Abstract
Many reports conclude nanoparticle (NP) brain entry based on bulk brain analysis. Bulk brain includes blood, cerebrospinal fluid and blood vessels within the brain contributing to the blood–brain and blood–cerebrospinal fluid barriers. Considering the brain as neurons, glia and their extracellular space (brain parenchyma), most studies did not show brain parenchymal NP entry. Blood–brain and blood–cerebrospinal fluid barriers anatomy and function are reviewed. Methods demonstrating brain parenchymal NP entry are presented. Results demonstrating bulk brain versus brain parenchymal entry are classified. Studies are reviewed, critiqued and classified to illustrate results demonstrating bulk brain versus parenchymal entry. Brain, blood and peripheral organ NP timecourses are compared and related to brain parenchymal entry evidence suggesting brain NP timecourse informs about brain parenchymal entry.
The issue addressed in this guide
When considering nanoparticle (NP) distribution, a question that is often raised is whether they enter the brain. With the goal to use NPs to deliver drugs to the brain, many researchers are hopeful that their NPs do enter the brain. Others are concerned about unintended NP brain entry and potential adverse effects. Many have oversimplified the concept of brain entry. They have not considered the extensive vasculature that permeates the brain, and the potential for an NP to be within the vasculature (blood), associated with components of the blood–brain barrier (BBB) or blood–cerebrospinal fluid barrier (BCSFB), or in cerebrospinal fluid (CSF), rather than in brain parenchyma that houses the brain cells and their extracellular (interstitial) space. This article aims: to inform about the distinction between distribution into the brain (bulk brain) versus brain parenchyma, present methods that can demonstrate brain parenchymal distribution, present a tiered classification of methods and results that demonstrate bulk brain versus brain parenchymal distribution, critically review some reports that claimed brain or brain parenchymal NP distribution and rate them according to the tiered classification, and suggest that NP residence time in the brain often informs about brain parenchymal entry.
BBB anatomy & function
To justify the claim that an NP crossed the BBB and entered brain parenchyma requires an understanding of brain anatomy, the vasculature within it and research methods that demonstrate brain parenchymal entry. The primary route to enable NP brain parenchymal entry is across the BBB. The BBB separates the vascular compartment from brain cells that are surrounded by extracellular/interstitial fluid that occupies approximately 20% of the parenchymal space. The BBB includes brain microvascular endothelial cells (BMECs) that line microvessels within the brain, forming the luminal (blood) side of the BBB (). BMECs have efflux transporters that effectively prevent brain parenchymal entry of many substances and metabolic enzymes to maintain brain homeostasis. On the other hand, BMECs have influx transporters that deliver essential nutrients, elements and factors to brain cells from the blood, supporting brain nutrition. Influx transporters have been utilized in a Trojan horse approach to hitchhike NPs through the BBB to brain parenchyma. BMECs differ from endothelial cells that line the vasculature outside of the brain by their near total absence of fenestrations (windows) through which NPs might diffuse and the presence of tight junctions between adjacent BMEC surfaces. Intact tight junctions prevent passage of ionic substances as small as lanthanum (hydrated ionic diameter ∼0.8 nm) through this paracellular barrier [Citation1]. Microvessels, the blood vessels between arteries and veins, are approximately 7.5 μm in diameter [Citation2], barely large enough for a red blood cell to squeeze through. Microvessels deliver blood to the brain from within the brain [Citation3].
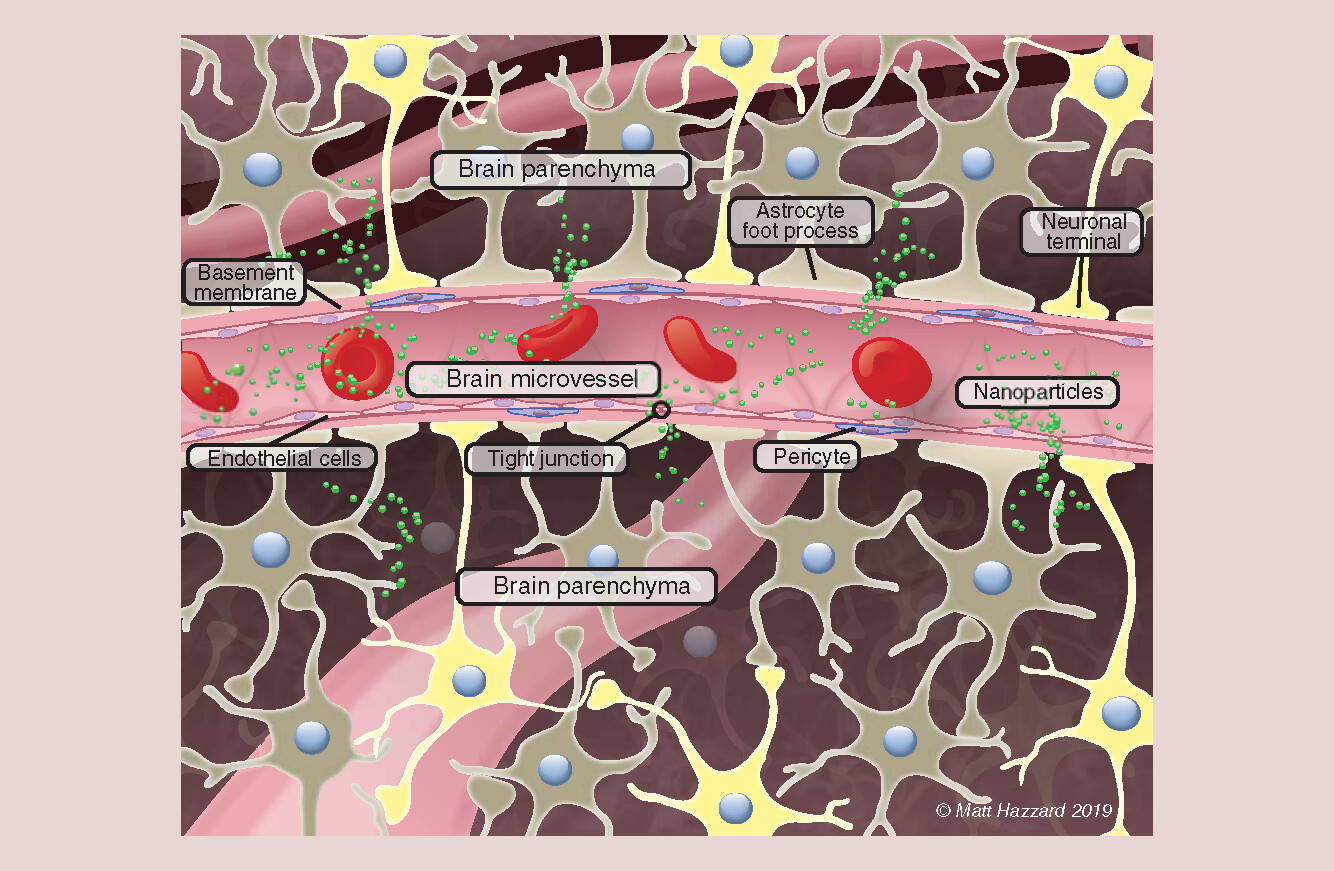
There are 100 billion capillaries, approximately 600 km long, with a surface area of 20 m2 within the human brain. These microvessels provide a blood supply within approximately 10 μm of every brain cell [Citation4], as illustrated in by the foreground and two background microvessels. They occupy approximately 2% of cortical brain volume and a greater space in some other brain regions [Citation5,Citation6]. Pericytes extend over approximately 30% of the BMEC surface. Surrounding the BMECs and pericytes is a basement membrane, covering the abluminal (brain-side) BMEC surface (). Foot processes of astrocytes, one of the brain’s glial cells, cover more than 90% of the abluminal basement membrane surface (). Neurons interact with this cell complex to form the neurovascular unit (). For an NP to enter brain parenchyma from blood, it must either distribute through a BMEC (transcellular) or between BMECs (paracellular), as suggested in . The latter is not expected when the BBB is intact but can occur when the BBB is opened (leaky, e.g., there is increased space between adjoining BMEC surfaces). This occurs in cancer (at the blood–tumor barrier), Alzheimer’s disease, traumatic brain injury, multiple sclerosis, infections, encephalitis, stroke, liver failure and other less common conditions [Citation7,Citation8]. BBB opening can be produced by osmotic insult, for example, 25% mannitol infusion into a carotid artery that delivers blood directly to its ipsilateral hemisphere [Citation9] or focused ultrasound in conjunction with systemically circulating microbubbles [Citation10].
