Abstract
Aim: This subanalysis of the OPTIPARK study aimed to confirm the effectiveness and safety of opicapone in patients with Parkinson’s disease and motor fluctuations in clinical practice specifically in the UK and to assess the impact of opicapone on treatment costs. Methods: Patients received opicapone added to levodopa for 6 months. Clinical outcomes were assessed at 3 and 6 months and treatment costs at 6 months. Results: Most patients’ general condition improved at 3 months, with sustained improvements reported at 6 months. Opicapone improved motor and non-motor symptoms at both timepoints, was generally well tolerated and reduced total treatment costs by GBP 3719. Conclusion: Opicapone added to levodopa resulted in clinical improvements and reduced treatment costs across UK clinical practice.
Plain language summary
Patients with Parkinson’s disease (PD) often experience motor fluctuations (reduced and variable response to medication) following prolonged treatment with levodopa, which is currently the most effective treatment for the symptoms of PD. Opicapone has been developed for use in combination with levodopa to reduce the occurrence of motor fluctuations and was shown to be effective in two large clinical trials. This study describes the effectiveness, safety and cost-saving impact of opicapone when used to treat patients with PD and motor fluctuations across everyday clinical practice in the UK. Six months’ treatment with opicapone was generally well tolerated, resulted in an improvement of the patients’ overall PD condition and reduced treatment costs.
Clinical trial registration: Registered in July 2016 at NCT02847442 (ClinicalTrial.gov)
Levodopa (also known as l-DOPA) is the most effective symptomatic treatment for Parkinson’s disease (PD); however, its long-term use is often associated with wearing-off symptoms [Citation1–4]. Following its oral administration, it is catabolized in the periphery by dopa decarboxylase (DDC) and catechol-O-methyltransferase (COMT), with only 1% of an oral dose penetrating the brain [Citation5,Citation6]. Peripheral inhibitors of DDC (DDCIs) and COMT (COMTIs) are commonly used as adjunct therapy in order to increase bioavailability and enhance its delivery to the brain, with the aim of ameliorating wearing-off symptoms [Citation5,Citation7].
Opicapone was developed to fulfill the need for a more potent, longer-acting COMTI, with a good safety profile [Citation5,Citation6,Citation8,Citation9]. It has been shown to be well tolerated and efficacious in reducing OFF-time in two large, randomized, placebo-controlled, multinational, pivotal trials (BIPARK-I and II) of patients with PD and end-of-dose motor fluctuations [Citation10,Citation11]. On the basis of these pivotal trials, opicapone was approved in the European Union as adjunct therapy to preparations of levodopa/DDCIs in adult patients with PD and end-of-dose motor fluctuations [Citation12]. It has now also been approved in the USA, Japan, South Korea, Australia and other countries, with slight variations in its approved indications in each country [Citation13,Citation14].
Many regulators and payers encourage the supplementation of randomized trial data with real-world study evidence [Citation15,Citation16]. OPTIPARK was a prospective, open-label, single-arm trial conducted in the UK and Germany [Citation17], with the primary intention of evaluating the change in the clinicians’ perception of their patients’ overall PD condition (as assessed by Clinician’s Global Impression of Change [CGI-C]) after 3 months of treatment in routine clinical practice with once-daily opicapone 50 mg [Citation17]. While patients in Germany were treated for 3 months only, patients in the UK were treated for 6 months to assess health economic costs. This subanalysis of OPTIPARK reports on the effectiveness and safety of opicapone after 3 and 6 months of treatment in the UK patients only and investigates the influence of opicapone on overall treatment costs for patients with PD in real-world settings across the UK.
Materials & methods
Study design
The study design of OPTIPARK has been described previously and the methods description partly reproduces their wording [Citation17]. In brief, a prospective, open-label, single-arm, multicenter trial evaluating opicapone 50 mg effectiveness in levodopa-treated patients with PD who experience motor fluctuations was conducted from November 2016 to July 2018 at 68 specialist neurology centers across the UK and Germany (EudraCT number: 2016-002391-27) [Citation17]. This subanalysis focuses on the outcomes from the patients in the UK centers only.
Patients received opicapone 50 mg capsules once daily at bedtime, at least 1 h before or after the last daily dose of levodopa/DDCI. Whereas patients in the German cohort were treated for 3 months, patients in the UK were treated for 6 months to investigate the influence of opicapone treatment on the health economic costs caused by PD. At the end of the study, patients could continue opicapone in line with local standard practice.
Study population
Patients with idiopathic PD aged ≥30 years were eligible if they reported symptoms of motor fluctuations as identified by at least one symptom on the 9-Symptom Wearing-off Questionnaire (WOQ-9) [Citation18]. They also had to be Hoehn and Yahr stages I–IV (during ON-time) and treated with 3–7 daily doses of levodopa/DDCI. Patients with symptoms and signs suggestive of atypical Parkinsonism, severe unpredictable OFF periods (investigator judgment) and severe hepatic impairment (Child-Pugh Class C) were excluded. The full list of inclusion and exclusion criteria has been detailed previously [Citation17].
