Abstract
Aim: Evaluate timing of motor improvement with carbidopa/levodopa (CD/LD) and apomorphine sublingual film (SL-APO) in patients with Parkinson’s disease and OFF episodes. Methods: A post hoc pooled analysis from two studies assessed Movement Disorder Society Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS-III) scores and investigator-rated FULL ON. Results: At 15 and 30 min following the prescribed first daily CD/LD dose, mean improvements in MDS-UPDRS-III scores were -6.7 and -16.3, respectively, and FULL ON was achieved by 6.5 and 41.8% of patients. Following an optimized SL-APO dose, mean improvements in MDS-UPDRS-III scores were -13.9 and -22.9, and FULL ON was achieved by 34.7 and 81.0% of patients. Conclusion: Concomitant administration of SL-APO with carbidopa/levodopa may be useful for delayed ON.
Tweetable abstract
Delayed ON with the first morning dose of levodopa (LD) is common in patients with Parkinson’s disease. Apomorphine sublingual film improves motor function at 15 min postdose. Concomitant use with LD may allow for faster onset of improvement when delayed ON occurs.
Carbidopa/levodopa (CD/LD) and other combinations of levodopa and decarboxylase inhibitors are the cornerstone of Parkinson’s disease (PD) treatment [Citation1]. As PD progresses, benefit from oral CD/LD doses may be delayed and/or reduced owing to gastrointestinal dysmotility and the effect of dietary protein on absorption [Citation1]. When CD/LD doses are not providing benefit (OFF time), motor and/or nonmotor symptoms re-emerge or worsen until onset of benefit occurs with the next oral CD/LD dose [Citation1,Citation2]. OFF time is typically associated with either a shortened duration of action of CD/LD before the next scheduled dose (wearing OFF) or delayed time to onset of effect after a dose of CD/LD is taken (delayed ON; dose failure/no ON) [Citation2]. Morning OFF is characterized by both morning akinesia, due to a wearing OFF of the last dose of CD/LD and a delayed ON response to the first morning dose [Citation2]. Delayed ON is common, with studies showing at least 50% of patients with PD and OFF episodes experience delayed ON at least one morning per week [Citation3] and an average time to ON with the first morning dose of CD/LD in patients with known delayed ON of ∼60 min [Citation4]. Dose failures also occur with nearly half of morning CD/LD doses in patients with known delayed ON [Citation4]. Based on continuous objective monitoring technology, persistent morning bradykinesia after the first daily dose of CD/LD is very common and may be underrecognized by patients and physicians [Citation5]. To date, limited data exist quantifying motor responses in patients who experience delayed ON with their first morning dose of CD/LD using functional scales, including the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III score.
While optimization of oral CD/LD dose, frequency and formulation as well as the addition of ‘ON-extender’ medications (catechol-O-methyltransferase inhibitors, monoamine oxidase-B [MAO-B] inhibitors, dopamine agonists, adenosine A2A receptor antagonists, etc.) are commonly used to address OFF time related to wearing OFF, they do not address OFF time related to delayed ON, particularly in regard to the first CD/LD dose of the day [Citation6]. Time spent waiting to turn ON may constitute the majority of daily OFF time, and may represent double the amount of OFF time that can be attributed to wearing OFF [Citation7]. Thus, many patients continue to experience troublesome OFF time after the addition of ‘ON-extenders’ [Citation1,Citation8,Citation9].
Acute, intermittent treatments, or on-demand treatments, that bypass the gastrointestinal system provide an alternative approach to address OFF episodes, particularly those related to delayed onset of action of CD/LD [Citation6,Citation10]. Due to their routes of administration and absorption, on-demand treatments can address OFF episodes more rapidly and reliably with concomitant oral CD/LD [Citation6]. Apomorphine sublingual film (SL-APO) is approved for the acute, intermittent treatment of OFF episodes associated with PD [Citation11]. A post hoc analysis of the SL-APO pivotal study demonstrated a motor function improvement with SL-APO based on the MDS-UPDRS Part III score of comparable magnitude to CD/LD but peaking at earlier time points, with an approximately twofold higher magnitude of response at 15 min postdose with SL-APO (n = 109) compared with CD/LD (n = 108) and a greater mean response observed for SL-APO versus CD/LD through 45 min postdose [Citation12]. These data suggest that SL-APO is as effective as CD/LD as far as magnitude of motor improvement but has a faster onset of action and peak effect, and thus could address OFF time related to delayed ON while patients wait for CD/LD to take effect.