Potential pitfalls of experimental result misinterpretation
Given the intimate spatial relationship between the microvessels of the vascular compartment and brain parenchyma, visual demonstration of brain parenchymal entry in vivo or in situ requires ≤10 μm resolution. Alternatively, one could use methods that separate BBB components from brain parenchymal components. Failure to distinguish brain parenchymal NP entry from bulk brain can result in the interpretation that an NP has crossed the BBB when it may not have or did not to the extent implied. For example, IVIS demonstration of increased fluorescence in the head region from an NP component does not differentiate whether the NP is in the skull; the blood in the vessels surrounding and within the brain; associated with the luminal side of BMECs; within or between the BMECs; between the BMECs and basement membrane; within the basement membrane, pericytes or astrocyte foot processes; or in brain parenchyma.
There have been many studies addressing brain entry of many different NPs that concluded they entered the brain, without demonstration that they crossed the BBB and entered brain parenchyma. Considering the brain as the cranial contents, the claim of NP brain entry can be accepted if it is shown that the NP is in bulk brain. But this does not demonstrate brain parenchymal entry. For most studies, the methods employed were not able to differentiate NPs in the blood versus associated with BBB components versus residing in brain parenchyma, as differentiated in .
NPs in or associated with the vascular compartment contribute to bulk brain but not brain parenchymal content
Rats perfused to remove blood (from the vascular compartment) 4 h after intravenous injection of gold glyconanoparticles had only approximately 4% as much NP component in bulk brain as rats that had not been perfused [Citation11]. Similar perfusion reduced gold in three bulk brain regions to 7–18% of that seen in nonperfused rats [Citation12]. This demonstrates that a great percentage of NPs in bulk brain may not be in brain parenchyma. Removal of blood from the entire body can be accomplished in the deeply anesthetized rodent by transcardial perfusion; introducing a perfusate into the left ventricle and ligating the right auricle to allow blood and perfusate drainage. Perfusate can be 0.9% sodium chloride or 0.1 mM phosphate @ pH 7.4. For electron microscopy, 4% paraformaldehyde and 3.5% glutaraldehyde in cacodylate/phosphate-buffered saline/saline buffer should be used. Gage et al. provide a protocol [Citation13]. Some studies accounted for the contribution of blood NPs within the brain to their bulk brain level [Citation6,Citation14–18]. NPs in bulk brain blood were estimated from the product of the peripheral blood NP concentration × brain vascular volume. However, this does not account for NPs adsorbed to the BMEC luminal wall or in BBB cellular and membrane components. After intravenous injection to rodents, NPs were observed adhered to the luminal wall of blood vessels in the brain with little to no evidence of brain tissue entry. This was seen for lipid (stearic acid and polysorbate 80) drug conjugate NPs [Citation19], 100-nm wide and 300-nm long poly(ethylene glycol) (PEG)-polyethylenimine-conjugated mesoporous silica NPs [Citation20], and a 5-nm polyhedral citrate-coated ceria NP that had a surface charge of -53 mV [Citation21]. In Dan et al.’s study, blood was removed from the brain by ceria-free perfusion. The preponderance of NPs in guinea pig brain that had been infused for 120 min with approximately 8-nm amphipathic hydrophilic/hydrophobic-coated gold/iron core NPs followed by 30 min perfusate washout was in endothelial cells [Citation22]. These observations are in agreement with a kinetic study that reported a negatively charged NP associated with cell surfaces within seconds, by Langmuir adsorption through electrostatic interaction [Citation23]. These results demonstrate the potential contribution of BBB-associated NPs to bulk brain content, even after removal of blood from the brain.
The importance of distinguishing among NP distribution in the vascular compartment, the cells comprising the BBB and brain parenchyma is nicely illustrated by the results of Fiandra et al. [Citation24]. Employing fluorometric dye fluorescein isothiocyanate (FITC)-labeled amphiphilic polymer-coated iron oxide NPs containing a fluorometric dye (AF660)-labeled antiretroviral drug, they differentiated localization of the drug from the nanoconstruct. Confocal laser scanning microscopy (CLSM) viewing of mouse brain sections stained to identify the BMECs demonstrated colocalization of the NPs and BMECs, interpreted as NP residence in the vascular compartment. Antiretroviral drug was seen within and outside of the vascular compartment, interpreted as having been released from the NPs. Similarly, using a GFAP antibody to identify blood vessels by the astrocyte foot processes that cover them, Dal Magro et al. saw considerable NPs surrounded by the GFAP antibody, but some particles that were not, the latter interpreted as having entered brain parenchyma [Citation25].
BCSFB anatomy & function
The CSF compartment is comprised of four ventricles within the brain (two lateral, a third and a fourth) and the CSF-filled subarachnoid space that surrounds the entire CNS. CSF is produced by choroid plexuses in each of the four ventricles and diffusion of extracellular fluid from the brain. Each lateral ventricle has a single outlet, to the third ventricle, that drains into the fourth ventricle, that drains into the subarachnoid space, creating one-directional flow out of the brain. CSF in the human and rat turns over approximately four- and ten-times daily, respectively. It exits from this compartment through arachnoid granulations into dural sinuses then into blood (via the jugular veins). The epithelial cells of the choroid plexuses have tight junctions, efflux transporters and metabolic properties similar to the BBB, creating the BCSFB between blood and CSF. There is little barrier to distribution between CSF and the brain, so substances injected into the CSF compartment, or able to cross the BCSFB, readily diffuse into the brain proximal to the CSF compartment.
Material entry into the nervous system, including the brain, might also be achieved by its introduction into the CSF compartment. In the human, this is most often accomplished by injection into CSF in the spinal region. Given CSF flow, NP injection into CSF would not be expected to persist for a long time compared with introduction into brain parenchymal space. As distribution from CSF into the CNS (brain and spinal cord) is by diffusion, and against extracellular fluid flow toward CSF, significant distribution very far into the brain would not be expected. Demonstration of successful NP distribution into brain parenchyma via this route would require similar techniques as apply to NP introduction into blood.
Convection-enhanced delivery of solutions into the extracellular brain space
A method to directly deliver solutions into the brain is convection-enhanced delivery (CED). In CED, the tip of a catheter is stereotaxically inserted into the brain through a hole in the cranium. A solute (drug or NP)-containing solution is infused through the catheter by a pump. Solute release from the catheter tip into brain extracellular space displaces extracellular fluid. The solute distributes through brain extracellular space surrounding the catheter tip by bulk flow to create a radial distribution up to a few centimeters. After termination of the infusion, the solute may continue to redistribute by bulk flow and diffusion as it is being locally cleared. CED has advantages of bypassing the BBB enabling brain parenchymal delivery of drugs that do not cross the BBB, delivery to a target site that can be deep within the brain and creation of much higher regional than distal brain and systemic drug concentration. It is most commonly used to treat glioblastoma multiforme, a brain cancer that has a poor prognosis [Citation26–28]. Small molecules are cleared within 72 h [Citation29], whereas some NPs have been found to persist for much longer ( & Supplementary Figure 1).
Table 1. Studies that reported the brain level of the nanoparticle or component(s) at multiple times after convection enhanced delivery. Mean residence time values were determined as described in nanoparticle kinetics in the brain.