Study assessments
End points were assessed at baseline and at 1, 3 and 6 months or at any early discontinuation visit. The primary outcome was CGI-C (7-point scale, ranging from ‘very much improved’ to ‘very much worse’), which assessed the clinicians’ perception of their patients’ illness after 3 months’ treatment with opicapone 50 mg; the same rater assessed CGI-C throughout the study and before the patient made his/her own assessment. Secondary assessments included CGI-C after 6 months of treatment and Patient’s Global Impression of Change (PGI-C), WOQ-9 assessments, the Unified Parkinson’s Disease Rating Scale (UPDRS) sections I–IV [Citation19], the Parkinson’s Disease Questionnaire (PDQ-8) [Citation20], the Non-Motor Symptoms Scale (NMSS) [Citation21] and change from baseline in total daily levodopa dose and dosing frequency after 3 and 6 months.
Health economic costs were evaluated using the Client Service Receipt Inventory (CSRI) questionnaire, which was adapted to assess the effect of 6 months’ opicapone treatment on the costs for the care of patients with PD in the UK [Citation22,Citation23]. CSRI questionnaires have also been used to assess resource use and costs for patients with other movement disorders, such as multiple sclerosis and Huntington’s disease [Citation24,Citation25]. The questionnaire was grouped into four sections. Section 1 contained the categories ‘hospital and residential services’ and ‘primary and community care services’. Section 2 contained ‘investigations/diagnostic tests’, ‘aids or devices’ and ‘adaptations to the home’. Section 3 contained ‘informal care’, ‘care because of multiple system atrophy (MSA)’ and ‘journeys’. Section 4 contained ‘occupation’ and included questions regarding full- and part-time gross wage, stopped or reduced working and unemployed/retired time. Categories from sections 1 and 2 were considered ‘formal service’, whereas categories from sections 3 and 4 were considered ‘unpaid care’ for the purposes of the cost evaluation. The questionnaire was completed by the investigator at baseline and after 6 months of treatment. All economic calculations were made in pounds sterling.
Treatment compliance was calculated based on the numbers of dispensed and returned opicapone capsules, and treatment duration excluding interruptions to study medication.
Safety was assessed through the specific reporting of treatment-emergent adverse events (TEAEs) as well as vital signs and regular physical and neurological examinations. Pre-specified subgroup analyses also evaluated change from baseline in levodopa total daily dose in patients who reported dopaminergic adverse events (AEs), i.e., dyskinesia, nausea, vomiting, orthostatic hypotension and any hallucination, illusion, delusion or disturbance in attention.
Statistical analysis
No sample size estimation was performed for this open-label study. The safety population included all patients who received ≥1 dose of opicapone. Effectiveness was assessed in the full analysis set (FAS), which included all patients in the safety population who had ≥1 CGI-C recorded post-baseline. The per-protocol set (PPS) included all patients of the FAS without any major protocol deviation. Analyses were descriptive; any missing values for the primary outcome measure (CGI-C) at 3 months were imputed using the last observation carried forward method. For UPDRS II (at ON and OFF), UPDRS III (at ON), UPDRS II plus III (at ON), PDQ-8 and NMSS, the means of changes from baseline were analyzed using Student’s t-test.
The formal service costs and unpaid care costs within the economic evaluation were determined using generalized linear models with a gamma distribution and log-link function at baseline and at month 6. The dependent variables of the model were the total formal service costs or total unpaid care service costs; explanatory variables of the model were age, gender, duration of PD, duration of wearing-off motor fluctuations, UPDRS I, UPDRS II activities of daily living (ADL) score at OFF stage, UPDRS II (ADL) plus III (motor function) during the ON stage, UPDRS IV, total unpaid care service cost (if dependent variable was total formal service cost) and total formal service cost (if dependent variable was total unpaid care service cost). Mean values at baseline and 6 months were calculated for all patients who had costs reported at the respective timepoints. The change from baseline was calculated per patient and for patients that had costs at both baseline and 6-month end point. In order to compare two regression models at baseline and at month 6, a sensitivity analysis was performed. Regression analyses at baseline and at month 6 were performed only for patients with all model data available at both visits. Significance level in order to decide whether there is a significant relationship between the variables in the regression model of the dataset was 0.05.
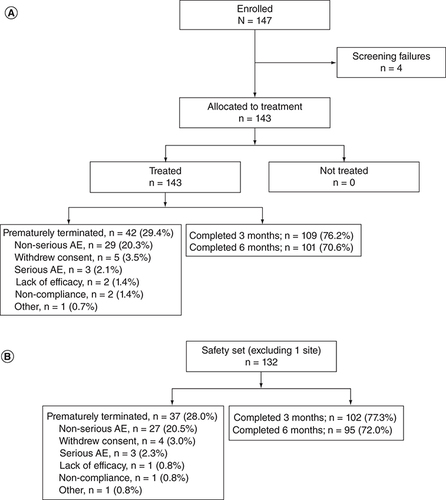
Data from 13 enrolled patients (including 11 treated patients) at one UK site were incomplete and inconsistent and were therefore excluded from the final analysis before the database was locked.
