Abstract
Background: How clinicians conduct diagnostic reasoning is a major issue.
Aim: To evaluate whether intuitive and analytic processes (differential diagnosis checklist, DDXC; general de-biasing checklist, GDBC) might improve diagnostic performance.
Methods: We enrolled 188 medical students (4th–6th grades) who were divided into two groups and assigned the five cases scenarios. Group 1 (n = 91) were instructed to provide the three most likely diagnoses immediately after reading the scenarios (intuitive diagnosis), then after reading GDBC (diagnosis by GDBC), and finally, after reading DDXC (diagnosis by DDXC). Conversely, group 2 (n = 97) were instructed to provide intuitive diagnoses, by DDXC, and by GDBC.
Results: Among the group 1, there was significant difference of total scores (p = 0.01 by ANOVA) between intuitive (8.25) and DDXC (8.77). Among the group 2, we noted significant difference of total scores (p = 0.001 by ANOVA) between intuitive (7.21) and DDXC (7.96). Among the difficult cases, the proportions of correct diagnosis increased after reading DDXC, although among the simple cases, the proportions of correct diagnosis decreased after reading DDXC.
Conclusion: The use of DDXC, not GDBC, may improve the diagnostic performance in difficult cases, while intuitive process may still be better for simpler cases.
Introduction
One of crucial factors in physicians’ clinical performances is diagnostic reasoning skill (Graber et al. Citation2005). Recent studies suggested that actual processes of diagnostic reasoning can be classified into two processes referred to as the intuitive process (system 1) and analytic process (system 2; Stanovich Citation1999; Evans Citation2008; Croskerry Citation2009). This “dual-processing” model implied that physicians would use both systems interchangeably during their diagnostic reasoning. Intuitive process is fast and unconscious mental simulation matching of a given clinical case to a prior experience based on the use of heuristics. Analytical process is slow and conscious analysis of a case based on the use of probability theory and outer resource like textbooks (Kahneman et al. Citation1982; Norman and Eva Citation2010).
A flaw of diagnostic reasoning could lead to diagnostic errors linked to higher morbidity and mortality in affected patients (Brennan et al. Citation1991; Wilson et al. Citation1995; Thomas et al. Citation2000; Kostopoulou et al. Citation2008; Schiff et al. Citation2009; Tokuda et al. Citation2011). Multiple studies suggested that most flaws of diagnostic reasoning might derive from error of intuitive process and that a variety of measures has been proposed to reduce the failure of intuitive process (Kahneman et al. Citation1982; Graber et al. Citation2005; Gandhi et al. Citation2006). The candidate measures included reflective feedback, cognitive forcing training, electronic differential diagnosis generators, or checklists (Croskerry Citation2000a,Citationb, Citation2003a,Citationb; Mamede & Schmidt Citation2004; Mamede et al. Citation2007a,Citationb, Citation2008a,Citationb, Citation2010; Singh et al. Citation2007; Evans Citation2008; Schiff Citation2008; Graber Citation2009; Ely et al. Citation2011).
Recently, Ely et al. (Citation2011) suggested that, based on the theory of intuitive error, using a general de-biasing checklist (GDBC) and a differential diagnosis checklist (DDXC) may be effective for reducing diagnostic errors, since these may aid physicians as the measures enhancing analytical process. GDBC can be developed to prompt physicians to optimize their cognitive approach by providing a reproducible approach to diagnosis (Appendix 2; Ely et al. Citation2011). DDXC can be developed to help avoid the failure to consider an alternative diagnosis from a comprehensive list for the common complaints (Ely et al. Citation2011). A checklist could highlight the diagnoses that should not be missed and those that are, in fact, commonly missed (Ely et al. Citation2011).
However, to our knowledge, few studies have been conducted for examining the effects of checklists, such as GDBC and DDXC, for enhancing analytic process on diagnostic performance. Thus, in this study, we evaluated the influence of these checklists on diagnostic performance by comparing diagnostic accuracy with intuitive and analytic processes. We also examined the differential effectiveness of the checklists by the relative difficulty of case scenarios.