Nasal cavity route of delivery directly to the CNS
There is only one site where the nervous system is directly exposed to the external environment of mammals, where direct uptake of NPs into the nervous system might occur. This is the roof of the nasal cavity where the olfactory neuron and the maxillary branch of the trigeminal neuron have terminals with receptors that mediate the perception of sensory stimuli, including smell. This route of administration, introducing test material into the nose (intranasal), is being investigated for NP entry into the brain, to bypass the BBB. Demonstration of NPs in these sensory neurons ex vivo (e.g., by electron microscopy visualization or elemental analysis under conditions that rule out dissolution or degradation that releases NP components), or of NPs within the nerve when it is in situ, provides confidence of nerve entry. The olfactory and trigeminal neurons are surrounded by CSF, so suggestion of NP in the region of the nerve, for example, from an NP fluorescent molecule, does not unequivocally show nerve entry. Although neuropeptides have been shown to enter CSF directly from the nasal cavity, this route was shown to not contribute to uptake of 110 nm 6-coumarin-loaded (for visualization) PEG-poly(lactic acid) NPs [Citation30]. There have been demonstrations of NP uptake by the olfactory or trigeminal nerve. NPs often accumulate in the olfactory bulb, with little or no distribution across the synapse between the olfactory bulb and the next neuron of the olfactory system that would enable distribution to distal brain regions (e.g., the pyriform cortex, amygdala, thalamic and hypothalamic nuclei, and hippocampus) after intranasal administration. Olfactory bulb accumulation was shown with colloidal gold [Citation31,Citation32], 13C carbon [Citation33], nanoscale manganese oxide [Citation34] and quantum dots [Citation35]. Distribution of quantum dots from the nasal cavity to the olfactory bulb was attributed to microtubule-mediated fast axonal transport (200–400 mm/day), because axonal flow is too slow to account for the timecourse of its appearance in the olfactory bulb [Citation35]. Based on the olfactory nerve length from the nasal cavity to the olfactory bulb (∼5 and 8 mm in mice and rats, respectively) fast axonal transport could traverse this with an NP in approximately 26–40 min. Much more time would be required for neuronal NP transport from the nasal cavity to distant brain regions, such as the striatum. Rapid appearance of NPs or NP components in distant sites can be attributed to absorption from the nasal cavity into systemic circulation followed by distribution into the brain. Translocation of NPs from the nasal cavity to the brain is low. Gamma counting of whole brain 24 h after intranasal 111In-labeled protein NP suggested approximately 0.2% of the dose was in the brain, assuming stability of the 111In-NP complex [Citation36]. No studies were found demonstrating NP entry into brain parenchyma beyond the olfactory bulbs after intranasal administration.
Methods to demonstrate NP brain parenchymal entry
To definitively claim that an NP has entered brain parenchyma requires methods that differentiate NP distribution in brain parenchyma from blood within the brain and BBB components. One approach is to use visual methods.
Visual methods to demonstrate NP brain parenchymal entry
lists some visual methods and their optimal resolution. High-resolution brain MRI does not provide, even under optimal conditions, sufficient resolution to differentiate NP localization between parenchymal versus nonparenchymal sites. It would not be able to characterize NP morphology, size or chemical identity.
Table 2. Visual methods that might be used to image brain nanoparticle distribution and their reported resolution.
Photoacoustic imaging (tomography) is a hybrid imaging modality that integrates optical contrast with high-ultrasonic spatial resolution in deep tissue. It can provide noninvasive images of the entire brain with less than msec temporal resolution [Citation37], based on the light absorption properties of the tissue and material (e.g., NPs) within it. Although useful for NP-assisted imaging, for example, of brain tumors, it lacks sufficient resolution to differentiate NP localization between parenchymal versus nonparenchymal sites.
The IVIS spectrum in vivo imaging system combines 2D and 3D optical tomography into a single platform to visualize fluorescence and bioluminescence. It has been extensively used to demonstrate the intensity of fluorescent NPs in organs in vivo and ex vivo. Intensity in the head region has been claimed by many as evidence of the NP in the brain. It is noninvasive and can be used with living organisms. However, even if used to localize NPs in ex vivo tissue, it does not have sufficient resolution to differentiate NP localization between parenchymal versus nonparenchymal sites.
Mass spectrometry imaging (MSI) includes many techniques, such as laser desorption/ionization mass spectrometry, matrix-assisted laser desorption ionization, (time-of-flight) secondary ion mass (SIM) spectrometry and desorption electrospray ionization. It has been used with surface-functionalized gold- or gadolinium-containing NPs to localize organics such as lipids, metabolites and proteins in the brain. Although some forms of MSI have submicron resolution (e.g., SIM), no reports were found using MSI to verify brain parenchymal NP localization.
Super-resolution microscopy utilizes fluorescent microscopes in a variety of approaches, categorized as those that approach the diffraction limit (0.25 μm in the focal plane) and those that break the diffraction limit by turning fluorescent markers on and off. In the latter rather than all fluorescent molecules emitting simultaneously (as occurs in conventional fluorescence microscopy), a small subpopulation is excited so that emission from neighbors does not overlap, enabling isolation of individual emitters [Citation38]. Approaches include stochastic optical reconstruction microscopy, direct stochastic optical reconstruction microscopy, photoactivated localization microscopy, fluorescence photoactivated localization microscopy and ground-state depletion with individual molecule return. Super-resolution microscopy has been used to image the NP protein corona [Citation39,Citation40] and localize NPs in cells [Citation41,Citation42], but no reports were found of in vivo, in situ or ex vivo NP localization.
Hyperspectral imaging combines imaging and spectrophotometry by capturing spectral data of infrared and visible light reflected by samples at each pixel in an enhanced dark-field microscopic image. For this to differentiate vascular versus parenchymal NP localization, an enhanced dark-field microscopic image needs to be interpreted first to identify the region of interest, then hyperspectral imaging employed to provide spectrophotometric data. This was demonstrated in studies that showed cellular sites of functionalized gold NPs [Citation43]; studies that included cellular, C. elegans, and Japanese medaka NP distribution cited by Roth [Citation44]; and porcine skin uptake of metal oxide NPs [Citation45]. This approach might enable differentiation of NP localization between brain parenchymal versus vascular sites, but no such studies were found.
Fluorescence microscopy visualizes fluorescent tags or incorporated molecules (e.g., doxorubicin and paclitaxel). With the exception of transmission electron microscopy (TEM), the only studies using a method in that appear to have differentiated NP parenchymal from vascular space distribution used fluorescence microscopy and CLSM. Using fluorescence microscopy, Mulik et al. saw fluorescent-labeled NPs within brain cells [Citation46]. Åslund et al. used CLSM to image fluorescent dye-containing NPs, and tomato lectin-labeled blood vessels, and saw NP fluorescent dye outside of the brain microvasculature [Citation47]. Some details of these studies and those reported by others who used CLSM are below and/or in [Citation22,Citation24,Citation25,Citation47–55].
Electron microscopy, typically TEM, is not only the best technique to characterize prepared NPs, it is also amenable to physicochemically identify and characterize NPs in samples obtained from biological environments [Citation56–58]. It provides the greatest resolution among the options in , and when equipped with capabilities such as high-angle annular dark-field detection, energy dispersive x-ray spectroscopy or electron energy loss spectroscopy, it enables chemical characterization.
The benefits of light microscopy (including identification of biological structures and location of regions of interest and fluorescent labels) have been combined with the benefits of electron microscopy (to provide NP localization and physicochemical information) in correlative light and electron microscopy (CLEM) [Citation59]. The two approaches were independently used to locate NPs in a murine brain tumor model [Citation60]. CLEM has been developed in a single instrument and used to localize NPs in cultured non-neuronal cells [Citation61]. No studies were found utilizing CLEM to investigate NP brain parenchymal entry.
Several of the above methods are based on detection of a label added to the NP, often a fluorescent molecule or metal/metal oxide. Correct interpretation of NP localization, when based on an added label, requires NP preparation that does not contain free label (which can be removed by dialysis) and understanding the label stability in vivo. Fluorescent molecules are susceptible to loss of their signal due to quenching (due to interaction with local molecular environmental components) and photobleaching (excitation light-induced destruction of the excited fluorophore). Persistent association (stability) of the label and the NP in vivo is required to attribute label signal to NP localization. Label dissociated from the NP will yield a different distribution than the label-NP complex. Covalent binding of label to an NP decreased label release, compared with encapsulation of the label [Citation62]. Incorporation of the label in the NP core did not produce changes in the NP surface properties [Citation63]. Label dissociation from a biodegradable polymer NP would be expected over time. Label dissociation can be assessed in vitro by incubation of the labeled NP with brain homogenate [Citation30] or separation of the NP and brain components from the released label, for example, by NP centrifugation, and detection of label in the supernatant. Other considerations include the potential for the label to alter the physicochemical properties of the NP and its resultant distribution and biological tolerance to the label. More in-depth discussions can be found in [Citation64,Citation65].