Results
Patient disposition & baseline characteristics
This subanalysis included 147 patients enrolled at 19 centers across the UK, 143 (97.3%) of whom received ≥1 dose of opicapone (A). The final safety set comprised 132 patients, excluding the 11 patients treated at the excluded site (B). Of these, 128 (97.0%) patients had ≥1 post-baseline CGI-C assessment and were included in the FAS. In the final safety set, there were 37 patients (28.0%) who terminated the trial prematurely and stopped treatment with opicapone. A total of 30 patients (22.7%) withdrew due to an AE, including 27 patients (20.5%) with a non-serious TEAE and 3 patients (2.3%) with a serious AE. Treatment compliance was high, with a mean ± standard deviation (SD) treatment compliance rate of 98.1 ± 7.88%. The majority of patients complied with ≥80% of doses. Most of the patients who completed the trial continued to receive opicapone on prescription (84/95; 88.4%).
Baseline characteristics of the safety analysis set are shown in . The study population comprised more men (>60%) than women, with a mean ± SD age of 67.3 ± 8.4 years. The mean ± SD time since diagnosis was 8.93 ± 5.15 years and the mean ± SD duration of motor fluctuations was 2.62 ± 2.8 years. At study entry, the majority of patients (73.5%) received concomitant PD treatment, including rasagiline (30.3%), pramipexole (23.5%) and ropinirole (23.5%).
Table 1. Baseline characteristics (safety analysis set).
CGI-C & PGI-C
After 3 months of opicapone, clinical improvement on the CGI-C scale was observed for 72.6% of patients, including 48% who were judged by the investigator to be ‘much improved’ or ‘very much improved’ (A). Patient self-rated levels (PGI-C) also improved, with the majority of patients (78.4%) reporting an improvement after 3 months of treatment, including 52% who reported as ‘much improved’ or ‘very much improved’ (B). After 6 months of treatment, the proportion of patients with clinical improvement remained high as judged by the investigators (CGI-C of 85.3%; C) and the patients (PGI-C of 79.8%; D), with 58% and 53% reporting as ‘much improved’ or ‘very much improved’ for CGI-C and PGI-C, respectively.
(A) Investigator-rated after 3 months/last observation carried forward (Clinicians’ Global Impression of Change [CGI-C], primary end point, n = 128). (B) Self-rated by the patient after 3 months (Patients’ Global Impression of Change [PGI-C], n = 102). (C) Investigator-rated after 6 months (CGI-C, n = 95). (D) Self-rated by the patient after 6 months (PGI-C, n = 94).
![Figure 2. Global Impression of Change following treatment with opicapone 50 mg. (A) Investigator-rated after 3 months/last observation carried forward (Clinicians’ Global Impression of Change [CGI-C], primary end point, n = 128). (B) Self-rated by the patient after 3 months (Patients’ Global Impression of Change [PGI-C], n = 102). (C) Investigator-rated after 6 months (CGI-C, n = 95). (D) Self-rated by the patient after 6 months (PGI-C, n = 94).](/cms/asset/2385bd0a-468a-44e6-b4dd-8a4ce2e6fe60/inmt_a_12343725_f0002.jpg)
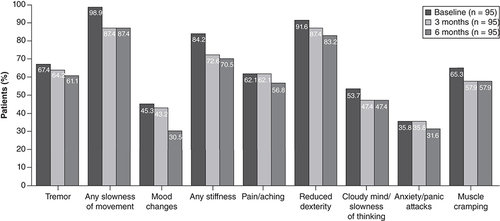
Wearing-off symptoms
The number of patients experiencing individual wearing-off symptoms, as assessed using the WOQ-9, was lower after 3 months of treatment with opicapone compared with baseline and remained low after 6 months of treatment ().
Rating scale outcomes
UPDRS I scores demonstrated no change in mentation, behavior and mood after 3 months (0.0 ± 1.7 points) and a slight increase from baseline after 6 months of treatment (0.2 ± 1.8 points; ). Improvements in ADL (UPDRS II) during OFF time, motor function (UPDRS III) during ON-time and total scores (UPDRS II + III) during ON-time were observed at both 3 and 6 months. After 3 months, UPDRS II and III scores decreased by 2.2 ± 4.9 points and 2.7 ± 8.3 points, respectively, with similar reductions observed after 6 months of treatment (UPDRS II: 2.4 ± 5.8 points, p = 0.0002; UPDRS III: 3.3 ± 10.1 points, p = 0.0002). Combined UPDRS II and III scores during ON-time decreased by 3.7 ± 10.7 after 3 months and remained low after 6 months (3.4 ± 22.2 points, p = 0.0007). UPDRS II scores during ON-time decreased by 0.5 ± 4.49 points and by 0.1 ± 4.6 points after 3 and 6 months, respectively. UPDRS IV scores (complications of therapy in the past week) were reduced by 0.6 ± 2.3 points after 3 months and by 0.1 ± 2.5 points after 6 months of treatment. PDQ-8 and NMSS assessments demonstrated improvements in quality of life and non-motor symptoms, respectively, with a decrease of -4.4 ± 13.6 points for PDQ-8 and -4.1 ± 22.7 points for NMSS after 3 months, and -2.4 ± 15.2 points (p = 0.1747) for PDQ-8 and -1.5 ± 25.8 points (p = 0.3274) for NMSS after 6 months of opicapone treatment.