Methods
We conducted a study of diagnostic quiz cases at several universities in Okinawa, Osaka, Nara, Tokyo, Toyama, and Ibaraki, Japan, from August 2011 to January 2012. Our diagnostic quiz cases included five cases; case 1, acute coronary syndrome; case 2, subarachnoid hemorrhage; case 3, Fitz-Hugh–Curtis syndrome; case 4, aortic dissection; and case 5, obturator hernia (Appendix 1). These case scenarios were developed from actual patients by a group of experienced teaching physicians (three associate professors of Medicine) in Department of Medicine, Toho University School of Medicine, Tokyo, Japan. The cases were arranged based on diagnostic difficulty estimated by the group, being the more difficult cases by the increasing case number (case 1, the least difficult; case 5, the most difficult) based on the consensus of the group of experienced teaching physicians on the contents of scenarios. The case scenario review was conducted and the content validity was confirmed by an independent panel of the university (three professors of Medicine).
Each case scenario was followed by three parentheses, in which participants were required to write down the first most, the second most, and the third most likely diagnoses in order. First, as intuitive diagnosis, participants were asked to write three likely diagnoses by quickly reading the case scenarios within five minutes after reading each case. Next, for group 1 students (n = 91), as diagnosis by GDBC, after receiving and reading GDBC, developed by Ely et al. (Citation2011; Appendix 2), they were also asked to write three likely diagnoses within five minutes for each case. Third, as diagnosis by DDXC, after receiving and reading DDXC, suggested by Ely et al. (Citation2011) and developed by our investigators based on multiple textbooks of differential diagnosis (Appendix 3), they were again asked to write three likely diagnoses within five minutes for each case. The DDXC included marking highlights for “Do not miss diagnosis” and “Frequently missed diagnosis.” Conversely, for group 2 students (n = 97), they were asked to write three likely diagnoses (diagnosis by DDXC) after receiving and reading DDXC and after receiving and reading GDBC (diagnosis by GDBC).
We used existing medical school conferences for implementing the study. These conferences were the regular conferences. All participants read and signed informed consent forms before the study. Participants were assured of confidentiality and anonymity. We allocated medical students of Okinawa, Osaka, and Ibaraki into the group 1 and those of Nara, Tokyo, and Toyama into the group 2. The study was approved by the Institutional Review Board of Mito Kyodo General Hospital, University of Tsukuba, Mito City, Ibaraki, Japan.
We analyzed the mean scores by allocating scores of score 3 for a correct diagnosis as the first most likely diagnosis, score 2 as the second most likely diagnosis, and score 1 as the third most likely diagnosis. We compared the mean total scores between intuitive diagnosis, diagnosis by GDBC and diagnosis by DDXC, using repeated measures of ANOVA adjusted for gender and grade, and the comparisons were performed using Sidak's post hoc analysis. Proportions of correctness of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC were also calculated. All statistical analyses were performed using SPSS-J 17.0 (Tokyo, Japan) and two-tailed p-value of less than 0.05 was considered as statistically significant.
Results
A total of 188 medical students (128 men and 60 women) participated in this study. They included 35 of 4th grade, 77 of 5th grade, and 76 of 6th grade students. shows score distribution of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC by groups 1 and 2.
Table 1 Score distribution of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC (n = 188)
reveals mean score of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC by the group 1. Among cases 3–5 (hard cases), in which mean baseline scores were relatively lower than those of cases 1 and 2 (easy cases), the mean scores of diagnosis by DDXC were higher than those of intuitive diagnosis and diagnosis by GDBC. However, among the easy cases, the mean scores of diagnosis by DDXC were lower than those of intuitive diagnosis and diagnosis by GDBC. Repeated measures of ANOVA indicated significant difference of the total scores among intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC (p = 0.003). By Sidak's post hoc analysis, the total scores between intuitive diagnosis and diagnosis by GDBC were not significantly different (p = 0.218), while total scores between intuitive diagnosis and diagnosis by DDXC were significantly different (p = 0.01).
