is, the most suitable people for ART are NSCLC patients with large tumor lesions and high radiosensitivity. However, how can NSCLC patients with these characteristics be prescreened? Researchers may need to further develop individualized gene phenotypes or biomarkers related to radiosensitivity, or construct prediction models based on these markers.
Selection of the appropriate time for ART: ‘when’
Tumor regression, tumor displacement, pleural effusion and/or atelectasis could cause anatomical changes, which would lead to significant differences between the planned prescription dose coverage and the actual dose coverage [Citation14,Citation15]. Jan et al. found that anatomical structure changes caused an average change of 0.5 Gy in MLD, 0.9% in lung V20, 1.2 Gy in mean heart dose and 1.4% in heart V40 [Citation15]. A total of 50.1% of NSCLC patients receiving CCRT had GTV reduction caused by tumor regression during radiotherapy and chemotherapy. It will significantly increase the average volume receiving 5 Gy (V5), volume receiving 20 Gy (V20), volume receiving 30 Gy (V30) and MLD of the lung, and the incidence of RP, if the radiotherapy plan is not adjusted in time according to the new GTV [Citation24]. The main emphasis of ART research should focus on tracing tumor regression and changes in the surrounding anatomical structures over time to schedule ART at the appropriate time point. At present, CBCT is the most common strategy to monitor changes in anatomical location and dose distribution. If the changes exceed the standard judged acceptable by the clinical oncologist, the adaptive plan will be recalculated according to the newly delineated GTV and PTV. However, this trigger standard is often subjective, and there is no uniformly applicable standard.
In 70 NSCLC patients who received conventional radical radiotherapy, daily CBCT before radiotherapy was obtained to outline the new tumor target on the images. Next, an ART plan was recalculated after fusing the daily CBCT image with the primary CT simulation image. Significant benefits from ART were found, with GTV decreased by more than 30% at any time point during the first 20 fractions of radiotherapy [Citation31]. Lim et al. analyzed the respiration-related CBCT of patients with locally advanced NSCLC, obtained weekly during radiotherapy. They found that tumor volume was on average decreased by 50.1% after radical radiotherapy and by 40.2% after 15 fractions. Furthermore, 60% of patients showed a volume reduction of more than 30% after 15 fractions, suggesting that 15 fractions of radiotherapy might be the appropriate time point for ART implementation [Citation32]. Lee et al. collected weekly CBCT images for 32 NSCLC patients who received radiotherapy. The average reduction in GTV was approximately 50%, and the most marked change in GTV occurred during radiotherapy sessions 15–20. This implied that a new CT scan and adaptive plan should be updated to revise the actual dose coverage deviation on tumors and surrounding OAR during this period [Citation33]. Berkovic et al. found that the tumor volumes in NSCLC patients treated with CCRT and sequential chemoradiotherapy decreased by 50.1 and 33.7%, respectively, by quantitatively analyzing daily CBCT. Patients who were treated with CCRT with a large initial tumor volume were recommended to receive ART at the 15th fraction of radiotherapy [Citation24]. To evaluate the percentage of tumor volume reduction and OAR-related dose decreases, Fox et al. arranged new CT simulations after 30 and 50 Gy of irradiation in 22 NSCLC patients receiving conventional radiotherapy, and new CT images were fused with the initial CT images. The average GTVs were reduced by 24.7% after 30 Gy irradiation and by 44.3% after 50 Gy irradiation, suggesting that the period of 30–50 Gy irradiation might be the optimum time point for ART [Citation34]. Our results also demonstrated that ART routinely scheduled after 42–44 Gy in 20 fractions of radiotherapy contributed to the reduced dosimetric parameters of OAR and radiotoxicity occurrence in patients with unresectable stage III NSCLC who received radical CCRT [Citation23].