Table 2. Scale assessments.
CSRI questionnaire
Total service costs during the 6 months prior to study participation were GBP 183,476.70, mainly driven by primary and community care (66.75%) and hospital and residential (22.96%) services costs (). Average total costs were GBP 13,537.62, including GBP 2334.97 for formal service and GBP 12,806.51 for unpaid care costs. After 6 months of opicapone treatment, total service costs, including costs for hospital and residential services, primary and community care services, investigations/diagnostic tests, aids or devices and adaptations to the home, had reduced to GBP 121,280.83, mainly driven by hospital and residential (46.75%) and primary and community care (36.30%) services costs. The cost reduction at 6 months is further confirmed by the decrease in the cost of care per individual patient throughout the different domains, apart from the costs for investigations or diagnostic tests, which increased slightly after 6 months of opicapone treatment (). Mean total costs decreased at month 6 by GBP 3718.60, including a reduction of £987.41 for formal service and GBP 2920.96 for unpaid care costs.
Table 3. Service costs at start and end of study (full analysis set)Table Footnote†.
A regression analysis demonstrated a statistically significant relationship for formal service costs and UPDRS I (estimate ± standard error [SE] = 0.134 ± 0.064; 95% CI: 0.008–0.259; p = 0.036) and UPDRS IV scores (estimate ± SE = 0.098 ± 0.040; 95% CI: 0.020–0.177; p = 0.014) at baseline (FAS). A similar analysis in the PPS confirmed the significance for UPDRS IV (estimate ± SE = 0.100 ± 0.034; 95% CI: 0.016–0.183; p = 0.02) but not UPDRS I scores at baseline. In addition, the sensitivity analysis (FAS) performed only for patients with all model data available at both visits supported the significant relationship for formal service costs and UPDRS I at baseline (estimate ± SE = 0.206 ± 0.083; 95% CI: 0.044–0.368; p = 0.013). No significant relationship for formal service costs was observed for any of the explanatory variables at 6 months.
Statistically significant relationships for unpaid care costs were observed for the duration of wearing-off motor fluctuations (estimate ± SE = -0.017 ± 0.005; 95% CI: -0.027–-0.006; p = 0.002), UPDRS I (estimate ± SE = 0.207 ± 0.101; 95% CI: 0.010–0.404; p = 0.040), UPDRS II at OFF stage (estimate ± SE = 0.099 ± 0.040; 95% CI: 0.020–0.177; p = 0.014) and UPDRS IV scores (estimate ± SE = 0.228 ± 0.078; 95% CI: 0.074–0.381; p = 0.004) at baseline and for females (estimate ± SE = 1.527 ± 0.595; 95% CI: 0.362–2.693; p = 0.010), as well as UPDRS I (estimate ± SE = 0.325 ± 0.116; 95% CI: 0.098–0.552; p = 0.005) at month 6 (FAS). The PPS supported these results with the exception of the UPDRS I scores at baseline, which demonstrated a non-significant relationship. At month 6, the regression analysis in the PPS showed significant relationships for the same variables as in the FAS as well as for UPDRS II at OFF stage. The sensitivity analysis (FAS) also supported the results; only for UPDRS I and UPDRS IV scores at baseline were no statistically significant differences observed.
Levodopa dosing
Treatment with opicapone for 3 months appeared to have little effect on the total daily levodopa dose and the number of daily doses. The majority of patients (72.5%) reported no change in the total daily dose, 16.7% had a dose increase and 10.8% had a dose decrease. With regard to levodopa dosing frequency, 69.6% of patients reported no change, 11.8% had an increase and 18.6% had a decrease in dosing frequency. At 3 months, the overall mean ± SD change in total daily dose from baseline was approximately -26.2 ± 130.04 mg levodopa per day. Similar observations were made after 6 months of treatment, with the majority of patients (71.6%) remaining on the same total daily levodopa dose, 16.8% of patients reporting a dose increase and 11.6% reporting a dose decrease. A total of 67.4% of patients reported no change in levodopa dosing frequency, 14.7% showed an increase and 17.9% showed a decrease. At 6 months, the overall mean ± SD change of total daily dose from baseline was approximately -25.5 ± 133.24 mg levodopa per day.
For patients who reported dopaminergic AEs (FAS), most patients (55.8%) remained on the same total daily levodopa dose, 16.3% had a dose increase and 27.9% had a dose decrease, resulting in an overall mean ± SD change of -30.8 ± 158.85 mg/day. After 6 months of treatment, an overall mean ± SD change of -28.1 ± 167.87 mg/day was reported in patients with dopaminergic AEs. For patients who experienced dyskinesia (FAS) and ended the 6 months of treatment, most patients (47%) had a dose decrease, resulting in an overall mean ± SD change of -183.9 ± 135.7 mg/day. Overall, 27 (28%) out of 95 patients had a levodopa reduction and ended the 6 months of treatment with an overall mean ± SD change of approximately -178.7 mg ± 129.1 mg levodopa per day. Out of these 27 patients, 14 (52%) reported dyskinesia and 10 (71%) were resolved by the end of the 6 months of treatment.
