Abstract
Aim: This study aimed to assess the usability of a specific EU-available application device for Sativex® (US adopted name: nabiximols) cannabinoid-based oromucosal spray in patients with multiple sclerosis (MS) and spasticity-related upper limb and hand impairment in routine daily practice. Methods: MS patients with upper limb and hand impairment evaluated the usability of the device using an ad hoc 18-item questionnaire. Results: 60 patients were included. The comprehensibility of the instructions for use, practical handling and ergonomics of the device were rated as optimal (mean scores ≥8.9/10 across questions). Assisting trained nurses also rated the device as easy to use and helpful for drug administration (mean scores 10/10). Conclusion: The application device may assist MS patients with upper limb impairment self-administer nabiximols oromucosal spray.
Plain language summary
Many patients with multiple sclerosis lose some function in their upper limbs (arms) and hands because of spasticity, which can make it difficult to take their medication at the required times each day. Patients taking nabiximols oromucosal spray may not have the strength or coordination needed to press the spray nozzle into their mouth. To support delivery of the medicine in these patients, a specific application device has been developed that reduces the strength necessary to administer the spray. 60 patients with upper limb/hand impairment tested the device and completed an 18-item questionnaire. Patients rated the instructions for use, ease of use and ergonomic features of the device as optimal, with average scores of ≥8.9/10 across questions.
Spasticity is a common and frequently disabling symptom of multiple sclerosis (MS) that interferes with patients’ ability to carry out daily life activities [Citation1,Citation2,Citation3,Citation4]. The loss of autonomy and associated distress can have a negative impact on patients’ quality-of-life [Citation5,Citation6]. Many patients with MS spasticity are poorly responsive to conventional oral antispasticity medications and continue to experience muscle rigidity and spasms despite standard first-line treatment. Add-on therapy may be required to control spasticity-related symptoms in these patients.
Sativex® (US adopted name: nabiximols) is a cannabinoid-based oromucosal spray containing balanced quantities of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) plus other plant-derived cannabinoids and non-cannabinoid components [Citation7]. Nabiximols is licensed across EU countries and other world regions as add-on therapy for moderate to severe MS spasticity poorly responsive to first-line treatments [Citation8]. In clinical trials, add-on nabiximols was shown to be superior to placebo [Citation9,Citation10,Citation11] and more effective than readjusting underlying antispasticity medications alone [Citation12] in reducing the severity of MS spasticity. Large observational and registry studies have confirmed its effectiveness during real-world use [Citation13,Citation14,Citation15,Citation16,Citation17]. Nabiximols is well tolerated with no evidence of unexpected adverse events or safety signals during routine use [Citation18], and with no untoward effects on cognition [Citation19,Citation20] or driving ability [Citation21].
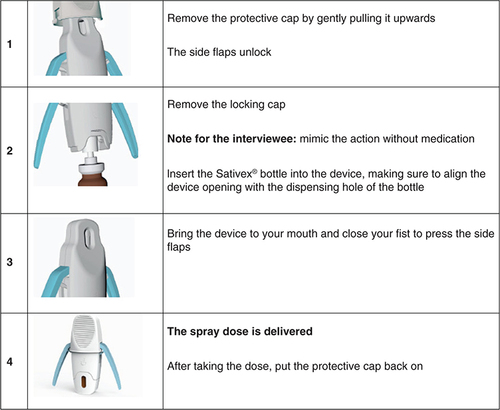
Nabiximols is formulated for self-administration to the inner mucosa of cheeks and under the tongue up to 12 times per day as required [Citation8]. However, patients with upper limb/hand spasticity may have difficulty actuating the spray bottle [Citation2]. To address this inconvenience, a specific application device (Almirall and Anima Barcelona, Barcelona, Spain) has been developed to support patients in self-administering their nabiximols dose. The application aid is available in the EU as a class I medical device per the applicable European Commission guidance [Citation22]. Designed for exclusive use with nabiximols oromucosal spray, the device consists of a locking cap, a central body in which to position the spray bottle, side wings to facilitate spraying and a base cap. The device delivers the complete dose of a single spray while reducing the strength required to actuate the spray nozzle. In pre-production testing involving 15 MS patients who were current nabiximols users, 73% of participants reported better application using the device, and 54% felt that less strength was required. Patients’ willingness to use the device was highest among those with greater upper arm/hand disability [Citation23]. Following approval of the device by the EU competent authority in 2019, it became available free of charge in MS units across EU countries aiming to support patients with MS spasticity and upper limb impairment who were receiving add-on nabiximols.
The current study was undertaken to validate the findings of the pre-production study in a larger sample of MS patients in the daily practice setting.