The present analysis represents the largest evaluation to date of motor responses, based on MDS-UPDRS Part III score, to the first morning dose of CD/LD in patients demonstrating known morning OFF, and aims to expand upon the initial observation that SL-APO may provide benefit for patients who experience delayed ON. Data from two Phase III studies were pooled to compare magnitude of motor improvement and time to onset of benefit with oral CD/LD and SL-APO.
Materials & methods
Participants
This post hoc analysis included screening and dose-optimization phase data pooled from the pivotal SL-APO study (CTH-300; double-blind, placebo-controlled, Phase III study [NCT02469090]) and an ongoing long-term safety and efficacy study (CTH-301; open-label, Phase III study [NCT02542696]; data cutoff date May 2019). The analysis included only those patients with no prior SL-APO exposure. Full eligibility criteria for the pivotal study were previously published [Citation11,Citation12]. Eligibility criteria for the current analysis are provided in . Key inclusion criteria were idiopathic PD responsive to and being treated with stable doses of CD/LD (immediate or controlled release) or LD/DOPA decarboxylase inhibitors and any additional PD medications for ≥4 weeks (≥8 weeks for MAO-B inhibitors), ≥1 OFF episode/day and ≥2 h of total daily OFF time. Key exclusion criteria included atypical or secondary parkinsonism, major psychiatric disorder, exposure to deep brain stimulation, intraduodenal LD or other apomorphine formulations ≤7 days of the first screening visit.
Table 1. Eligibility criteria for the pivotal and long-term studies.
The studies were designed, conducted and monitored in accordance with the World Medical Association Declaration of Helsinki and International Council for Harmonisation guidelines. The study protocols and patient informed consent form were approved by an institutional review board.
Study design
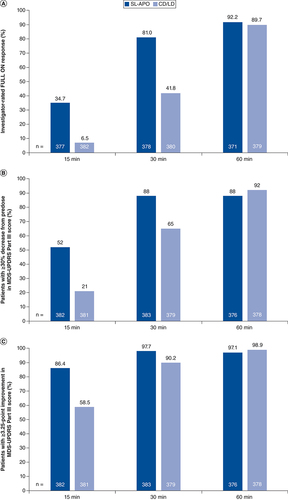
The SL-APO pivotal study (CTH-300) was a multicenter, randomized, double-blind, placebo-controlled, Phase III study, which was followed by an ongoing, open-label, long-term Phase III study (CTH-301). Both studies included a screening period, in which the open-label response to the prescribed first oral CD/LD dose of the day was assessed, and a phase in which patients underwent open-label SL-APO dose optimization () [Citation11]. In the pivotal study, these periods were followed by a double-blind, placebo-controlled maintenance phase; in the long-term study, these periods were followed by a long-term open-label treatment phase. However, this analysis includes data only from the screening and dose-optimization phases of both studies.
At screening, patients attended morning office visits when OFF and received their prescribed first morning CD/LD dose without adjunctive PD medications (levodopa challenge; ). CD/LD was administered to patients at doses that were previously identified by their movement disorder specialist physicians, and patients had been on stable doses for ≥4 weeks. Dose optimization of SL-APO began with a 10-mg dose and was increased in 5-mg increments (maximum 35 mg) at subsequent visits until an effective and tolerable FULL ON, assessed by both patient and investigator, occurred within 45 min.
Evaluations
Motor responses were evaluated using MDS-UPDRS Part III and investigator-rated FULL ON, performed predose and 15, 30 and 60 min postdose. FULL ON was defined as medication-provided benefit with regard to mobility, stiffness and slowness and adequate motor function allowing for performance of normal daily activities [Citation11,Citation13]. Post hoc analyses evaluated motor response to the prescribed morning CD/LD dose during screening and the response to the final effective, tolerable SL-APO dose identified during dose optimization. Responder analyses included the proportion of patients who achieved a ≥30% decrease and a minimal clinically important difference (≥3.25-point reduction) [Citation14] in MDS-UPDRS Part III score from predose at 15, 30 and 60 min postdose.