Safety & tolerability
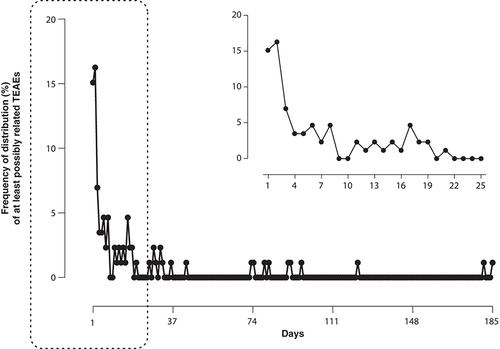
TEAEs occurred in 119 (90.2%) patients and were mostly mild or moderate (). TEAEs that were considered to be at least possibly related to treatment were observed in 86 (65.2%) patients, with dyskinesia (27.3%) and dry mouth (12.1%) being the most frequent of these (≥3%), in line with previous reports. No patient experienced explosive diarrhea after 6 weeks of treatment, which is a common side effect associated with other COMTIs. Most side effects were reported during the first week of treatment and the incidence was very low from the third to fourth week onward (). At least possibly related serious TEAEs were uncommon, with a reported frequency of 1.5%. A total of 30 (22.7%) patients terminated prematurely due to TEAEs, the two most common being dyskinesia in 5 (3.8%) patients and abnormal dreams in 4 patients (3.0%). Two out of the 5 patients that discontinued due to dyskinesia had a levodopa dose reduction during the study. No patient demonstrated any relevant changes in vital signs and physical and neurological examinations throughout the study.
Table 4. Incidence of treatment-emergent adverse events.
Discussion
This OPTIPARK subanalysis confirmed the effectiveness, safety and tolerability of opicapone in UK clinical practice, with a demonstrable cost-saving impact. Similar to what has been reported for the overall study population [Citation17], most patients’ global PD condition was improved with opicapone (≥72.5% as judged by clinicians and the patients themselves) 3 months after they started treatment. In the UK cohort, sustained clinical improvements were reported after 6 months of treatment. Given the progressive nature of the disease, the stable, sustained effect observed with opicapone after 6 months of treatment is an important finding. Opicapone was generally well tolerated, with observed AEs in line with those reported in the overall OPTIPARK population and those reported for other dopaminergic treatments.
PDQ-8 assessments demonstrated that opicapone was also associated with an improvement in overall quality of life at both 3 and 6 months of treatment. UPDRS ADL scores (UPDRS II) during OFF-time and motor scores (UPDRS III) during ON-time also improved after 3 and 6 months of opicapone treatment. UPDRS scores for mentation, behavior and mood (UPDRS I) remained low after 3 months and slightly increased after 6 months of treatment compared with baseline. Given the already very low scores at baseline, the likelihood of observing any improvement in these scores is relatively low compared with that of observing no change or worsening of effect. As shown for the overall population [Citation17], treatment with opicapone was associated with not only a reduction in OFF-time and an increase in ON-time but also an improvement in the quality of ON-time in patients in the UK. Non-motor symptoms are another important source of disability and contribute to a worse quality of life for people living with PD [Citation26,Citation27]. The pivotal BIPARK-I and II studies as well as the overall OPTIPARK study suggested an overall improvement in non-motor symptoms [Citation10,Citation11,Citation17], which was further confirmed in this OPTIPARK subanalysis.
Opicapone 50 mg was generally well tolerated in patients with PD in routine clinical practice in the UK, with the most frequently reported TEAEs in keeping with the known safety profile of opicapone [Citation10,Citation11,Citation13,Citation17]. The majority of AEs were mild to moderate in severity and occurred primarily during the first week of treatment. A large proportion of patients (22.7%) withdrew from the study due to AEs but causative types of AEs were diverse. The most frequent AE leading to study discontinuation was dyskinesia, affecting only 5 patients (3.8%). Diarrhea has been considered a common side effect of COMTIs [Citation28], but there were no TEAEs or serious AEs related to this event in this UK cohort and there are accumulating data to suggest that this is a rare complication with opicapone [Citation29,Citation30]. Orange urine discoloration, which is frequently reported with entacapone use, was also not observed with opicapone treatment.
This OPTIPARK subanalysis also assessed the impact of opicapone on overall treatment costs in patients with PD treated in routine clinical practice in the UK. As PD advances, medical needs increase, resulting in higher costs in the later stages of the illness. A recently completed study on the economic cost of PD in the UK has shown a financial burden amounting to over GBP 16,500 per patient household per year. The annual cost of treatment to the National Health Service was estimated at GBP 2118 per patient and the total annual economic burden at GBP 20,123 per household [Citation31]. Other studies have estimated that PD is the third most costly physically debilitating neurological disease after multiple sclerosis and stroke, on a cost per case basis [Citation32]. The recurring financial expenses and reduced income of the patients and their carers can directly impact the living/health conditions and quality of life of those involved, who often find themselves in financial distress [Citation33]. Several studies have shown that costs increase as PD advances [Citation34,Citation35], and one UK-based study indicated that some of this increased cost is related to time spent in the OFF state [Citation34]. Average 12-monthly total costs increased according to the time spent in the OFF state from GBP 25,630 in patients spending less than 25% of their waking hours in OFF to GBP 62,147 for patients spending more than 75% of their time in OFF. New treatment strategies that can alleviate the impact of the disease without adding considerably to the already high-cost burden of the disease are to be welcomed.