Patients & methods
The study was conducted between September and December 2020 at the Multiple Sclerosis Center of the Istituto Neurologico Mediterraneo Neuromed in Pozzilli, Italy. A within-subject design was used whereby participants served as their own controls. Italian-speaking MS patients with upper limb spasticity and impaired finger dexterity of both hands, who had been receiving nabiximols oromucosal spray for at least 2 years, were invited to test the device. Disability was assessed by the 0–10 Extended Disability Status Scale (EDSS) [Citation24]. Impairment of hand motility was diagnosed and assessed by the Nine Hole Peg Test (9HPT) [Citation25]. All patients provided written informed consent for participation in the study.
In view of the need for clear instructions and ease of use of any medical device, the evaluation involved structured interviews and ad hoc tests for handling ability in participating patients. At the time of testing, patients were provided with approved instructions for use of the device consisting of images and a written description of each phase of the procedure (). Patients also received an oral explanation from informed nurses about use of the application device. Patients then underwent a structured interview in Italian consisting of 18 questions about the comprehensibility of instructions for use of the device; their experience with the device based on two handling sessions before and after oral explanation with demonstration by the interviewer in the absence of drug administration (empty device); their opinions about the device characteristics; and their propensity (willingness) to use the device. Answers were scored on a 10-point qualitative scale (from 1 = not at all to 10 = very much), and, where applicable, scores were categorically labeled as insufficient (0–5), sufficient (6), good (7–8) or optimal (9–10). The questionnaire was administered by trained nurses with experience in nabiximols use in patients with MS spasticity. In addition to recording patients’ experience with the device, nurses recorded patients’ main demographic and clinical features and whether patients had any doubts about the written instructions. Nurses also provided their own opinions about patients’ handling of the device after reading the instructions, their opinions about patients’ handling of the device after oral explanation and practical demonstration and an overall opinion about the application device. Interpretation of scores was pre-specified. The questionnaire is provided in Supplementary Table 1.
Results
Participants’ demographic data and relevant clinical characteristics are summarized in . 60 MS spasticity patients treated with nabiximols, 29 males and 31 females, aged between 36 and 68 years, participated in the study. Mean MS duration was 22 years, and mean spasticity duration was 8 years. Average duration of treatment with nabiximols oromucosal spray was 3.3 (range: 2–5) years, and the average daily dose was 6.3 (range: 4–8) sprays/day. As a group, patients had moderate to severe disability (mean EDSS score of 6.0). All patients had moderate to severe upper limb spasticity; mean 9HPT scores were 58 s (range: 47–69) for the dominant hand and 68.5 s (range: 61–76) for the non-dominant hand. Most patients (80%) reported difficulties in daily use of nabiximols: 11% required a caregiver’s help, and 8.3% reported that several administrations were missed due to difficulties in using the spray bottle.
Table 1. Patients’ demographic and clinical features (n = 60).
Medical device usability test
The results of the medical device usability test are summarized in . Patients rated the comprehensibility of the instructions for use as optimal, with mean scores ≥9.7 (range: 8–10) for all items. Patients also rated the device’s usability as optimal (mean scores ≥9.5; range: 7–10) based on an initial practice test using the instructions alone and on a repeat practice test after a verbal explanation and demonstration of the instructions by the interviewer. All participating patients were able to assemble and disassemble the application device. Ergonomic aspects such as handling, carrying and holding of the device were rated as optimal (mean scores ≥8.9; range: 7–10), as were the ease of operation and general ability of the device to simplify self-administration of nabiximols (mean scores ≥9.4; range: 7–10). Overall, participating patients reported being highly likely to use the application device themselves (mean score 9.8; range: 8–10) and to advise friends to use the device (mean score 9.9; range: 9–10).
Table 2. Results of the medical device usability test.
The trained nurses who administered the questionnaire rated the device’s ease of use (mean score: 10) and its potential to help patients self-administer nabiximols oromucosal spray (mean score: 10) as optimal.
Discussion
This article reports a usability test carried out by MS spasticity patients with upper limb/hand impairment on an application device developed to support self-administration of nabiximols oromucosal spray. To the authors’ knowledge, this is the first study to investigate the usability of the device under routine clinical practice-like conditions. Despite its simple observational design, the study is informative as it reflects the perception of experienced nabiximols users.
Using a questionnaire, patients rated the clarity of instructions for use, practical handling of the device and ergonomics of the device as optimal. The homogeneity of the participating patients’ responses supports their acceptance of the utility of the device. There was close agreement between patients’ opinions and those of trained nurses assisting with the evaluation who scored the device ‘10 out of 10’ for ease of use and potential in helping patients self-administer nabiximols oromucosal spray. Overall, feedback about the device in the real-world setting was more favorable than that received during pre-production testing carried out in Spain [Citation23], which was highly encouraging given that participating patients represented the group most likely to need the device. The absence of malfunctioning issues such as those encountered during prototype testing likely supported the positive opinions of the patients and healthcare professionals. Although safety of the application device was not formally assessed during testing, no issues were reported.