The pivotal study analysis population included all randomized patients who received ≥1 postrandomization dose of study medication. The long-term study analysis population included all new patients who were successfully dose optimized and administered ≥1 dose of study medication. All assessments described herein were analyzed post hoc and descriptively.
Results
Patients
A total of 384 patients (pivotal study, n = 109; long-term study, n = 275) were included in this analysis. The mean age of patients was 63.8 years, 64.8% were male and 95.1% were White. An average of 82.8% of patients had a diagnosis of PD for at least 5 years and 81.8% had a Hoehn and Yahr score of 2 to 2.5 (). Mean (standard deviation [SD]) predose MDS-UPDRS Part III scores were similar before both the prescribed first morning dose of CD/LD and the optimized dose of SL-APO (42.2 [13.9] and 41.9 [15.0], respectively).
Table 2. Demographic and baseline characteristics.Table Footnote†
Delayed ON response with CD/LD
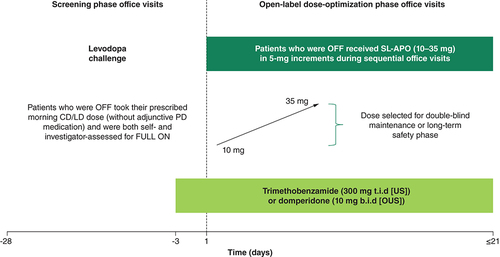
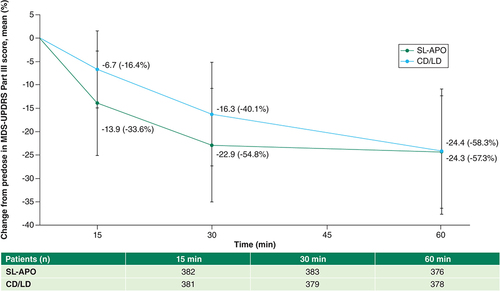
The mean (SD) change from predose in MDS-UPDRS Part III score with the prescribed first morning CD/LD dose at 15-, 30- and 60-min time points was -6.7 (8.2), -16.3 (11.1) and -24.4 (12.1), respectively (). The percent change from predose for CD/LD at 15, 30 and 60 min postdose was 16.4, 40.1 and 58.3%, respectively (). Investigator-rated FULL ON was achieved by 6.5, 41.8 and 89.7% of patients with CD/LD at 15, 30 and 60 min postdose, respectively (A). After receiving their prescribed first daily CD/LD dose, the proportions of patients achieving a ≥30% decrease in MDS-UPDRS Part III score from predose were 21, 65 and 92% at 15, 30 and 60 min postdose, respectively (B). Clinically meaningful improvement in MDS-UPDRS Part III score (≥3.25-point reduction) was achieved by 58.5, 90.2 and 98.9% of patients treated with CD/LD at 15, 30 and 60 min, respectively (C).
Error bars represent standard deviations.
CD/LD: Carbidopa/levodopa; MDS-UPDRS: Movement Disorder Society Unified Parkinson’s Disease Rating Scale; SL-APO: Apomorphine sublingual film.
Patients with (A) investigator-rated FULL ON, (B) ≥30% decrease from predose in MDS-UPDRS Part III score and (C) clinically meaningful improvement (≥3.25-point reduction) in MDS-UPDRS Part III score with the prescribed first CD/LD dose at screening and final SL-APO dose during dose optimization.
CD/LD: Carbidopa/levodopa; MDS-UPDRS: Movement Disorder Society Unified Parkinson’s Disease Rating Scale; SL-APO: Apomorphine sublingual film.
Experience with SL-APO
The mean (SD) change from predose in MDS-UPDRS Part III score for the optimized dose of SL-APO was -13.9 (11.1), -22.9 (12.1) and -24.3 (13.4) at 15, 30 and 60 min postdose, respectively (). The percent change from predose for SL-APO at 15, 30 and 60 min postdose was 33.6, 54.8 and 57.3%, respectively (). Investigator-rated FULL ON was achieved by 34.7, 81.0 and 92.2% of patients with SL-APO at 15, 30 and 60 min postdose, respectively (A). In patients receiving an optimized dose of SL-APO, the proportions of patients achieving a ≥30% decrease in MDS-UPDRS Part III score from predose were 52, 88 and 88% at 15, 30 and 60 min postdose, respectively (B). Clinically meaningful improvement in MDS-UPDRS Part III score was achieved by 86.4, 97.7 and 97.1% of patients treated with SL-APO at 15, 30 and 60 min postdose, respectively (C).