In this analysis of the UK cohort, an apparent cost-saving impact was observed after 6 months of treatment, with a mean total service cost decrease of GBP 3719. This constituted a saving of approximately GBP 987 in formal service costs and, importantly, a GBP 2921 saving in unpaid care costs. Unpaid care costs included work loss-related costs and informal care, which would primarily affect the household cost to the patient and their carer. These findings point toward the potential for opicapone to reduce the financial burden of PD to patients and their carers, as well as to healthcare and social care services. In an analogous situation, the addition of opicapone to levodopa infusion therapy in advanced PD has also shown cost savings [Citation36]. Additional studies will be required to explore this possibility over the longer term and across different settings.
The limitations of the study include those inherent to open-label studies without placebo control, where both the clinician and the patient have expectations of the treatment and are therefore at risk of placebo effects and investigator bias. As the population by definition included patients struggling in the period before recruitment, there is a possibility that some of the change may simply reflect regression to the mean. A formal protocol of levodopa dose adjustment may have further improved tolerability requires further study. However, despite these limitations, these real-world data complement the evidence from clinical trials, confirming that opicapone is generally well tolerated, with a positive impact on quality of life and potential important net cost savings.
Conclusion
Once-daily opicapone 50 mg added to levodopa therapy in patients with PD and motor fluctuations was effective and generally well tolerated in routine clinical practice across the UK. The patients’ overall PD condition as judged by the clinicians and the patients was clinically improved after 3 and 6 months of treatment. Opicapone was associated with an improvement in both motor and non-motor symptoms and quality of life after 3 and 6 months of treatment and had a cost-saving impact that could benefit patients, their carers as well as healthcare and social care systems.
Opicapone is a catechol-O-methyltransferase inhibitor that proved effective in treating wearing-off symptoms when given as an adjunct to levodopa therapy in patients with Parkinson’s disease (PD) and motor fluctuations in two large, randomized, placebo-controlled trials (BIPARK-I and II).
The OPTIPARK study, a prospective, open-label, single-arm study conducted in clinical practices across the UK and Germany, confirmed the effectiveness and safety of 3-month treatment with opicapone in real-world settings.
While patients in Germany were treated for 3 months only, patients in the UK were treated for 6 months to assess health economic costs.
This OPTIPARK subanalysis reports the clinical outcomes after 3 and 6 months of opicapone treatment in the UK patients only and the impact of opicapone on overall treatment costs for patients with PD in clinical practice in the UK.
In the UK cohort, patients’ overall PD condition as judged by the clinicians and the patients was clinically improved after 3 months, with further improvements observed after 6 months of treatment.
Opicapone was associated with an improvement in both motor and non-motor symptoms and quality of life after 3 and 6 months of treatment.
In line with the overall OPTIPARK population, treatment with opicapone was generally well tolerated, with the majority of the adverse events reported being of mild or moderate severity.
Opicapone had an apparent cost-saving impact after 6 months of treatment in patients with PD in the UK, with a mean reduction in total service costs of GBP 3719.
Author contributions
C Schofield, KR Chaudhuri, C Carroll, JC Sharma, N Pavese, J Evans, T Foltynie and A Lees were study investigators in the UK, were involved in the study design, data collection and data interpretation, and contributed equally to the first draft. P Soares-da-Silva participated in the study design, data collection, data management and data analysis. All authors provided critical review of the manuscript and read and approved the final draft.
Ethical conduct of research
Institutional review boards at the participating sites approved the protocol and the trial was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines. All patients provided written informed consent.
Acknowledgments
The authors thank the patients and the study staff involved in the trial, including the research nurses at the UK study sites and all OPTIPARK study UK investigators: J Alty, R Amin, M Boca, S Butterworth, C Carroll, G Charlesworth, KR Chaudhuri, R Chinnadurai, J Collins, JS Cosgrove, S Cravey, D Damodaran, N Dimitrov, R Durcan, S Ellis, A Elmarimi, J Evans, J Fisher, D Grosset, S Jamieson, C Kobylecki, SH Lim, V Lyell, B Mohamed, S Molloy, N Pavese, D Paviour, M Purchas, K Rashed, C Rickards, T Saifee, G Sare, C Schofield, N Setty, J Sharma, R Sheridan, SL Shu, M Silverdale, R Sophia, S Statton, M Steiger, C Thomas, R Walker and TY Foung.