The first-line pharmacological treatment of MS spasticity centers mainly around the oral muscle relaxants baclofen and tizanidine, used alone or in combination [Citation26]. However, some patients are unable to achieve effective doses of these medications due to poor tolerability and in others, initial efficacy may wane over time. Since add-on nabiximols provides these patients with another opportunity for symptomatic relief of spasticity, efforts should be made to maintain treatment for as long as possible. The oromucosal delivery system of nabiximols, although an uncommon route, allows for proper absorption of the active principles while avoiding plasma concentrations likely to be associated with THC adverse events [Citation27]. In recognizing that spasticity-related impairment in finger motor activity may limit patients’ ability to self-administer nabiximols spray, the application device has the potential to facilitate treatment adherence and, by extension, treatment effectiveness.
Limitations
Study limitations include the relatively modest sample size and potential for bias in terms of patient selection. Survey methodology tends to result in a higher proportion of participants in relatively good health, without impaired cognition, who may not be truly representative of the overall target population. Although lacking a control group, the within-subject design (i.e., direct patient experience without vs with the application device) was considered to be more appropriate for study purposes. No correlation analysis was performed with regard to degree of upper limb disability and level of satisfaction with the device, as this would be non-informative given the high or very high scores for all survey questions. Other limitations relate to the survey itself, which did not undergo construct validity evaluation or pilot testing before use. Although survey questions were designed in lay language to be easy to understand, participants may have misunderstood certain questions or failed to consider their responses carefully. Answering survey questions in the presence of an assisting nurse (vs anonymously) may have influenced the responses if patients were reluctant to express any concerns. On the other hand, limiting study participation to patients with upper limb/hand impairment who were experienced nabiximols users strengthened the findings, as this is the group most likely to benefit from use of the application device.
Conclusion
The clearly positive feedback from patients with MS-related upper limb/hand impairment and healthcare professionals about the usability of the nabiximols application device supports its use in suitable patients in everyday clinical practice. While the descriptive nature of the study has limitations, it also provides simplicity in terms of implementation and analyses, and thus is eminently relatable to the broader team of allied health professionals involved in the care of patients with MS spasticity. It might be speculated that the application device can enhance adherence to nabiximols treatment in the mid/long term, facilitating the intake of medication as prescribed and reducing the risk of missed doses, although this would require confirmation in a well-controlled, long-term follow-up study.
Background
Nabiximols (Sativex®) cannabinoid-based oromucosal spray is licensed across EU countries and other world regions as add-on therapy for moderate to severe multiple sclerosis (MS) spasticity poorly responsive to first-line oral medications.
As some patients with MS spasticity-related upper limb/hand impairment may have difficulty actuating the spray bottle, a specific application device has been developed to support self-administration of nabiximols oromucosal spray.
Aim
The study aimed to assess the instructions for use and usability of the application device in routine daily practice.
Methods
MS patients with upper limb/hand impairment who were experienced users of nabiximols evaluated the usability of the application device by completing an 18-item questionnaire.
Results
Patients (n = 60) rated the comprehensibility of the instructions for use, practical handling of the device and ergonomics of the device as optimal.
Trained nurses who assisted with the questionnaire also rated the device as easy to use and helpful for taking the medicine.
Conclusion
The application device has the potential to facilitate self-administration of nabiximols oromucosal spray in patients with MS spasticity-related upper limb/hand impairment.
Whether the application device supports adherence to nabiximols oromucosal spray merits investigation in appropriately designed studies.
Author contributions
D Centonze conceived and designed the study. A Creta, L Gilio and R Fantozzi performed the experiments, and A Creta and R Fantozzi acquired the data. All authors analyzed and interpreted the data, prepared and made corrections and modifications to the manuscript and read and approved the final version.
Supplemental Text 1
Download MS Word (17.9 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at:www.tandfonline.com/doi/full/10.2217/nmt-2022-0014
Financial & competing interests disclosure
D Centonze is an Advisory Board member or has given advice to Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva; has received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva; and is the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme and Teva. His pre-clinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. A Creta, L Gilio and R Fantozzi have no conflicts of interest to report. The study was funded by Almirall, S.A. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance was provided by M De Simone and A Shah (Polistudium SRL, Milan, Italy). Additional editorial assistance was provided by Content Ed Net (Madrid, Spain). All editorial assistance was supported unconditionally by Almirall.
Data sharing statement
Data are available upon request to the corresponding author.
References
- Zwibel HL . Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv. Ther., 26(12), 1043–1057 (2009).
- Bensmail D , VermerschP. Epidemiology and clinical assessment of spasticity in multiple sclerosis. Rev. Neurol. (Paris), 168(Suppl. 3), S45–S50 (2012).