Evaluation of treatment onset for CD/LD versus SL-APO
Mean change from predose in MDS-UPDRS Part III score for the optimized dose of SL-APO was approximately double at 15 min postdose and remained greater at 30 min postdose compared with the prescribed first morning CD/LD dose (). Investigator-rated FULL ON was achieved by a greater proportion of patients with SL-APO versus CD/LD at 15 and 30 min postdose (A). Treatment with SL-APO resulted in greater proportions of patients with a ≥30% decrease in MDS-UPDRS Part III score from predose and with a clinically meaningful improvement in MDS-UPDRS Part III score from predose than with CD/LD at 15 and 30 min postdose (B & C).
Discussion
In the largest evaluated dataset of patients with demonstrated morning OFF, full motor benefit did not occur for most patients until 60 min after their prescribed first morning dose of CD/LD. At 15 and 30 min postdose, 6.5 and 41.8% of patients treated with CD/LD achieved a FULL ON and patients experienced changes from predose in MDS-UPDRS Part III scores of -6.7 and -16.3 points, respectively. At 15 and 30 min postdose, 34.7 and 81.0% of patients treated with SL-APO achieved a FULL ON and patients experienced changes from predose in MDS-UPDRS Part III scores of -13.9 and -22.9 points, respectively.
LD is the most effective treatment for motor symptoms associated with PD. Morning akinesia is common and patients often experience prolonged morning OFF due to a delayed ON response to the first morning dose of CD/LD. In a study of patients with advanced PD, mean time to ON with a single LD dose was 46 ± 21 min, with delayed ON representing approximately 68% (197 ± 106 min) of total daily OFF time (261 ± 97 min) and the remaining time (64 ± 43 min) attributed to wearing OFF [Citation7]. In another study of patients treated with CD/LD for at least 1 year, delayed ON with the first morning CD/LD dose was reported by 51% of patients at least one time in the last 7 days and daily by 21% of patients, with a delayed ON with lunchtime doses reported on 2.3 days/week [Citation3]. In this population, 56% of patients experienced a time to ON of >30 min, with a mean time to ON after the morning dose of CD/LD of 35–40 min. Dose failures with the first morning CD/LD dose were reported as at least once per week by 14% of patients and at least four times per week by 10% of patients [Citation3]. At baseline in the current analysis, 69.5% and 43.5% of patients reported experiencing delayed ON and dose failure, respectively. The prevalence of delayed ON with the first morning dose of CD/LD can represent a substantial negative impact on patients’ functioning [Citation15].
Strategies featured in treatment algorithms to address the occurrence of OFF episodes typically include two approaches [Citation13,Citation16]. The first is to adjust the dose, frequency and formulation of the LD regimen. The second is to initiate an adjunctive treatment to extend the duration of ON responses produced by LD. These so called ‘ON-extenders’ may be associated with up to an average of 2 h of decreased net OFF time; however, patients may still experience as much as 4 h of OFF time per day [Citation8,Citation9,Citation17–23]. Furthermore, ‘ON-extenders’ have been evaluated for their ability to extend the duration of ON time, but they are unable to acutely turn patients from OFF to ON, which is particularly problematic when associated with the delay in benefit of the first CD/LD dose of the day. On-demand therapies can be used complementary to or between doses of CD/LD to turn patients from OFF to ON to address this unmet need.