Financial & competing interests disclosure
The study was funded by BIAL. P Soares-da-Silva was employed by the funder and participated in the study design, data collection, data management and data analysis. The funder of the study had no other role in data interpretation or in the decision to submit the manuscript for publication. KR Chaudhuri has served as an advisory board member for AbbVie, UCB, GKC, BIAL, Cynapsus Therapeutics, Lobsor Pharmaceuticals, STADA, Medtronic, Zambon, Profile, Sunovion, Roche, Theravance, Scion, Britannia, Acadia and 4D. He has received honoraria for lectures from AbbVie, Britannia, UCB, Zambon, Novartis, Boehringer Ingelheim, BIAL, Kyowa Kirin and SK Pharma. He has obtained investigator-initiated grants from Britannia, AbbVie, UCB, GKC and BIAL and academic grants from the EU, IMI EU, Horizon 2020, Parkinson’s UK, NIHR, PDNMG, EU (Horizon 2020), Kirby Laing Foundation, NPF, MRC and Wellcome Trust. He also reports royalties or licenses from Oxford and Cambridge publishers and the Mapi Institute (KPPS, PDSS 2) and payment for expert testimony from GMC. C Carroll has received a salary from University of Plymouth, University Hospitals Plymouth NHS Trust and National Institute of Health Research. She has also obtained advisory, consulting and lecture fees from AbbVie, BIAL, Lundbeck, Britannia, Global Kinetics and Medscape and research funding from Parkinson’s UK, National Institute of Health Research and Cure Parkinson’s. N Pavese has received advisory, consulting and lecture fees from BIAL, Britannia, Boston Scientific, Roche and Abbvie and research funding from Independent Research Fund Denmark, Danish Parkinson’s disease Association, Parkinson’s UK, Center of Excellence in Neurodegeneration (CoEN) network award, GE Healthcare Grant, Multiple System Atrophy Trust, EU Joint Program Neurodegenerative Disease Research (JPND), EU Horizon 2020 research and innovation programme, Italian Ministry of Health and the MJFF. J Evans has served as an advisory board member for BIAL. T Foltynie has received grants from National Institute of Health Research, Michael J. Fox Foundation, John Black Charitable Foundation, Cure Parkinson’s Trust, Innovate UK, Janet Owens Research Fellowship, Rosetrees Trust, Van Andel Research Institute and Defeat MSA. He has served on advisory boards for Peptron, Voyager Therapeutics, Handl Therapeutics, Veeva Systems, Inc., Living Cell Technologies, BIAL and Profile Pharma. He has received honoraria for talks sponsored by BIAL, Profile Pharma and Boston Scientific. H Reichmann participated on advisory boards, gave lectures and received research grants from Abbott, Abbvie, Bayer Health Care, BIAL, Boehringer/Ingelheim, Britannia, Cephalon, Desitin, GSK, Lundbeck, Medtronic, Merck-Serono, Novartis, Orion, Pfizer, TEVA, UCB, Valeant and Zambon. L Zurowska and P Soares-da-Silva are employed by BIAL. A Lees is funded by the Reta Lila Weston Institute of Neurological Studies, University College London, Institute of Neurology and reports consultancies from Britannia Pharmaceuticals and BIAL. He also reports grants and/or research support from the Frances and Renee Hock Fund and honoraria from Britannia Pharmaceuticals, Profile Pharma, UCB, Roche, BIAL, STADA, Nordiclnfu Care and NeuroDerm. C Schofield and JC Sharma have no conflict of interests to declare. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance for the development of this manuscript was funded by BIAL. Editorial assistance was provided by K Male from mXm Medical Communications.
Data sharing statement
The dataset supporting the conclusions of this article is included within the article. The study sponsor (BIAL) undertakes to share, upon request, anonymized patient-level, study-level clinical trial data (analyzable datasets) and other information (such as protocols) from this clinical trial to qualified researchers as necessary for conducting legitimate research. Information is provided at www.bial.com.
References
- Fox SH , KatzenschlagerR, LimSYet al. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord., 33(8), 1248–1266 (2018).
- National Institute for Health and Care Excellence . Parkinson’s disease in adults: diagnosis and management (2022). https://www.nice.org.uk/guidance/ng71/evidence/full-guideline-pdf-4538466253
- Lane EL . L-DOPA for Parkinson’s disease – a bittersweet pill. Eur. J. Neurosci., 49(3), 384–398 (2019).
- Muller T , MohrJD. Long-term management of Parkinson’s disease using levodopa combinations. Expert. Opin. Pharmacother., 19(9), 1003–1011 (2018).
- Almeida L , RochaJF, FalcaoAet al. Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel catechol-O-methyltransferase inhibitor, in healthy subjects: prediction of slow enzyme-inhibitor complex dissociation of a short-living and very long-acting inhibitor. Clin. Pharmacokinet., 52(2), 139–151 (2013).
- Kiss LE , FerreiraHS, TorraoLet al. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J. Med. Chem., 53(8), 3396–3411 (2010).
- Montioli R , VoltattorniCB, BertoldiM. Parkinson’s disease: recent updates in the identification of human dopa decarboxylase inhibitors. Curr. Drug. Metab., 17(5), 513–518 (2016).
- Fabbri M , FerreiraJJ, LeesAet al. Opicapone for the treatment of Parkinson’s disease: a review of a new licensed medicine. Mov. Disord., 33(10), 1528–1539 (2018).
- Scott LJ . Opicapone: a review in Parkinson’s disease. CNS Drugs, 35(1), 121–131 (2021).
- Ferreira JJ , LeesA, RochaJFet al. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol., 15(2), 154–165 (2016).