Table 2 Mean scores of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC (n = 188)
also reveals the mean score of intuitive diagnosis, diagnosis by DDXC, and diagnosis by GDBC by the group 2. Among cases 3–5 (hard cases), in which mean baseline scores were relatively lower than those of cases 1 and 2 (easy cases), the mean scores of diagnosis by DDXC were higher than those of intuitive diagnosis. However, among the easy cases, the mean scores of diagnosis by DDXC were lower than those of intuitive diagnosis. Repeated measures of ANOVA indicated significant difference of total score among intuitive diagnosis, diagnosis by DDXC, and diagnosis by GDBC (p = 0.001). By Sidak's post hoc analysis, total scores between intuitive diagnosis and diagnosis by DDXC were significantly different (p = 0.001), while total scores between diagnosis by DDXC and GDBC were not significantly different (p = 0.25).
shows proportions of correct diagnosis of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC by the group 1. Among cases 3–5, in which the proportions were relatively lower than those of cases 1 and 2, the proportions of correct diagnosis by DDXC were greater than those of intuitive diagnosis and diagnosis by GDBC. However, among cases 1 and 2 with relatively greater proportions of correct diagnosis, the proportions of correct diagnosis by DDXC were lower than those of intuitive diagnosis and diagnosis by GDBC. For all cases combined, 60% of correct diagnosis was observed for intuitive diagnosis, 62% for diagnosis by GDBC, and 67% for diagnosis by DDXC.
Figure 1. Proportions of correct diagnosis of intuitive diagnosis, diagnosis by GDBC, and diagnosis by DDXC by the groups 1 and 2. Notes: The horizontal axis indicates the proportions of correct diagnosis. GDBC, general de-biasing checklist and DDXC, differential diagnosis checklist.
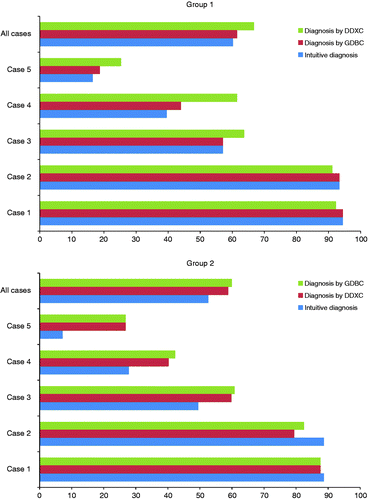
also shows proportions of correct diagnosis of intuitive diagnosis, diagnosis by DDXC, and diagnosis by GDBC by the group 2. Among cases 3–5, in which the proportions of correct diagnosis were relatively lower than those of cases 1 and 2, the proportions of diagnosis by DDXC were greater than those of intuitive diagnosis, and only minimum difference of the proportions was noted between diagnosis by DDXC and GDBC. However, among cases 1 and 2 with relatively greater proportions of correct diagnosis, the proportions of diagnosis by DDXC were actually lower than those of intuitive diagnosis, and only minimum difference of the proportions was noted between diagnosis by DDXC and GDBC. For all cases combined, 53% of correct diagnosis was observed for intuitive diagnosis, 59% for diagnosis by DDXC, and 60% for diagnosis by GDBC.
Discussion
Our results using case scenarios for medical students suggested that the use of DDXCs improved diagnostic performance, but that the use of a GDBC did not. Although total scores in all cases combined were significantly greater in reading a DDXC than without it, there was difference in the effects of a DDXC on diagnostic performance between difficult and simple cases. The use of a DDXC led to better diagnostic performance among difficult cases. However, it might lead to lower performance among simple cases.
To our knowledge, this is the first study to show that DDXCs worked effectively for improving diagnostic performance among difficult cases. Our results also suggested that GDBC did not work well as a tool for better diagnostic performance. There are several reasons that could explain these results. First, each item of the de-biasing checklist was not designed to hint any specific differential diagnosis, while it provided overall advice to make differential diagnosis. Hence this checklist might work little in each specific clinical scenario. Second, because the participants of this study were all medical students, who were novice and had little clinical experience, the use of GDBC might not have contributed to the baseline performance level without heuristic thinking among students.
Based on the different levels of difficulty among case scenarios, there was a trend suggesting that the proportions of correct diagnosis after reading DDXCs were lower in simpler cases. According to the dual processing theory, analytical process including a checklist, works better for difficult cases and intuitive process may be more effective for solving easy cases (Klein Citation2004). Thus, the DDXC, which is involved with the analytical process, displays its effectiveness for difficult cases rather than for easy cases. In contrast, there may be an advantage for intuitive process for easy cases in terms of its swiftness and efficiency. It is important to reappraise the importance of our intuition for diagnosis of common conditions (Kahneman Citation2011).