On-demand therapies, including sublingual and subcutaneous apomorphine formulations and inhaled LD, are approved for the acute, intermittent treatment of OFF episodes associated with PD. Studies using subcutaneous formulations of apomorphine have shown results consistent with the current sublingual analysis. An open-label study of 88 patients with PD demonstrated that subcutaneous apomorphine injection (SC-APO) administered in place of the morning CD/LD dose significantly decreased time to ON versus CD/LD (24 min vs 61 min). Most patients experienced improvement in time to ON (∼96%) with SC-APO and there were substantially fewer dose failures (time to ON >60 min) versus CD/LD (7 vs 46%) [Citation4]. The data reported herein are consistent with findings from SC-APO studies, suggesting that concomitant dosing of apomorphine with CD/LD could allow patients to achieve a faster ON while waiting for CD/LD to take effect. To date, only one study has directly compared patient preference for and satisfaction with SL-APO and SC-APO. In an open-label, randomized, crossover study of these two treatments, more patients reported an overall preference for SL-APO (72%) versus SC-APO/no preference [Citation24] and greater global satisfaction with SL-APO versus SC-APO based on convenience and global satisfaction [Citation25]. These results may be associated with the challenges of assembling the SC-APO device and/or giving oneself an injection in a state of reduced motor function, as well as potential needle phobia [Citation10]. Results of studies of inhaled LD yield similar results to those of SL-APO and SC-APO. In a randomized, crossover study of inhaled LD administered with patient’s first morning dose of CD/LD, a significantly greater proportion of patients turned ON at 30 min postdose with inhaled LD plus CD/LD compared with CD/LD alone (66.7 vs 44.5%, respectively) [Citation26]. Collectively, these data suggest that on-demand therapies could be used concomitantly with CD/LD (particularly with the first CD/LD dose of the day) to turn patients from OFF to ON faster and more reliably than with CD/LD alone.
Several limitations of this analysis should be considered. Patients took CD/LD doses that were prescribed by a movement disorder specialist as part of their routine clinical care and may not have been optimized in a similar fashion as SL-APO. However, most patients had PD for ≥5 years and were on stable doses of CD/LD for ≥4 weeks (mean total daily dose, 1060.1 mg). Open-label administration of SL-APO and CD/LD, along with investigator-assessed FULL ON, may have introduced bias. Safety and tolerability were not analyzed in this specific subpopulation. However, previously published data have demonstrated that SL-APO is generally safe and well tolerated; the most frequently reported adverse events with SL-APO were nausea, dizziness and somnolence, which are known side effects of dopamine agonists [Citation11,Citation27]. Lastly, all analyses were post hoc, and the studies were not designed as prospective comparisons of SL-APO with CD/LD versus CD/LD alone.
Conclusion
In this post hoc analysis of pooled data from two Phase III studies, many patients experienced a delayed ON response with their prescribed first morning dose of CD/LD. The results of the current analysis demonstrated more rapid benefit with the optimized dose of SL-APO, suggesting that patients taking SL-APO concomitantly with their oral CD/LD doses may be able to achieve a faster onset of improvement in motor function versus their prescribed oral CD/LD dose alone. This finding could be important considering the burden of delayed onset of CD/LD. Given the prevalence of delayed ON/dose failure, using SL-APO in combination with CD/LD may provide a more rapid ON in patients reporting a delayed onset of benefit with CD/LD.
CD/LD and other combinations of LD and decarboxylase inhibitors are the cornerstone of PD treatment.
As PD progresses, benefit from oral CD/LD doses may be delayed and/or reduced and many patients experience delayed time to effect of CD/LD, including delayed ON and dose failures (no ON).
The current study was a post hoc analysis of pooled data from two studies gathering motor response to CD/LD and SL-APO.
Many patients with PD experienced a delayed ON response to their prescribed morning oral dose of CD/LD, with full benefit of CD/LD occurring by 60 min.
Investigator-rated FULL ON was achieved by 6.5% and 41.8% of patients with CD/LD at 15 and 30 min postdose, respectively, and investigator-rated FULL ON was achieved by 34.7 and 81.0% of patients with SL-APO at 15 and 30 min postdose, respectively.
SL-APO resulted in comparable but faster improvement in motor function compared with the prescribed oral CD/LD dose.
These findings support the potential clinical utility of adding SL-APO concomitantly to CD/LD in patients with delayed ON or dose failures.
Author contributions
Study concept and design: SH Isaacson, A Bowling, E Pappert and F Stocchi. Data acquisition, analysis or interpretation: SH Isaacson, A Bowling, I Zhang, E Pappert and F Stocchi. Statistical analysis: I Zhang. Drafting of the manuscript: SH Isaacson, A Bowling, I Zhang, E Pappert and F Stocchi. Critical review of the manuscript: SH Isaacson, A Bowling, I Zhang, E Pappert and F Stocchi. Approval of the final manuscript: SH Isaacson, A Bowling, I Zhang, E Pappert and F Stocchi.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval and have followed the principles outlined in the World Medical Association Declaration of Helsinki and International Council for Harmonisation guidelines for all human experimental investigations. In addition, informed consent has been obtained from the participants involved.