- Zettl UK , HenzeT, EssnerU, FlacheneckerP. Burden of disease in multiple sclerosis patients with spasticity in Germany: mobility improvement study (Move I). Eur. J. Health Econ., 15(9), 953–966 (2014).
- Vermersch P . MObility ImproVEment with spasticity in multiple sclerosis in Europe: the MOVE 1 EU study. Neurodegener. Dis. Manag., 4(6), 407–415 (2014).
- Arroyo R , MassanaM, VilaC. Correlation between spasticity and quality of life in patients with multiple sclerosis: the CANDLE study. Int. J. Neurosci., 123, 850–858 (2013).
- Flachenecker P , HenzeT, ZettlUK. Spasticity in patients with multiple sclerosis-clinical characteristics, treatment and quality of life. Acta Neurol. Scand., 129, 154–162 (2014).
- Russo E , GuyGW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses, 66(2), 234–246 (2006).
- Electronic medicines compendium (emc) Sativex® oromucosal spray. Summary of product characteristics (2018). www.medicines.org.uk/emc/product/602/smpc [ Sativex Oromucosal Spray – Summary of Product Characteristics (SmPC) – (emc) (medicines.org.uk).
- Collin C , DaviesP, MutibokoIK, RatcliffeS, Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol., 14(3), 290–296 (2007).
- Collin C , EhlerE, WaberzinekGet al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res., 32, 451–459 (2010).
- Novotna A , MaresJ, RatcliffeSet al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol., 18, 1122–1131 (2011).
- Markovà J , EssnerU, AkmazBet al. Sativex® as add-on therapy vs. further optimized first-line antispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int. J. Neurosci., 129, 119–128 (2019).
- Flachenecker P , HenzeT, ZettlUK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice – results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur. Neurol., 71, 271–279 (2014).
- Flachenecker P , HenzeT, ZettlUK. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur. Neurol., 72(1-2), 95–102 (2014).
- Vermersch P , TrojanoM. Tetrahydrocannabinol:cannabidiol oromucosal spray for multiple sclerosis-related resistant spasticity in daily practice. Eur. Neurol., 76, 216–226 (2016).
- Patti F , MessinaS, SolaroCet al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J. Neurol. Neurosurg. Psychiatry, 87(9), 944–951 (2016).
- Ferrè L , NuaraA, PavanGet al. Efficacy and safety of nabiximols (Sativex®) on multiple sclerosis spasticity in a real-life Italian monocentric study. Neurol. Sci., 37(2), 235–242 (2016).
- Prieto González JM , Vila SilvánC. Safety and tolerability of nabiximols oromucosal spray: a review of real-world experience in observational studies, registries and case reports. Exp. Rev. Neurother., 21(5), 547–558 (2021).
- Vachová M , NovotnáA, MaresJet al. A multicentre, double-blind, randomised, parallel-group, placebo-controlled study of effect of long-term Sativex® treatment on cognition and mood of patients with spasticity due to multiple sclerosis. J. Mult. Scler., 1, 122 (2014).
- Alessandria G , MeliR, InfanteMT, VestitoL, CapelloE, BandiniF. Long-term assessment of the cognitive effects of nabiximols in patients with multiple sclerosis: a pilot study. Clin. Neurol. Neurosurg., 196, 105990 (2020).
- Freidel M , Tiel-WilckK, SchreiberH, PrechtlA, EssnerU, LangM. Drug-resistant MS spasticity treatment with Sativex(®) add-on and driving ability. Acta Neurol. Scand., 131(1), 9–16 (2015).
- European Commission . DG health and consumer. Directorate B, unit B2 “cosmetics and medical devices”. Medical devices: guidance document – classification of medical devices. Guidelines relating to the application of the council directive 93/42/EEC on medical devices. (MEDDEV 2. 4/1 rev). (2010). https://pdf4pro.com/amp/view/medical-devices-guidance-document-2bddb.html
- Montero-Escribano P , Vila SilvánC. Application device for THC:CBD oromucosal spray in the management of resistant spasticity: pre-production testing. Expert Rev. Med. Devices, 16(9), 835–840 (2019).
- Kurtzke JF . Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology, 33(11), 1444–1452 (1983).
- Solaro C , GrangeE, DiGiovanni Ret al. Nine Hole Peg Test asymmetry in refining upper limb assessment in multiple sclerosis. Mult. Scler. Relat. Disord., 45, 102422 (2020).
- Otero-Romero S , Sastre-GarrigaJ, ComiGet al. Pharmacological management of spasticity in multiple sclerosis: systematic review and consensus paper. Mult. Scler., 22(11), 1386–1396 (2016).
- Lucas CJ , GalettisP, SchneiderJ. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol., 84(11), 2477–2482 (2018).