Methods to demonstrate NP brain parenchymal entry
Methods are available to assess whether NPs introduced into the vasculature perfusing the brain are associated with BBB components and/or crossed the BBB to enter brain parenchyma. The in situ brain perfusion technique can be used to determine NP entry rate and extent of distribution into bulk brain, multiple bulk brain regions and/or the choroid plexus, ipsilateral to the carotid artery perfused after short-term (few minutes or less) intracarotid infusion [Citation66]. An intracarotid perfusion rate sufficiently high to prevent blood from entering the perfused carotid artery is used, therefore preventing blood from entering the brain hemisphere perfused by that artery. This enables control of the chemical environment of the material tested (therefore its chemical form [speciation]), based on the perfusion fluid composition and avoidance of blood exposure. Preventing blood exposure avoids potential NP biotransformation (e.g., corona coating by plasma proteins, dissolution or particle breakdown) that might change its surface properties and brain uptake. Although the in situ brain perfusion technique removes blood from the brain vasculature, it does not distinguish NP distribution associated with the BBB versus brain parenchyma, nor choroid plexuses from brain unless they are separated.
The capillary depletion method is intended to separate BBB components from brain parenchyma, to produce capillary-depleted brain parenchyma [Citation67]. This is often conducted with brain tissue obtained using the in situ brain perfusion technique but can be used with brain tissue obtained by other methods that remove blood from the brain. The methods are described in detail in [Citation68]. The capillary depletion method uses centrifugation of brain homogenate in dextran that separates the BMECs into the pellet (along with brain nuclei and erythrocytes if blood was not perfused from the brain) from the supernatant that contains brain cells and brain extracellular fluid. Presence of the test material in the supernatant is taken as evidence that it has transcytosed the BBB into brain parenchyma. This method was employed to ascertain the fraction of 190-nm poly(n-butyl cyanoacrylate) (PBCA)-coated doxorubicin-loaded NPs that entered brain parenchyma [Citation69]. Doxorubicin was seen in the brain fraction after intravenous administration in NPs but not when doxorubicin solution was administered with 1% polysorbate 80 (intended to open the BBB). Coating the NPs with 1% polysorbate 80 increased the doxorubicin supernatant to pellet ratio, suggesting the surfactant-coated PBCA NPs entered brain parenchyma. Employing the capillary depletion method after whole body perfusion to remove blood, it was shown that the percentage of the injected dose of 111In-DTPA-multiwalled carbon nanotubes (median diameter: 18.9 and length: 500 nm) decreased in brain capillaries but not brain parenchyma over 24 h [Citation70]. The capillary depletion method was used to obtain curcumin for LC–MS/MS analysis from mouse brains after oral dosing of a curcumin-containing NP. The report does not state if the brain was perfused prior to harvest. If not the supernatant fraction would be expected to include blood plasma contents that could have contained some/all of the curcumin [Citation71]. The capillary depletion method was used to demonstrate that cationic bovine serum albumin-coated biodegradable polymersomes (∼100 nm, composed of PEG- and maleimide-PEG-poly(ε-caprolactone), loaded with coumarin-6) entered brain parenchyma. The supernatant fraction increased from a half to 1 h and was fairly stable for 4 h, and increased over the 4 h in relation to the pellet (vascular fraction) [Citation72]. Capillary depletion was used in transcardially perfused mice to assess the influence of transferrin-receptor antibody density on gold NPs and platinum-containing liposomes to enter brain parenchyma [Citation73]. 2.5 h after intravenous injection, gold and platinum in the parenchymal fraction were approximately 27 and 22% of the combined parenchymal and capillary fractions, respectively. In contrast to demonstrating brain parenchymal entry, the capillary depletion method suggested nearly all of 5-nm ceria in the brain was associated with the capillary endothelial cells, consistent with EM observations of nanoceria adhered to the luminal side of blood vessel walls in the brain, noted above [Citation21].
NP components have been sampled from the brain using microdialysis probes [Citation74–77]. In the presence of an intact BBB, it is assumed that this technique samples brain extracellular space. Microdialysis probes with 30 and 100 kDa molecular weight cutoff membranes would be expected to allow penetration of up to 5–8 and 8–14 nm (depending on membrane composition) diameter spherical particles and molecules released from NPs. The appearance of an NP component in the dialysate from a probe would not conclusively demonstrate NP distribution into brain parenchyma. None of the above studies demonstrated that intact NPs were recovered in the dialysate.
Level of demonstration of NP brain or brain parenchymal entry & critical review of study claims
Convincing demonstration of brain parenchymal entry is limited to in vivo studies. In vitro BBB models do not recapitulate BBB complexity. They lack all of its cell composition, cell interactions, cell spatial relationships and magnitude of resistance to distribution across them. Therefore, this report is limited to in vivo, in situ and ex vivo brain studies. Many reports published within the past 5 years were identified by a SciFinder search of the terms NP and brain, a SciFinder search of the terms NP and BBB for reports published in 2019, and a PubMed search of NPs, drug delivery systems and brain parenchyma. The reports were reviewed, summarized and critiqued for evidence supporting claims in the report and classified for the level of demonstration of NP brain or brain parenchymal entry, as defined in . Some reports published more than 5 years ago that are informative for their demonstration of NP brain parenchymal entry and/or reported brain NP concentration at multiple times are included. The results are in .
Table 3. A classification of results demonstrating brain versus. brain parenchymal entry.
Table 4. Brain nanoparticle distribution study claims, summary, critique, support for the claims and level of brain or brain parenchymal entry.
NP kinetics in the brain
Endocytosis is the prevalent mechanism that mediates NP transport across the intact BBB. It would not be anticipated that after NPs cross the BBB and enter brain parenchyma they would be rapidly cleared from that compartment [Citation78]. After NP infusion directly into the brain by CED, HPLC determination of two components showed clearance half-lives of 250–400 h () [Citation79]. MRI-determined NP distribution volume after CED decreased 50% in approximately 250 h () [Citation80]. Some details of these and other studies that reported brain NP or component levels at multiple times after CED are in . Brain clearance half-life was shown to be dose dependent, increasing from less than 96 h after 60 μg to approximately 480 h after 1600 μg [Citation81], suggesting the quantity of NPs in brain parenchyma influence their duration of persistence in that compartment.
Some studies reported the brain level of the NP or its components at multiple times after its peripheral administration. Some studies also reported blood and/or peripheral organ levels. The reported values are shown in Supplementary Figure 1. To be able to visually compare the rates of NP/component increase or decrease over time given the wide range of levels and timecourses among the studies, the results were normalized to the dose for CED studies or first reported value for other studies and shown as double log figures. It is expected that NPs that entered brain parenchyma would persist longer in the brain than NPs that did not cross the BBB, and longer than in the blood. Most reports of brain NP or component determination over time did not have many values (range for the non-CED and non-microdialysis studies: 2–16, median: 5) discouraging rigorous pharmacokinetic analysis or estimate of NP residence to infinity. Noncompartmental mean residence time (MRT) to the last sample was determined using Phoenix 8.1 WinNonLin. The MRT of NPs or their components after peripheral or intranasal administration is generally less than after CED, suggesting less than 100% of the NPs or components entered brain parenchyma. Good agreement was obtained between the MRT results and author reported half-lives for the same datasets ( & ). The MRT was similarly calculated for NP, or its component, in blood from the studies that determined them at multiple times after NP administration. The results are shown in parentheses in . The timecourse and MRTs for the NP or its component in the brain, blood and peripheral organs were often similar (Supplementary Figure 1 & ). This could be due to most or all of the NP/component in the brain residing in blood within the brain or adhering to the luminal wall of BMECs and being cleared from brain at the same rate as blood clearance from peripheral circulation. Exceptions were seen in two reports where the brain MRT was considerably longer than blood MRT [Citation17,Citation58], suggesting NP distribution beyond the vascular compartment. However, evidence of brain parenchymal entry was not provided.
