Welcome to the ninth volume of Neurodegenerative Disease Management. The start of a new volume is a great time to look back at past content and think about how the field has progressed over the last year. Below, I will discuss some of 2018’s most popular articles, and also look forward to what 2019 has to bring, in this issue and beyond.
In this issue
The first issue of 2019 features a variety of topics, and starts with three reviews evaluating specific treatments. The first, from Kristen et al., examines patisiran; a double-stranded small interfering RNA, for the treatment of hereditary ATTR amyloidosis [Citation1]. The authors review the efficacy and safety data from the patisiran clinical trial program. Next, Benson et al. review the use of inotersen, a second-generation antisense oligonucleotide, for the treatment of transthyretin amyloidosis [Citation2]. Finally in this section, Richard and Frank look at the use of deutetrabenazine in the treatment of chorea in Huntington’s disease; specifically the drug’s pharmacological properties, interactions, administration and efficacy [Citation3].
Two papers presenting original research are also included in this issue; the first is from Poewe et al., in which the authors conduct a post hoc analysis of the GLORIA registry to examine the long-term effect of levodopa–carbidopa intestinal gel on dyskinesia burden in Parkinson’s disease [Citation4]. Finally, Lee et al. present a study piloting the Person-centred Risk Assessment Framework (PCRAF) – a framework managing risk among persons living with dementia in primary care [Citation5].
Content highlights of 2018
As in previous years, we have taken a look back over the content published in 2018 to see what topics have proved most popular with our readers ().
Table 1. Top ten Neurodegenerative Disease Management 2018 articles by readership†.
The most-read article of 2018 (at the time of writing), is a Case Report from Hartzell et al., discussing the difficult topic of suicide in early-onset Alzheimer’s disease [Citation6]. The authors conclude that: ‘for clinicians who treat and evaluate patients with neurodegenerative disorders, safety and risk assessment must become integral components of ongoing care’.
Interview content has proven particularly popular with our readers in 2018, with four articles in the top ten being interviews with experts in their field [Citation7,Citation9–11]. These interviews took place following the ECTRIMS–ACTRIMS conference in October 2017, and highlighted some of the key research presented at that conference by F Hoffmann-La Roche (Switzerland), Biogen (MA, USA), Celgene (Switzerland) and Merck KGaA (Germany). It was great to speak with these individuals, and learn about the exciting developments that are underway in the management of multiple sclerosis.
Editorials also proved popular features in 2018; in the June issue, Modrego discusses the unmet needs in the management of myasthenia gravis [Citation8]. And back in January, Popugaeva and Bezprozvanny discussed STIM proteins as regulators of neuronal store-operated calcium influx [Citation12].
The journal’s most popular original research this year came from Ogino et al. [Citation13] and Ripamonti et al. [Citation15]. Real-world data are increasingly being used in medical research, and in the first article the authors used real-world data from a Japanese health insurance claims database to investigate the treatment, comorbidities and prevalence of multiple sclerosis in an employed Japanese population. In the second, the authors investigate the neglected area of respiratory function decline in Duchenne muscular dystrophy.
Rounding out the top ten from 2018 is a Special Report article from Fackrell et al., in which the authors present a clinical consensus outlining practical considerations for managing motor fluctuations in patients with Parkinson’s disease that complement current national guidelines from NICE (England, Wales and Northern Ireland) and SIGN (Scotland) [Citation14].
Demographics of contributors
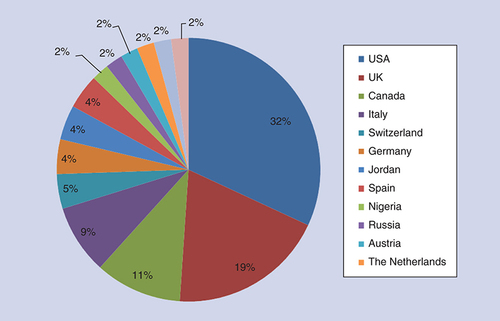
It is interesting to consider the global nature of neurodegenerative disease research, as well as the topics being discussed. illustrates the countries of the lead authors who published in Neurodegenerative Disease Management in 2018. As usual, the USA and the UK account for around half of our authorship; however, the remainder is varied, including Canada, Italy and Switzerland.
Editorial leadership
In 2018, I was delighted to welcome a new Senior Editor to the journal – Professor Theresa A Zesiewicz, a previous Editorial Board member. Professor Zesiewicz is the Director of the University of South Florida (USF) Ataxia Research Center (FL, USA), the PADRECC Veterans’ Parkinson’s Disease Clinic and the Frances J Zesiewicz Foundation for Parkinson’s Disease at USF. Professor Zesiewicz specializes in movement disorders, including Parkinson’s disease, essential tremor, ataxia, restless legs syndrome and dystonia.