In summary, the most significant change in tumor volume occurred at 30–50 Gy/15–25 fractions during conventionally fractionated radiotherapy in NSCLC patients receiving CCRT. Therefore, this period may be the most appropriate time for ART arrangement. However, how can we select an individualized time point for ART in each patient? There is a need to develop a monitoring model characteristic that is easy to operate and easy to track based on the tumor response–radiotherapy dose model.
Selection of the optimal mode for ART: ‘how’
The changing of target tumors during radiotherapy has been fully revealed since image-guided radiation therapy has been widely applied. At present, ART research in NSCLC patients mainly focuses on the application of different forms of imaging guidance, such as CBCT, MRT and PET-CT, in the investigation of GTV reduction, dosimetric parameters of OAR and radiotoxicity risk. All of them have their advantages and disadvantages, as shown in .
Table 4. Comparison of different forms of image guidance.
CBCT-guided ART
Woodford et al. used CBCT to monitor the variation in GTV and then adjusted the target delineation and radiotherapy plan by merging it with the images from CT simulation. They found that the tumor volume of NSCLC patients was reduced by 30%, resulting in a significant decrease in the dose to the surrounding normal tissue [Citation31]. Kwint et al. proposed a decision support system named the traffic light protocol, which was retrospectively applied to CBCT scans from 177 lung cancer patients treated with radical radiotherapy. The support system procedure is as follows. Red: once the GTV was outside the PTV because of intrathoracic anatomical changes (ITACs), ART needed to be performed immediately before treatment. Orange: the GTV was changed but remained inside the PTV; ART should be acted on before the next fraction. Yellow: although an ITAC was observed, the GTV was still well inside the PTV, and no action was required. Green: no change, and no action was required. The authors discovered that patients with ITACs accounted for 72% during the treatment [Citation14]. In another study, CBCT was performed before daily radiotherapy to track the anatomical changes in 233 patients with advanced lung cancer. Once the anatomical changes exceeded the preset reference, patients underwent new CT simulation, delineations and adaptive plans. Sixty-three patients (27%) finally received an adaptive plan, ten of whom were adaptively adjusted twice, while three patients were adaptively adjusted three times. Moreover, 75% of all patients with adaptation plans correctly adjusted for tumor dose decrease, contributing to a reduction in OAR dose [Citation18].
Mid-term localized CT-guided ART
Although increasing evidence is accumulating in studies about monitoring the timing of ART by CBCT, there are still many limitations in the prediction of the initiation time for ART. Megavoltage energy photon-based CBCT has poor image quality, causing some difficulty in the quantitative analysis of tumor and anatomical changes in patients. Radiation oncologists need to compare the CBCT images with the initial CT images frequently, which means more working hours are needed. CT simulation and adaptive planning can only be arranged after observed changes by CBCT. Mid-term localized CT scanning is planned to be performed at the predetermined time point, and whether ART is conducted depends on the scanning results. It may help to implement resource preallocation and ART effectively. In a prospective study, 217 patients with stage III NSCLC received weekly CT scans during CCRT. Fifty of these patients were replanned for radiotherapy, and significant tumor volume changes were mostly observed in the CT localization in the middle of treatment [Citation19]. In our retrospective study, 64 patients with unresectable stage III NSCLC also underwent CT localization in the middle of treatment. The tumor volumes were reduced by a median of 38.2%, and even the top quartile of patients (those with the least tumor shrinkage) benefited from the ART-induced decrease in the key OAR dose [Citation23].