Studies classified according to the criteria in as demonstrating brain parenchymal entry (levels 5 and 6) that reported brain NP or component levels at multiple times are shown in Supplementary Figure 1D & E. Brain levels often increased over time or decreased more slowly than in studies classified as not showing brain parenchymal entry. Brain levels often decreased more slowly than blood levels in studies shown in Supplementary Figure 1D and E than B or C. There is some concordance between the classification levels and NP/component timecourse in the brain, suggesting the rate of NP/component clearance from the brain reflects the absence or presence of brain parenchymal entry.
The evidence of NP parenchymal entry may not be from the same measure as that providing the brain timecourse (e.g., TEM showing NP in brain cells, whereas NP/marker residence determined by HPLC). When NP or component was determined by radioactivity, neutron activation analysis, IVIS imaging, fluorescence, HPLC, atomic absorption spectroscopy, inductively coupled plasma–optical emission spectroscopy, inductively coupled plasma–mass spectrometry or LC–MS, it could be that only a fraction was in brain parenchyma. A risk of concluding NP brain entry or brain parenchymal entry from a component of the NP, such as an incorporated fluorescent dye or a metal component of a metal- or metal oxide-containing NP, is that the dye or metal might be released and its distribution does not represent the NP. For example, it was concluded that dye clearance from the brain in 24 h was ‘most likely due to degradation of the dye itself rather than actual clearance of the NPs from the brain’ [Citation82]. Similarly, metal analysis by neutron activation analysis, atomic absorption spectroscopy, inductively coupled plasma–optical emission spectroscopy or inductively coupled plasma–mass spectrometry may be informing about released ion, particularly from readily soluble NPs such as those containing copper, silver and zinc.
In contrast, the results of many studies which did not demonstrate NP distribution in brain parenchyma showed more rapid clearance of brain-associated NPs ( & Supplementary Figure 1). Many studies did not remove blood from the brain. The decline of NPs within a few hours in the brain, at a rate similar to its decline in blood, and more rapidly than the decline from peripheral organs, may be due to lack of brain parenchymal entry. Such results suggest that only some, or none, of the NPs crossed the BBB. The NPs may have adhered to the luminal wall of brain vasculature or localized in cellular or membrane components of the BBB, and subsequently distributed away from these sites into blood circulating through the brain. In studies that did not use sufficient methods to demonstrate NP brain parenchymal entry, the NPs may indeed have crossed the BBB. However, the methods do not verify that. If procedures are used to rule out the contribution of NPs in blood and adherence to BMECs, NP persistence in the brain may indicate successful distribution across the BBB.
Conclusion
The BBB and BCSFB provide a significant challenge to deliver substances into the brain to access neural, glial and cancer cells. Verification that the substance has entered into the compartment that houses these cells (brain parenchyma) is not trivial. Experimental results are sometimes overinterpreted to suggest successful delivery of substances that might directly act on cells in the brain when verification of brain parenchymal entry is lacking. An understanding of the anatomy and location of the barriers (BBB and BCSFB) to brain parenchymal entry helps one interpret if a substance has entered brain parenchyma. Visual and procedural methods have been used to verify brain parenchymal entry. In this article, methods have been classified as to the level of verification of brain parenchymal entry and then critically applied to studies. The BBB can be bypassed by direct substance introduction into the brain by CED that often results in prolonged residence of the substance in the brain. It is suggested that prolonged substance residence in the brain after its peripheral (e.g., iv.) administration suggests brain parenchymal entry. It is hoped that this guide will help researchers and reviewers be more precise in their demonstration and interpretation of substance distribution in the brain.
Future perspective
As the study of NP biological interaction matures, there is greater understanding of how to critically assess NP effects and their distribution. If the criteria and methods that demonstrate bulk brain and brain parenchymal entry proposed herein are employed by researchers and reviewers, there will be more precise reporting of brain NP distribution. More realistic result interpretation will enable more realistic clinical benefit expectation. This will enhance the credibility of this endeavor and help investigators engage in best practice research.
The anatomy and function of the blood–brain (BBB) and blood-cerebrospinal fluid barriers are reviewed.
Exposure routes to bypass the BBB (convection-enhanced delivery and uptake from the nasal cavity by cranial nerves) are described.
Visual and procedural methods to determine nanoparticle (NP) brain and brain parenchymal entry are presented.
Criteria are proposed to classify levels of experimental result evidence that demonstrate NP bulk brain or brain parenchymal entry.
Studies of NP brain entry or distribution across the BBB are summarized, critiqued and evaluated using the classification levels to interpret if the study shows NP bulk brain or brain parenchymal entry.
NP or component persistence in the brain after systemic or intranasal administration compared with blood and peripheral organs and direct infusion by convection-enhanced delivery into the brain are compared with interpret support for NP distribution beyond the brain vasculature, into brain parenchyma.
Supplemental document 1
Download Zip (468.7 KB)Acknowledgments
The author thanks Uschi M Graham for her excellent suggestion on the tenor of this review and suggestion of some of the reviews cited in , and Matt Hazzard, medical illustrator, University of Kentucky College of Medicine for creating .
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/nnm-2019-0169
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Sanovich E , BartusRT, FridenPMet al. Pathway across blood–brain barrier opened by the bradykinin agonist, RMP-7. Brain Res.705(1–2), 125–135 (1995).
- Burke MJC , NelsonL, SladeJYet al. Morphometry of the hippocampal microvasculature in post-stroke and age-related dementias. Neuropathol. Appl. Neurobiol.40(3), 284–295 (2014).
- Zlokovic BV , ApuzzoML. Strategies to circumvent vascular barriers of the central nervous system. Neurosurgery43(4), 877–878 (1998).
- Boström M , HellstroemErkenstam N, KaluzaDet al. The hippocampal neurovascular niche during normal development and after irradiation to the juvenile mouse brain. Int. J. Radiat. Biol.90(9), 778–789 (2014).
- Ohno K , PettigrewKD, RapoportSI. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am. J. Physiol.235(3), H299–H307 (1978).
- Calvo P , GouritinB, ChacunHet al. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm. Res.18(8), 1157–1166 (2001).
- Weiss N , MillerF, CazaubonS, CouraudP-O. The blood–brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta, Biomembr.1788(4), 842–857 (2009).
- Rosenberg GA . Neurological diseases in relation to the blood–brain barrier. J. Cereb. Blood Flow Metab.32(7), 1139–1151 (2012).
- Bellavance M-A , BlanchetteM, FortinD. Recent advances in blood–brain barrier disruption as a CNS delivery strategy. AAPS J.10(1), 166–177 (2008).
- Burgess A , HynynenK. Microbubble-assisted ultrasound for drug delivery in the brain and central nervous system. Adv. Exp. Med. Biol.880, 293–308 (2016).
- Frigell J , GarciaI, Gomez-VallejoV, LlopJ, PenadesS. 68Ga-labeled gold glyconanoparticles for exploring blood–brain barrier permeability: preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J. Am. Chem. Soc.136(1), 449–457 (2014).
- Sela H , EliaP, ZachRet al. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnol.13, 71 (2015).
- Gage GJ , KipkeDR, ShainW. Whole animal perfusion fixation for rodents. J. Vis. Exp.65(65), 3564 (2012).
- Buzulukov YP , ArianovaEA, DeminVFet al. Bioaccumulation of silver and gold nanoparticles in organs and tissues of rats studied by neutron activation analysis. Biol. Bull.41(3), 255–263 (2014).
- Schleh C , Semmler-BehnkeM, LipkaJet al. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology6, 36–46 (2012).
- Yokel RA , TsengMT, DanMet al. Biodistribution and biopersistence of ceria engineered nanomaterials: size dependence. Nanomedicine9(3), 398–407 (2013).
- Schäffler M , SousaF, WenkAet al. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials35(10), 3455–3466 (2014).
- Hirn S , Semmler-BehnkeM, SchlehCet al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm.77(3), 407–416 (2011).
- Gessner A , OlbrichC, SchroderW, KayserO, MullerRH. The role of plasma proteins in brain targeting: species dependent protein adsorption patterns on brain-specific lipid drug conjugate (LDC) nanoparticles. Int. J. Pharm.214(1–2), 87–91 (2001).