Supplemental Text 1
Download MS Word (57.6 KB)Acknowledgments
The authors would like to thank W Neeson of Sunovion Pharmaceuticals Canada Inc. (Ontario, Canada) and B Navia, formerly of Sunovion Pharmaceuticals Inc. (MA, USA) for their contributions to the conceptual design and data analysis. The authors also thank all coinvestigators for their participation in the clinical studies (see Supplementary Table 1).
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at:www.tandfonline.com/doi/full/10.2217/nmt-2022-0038
Financial & competing interests disclosure
The study was supported by funding from Sunovion Pharmaceuticals Inc. (MA, USA). SH Isaacson reports honoraria for CME, consultant, research grants, and/or promotional speaker on behalf of AbbVie, Acadia Pharmaceuticals Inc, Acorda Therapeutics, Inc., Adamas Pharmaceuticals, Inc., Addex Therapeutics, AFFiRiS AG, Alexza Pharmaceuticals, Allergan, Amarantus BioScience, Amneal Pharmaceuticals, Aptinyx, Axial Therapeutics, Inc., Axovant Gene Therapies, BenevolentAI, Biogen, Britannia Pharmaceuticals, Cadent Therapeutics, Cala Health, Cerecor, Inc., Cerevel Therapeutics, Cipla, Eli Lilly, Enterin Inc., GE Healthcare, Global Kinetics Pty Ltd, Impax Laboratories, Impel NeuroPharma, Intec Pharma, Ipsen, Jazz Pharmaceuticals, Kyowa Kirin, Lundbeck, Merz Pharmaceuticals, Michael J. Fox Foundation, Mitsubishi Tanabe Pharma, Neuraly, Neurocrine Biosciences, NeuroDerm, Parkinson Study Group, Pharma Two B, Prilenia Therapeutics, Promentis Pharmaceuticals, Inc., Revance, Roche, Sanofi, Sunovion Pharmaceuticals Inc., Sun Pharma, Supernus Pharmaceuticals, Inc., Teva, Theravance Biopharma and UCB. A Bowling, I Zhang and E Pappert are currently or were employees of Sunovion Pharmaceuticals Inc. F Stocchi has received compensation for employment, consulting, advisory board participation and/or speaking engagements with Bial Chiesi, Neuroderm, Zambon, Britannia, and Sunovion Pharmaceuticals Inc. and has received research support from Zambon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance was provided by J Deckman and M Wallace-Nadolski of The Lockwood Group (CT, USA) and was supported by funding from Sunovion Pharmaceuticals Inc. (MA, USA).
Data sharing statement
The authors certify that this manuscript reports clinical trial data: NCT02469090; NCT02542696.
Access to deidentified participant data will be provided after a research proposal is submitted online (https://vivli.org) and receives approval from the Independent Review Panel and after a data sharing agreement is in place. Access will be provided for an initial period of 12 months after approval of the data sharing request, but an extension can be granted, when justified, for up to an additional 12 months.
References
- Olanow CW , SternMB, SethiK. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology, 72(4 Suppl. 21), S1–S136 (2009).
- Chou KL , StacyM, SimuniTet al. The spectrum of “off” in Parkinson’s disease: what have we learned over 40 years? Parkinsonism Relat. Disord., 51, 9–16 (2018).
- Stocchi F , ColettiC, BonassiS, RadicatiFG, VaccaL. Early-morning OFF and levodopa dose failures in patients with Parkinson’s disease attending a routine clinical appointment using Time-to-ON Questionnaire. Eur. J. Neurol., 26(5), 821–826 (2019).
- Isaacson S , LewM, OndoW, HubbleJ, ClinchT, PaganF. Apomorphine subcutaneous injection for the management of morning akinesia in Parkinson’s disease. Mov. Disord. Clin. Pract., 4(1), 78–83 (2017).