FDG-PET/CT-guided ART
The local recurrence rate is found to be high in NSCLC patients receiving conventionally fractionated radiotherapy, and the recurrent tumor region is primarily distributed in the area with higher FDG metabolism [Citation49,Citation50]. Feng et al. compared patients with stage I–III NSCLC before radiotherapy and in mid-radiotherapy (after 40–50 Gy) by FDG-PET/CT. Average reductions of 26% (+15 to -75%) and 44% (+10 to -100%) were found in CT-based and PET-based tumor volumes after 40–50 Gy of radiotherapy, respectively. By GTV reduction after 40–50 Gy, PET-guided ART mid-radiotherapy contributed to boosting tumor dose (mean; 58 Gy) or reducing NTCP (mean: 2%) [Citation35]. By PET-guided ART, a phase II clinical study including 20 patients with locally advanced NSCLC showed that 15 of them could be escalated to at least 72 Gy in the primary tumor or high-FDG-uptake location (>50% maximum standard uptake value) without exceeding the predefined dose limits of OAR [Citation36]. Another FDG-PET/CT-guided ART trial enrolled 42 stage II or III patients with inoperable NSCLC. FDG-PET/CT was conducted in all patients after a dose equivalent to 2 Gy per fraction of 40–50 Gy, and the radiation dose was adaptively escalated to the FDG-indicated tumor region detected by a mid-treatment PET scan. The delivered tumor dose could be up to 86 Gy while keeping lung NTCP to 17.2% or less (MLD approximately equal to 20 Gy) [Citation37].
MRI-guided ART
The application of online real-time ART can track various systemic and random anatomical changes followed by plan adjustment. Online MRI-guided replanning, for instance, is good for reviewing changes in tumor and anatomical structures and motility changes in patients during radiotherapy [Citation51]. However, it is still a great challenge to apply online MRI-guided ART safely and reliably in the clinic [Citation52]. The latest research indicates that the time delay of real-time MRI localization is 300–500 ms [Citation53]. MRI-guided real-time ART has not been widely used in NSCLC. There are reports of feasible utilization of MRI-guided stereotactic body radiation therapy for target lesion irradiation in patients with early-stage and metastatic NSCLC [Citation38,Citation39]. MRI-guided ART applied in patients with unresectable primary lung cancer or lung metastases could encourage local control while avoiding OAR toxicity [Citation40–42]. Nevertheless, its impact on tumor control and survival remains to be elucidated. Moreover, functional MRIs such as diffusion-weighted MRI play a role in the prognostic prediction of various tumors after radiotherapy [Citation54,Citation55]. Oxygen-enhanced MRI can map regional hypoxia and be used in NSCLC patients to guide ART as a result of the association between hypoxia and poor tumor response to radiotherapy and prognosis [Citation43]. Therefore the development of functional MRI biomarker-guided ART can potentially help individualized therapy. However, there is still a lack of evidence from prospective clinical trials to support the clinical benefit of MRI-guided real-time-localization ART.
Future perspective
Radiotherapy is one of the three major treatments for a variety of solid tumors, including NSCLC. The respiratory motions, improvement of tumor regression, involved lymph node shifts, atelectasis, or pleural effusion during radiotherapy result in alterations of the surrounding anatomical structure, leading to a deviation between the prescribed and actual dose coverage areas. However, the larger impact on the dose coverage is thought to be caused by anatomical changes caused by the tumor regression, the improvement of atelectasis, or pleural effusion than that of respiratory tumor motion in NSCLC patients [Citation56,Citation57]. Daily CBCT is a common strategy to monitor anatomical changes due to tumor shrinkage and localization during radiotherapy for NSCLC patients [Citation58]. However, phase offsets existed in the motion of the involved lymph nodes and primary tumor [Citation59]. Because of low contrast in CBCT and the inability to accurately determine lymph node shifts, Hoffmann et al. suggested that selected anatomical landmarks surrounding the lymph nodes with metastatic cancer could be used to track and evaluate the involved lymph node shifts during radiotherapy [Citation60]. Moller et al. reviewed CBCT scans of 163 lung cancer patients, 12% of whom required ART to optimize the dose coverage in conformity of target or normal tissue induced by atelectasis, pleural effusion or pneumonitis [Citation61].