- Baghirov H , KaramanD, ViitalaTet al. Feasibility study of the permeability and uptake of mesoporous silica nanoparticles across the blood-brain barrier. PLoS ONE11(8), e0160705 (2016).
- Dan M , TsengMT, WuPet al. Brain microvascular endothelial cell association and distribution of a 5 nm ceria engineered nanomaterial. Int. J. Nanomed.7, 4023–4036 (2012).
- Sanavio B , LibrizziL, PennacchioPet al. Distribution of superparamagnetic Au/Fe nanoparticles in an isolated guinea pig brain with an intact blood brain barrier. Nanoscale10(47), 22420–22428 (2018).
- Wilhelm C , GazeauF, RogerJ, PonsJN, BacriJC. Interaction of anionic superparamagnetic nanoparticles with cells: kinetic analyses of membrane adsorption and subsequent internalization. Langmuir18(21), 8148–8155 (2002).
- Fiandra L , ColomboM, MazzucchelliSet al. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine11(6), 1387–1397 (2015).
- Dal Magro R , AlbertiniB, BerettaSet al. Artificial apolipoprotein corona enables nanoparticle brain targeting. Nanomedicine14(2), 429–438 (2018).
- Healy AT , VogelbaumMA. Convection-enhanced drug delivery for gliomas. Surg. Neurol. Int.6(Suppl. 1), S59–S67 (2015).
- Lonser RR , SarntinoranontM, MorrisonPF, OldfieldEH. Convection-enhanced delivery to the central nervous system. J. Neurosurg.122(3), 697–706 (2015).
- Mehta AM , SonabendAM, BruceJN. Convection-enhanced delivery. Neurotherapeutics14(2), 358–371 (2017).
- Singh R , WangM, SchweitzerMEet al. Volume of distribution and clearance of peptide-based nanofiber after convection-enhanced delivery. J. Neurosurg.129(1), 10–18 (2018).
- Liu Q , ShenY, ChenJet al. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm. Res.29(2), 546–558 (2012).
- De Lorenzo AJD . The olfactory neuron and the blood–brain barrier. In: Taste and Smell in Vertebrates. WolstenholmeG, KnightJ ( Eds). Churchhill, London, UK, 151–176 (1970).
- Gopinath PG , GopinathG, KumarTCA. Target site of intranasally sprayed substances and their transport across the nasal mucosa: a new insight into the intransal route of drug-delivery. Curr. Ther. Res.23(5), 596–607 (1978).
- Oberdörster G , SharpZ, AtudoreiVet al. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol.16(6–7), 437–445 (2004).
- Elder A , GeleinR, SilvaVet al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect.114(8), 1172–1178 (2006).
- Hopkins LE , PatchinES, ChiuP-Let al. Nose-to-brain transport of aerosolised quantum dots following acute exposure. Nanotoxicology8(8), 885–893 (2014).
- Migliore MM , VyasTK, CampbellRB, AmijiMM, WaszczakBL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J. Pharm. Sci.99(4), 1745–1761 (2010).
- Wang D , WuY, XiaJ. Review on photoacoustic imaging of the brain using nanoprobes. Neurophotonics3(1), 010901 (2016).
- Baddeley D , BewersdorfJ. Biological insight from super-resolution microscopy: what we can learn from localization-based images. Annu. Rev. Biochem.87, 965–989 (2018).
- Feiner-Gracia N , BeckM, PujalsSet al. Super-resolution microscopy unveils dynamic heterogeneities in nanoparticle protein corona. Small13(41), 1701631 (2017).
- Clemments AM , BotellaP, LandryCC. Spatial mapping of protein adsorption on mesoporous silica nanoparticles by stochastic optical reconstruction microscopy. J. Am. Chem. Soc.139(11), 3978–3981 (2017).
- Van Der Zwaag D , VanparijsN, WijnandsSet al. Super resolution imaging of nanoparticles cellular uptake and trafficking. ACS Appl. Mater. Interfaces8(10), 6391–6399 (2016).
- Fumagalli G , MazzaD, ChristodoulouMSet al. Cyclopamine-paclitaxel-containing nanoparticles: internalization in cells detected by confocal and super-resolution microscopy. ChemPlusChem80(9), 1380–1383 (2015).
- Patskovsky S , BergeronE, RiouxD, MeunierM. Wide-field hyperspectral 3D imaging of functionalized gold nanoparticles targeting cancer cells by reflected light microscopy. J. Biophotonics8(5), 401–407 (2015).
- Roth GA , TahilianiS, Neu-BakerNM, BrennerSA. Hyperspectral microscopy as an analytical tool for nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.7(4), 565–579 (2015).
- Pena MDPS , GottipatiA, TahilianiSet al. Hyperspectral imaging of nanoparticles in biological samples: simultaneous visualization and elemental identification. Microsc. Res. Tech.79(5), 349–358 (2016).
- Mulik RS , BingC, Ladouceur-WodzakMet al. Localized delivery of low-density lipoprotein docosahexaenoic acid nanoparticles to the rat brain using focused ultrasound. Biomaterials83, 257–268 (2016).
- Åslund AKO , BergS, HakSet al. Nanoparticle delivery to the brain – by focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J. Control. Rel.220(Part A), 287–294 (2015).
- Yang T , MartinP, FogartyBet al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm. Res.32(6), 2003–2014 (2015).
- Han L , KongDK, ZhengM-Qet al. Increased nanoparticle delivery to brain tumors by autocatalytic priming for improved treatment and imaging. ACS Nano10(4), 4209–4218 (2016).
- Kundu P , DasM, TripathyK, SahooSK. Delivery of dual drug loaded lipid based nanoparticles across the blood–brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of parkinson’s disease. ACS Chem. Neurosci.7(12), 1658–1670 (2016).
- Ko YT . Nanoparticle-mediated delivery of oligonucleotides to the blood–brain barrier: in vitro and in situ brain perfusion studies on the uptake mechanisms. J. Drug Target.21(9), 866–873 (2013).
- Hu X , YangF, LiaoY, LiL, ZhangL. Cholesterol-PEG comodified poly(N-butyl) cyanoacrylate nanoparticles for brain delivery: in vitro and in vivo evaluations. Drug Deliv.24(1), 121–132 (2017).
- Liang J , GaoC, ZhuYet al. Natural brain penetration enhancer-modified albumin nanoparticles for glioma targeting delivery. ACS Appl. Mater. Interfaces10(36), 30201–30213 (2018).
- Tamba BI , StreinuV, FolteaGet al. Tailored surface silica nanoparticles for blood–brain barrier penetration: preparation and in vivo investigation. Arabian J. Chem.11(6), 981–990 (2018).
- Yang J-T , KuoY-C, ChenIYet al. Protection against neurodegeneration in the hippocampus using sialic acid- and 5-HT-moduline-conjugated lipopolymer nanoparticles. ACS Biomater. Sci. Eng.5(3), 1311–1320 (2019).
- Rasmussen K , GonzálezM, KearnsPet al. Review of achievements of the OECD Working Party on Manufactured Nanomaterials’ Testing and Assessment Programme. From exploratory testing to test guidelines. Regul. Toxicol. Pharmacol.74, 147–160 (2016).
- Lin P-C , LinS, WangPC, SridharR. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv.32(4), 711–726 (2014).
- Manaia EB , AbuçafyMP, Chiari-AndréoBGet al. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed.12, 4991–5011 (2017).
- Agronskaia AV , ValentijnJA, Van DrielLFet al. Integrated fluorescence and transmission electron microscopy. J. Struct. Biol.164(2), 183–189 (2008).
- Kempen PJ , KircherMF, DeLa Zerda Aet al. A correlative optical microscopy and scanning electron microscopy approach to locating nanoparticles in brain tumors. Micron68, 70–76 (2015).
- Han S , RaabeM, HodgsonLet al. High-contrast imaging of nanodiamonds in cells by energy filtered and correlative light-electron microscopy: toward a quantitative nanoparticle-cell analysis. Nano Lett.19(3), 2178–2185 (2019).