In contrast, a checklist of differential diagnosis worked effectively for making accurate diagnosis for difficult cases. The one reason for this effectiveness might be that they included the marking highlights on “Do not miss diagnosis” and “Frequently missed diagnosis.” Although the checklist may work effectively, in reality, it seems difficult to use checklists for every single patient in hectic clinical environments, such as emergency rooms or walk-in clinics (Klein Citation2004). The use of a DDXC may be effectively implemented for facing patients with difficult conditions.
In order to generalize the usefulness for a differential diagnosis, further studies are needed to evaluate the usefulness of the checklists in actual clinical settings and to examine the effects by the use of checklists on real clinical outcomes. It may be needed to evaluate a number of cases that involve a broad spectrum of disease, ages, and ethnicities. In addition, further studies are also needed to examine the performance among physicians, including residents and staff physicians.
In conclusion, among medical students, the use of DDXCs improved diagnostic performance in difficult cases but it might lead to lower performance in simple cases. GDBC did not help medical students for better diagnostic performance. Our results seem to be consistent with the dual processing theory implying that analytical process works better for difficult cases and intuitive process may be more effective for solving easy cases.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, Newhouse JP, Weiler PC, Hiatt HH. Incidence of adverse events and negligence in hospitalized patients: Results of the Harvard Medical Practice Study I. N Engl J Med 1991; 324(6)370–376
- Croskerry P. The cognitive imperative: Thinking about how we think. Acad Emerg Med 2000a; 7(11)1223–1231
- Croskerry P. The feedback sanction. Acad Emerg Med 2000b; 7(11)1232–1238
- Croskerry P. Cognitive forcing strategies in clinical decisionmaking. Ann Emerg Med 2003a; 41(1)110–120
- Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med 2003b; 78(8)775–780
- Croskerry P. A universal model of diagnostic reasoning. Acad Med 2009; 84(8)1022–1028
- Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med 2011; 86(3)307–313
- Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol 2008; 59: 255–278
- Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, Studdert DM. Missed and delayed diagnoses in the ambulatory setting: A study of closed malpractice claims. Ann Intern Med 2006; 145(7)488–496
- Graber ML. Educational strategies to reduce diagnostic error: Can you teach this stuff?. Adv Health Sci Educ Theory Pract 2009; 14(Suppl. 1)63–69
- Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med 2005; 165(13)1493–1499
- Kahneman D. Thinking, fast and slow. Farrar Straus & Giroux, New York 2011
- Kahneman D, Slovic P, Tversky A. Judgment under uncertainty: Heuristics and biases. Cambridge University Press, Cambridge 1982
- Klein GA. The power of intuition: How to use your gut feelings to make better decisions at work. Crown Business, New York 2004
- Kostopoulou O, Delaney BC, Munro CW. Diagnostic difficulty and error in primary care: A systematic review. Fam Pract 2008; 25(6)400–413
- Mamede S, Schmidt HG. The structure of reflective practice in medicine. Med Educ 2004; 38(12)1302–1308
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ 2008a; 42(5)468–475
- Mamede S, Schmidt HG, Rikers R. Diagnostic errors and reflective practice in medicine. J Eval Clin Pract 2007a; 13(1)138–145
- Mamede S, Schmidt HG, Rikers RM, Penaforte JC, Coelho-Filho JM. Breaking down automaticity: Case ambiguity and the shift to reflective approaches in clinical reasoning. Med Educ 2007b; 41(12)1185–1192
- Mamede S, Schmidt HG, Rikers RM, Penaforte JC, Coelho-Filho JM. Influence of perceived difficulty of cases on physicians' diagnostic reasoning. Acad Med 2008b; 83(12)1210–1216
- Mamede S, van Gog T, van den Berge K, Rikers RM, van Saase JL, van Guldener C, Schmidt HG. Effect of availability bias and reflective reasoning on diagnostic accuracy among internal medicine residents. JAMA 2010; 304(11)1198–1203
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ 2010; 44(1)94–100
- Schiff GD. Minimizing diagnostic error: The importance of follow-up and feedback. Am J Med 2008; 121(5 Suppl)S38–S42
- Schiff GD, Hasan O, Kim S, Abrams R, Cosby K, Lambert BL, Elstein AS, Hasler S, Kabongo ML, Krosnjar N, et al. Diagnostic error in medicine: Analysis of 583 physician-reported errors. Arch Intern Med 2009; 169(20)1881–1887
- Singh H, Thomas EJ, Khan MM, Petersen LA. Identifying diagnostic errors in primary care using an electronic screening algorithm. Arch Intern Med 2007; 167(3)302–308
- Stanovich KE. Who is rational?: Studies of individual differences in reasoning. Lawrence Erlbaum, Mahwah, NJ 1999
- Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Zeena T, Williams EJ, Howard KM, Weiler PC, Brennan TA. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care 2000; 38(3)261–271
- Tokuda Y, Kishida N, Konishi R, Koizumi S. Cognitive error as the most frequent contributory factor in cases of medical injury: A study on verdict's judgment among closed claims in Japan. J Hosp Med 2011; 6(3)109–114
- Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The quality in Australian health care study. Med J Aust 1995; 163(9)458–471
Appendix 1
Case 1
A 63-year-old male with a two-month history of “epigastralgia.”