The OS of NSCLC patients is associated with the local control rate, which can be enhanced by an increased radiotherapy dose to the tumor region. However, uniform dose escalation will eventually lead to increased NTCP without prognostic benefit due to the dose restriction of key OARs [Citation62]. ART was applied as a strategy to revise the dose distribution discrepancies caused by anatomical changes. ART by daily online tumor matching for patients with locally advanced lung cancer could ensure or increase TCP with decreased normal tissue-related irradiation [Citation18,Citation63,Citation64]. A learning program qualification was reported to correctly score deviations and anatomical changes due to lymph nodes, primary tumor, atelectasis and pleural effusion during ART based on soft-tissue matching for lung cancer [Citation65]. Moreover, in the process of radiotherapy, the large range of irradiation areas raises the risk of radiation-related toxicity, causing radiotherapy interruption and prolonged total treatment time, which seriously affects TCP [Citation66]. The TCP of NSCLC, one of the tumors most affected by radiotherapy interruption, decreased by 1.6% when the total treatment time was extended by 1 day [Citation67]. Due to poor TCP and a high rate of RP, almost half of stage III NSCLC patients could not complete the standard regimen of CCRT and sequential CIT [Citation8,Citation9].
Immunotherapy has attracted much attention because it kills tumor cells by activating antitumor immunity, but its effectiveness depends on normal T lymphocytes [Citation68]. Due to the high sensitivity of T lymphocytes to radiation, radiation-induced lymphopenia is found in 70% of NSCLC patients receiving CCRT or radiotherapy alone, and severe lymphopenia is related to poor prognosis [Citation69,Citation70]. The larger irradiation area of NSCLC patients led to a lower bottom value of radiation-induced lymphopenia and a worse prognosis [Citation69]. Choosing patients suitable for ART could reduce the risk of radiation-induced lymphopenia by adaptively reducing the irradiation area promptly [Citation71]. Therefore it is very important to conduct ART routinely during the radiotherapy of NSCLC patients.
As shown in , the effective implementation of ART involves the confirmation of the following three elements: the patient selection (who), the time point selection (when) and the mode selection (how) of ART. The different imaging mode-guided ARTs have advantages in timeliness, sensitivity, specificity and economic costs. The single or combined application of imaging modes should be flexibly selected according to the actual capability of the clinic. At present, the research results regarding the selection of sensitive patients and time points for ART are primarily based on retrospective analysis. The construction of mathematical prediction models in ART requires the acquisition of multiple images, which has the disadvantages of complex procedures, large amounts of time required and difficulty in tracking data.
Non-small-cell lung cancer patients with large tumor volume, earlier tumor response to radiotherapy and significantly decreased tumor volume are the eligible patients for adaptive radiotherapy (ART). The accomplishment of 30–50 Gy/15–25 fractions of radiation is the appropriate period for ART in non-small-cell lung cancer patients. Different forms of image guidance, including CBCT, MRI and PET-CT can be applied in the implementation of ART, and various image guidance techniques can be used separately or jointly. In the future, predictive and monitoring models based on circulating biomarkers and the radiomics characteristics will be useful to screen appropriate patients and determine the correct time for the ART schedule.
CBCT: Cone-beam computed tomography; CT: Computed tomography; F: Fraction; FDG: Fluorodeoxyglucose; MR: Magnetic resonance.
In addition, a variety of circulating biomarkers have been developed in many studies on the prognosis of radiotherapy. These biomarkers are easily collected from peripheral blood or body fluids and can be applied in monitoring and tracking at multiple time points. For example, extracellular vesicles are stable and efficient circulating makers that can transmit information among cells [Citation72]. Exosomal miRNAs can be easily detected in circulating body fluids and function as potential biomarkers for predicting and monitoring the response to radiotherapy in other solid tumors [Citation73]. Therefore, we can pay more attention to research on the application of these biomarkers based on the treatment response-dependent radiotherapy dose/time point model in ART in the future. Exploring biomarkers with high sensitivity and specificity, and developing prediction models based on these biomarkers, can simplify the prescreening of patients for ART and the prediction of an appropriate time frame for ART, which has the potential to guide the effective clinical application of ART.