- Isaacson SH , PahwaR, PappertEJ, Torres-RussottoD. Evaluation of morning bradykinesia in Parkinson’s disease in a United States cohort using continuous objective monitoring. Clin. Park. Relat. Disord., 6, 100145 (2022).
- Isaacson SH , PaganFL, LewMF, PahwaR. Should “on-demand” treatments for Parkinson’s disease OFF episodes be used earlier?Clin. Park. Relat. Disord., 7, 100161 (2022).
- Merims D , DjaldettiR, MelamedE. Waiting for ON: a major problem in patients with Parkinson disease and ON/OFF motor fluctuations. Clin. Neuropharmacol., 26(4), 196–198 (2003).
- Rascol O , BrooksDJ, MelamedEet al. Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet, 365(9463), 947–954 (2005).
- Lewitt PA , GuttmanM, TetrudJWet al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann. Neurol., 63(3), 295–302 (2008).
- Olanow CW , PoeweW, RascolO, StocchiF. On-demand therapy for OFF episodes in Parkinson’s disease. Mov. Disord., 36(10), 2244–2253 (2021).
- Olanow CW , FactorSA, EspayAJet al. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled Phase III study. Lancet Neurol., 19(2), 135–144 (2020).
- Hui JS , FoxSH, NeesonWet al. Open-label titration of apomorphine sublingual film in patients with Parkinson’s disease and “OFF” episodes. Parkinsonism Relat. Disord., 79, 110–116 (2020).
- Armstrong MJ , OkunMS. Diagnosis and treatment of Parkinson disease: a review. JAMA, 323(6), 548–560 (2020).
- Horváth K , AschermannZ, ÁcsPet al. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat. Disord., 21(12), 1421–1426 (2015).
- Hauser RA , LewittPA, ComellaCL. On demand therapy for Parkinson’s disease patients: opportunities and choices. Postgraduate Medicine, 133(7), 721–727 (2021).
- Fox SH , KatzenschlagerR, LimSYet al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord., 33(8), 1248–1266 (2018).
- Elmer LW , JuncosJL, SingerCet al. Pooled analyses of phase III studies of ADS-5102 (amantadine) extended-release capsules for dyskinesia in Parkinson’s disease. CNS Drugs, 32(4), 387–398 (2018).
- Ferreira JJ , LeesA, RochaJ-F, PoeweW, RascolO, Soares-Da-SilvaP. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol., 15(2), 154–165 (2016).
- Lieberman A , RanhoskyA, KortsD. Clinical evaluation of pramipexole in advanced Parkinson’s disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology, 49(1), 162–168 (1997).
- Pahwa R , StacyMA, FactorSAet al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology, 68(14), 1108–1115 (2007).
- Rajput AH , MartinW, Saint-HilaireMH, DorflingerE, PedderS. Tolcapone improves motor function in parkinsonian patients with the “wearing-off” phenomenon: a double-blind, placebo-controlled, multicenter trial. Neurology, 49(4), 1066–1071 (1997).
- Rinne UK , LarsenJP, SidenA, Worm-PetersenJ. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology, 51(5), 1309–1314 (1998).
- Thach A , ZichlinM, PeddleMet al. Systematic literature review of key outcomes used to assess adjunctive treatments for Parkinson’s disease [abstract]. Mov. Disord., 37 (2002).
- Stocchi F , RascolO, PoeweWet al. Efficacy of apomorphine sublingual film versus subcutaneous apomorphine for the treatment of OFF episodes in Parkinson’s disease [abstract]. Mov. Disord., 37(Suppl. 1), S356–S356 (2022).
- Schwarz J , CarrollC, EbersbachGet al. Apomorphine sublingual film versus subcutaneous apomorphine in the treatment of OFF episodes in Parkinson’s disease: Results from an assessment of patient satisfaction [abstract]. Mov. Disord., 37(Suppl. 1), S352–S352 (2022).
- Hauser RA , IsaacsonSH, EllenbogenAet al. Orally inhaled levodopa (CVT-301) for early morning OFF periods in Parkinson’s disease. Parkinsonism Relat. Disord., 64, 175–180 (2019).
- Hauser RA , OlanowCW, DzyngelBet al. Sublingual apomorphine (APL-130277) for the acute conversion of OFF to ON in Parkinson’s disease. Mov. Disord., 31(9), 1366–1372 (2016).