- Weiss B , SchaeferUF, ZappJet al. Nanoparticles made of fluorescence-labelled poly(L-lactide-co-glycolide): preparation, stability, and biocompatibility. J. Nanosci. Nanotechnol.6(9–10), 3048–3056 (2006).
- Zandanel C , VauthierC. Characterization of fluorescent poly(isobutylcyanoacrylate) nanoparticles obtained by copolymerization of a fluorescent probe during Redox Radical Emulsion Polymerization (RREP). Eur. J. Pharm. Biopharm.82(1), 66–75 (2012).
- Li K , LiuB. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev.43(18), 6570–6597 (2014).
- Reisch A , KlymchenkoAS. Fluorescent polymer nanoparticles based on dyes: seeking brighter tools for bioimaging. Small12(15), 1968–1992 (2016).
- Takasato Y , RapoportSI, SmithQR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol.247(3 Pt 2), H484–H493 (1984).
- Triguero D , BuciakJ, PardridgeWM. Capillary depletion method for quantification of blood–brain barrier transport of circulating peptides and plasma proteins. J. Neurochem.54(6), 1882–1888 (1990).
- Yokel RA . Methods to quantify nanomaterial association with, and distribution across, the blood–brain barrier in vivo. In: Nanotoxicity: Methods and Protocols. ZhangQ ( Ed.). Springer, NY, USA, 281–299 (2018).
- Wohlfart S , KhalanskyAS, GelperinaS, BegleyD, KreuterJ. Kinetics of transport of doxorubicin bound to nanoparticles across the blood-brain barrier. J. Control. Rel.154, 103–107 (2011).
- Kafa H , WangJT-W, RubioNet al. The interaction of carbon nanotubes with an in vitro blood–brain barrier model and mouse brain in vivo. Biomaterials53, 437–452 (2015).
- Ramalingam P , KoYT. Enhanced oral delivery of curcumin from n-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm. Res.32(2), 389–402 (2015).
- Pang Z , GaoH, ChenJet al. Intracellular delivery mechanism and brain delivery kinetics of biodegradable cationic bovine serum albumin-conjugated polymersomes. Int. J. Nanomed.7, 3421–3432 (2012).
- Johnsen KB , BakM, MelanderFet al. Modulating the antibody density changes the uptake and transport at the blood–brain barrier of both transferrin receptor-targeted gold nanoparticles and liposomal cargo. J. Control. Rel.295, 237–249 (2019).
- Bommana MM , KirthivasanB, SquillanteE. In vivo brain microdialysis to evaluate FITC-dextran encapsulated immunopegylated nanoparticles. Drug Deliv.19(6), 298–306 (2012).
- Zhang X , LiuL, ChaiG, ZhangX, LiF. Brain pharmacokinetics of neurotoxin-loaded PLA nanoparticles modified with chitosan after intranasal administration in awake rats. Drug Dev. Ind. Pharm.39(11), 1618–1624 (2013).
- Liu Z , OkekeCI, ZhangLet al. Mixed polyethylene glycol-modified breviscapine-loaded solid lipid nanoparticles for improved brain bioavailability: preparation, characterization, and in vivo cerebral microdialysis evaluation in adult Sprague Dawley rats. AAPS PharmSciTech15(2), 483–496 (2014).
- Zhu J , ZouJ, MuCet al. Intranasal administration of pullulan-based nanoparticles for enhanced delivery of adriamycin into the brain: in vitro and in vivo evaluation. Pharmazie74(1), 39–46 (2019).
- Al Zaki A , HuiJZ, HigbeeE, TsourkasA. Biodistribution, clearance, and toxicology of polymeric micelles loaded with 0.9 or 5 nm gold nanoparticles. J. Biomed. Nanotechnol.11(10), 1836–1846 (2015).
- Krauze MT , NobleCO, KawaguchiTet al. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro-Oncol.9(4), 393–403 (2007).
- Corem-Salkmon E , RamZ, DanielsDet al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int. J. Nanomed.6, 1595–1602 (2011).
- Noble CO , KrauzeMT, DrummondDCet al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res.66(5), 2801–2806 (2006).
- Oppong-Damoah A , ZamanRU, D’SouzaMJ, MurnaneKS. Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Horm. Behav.108, 20–29 (2019).
- Mackay JA , DeenDF, SzokaFC. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res.1035(2), 139–153 (2005).
- Saito R , KrauzeMT, NobleCOet al. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro-Oncol.8(3), 205–214 (2006).
- French JT , GoinsB, SaenzMet al. Interventional therapy of head and neck cancer with lipid nanoparticle-carried rhenium 186 radionuclide. J. Vasc. Interv. Radiol.21(8), 1271–1279 (2010).
- Weng KC , HashizumeR, NobleCOet al. Convection-enhanced delivery of targeted quantum dot-immunoliposome hybrid nanoparticles to intracranial brain tumor models. Nanomedicine (Lond.)8(12), 1913–1925 (2013).
- Sirianni RW , ZhengM-Q, PatelTRet al. Radiolabeling of poly(lactic-co-glycolic acid) (PLGA) nanoparticles with biotinylated F-18 prosthetic groups and imaging of their delivery to the brain with positron emission tomography. Bioconjug. Chem.25(12), 2157–2165 (2014).
- Arshad A , YangB, BienemannASet al. Convection-enhanced delivery of carboplatin PLGA nanoparticles for the treatment of glioblastoma. PLoS ONE10(7), e0132266 (2015).
- Chen EM , QuijanoAR, SeoY-Eet al. Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials178, 193–203 (2018).
- Stephen ZR , ReviaRA, WangKet al. Time-resolved MRI assessment of convection-enhanced delivery by targeted and non-targeted nanoparticles in a human glioblastoma mouse model. Cancer Res.79(18), 4776–4786 (2019).
- Stucht D , DanishadKA, SchulzePet al. Highest resolution in vivo human brain MRI using prospective motion correction. PLoS ONE10(7), e0133921 (2015).
- IVIS Imaging Systems (2019). www.Perkinelmer.Com/Lab-Solutions/Resources/Docs/Sht_011713c_01_Ivis_Comparison_Table.Pdf
- Barré FPY , HeerenRMA, PotočnikNO. Mass Spectrometry imaging in nanomedicine: unraveling the potential of MSI for the detection of nanoparticles in neuroscience. Curr. Pharm. Des.23(13), 1974–1984 (2017).
- Ries J , KaplanC, PlatonovaE, EghlidiH, EwersH. A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nat. Methods9(6), 582–584 (2012).
- Dudok B , BarnaL, LedriMet al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat. Neurosci.18(1), 75–86 (2015).
- Herrmannsdorfer F , FlottmanB, NanguneriSet al. 3D D STORM imaging of fixed brain tissue. Methods Mol. Biol.1538, 169–184 (2017).
- Wen C-J , ZhangL-W, Al-SuwayehSA, YenT-C, FangJ-Y. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int. J. Nanomed.7, 1599–1611 (2012).
- Singh-Moon RP , RoblyerDM, BigioIJ, JoshiS. Spatial mapping of drug delivery to brain tissue using hyperspectral spatial frequency-domain imaging. J. Biomed. Opt.19(9), 96003 (2014).
- Mitkovski M , Padovan-NetoFE, Raisman-VozariRet al. Investigations into potential extrasynaptic communication between the dopaminergic and nitrergic systems. Front. Physiol. Membr. Physiol. Biophys.3(Sept.), 372 (2012).
- Malatesta M . Transmission electron microscopy for nanomedicine: novel applications for long-established techniques. Eur. J. Histochem.60(4), 280–284 (2016).
- Tröster SD , MüllerU, KreuterJ. Modification of the body distribution of poly(methyl methacrylate) nanoparticles in rats by coating with surfactants. Int. J. Pharm.61(1–2), 85–100 (1990).
- Fundarò A , CavalliR, BargoniAet al. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol. Res.42(4), 337–343 (2000).
- Gao K , JiangX. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int. J. Pharm.310(1–2), 213–219 (2006).
- Chen Y-S , HungY-C, LinL-Wet al. Size-dependent impairment of cognition in mice caused by the injection of gold nanoparticles. Nanotechnol.21, 485102/485101–485102/485109 (2010).