- Lees AJ , FerreiraJ, RascolOet al. Opicapone as adjunct to levodopa therapy in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol., 74(2), 197–206 (2017).
- European Medicines Agency . Ongentys, INN-opicapone. Summary of product characteristics (2022). https://www.ema.europa.eu/en/documents/product-information/ongentys-epar-product-information_en.pdf
- U.S. Food and Drug Administration . ONGENTYS (opicapone) prescribing information (2022). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212489s000lbl.pdf
- Australian Government Department of Health and Therapeutic Goods . Australian public assessment report for opicapone (2022). https://www.tga.gov.au/sites/default/files/auspar-opicapone-210210.docx
- U.S. Food and Drug Administration . Submitting documents using real-world data and real-world evidence to FDA for drugs and biologics guidance for industry (2022). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/submitting-documents-using-real-world-data-and-real-world-evidence-fda-drugs-and-biologics-guidance
- Cave A , KurzX, ArlettP. Real-world data for regulatory decision making: challenges and possible solutions for Europe. Clin. Pharmacol. Ther., 106(1), 36–39 (2019).
- Reichmann H , LeesA, RochaJFet al. Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: the OPTIPARK open-label study. Transl. Neurodegener., 9(1), 9 (2020).
- Stacy M , HauserR, OertelWet al. End-of-dose wearing off in Parkinson disease: a 9-question survey assessment. Clin. Neuropharmacol., 29(6), 312–321 (2006).
- Fahn S (Ed.), EltonR. Unified Parkinson’s Disease Rating Scale. In: Recent Developments in Parkinson’s Disease (Volume 2).MarsdenCD, CalneDB, GoldsteinM ( Eds). MacMillan Healthcare Information, NJ, USA, 153–164 (1987).
- Jenkinson C , FitzpatrickR, PetoV, GreenhalfR, HymanN. The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychol. Health., 12, 805–814 (1997).
- Chaudhuri KR , Martinez-MartinP, BrownRGet al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov. Disord., 22(13), 1901–1911 (2007).
- Beecham J , KnappM. Costing psychiatric interventions. In: Measuring Mental Health Needs.ThornicroftG ( Ed.). Gaskell, London, UK, 200–224 (2001).
- Mccrone P , PayanCA, KnappMet al. The economic costs of progressive supranuclear palsy and multiple system atrophy in France, Germany and the United Kingdom. PLoS ONE, 6(9), e24369 (2011).
- Busse M , Al-MadfaiDH, KenkreJ, LandwehrmeyerGB, BentivoglioA, RosserA. Utilisation of healthcare and associated services in Huntington’s disease: a data mining study. PLoS Curr., 3, RRN1206 (2011).
- Mccrone P , HeslinM, KnappM, BullP, ThompsonA. Multiple sclerosis in the UK: service use, costs, quality of life and disability. Pharmacoeconomics, 26(10), 847–860 (2008).
- Chaudhuri KR , SchapiraAH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol., 8(5), 464–474 (2009).
- Chaudhuri KR , YatesL, Martinez-MartinP. The non-motor symptom complex of Parkinson’s disease: a comprehensive assessment is essential. Curr. Neurol. Neurosci. Rep., 5(4), 275–283 (2005).
- Rivest J , BarclayCL, SuchowerskyO. COMT inhibitors in Parkinson’s disease. Can. J. Neurol. Sci., 26(Suppl. 2), S34–S38 (1999).
- Lees A , FerreiraJJ, RochaJFet al. Safety profile of opicapone in the management of Parkinson’s disease. J. Parkinsons Dis., 9(4), 733–740 (2019).
- Greenwood J , PhamH, ReyJ. Opicapone: a third generation COMT inhibitor. Clin. Park. Relat. Disord., 4, 100083 (2021).
- Economic, social and financial cost of Parkinson’s on individuals, carers and their families in the UK: final report (2022). https://www.shu.ac.uk/research/specialisms/health-and-social-care-research/reports/economic-social-and-financial-cost-of-parkinsons-on-individuals-carers-and-their-families
- Andlin-Sobocki P , JonssonB, WittchenHU, OlesenJ. Cost of disorders of the brain in Europe. Eur. J. Neurol., 12(Suppl. 1), S1–S27 (2005).
- Gumber A . Effects of out-of-pocket (OOP) payments and financial distress on quality of life (QoL) of people with Parkinson’s (PwP) and their carers. Health Qual. Life Outcomes, 15(Suppl. 1), A33 (2017).
- Findley LJ , WoodE, LowinJ, RoederC, BergmanA, SchifflersM. The economic burden of advanced Parkinson’s disease: an analysis of a UK patient dataset. J. Med. Econ., 14(1), 130–139 (2011).
- Weir S , SamnalievM, KuoTCet al. Short- and long-term cost and utilization of health care resources in Parkinson’s disease in the UK. Mov. Disord., 33(6), 974–981 (2018).
- Leta V , Van WamelenDJ, SauerbierAet al. Opicapone and levodopa-carbidopa intestinal gel infusion: the way forward towards cost savings for healthcare systems? J. Parkinsons Dis., 10(4), 1535–1539 (2020).