Adaptive radiotherapy is a new radiotherapy
Adaptive radiotherapy (ART) is guided by various forms of imaging to avoid radiation overexposure due to the anatomical location changes of surrounding normal tissues during treatment.
Advantages of clinical application of ART in non-small-cell lung cancer
Patients with unresectable non-small-cell lung cancer (NSCLC) can gain dosimetric parameters and prognostic benefits from ART.
Three essential factors of ART for NSCLC
Who can gain more benefits from ART: NSCLC patients with large tumor lesions and high radiosensitivity.
When is the optimal time to conduct ART: the period of 30–50 Gy/15–25 fractions of radiotherapy.
How to better implement ART: all imaging guidances have their advantages and disadvantages.
Future perspective
To effectively implement ART, we require a prediction model based on individual biomarkers with high sensitivity and specificity, which can screen optimal patients and time point for ART.
Author contributions
S Zhou and Y Meng contributed to the data collection and to writing and editing of the manuscript. X Sun and Z Jin made the figures and tables. W Feng and H Yang designed and guided this study. All authors listed made a substantial, direct and intellectual contribution to the work and approved it for publication.
Financial & competing interests disclosure
This study was supported by National Natural Science Foundation of China (NSFC 81872458), Open project of Zhejiang Key Laboratory of Radiation Oncology (2022ZJCCRAD03). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Siegel RL , MillerKD, FuchsHE, JemalA. Cancer statistics, 2021. CA Cancer J. Clin.71(1), 7–33 (2021).
- Molina JR , YangP, CassiviSD, SchildSE, AdjeiAA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc.83(5), 584–594 (2008).
- Khatri A , GuJJ, McKernanCM, XuX, PendergastAM. ABL kinase inhibition sensitizes primary lung adenocarcinomas to chemotherapy by promoting tumor cell differentiation. Oncotarget10(20), 1874–1886 (2019).
- Kong FM , ZhaoJ, WangJ, Faivre-FinnC. Radiation dose effect in locally advanced non-small cell lung cancer. J. Thorac. Dis.6(4), 336–347 (2014).
- Yoon SM , ShaikhT, HallmanM. Therapeutic management options for stage III non-small-cell lung cancer. World J. Clin. Oncol.8(1), 1–20 (2017).
- Antonia SJ , VillegasA, DanielDet al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med.377(20), 1919–1929 (2017).
- Antonia SJ , VillegasA, DanielDet al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med.379(24), 2342–2350 (2018).
- Jung HA , NohJM, SunJMet al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer146, 23–29 (2020).
- Eichkorn T , BozorgmehrF, RegnerySet al. Consolidation immunotherapy after platinum-based chemoradiotherapy in patients with unresectable stage III non-small cell lung cancer – cross-sectional study of eligibility and administration rates. Front. Oncol.10, 586449 (2020).
- Machtay M , BaeK, MovsasBet al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small-cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys.82(1), 425–434 (2012).
- Bradley JD , PaulusR, KomakiRet al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol.16(2), 187–199 (2015).
- Shaverdian N , OffinMD, RimnerAet al. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiother. Oncol.144, 101–104 (2020).
- Wang D , BiN, ZhangTet al. Comparison of efficacy and safety between simultaneous integrated boost intensity-modulated radiotherapy and conventional intensity-modulated radiotherapy in locally advanced non-small-cell lung cancer: a retrospective study. Radiat. Oncol.14(1), 106 (2019).
- Kwint M , ConijnS, SchaakeEet al. Intrathoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother. Oncol.113(3), 392–397 (2014).
- Jan N , GuyC, ReshkoLB, HugoGD, WeissE. Lung and heart dose variability during radiation therapy of non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys.98(3), 683–690 (2017).
- Dawson LA , SharpeMB. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol.7(10), 848–858 (2006).