In 2018, we were also pleased to welcome four new members to the Editorial Board: Professor Tom Mercer (Queen Margaret University, UK), Professor Olivier Piguet (University of Sydney, Australia), Professor Till Sprenger (DKD Helios Klinik Wiesbaden, Germany) and Professor Joe Verghese (Albert Einstein College of Medicine, NY, USA).
Conclusion
In conclusion, I would like to thank all our readers, authors and Editorial Board members who contributed to the journal in 2018. I look forward to working with you all again in the coming year, and of course would welcome any feedback or article proposals you might have. Do follow the journal’s Twitter account to keep up with journal and other news [Citation16], or join our Neurology LinkedIn group [Citation17].
Financial & competing interests disclosure
L Dormer is an employee of Future Medicine Ltd. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Kristen AV , Ajroud-DrissSA, ConceiçãoI, GorevicP, KyriakidesT, ObiciL . Patisiran, an RNAi therapeutic for the treatment of hereditary ATTR amyloidosis . Neurodegener. Dis. Manag.9 ( 1 ), doi:10.2217/nmt-2018-0033 ( 2019 ) ( Epub ahead of print ).
- Benson MD , DasguptaNR, MoniaBP . Inotersen (transthyretin specific antisense oligonucleotide) for treatment of transthyretin amyloidosis . Neurodegener. Dis. Manag.9 ( 1 ), doi:10.2217/nmt-2018-0037 ( 2019 ) ( Epub ahead of print ).
- Richard A , FrankS . Deutetrabenazine in the treatment of Huntington’s disease . Neurodegener. Dis. Manag.9 ( 1 ), doi:10.2217/nmt-2018-0040 ( 2019 ) ( Epub ahead of print ).
- Poewe W , ChaudhuriKR, BergmannL, AntoniniA . Levodopa–carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry . Neurodegener. Dis. Manag.9 ( 1 ), doi:10.2217/nmt-2018-0034 ( 2019 ) ( Epub ahead of print ).
- Lee L , HillierLM, LuSKet al. Person-Centred Risk Assessment Framework: assessing and managing risk in older adults living with dementia . Neurodegener. Dis. Manag.9 ( 1 ), doi:10.2217/nmt-2018-0031 ( 2019 ) ( Epub ahead of print ).
- Hartzell JW , GearyR, GyureK, ChivukulaVR, HautMW . Completed suicide in an autopsy-confirmed case of early onset Alzheimer’s disease . Neurodegener. Dis. Manag.8 ( 2 ), 81 – 88 ( 2018 ).
- Belachew S . Advancing the understanding of progression in multiple sclerosis: an interview with Shibeshih Belachew . Neurodegener. Dis. Manag.8 ( 1 ), 9 – 12 ( 2018 ).
- Modrego PJ . Myasthenia gravis: the unmet needs of a paradigmatic autoimmune disease . Neurodegener. Dis. Manag.8 ( 3 ), 137 – 139 ( 2018 ).
- Sheikh SI . The exciting field of neuro-repair in multiple sclerosis: an interview with Sarah I Sheikh . Neurodegener. Dis. Manag.8 ( 1 ), 13 – 15 ( 2018 ).
- Koscielny V . Phase III SUNBEAM and RADIANCE PART B trials for Ozanimod in relapsing multiple sclerosis demonstrate superiority versus interferon-β-1a (Avonex®) in reducing annualized relapse rates and MRI brain lesions . Neurodegener. Dis. Manag.8 ( 3 ), 141 – 142 ( 2018 ).
- Rossetti L . Treating MS without continuous immunosuppression: an interview with Luciano Rossetti . Neurodegener. Dis. Manag.8 ( 2 ), 69 – 71 ( 2018 ).
- Popugaeva E , BezprozvannyI . STIM proteins as regulators of neuronal store-operated calcium influx . Neurodegener. Dis. Manag.8 ( 1 ), 5 – 7 ( 2018 ).
- Ogino M , ShiozawaA, OtaH, OkamotoS, HiroiS, KawachiI . Treatment and comorbidities of multiple sclerosis in an employed population in Japan: analysis of health claims data . Neurodegener. Dis. Manag.8 ( 2 ), 97 – 103 ( 2018 ).
- Fackrell R , CarrollCB, GrossetDGet al. Noninvasive options for ‘wearing-off’ in Parkinson’s disease: a clinical consensus from a panel of UK Parkinson’s disease specialists . Neurodegener. Dis. Manag.8 ( 5 ), 349 – 360 ( 2018 ).
- Ripamonti E , D’AngeloG . Measurement of respiratory function decline in patients with Duchenne muscular dystrophy: a conjoint analysis . Neurodegener. Dis. Manag.8 ( 2 ), 89 – 96 ( 2018 ).
- Neurodegenerative Disease Management Twitter . https://twitter.com/@fsgnmt .
- Future Science Group Neurology LinkedIn group . www.linkedin.com/groups/8204606/ .