- Guerrero S , ArayaE, FiedlerJLet al. Improving the brain delivery of gold nanoparticles by conjugation with an amphipathic peptide. Nanomedicine (Lond.)5, 897–913 (2010).
- Liu H-L , HuaM-Y, YangH-Wet al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl Acad. Sci. USA107(34), 15205-10– (2010).
- Tsai Y-M , ChienC-F, LinL-C, TsaiT-H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharm.416(1), 331–338 (2011).
- Wen C-J , YenT-C, Al-SuwayehSA, ChangH-W, FangJ-Y. In vivo real-time fluorescence visualization and brain-targeting mechanisms of lipid nanocarriers with different fatty ester:oil ratios. Nanomedicine (Lond.)6(9), 1545–1559 (2011).
- Wen Z , YanZ, HeRet al. Brain targeting and toxicity study of odorranalectin-conjugated nanoparticles following intranasal administration. Drug Deliv.18(8), 555–561 (2011b).
- Dziendzikowska K , Gromadzka-OstrowskaJ, LankoffAet al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol.32(11), 920–928 (2012).
- Prades R , GuerreroS, ArayaEet al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials33(29), 7194–7205 (2012).
- Martins SM , SarmentoB, NunesCet al. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur. J. Pharm. Biopharm.85(3PA), 488–502 (2013).
- Mazza M , NotmanR, AnwarJet al. Nanofiber-based delivery of therapeutic peptides to the brain. ACS Nano7(2), 1016–1026 (2013).
- Chaturvedi M , KaczmarekL, MolinoY, SreedharB, KhrestchatiskyM. Tissue inhibitor of matrix metalloproteinases-1 loaded poly(lactic-co-glycolic acid) nanoparticles for delivery across the blood-brain barrier. Int. J. Nanomed.9, 575–588 (2014).
- Chen Y-C , ChiangC-F, ChenL-Fet al. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials35(13), 4066–4081 (2014).
- Jose S , SowmyaS, CinuTAet al. Surface modified PLGA nanoparticles for brain targeting of bacoside-A. Eur. J. Pharm. Sci.63, 29–35 (2014).
- Joachim E , KimI-D, JinYet al. Gelatin nanoparticles enhance the neuroprotective effects of intranasally administered osteopontin in rat ischemic stroke model. Drug Del. Trans. Res.4(5–6), 395–399 (2014).
- Shilo M , MotieiM, HanaP, PopovtzerR. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale6(4), 2146–2152 (2014).
- Wang B , LvL, WangZet al. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis. Biomaterials35(22), 5897–5907 (2014).
- Wang JTW , FabbroC, VenturelliEet al. The relationship between the diameter of chemically-functionalized multi-walled carbon nanotubes and their organ biodistribution profiles in vivo. Biomaterials35(35), 9517–9528 (2014).
- Yang L , KuangH, ZhangWet al. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale7(2), 625–636 (2015).
- Yadav S , GattaccecaF, PanicucciR, AmijiMM. Comparative biodistribution and pharmacokinetic analysis of cyclosporine-A in the brain upon intranasal or intravenous administration in an oil-in-water nanoemulsion formulation. Mol. Pharm.12(5), 1523–1533 (2015).
- Joo J , KwonEJ, KangJet al. Porous silicon-graphene oxide core-shell nanoparticles for targeted delivery of siRNA to the injured brain. Nanoscale Horiz.1(5), 407–414 (2016).
- Zhang C , LiuQ, ShaoX, QianY, ZhangQ. Phage-displayed peptide-conjugated biodegradable nanoparticles enhanced brain drug delivery. Mater. Lett.167, 213–217 (2016).
- Ruan S , HuC, TangXet al. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation. ACS Nano10(11), 10086–10098 (2016).
- Shah B , KhuntD, MisraM, PadhH. Application of Box–Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route. Int. J. Biol. Macromol.89, 206–218 (2016).
- Belhadj Z , YingM, WeiXet al. Multifunctional targeted liposomal drug delivery for efficient glioblastoma treatment. Oncotarget8(40), 66889–66900 (2017).
- Betzer O , ShiloM, OpochinskyRet al. The effect of nanoparticle size on the ability to cross the blood–brain barrier: an in vivo study. Nanomedicine (Lond.)12(13), 1533–1546 (2017).
- Bouchoucha M , BéliveauÉ, KleitzF, CalonF, FortinM-A. Antibody-conjugated mesoporous silica nanoparticles for brain microvessel endothelial cell targeting. J. Mater. Chem. B5(37), 7721–7735 (2017).
- Ghadiri M , Vasheghani-FarahaniE, AtyabiFet al. Transferrin-conjugated magnetic dextran-spermine nanoparticles for targeted drug transport across blood-brain barrier. J. Biomed. Mater. Res. A105(10), 2851–2864 (2017).
- Velasco-Aguirre C , Morales-ZavalaF, Salas-HuenuleoEet al. Improving gold nanorod delivery to the central nervous system by conjugation to the shuttle Angiopep-2. Nanomedicine (Lond.)12(20), 2503–2517 (2017).
- Ishak RaH , MostafaNM, KamelAO. Stealth lipid polymer hybrid nanoparticles loaded with rutin for effective brain delivery – comparative study with the gold standard (Tween 80): optimization, characterization and biodistribution. Drug Deliv.24(1), 1874–1890 (2017).
- Kumar P , SharmaG, KumarRet al. Stearic acid based, systematically designed oral lipid nanoparticles for enhanced brain delivery of dimethyl fumarate. Nanomedicine (Lond.)12(23), 2607–2621 (2017).
- Ammar HO , GhorabMM, MahmoudAA, HigazyIM. Lamotrigine loaded poly-ε-(D,L-lactide-co-caprolactone) nanoparticles as brain delivery system. Eur. J. Pharm. Sci.115, 77–87 (2018).
- Chan TG , MorseSV, CoppingMJ, ChoiJJ, VilarR. Targeted delivery of DNA–Au nanoparticles across the blood–brain barrier using focused ultrasound. ChemMedChem13(13), 1311–1314 (2018).
- Dutta L , MukherjeeB, ChakrabortyTet al. Lipid-based nanocarrier efficiently delivers highly water soluble drug across the blood–brain barrier into brain. Drug Deliv.25(1), 504–516 (2018).
- Fernandes J , GhateMV, BasuMallik S, LewisSA. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm.547(1–2), 563–571 (2018).
- Li HY , ZhangB, ChanPSet al. Convergent synthesis and characterization of fatty acid-conjugated poly(ethylene glycol)-block-poly(epsilon-caprolactone) nanoparticles for improved drug delivery to the brain. Eur. Polym. J.98, 394–401 (2018).
- Graverini G , PiazziniV, LanducciEet al. Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: in vitro and in vivo evaluation. Colloids Surf. B161, 302–313 (2018).
- Najafabadi RE , KazemipourN, EsmaeiliA, BeheshtiS, NazifiS. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol.19(1), 59 (2018).
- Pandey PK , SharmaAK, RaniSet al. MCM-41 nanoparticles for brain delivery: better choline-esterase and amyloid formation inhibition with improved kinetics. ACS Biomater. Sci. Eng.4(8), 2860–2869 (2018).
- Chen Y , XuC, YuB, FanH, HuW. Efficient cholera toxin B subunit-based nanoparticles with MRI capability for drug delivery to the brain following intranasal administration. Macromol. Biosci.19(2), e1800340 (2019).
- Ha S-W , ChoA-S, KimTYet al. Ultrasound-sensitizing nanoparticle complex for overcoming the blood-brain barrier: an effective drug delivery system. Int. J. Nanomed.14, 3743–3752 (2019).
- Israel LL , BraubachO, GalstyanAet al. A combination of tri-Leucine and angiopep-2 drives a polyanionic polymalic acid nanodrug platform across the blood–brain barrier. ACS Nano13(2), 1253–1271 (2019).
- Zhang T , LipH, HeCet al. Multitargeted nanoparticles deliver synergistic drugs across the blood–brain barrier to brain metastases of triple negative breast cancer cells and tumor-associated macrophages. Adv. Healthcare Mat.8(18), e1900543 (2019).