- Yan D , ViciniF, WongJ, MartinezA. Adaptive radiation therapy. Phys. Med. Biol.42(1), 123–132 (1997).
- Moller DS , HoltMI, AlberMet al. Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose. Radiother. Oncol.121(1), 32–38 (2016).
- Ramella S , FioreM, SilipigniSet al. Local control and toxicity of adaptive radiotherapy using weekly CT imaging: results from the LARTIA trial in stage III NSCLC. J. Thorac. Oncol.12(7), 1122–1130 (2017).
- Witt JS , RosenbergSA, BassettiMF. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol.21(2), e74–e82 (2020).
- Shelley CE , BarracloughLH, NelderCL, OtterSJ, StewartAJ. Adaptive radiotherapy in the management of cervical cancer: review of strategies and clinical implementation. Clin. Oncol. (R. Coll. Radiol.)33(9), 579–590 (2021).
- Kong V , HansenVN, HafeezS. Image-guided adaptive radiotherapy for bladder cancer. Clin. Oncol. (R. Coll. Radiol.)33(6), 350–368 (2021).
- Meng Y , LuoW, XuHet al. Adaptive intensity-modulated radiotherapy with simultaneous integrated boost for stage III non-small cell lung cancer: is a routine adaptation beneficial? Radiother. Oncol. 158, 118–124 (2021).
- Berkovic P , PaelinckL, LievensYet al. Adaptive radiotherapy for locally advanced non-small-cell lung cancer, can we predict when and for whom? Acta Oncol. 54(9), 1438–1444 (2015).
- Chen M , YangJ, LiaoZet al. Anatomic change over the course of treatment for non-small-cell lung cancer patients and its impact on intensity-modulated radiation therapy and passive-scattering proton therapy deliveries. Radiat. Oncol.15(1), 55 (2020).
- Amugongo LM , Vasquez OsorioE, GreenAFet al. Early prediction of tumour-response to radiotherapy in NSCLC patients. Phys. Med. Biol. (2021).
- Tariq I , ChenT, KirkbyNF, JenaR. Modelling and Bayesian adaptive prediction of individual patients’ tumour volume change during radiotherapy. Phys. Med. Biol.61(5), 2145–2161 (2016).
- Duan C , ChaovalitwongseWA, BaiFet al. Sensitivity analysis of FDG PET tumor voxel cluster radiomics and dosimetry for predicting mid-chemoradiation regional response of locally advanced lung cancer. Phys. Med. Biol.65(20), 205007 (2020).
- Sunassee ED , TanD, JiNet al. Proliferation saturation index in an adaptive Bayesian approach to predict patient-specific radiotherapy responses. Int. J. Radiat. Biol.95(10), 1421–1426 (2019).
- Jin JY , WangW, TenHaken RKet al. Use a survival model to correlate single-nucleotide polymorphisms of DNA repair genes with radiation dose–response in patients with non-small-cell lung cancer. Radiother. Oncol.117(1), 77–82 (2015).
- Woodford C , YartsevS, DarAR, BaumanG, Van DykJ. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images. Int. J. Radiat. Oncol. Biol. Phys.69(4), 1316–1322 (2007).
- Lim G , BezjakA, HigginsJet al. Tumor regression and positional changes in non-small-cell lung cancer during radical radiotherapy. J. Thorac. Oncol.6(3), 531–536 (2011).
- Lee YH , KimYS, LeeHCet al. Tumour volume changes assessed with high-quality KVCT in lung cancer patients undergoing concurrent chemoradiotherapy. Br. J. Radiol.88(1052), 20150156 (2015).
- Fox J , FordE, RedmondKet al. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys.74(2), 341–348 (2009).
- Feng M , KongFM, GrossMet al. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int. J. Radiat. Oncol. Biol. Phys.73(4), 1228–1234 (2009).
- van Elmpt W , DeRuysscher D, vander Salm Aet al. The PET-boost randomised phase II dose-escalation trial in non-small-cell lung cancer. Radiother. Oncol.104(1), 67–71 (2012).
- Kong FM , TenHaken RK, SchipperMet al. Effect of midtreatment PET/CT-Adapted radiation therapy with concurrent chemotherapy in patients with locally advanced non-small-cell lung cancer: a phase 2 clinical trial. JAMA Oncol.3(10), 1358–1365 (2017).
- Finazzi T , Ronden-KianoushMI, SpoelstraFOBet al. Stereotactic ablative radiotherapy in patients with early-stage non-small cell lung cancer and co-existing interstitial lung disease. Acta Oncol.59(5), 569–573 (2020).
- Fischer-Valuck BW , HenkeL, GreenOet al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv. Radiat. Oncol.2(3), 485–493 (2017).
- Henke LE , OlsenJR, ContrerasJAet al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: results of a phase 1 trial. Adv. Radiat. Oncol.4(1), 201–209 (2019).
- Finazzi T , PalaciosMA, SpoelstraFOBet al. Role of on-table plan adaptation in MR-guided ablative radiation therapy for central lung tumors. Int. J. Radiat. Oncol. Biol. Phys.104(4), 933–941 (2019).
- Finazzi T , HaasbeekCJA, SpoelstraFOBet al. Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors. Int. J. Radiat. Oncol. Biol. Phys.107(2), 270–278 (2020).
- Salem A , LittleRA, LatifAet al. Oxygen-enhanced MRI is feasible, repeatable, and detects radiotherapy-induced change in hypoxia in xenograft models and in patients with non-small-cell lung cancer. Clin. Cancer Res.25(13), 3818–3829 (2019).
- Ramsey CR , LangenKM, KupelianPAet al. A technique for adaptive image-guided helical tomotherapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys.64(4), 1237–1244 (2006).
- Berkovic P , PaelinckL, GulybanAet al. Adaptive radiotherapy for locally advanced non-small cell lung cancer: dosimetric gain and treatment outcome prediction. Acta Oncol.56(11), 1656–1659 (2017).
- Guckenberger M , RichterA, WilbertJ, FlentjeM, PartridgeM. Adaptive radiotherapy for locally advanced non-small-cell lung cancer does not underdose the microscopic disease and has the potential to increase tumor control. Int. J. Radiat. Oncol. Biol. Phys.81(4), e275–282 (2011).
- van Diessen J , DeRuysscher D, SonkeJJet al. The acute and late toxicity results of a randomized phase II dose-escalation trial in non-small-cell lung cancer (PET-boost trial). Radiother. Oncol.131, 166–173 (2019).
- Palma DA , SenanS, TsujinoKet al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys.85(2), 444–450 (2013).
- Aerts HJ , van BaardwijkAA, PetitSFet al. Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy 18fluorodeoxyglucose-PET-CT scan. Radiother. Oncol.91(3), 386–392 (2009).
- Abramyuk A , TokalovS, ZophelKet al. Is pre-therapeutical FDG-PET/CT capable to detect high risk tumor subvolumes responsible for local failure in non-small-cell lung cancer? Radiother. Oncol. 91(3), 399–404 (2009).
- Sonke JJ , AznarM, RaschC. Adaptive radiotherapy for anatomical changes. Semin. Radiat. Oncol.29(3), 245–257 (2019).
- Thorwarth D , LowDA. Technical challenges of real-time adaptive MR-guided radiotherapy. Front. Oncol.11, 634507 (2021).
- Borman PTS , TijssenRHN, BosCet al. Characterization of imaging latency for real-time MRI-guided radiotherapy. Phys. Med. Biol.63(15), 155023 (2018).
- Mahmood F , JohannesenHH, GeertsenP, HansenRH. Repeated diffusion MRI reveals earliest time point for stratification of radiotherapy response in brain metastases. Phys. Med. Biol.62(8), 2990–3002 (2017).
- Leibfarth S , WinterRM, LyngH, ZipsD, ThorwarthD. Potentials and challenges of diffusion-weighted magnetic resonance imaging in radiotherapy. Clin. Transl. Radiat. Oncol.13, 29–37 (2018).
- Britton KR , StarkschallG, LiuHet al. Consequences of anatomic changes and respiratory motion on radiation dose distributions in conformal radiotherapy for locally advanced non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys.73(1), 94–102 (2009).
- Schmidt ML , HoffmannL, KandiM, MollerDS, PoulsenPR. Dosimetric impact of respiratory motion, interfraction baseline shifts, and anatomical changes in radiotherapy of non-small cell lung cancer. Acta Oncol.52(7), 1490–1496 (2013).
- Knap MM , HoffmannL, NordsmarkM, VestergaardA. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol.49(7), 1077–1084 (2010).
- Pantarotto JR , PietAH, VincentA, vanSornsen de Koste JR, SenanS. Motion analysis of 100 mediastinal lymph nodes: potential pitfalls in treatment planning and adaptive strategies. Int. J. Radiat. Oncol. Biol. Phys.74(4), 1092–1099 (2009).
- Hoffmann L , HoltMI, KnapMM, KhalilAA, MollerDS. Anatomical landmarks accurately determine interfractional lymph node shifts during radiotherapy of lung cancer patients. Radiother. Oncol.116(1), 64–69 (2015).
- Moller DS , KhalilAA, KnapMM, HoffmannL. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother. Oncol.110(3), 517–522 (2014).
- Scott JG , SedorG, ScarboroughJAet al. Personalizing radiotherapy prescription dose using genomic markers of radiosensitivity and normal tissue toxicity in NSCLC. J. Thorac. Oncol.16(3), 428–438 (2021).
- Guckenberger M , WilbertJ, RichterA, BaierK, FlentjeM. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys.79(3), 901–908 (2011).
- Tvilum M , KhalilAA, MollerDS, HoffmannL, KnapMM. Clinical outcome of image-guided adaptive radiotherapy in the treatment of lung cancer patients. Acta Oncol.54(9), 1430–1437 (2015).
- Boejen A , VestergaardA, HoffmannLet al. A learning programme qualifying radiation therapists to manage daily online adaptive radiotherapy. Acta Oncol.54(9), 1697–1701 (2015).
- Inal A , DumanE. Adaptive time management for patients who have non-small-cell lung cancer and underwent definitive radiotherapy: a dosimetric study of different gap duration scenarios. Int. J. Radiat. Biol.97(2), 219–227 (2021).
- Fowler JF , ChappellR. Non-small-cell lung tumors repopulate rapidly during radiation therapy. Int. J. Radiat. Oncol. Biol. Phys.46(2), 516–517 (2000).
- Ribas A . Tumor immunotherapy directed at PD-1. N. Engl. J. Med.366(26), 2517–2519 (2012).
- Tang C , LiaoZ, GomezDet al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small-cell lung cancer patient outcomes. Int. J. Radiat. Oncol. Biol. Phys.89(5), 1084–1091 (2014).
- Cho O , OhYT, ChunM, NohOK, LeeHW. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol.37(1), 971–978 (2016).
- Ellsworth SG , YalamanchaliA, ZhangHet al. Comprehensive analysis of the kinetics of radiation-induced lymphocyte loss in patients treated with external beam radiation therapy. Radiat. Res.193(1), 73–81 (2020).
- Shao H , ImH, CastroCMet al. New technologies for analysis of extracellular vesicles. Chem. Rev.118(4), 1917–1950 (2018).
- Malla B , ZauggK, VassellaE, AebersoldDM, DalPra A. Exosomes and exosomal microRNAs in prostate cancer radiation therapy. Int. J. Radiat. Oncol. Biol. Phys.98(5), 982–995 (2017).