Patient data ➀ (History)
History of the present illness (HPI): The patient, a 63-year-old male, was found to have diabetes about five years ago. The patient modified his diet but otherwise he had not come seen to his primary care physician for regular checkups. For the latest two months, the patient had experienced a dull pain in his epigastrium after meals. The patient had just observed his condition. The previous day, he experienced an epigastric pain for about 10 minutes while on his way to work in the morning. The pain was not associated with his meal. On the day of the visit to the hospital, the patient suddenly experienced more intense pain than usual in the epigastrium while a conference at his work. He also felt like fainting. The pain finally subsided in about 30 minutes, but the patient became concerned and came to this hospital on foot.
Past medical history (PMH): Diabetes.
Social history (SH): The patient did not drink but smoked one pack a day.
Patient data ➁ (Interview 1)
On his epigastric pain
O: Sudden onset.
P: No aggravating factor. Lasted for 30 minutes, subsided spontaneously by rest.
Q: Denies diaphoresis, but had severe pain. This was more severe pain than he had ever had.
R: Located at epigastrium. No pain or
S: Felt like fainting. No loss of consciousness, nausea, and vomiting. Accompanied palpitation.
T: Sometimes had experienced postprandial epigastric discomfort lasting for one hour since two months before.
Experienced an epigastric pain for 10 minutes when he was walking in the morning the previous day.
Patient data ➂ (Interview 2)
He had experience several episodes of epigastralgias even in walking for the last week. He reported that those pain were more severe than previous postprandial pains seen in the last two months.
On the day of the presentation, he experienced an epigastric pain when he was sitting during the meeting. He also had palpitation and fainting sensation. He was not standing up, but sitting still at the moment he felt fainting.
There was no loss of consciousness. No nausea and vomiting.
Patient data ➃ (Physical findings)
Vital signs: BP 162/98 mmHg, Pulse 96 beats/min regular, BT 36.8°C, RR 18/min.
Physical findings: Alert and oriented; no anemia; no icterus; no abnormalities in his mouth and pharynx; no thyroid enlargement; no juglar venous distention. Heart: No rub/murmur/gallop. Lung: Clear to auscultation bilaterally. Abdomen: Soft and flat, no tenderness; no edema on extremities.
No bilateral difference in his blood pressure. No hypotension in the legs. Tilt test negative.
Case 2
A 56-year-old female with two-day history of “headache.”
Patient data ➀ (History)
HPI: The patient, a 56-year-old female, had not initially suffered from headaches. Two days before the visit to our hospital, the patient developed headaches and nausea while watching soccer live on TV and was seen by a nearby physician. Her physical findings and his head CT were normal. The patient was diagnosed with an upper respiratory tract infection and was sent to his home with common cold medications. She took the medicine and rested at home, but still had headaches and came to this hospital on foot. She was alert and oriented.
PMH: The patient has been treated by a nearby physician for hypertension since her 50s.
SH: The family history included a father who died of a subarachnoid hemorrhage.
Patient data ➁ (Interview)
Two days prior to his visit, the patient suddenly suffered headaches unlike any she had ever had before while watching the team she supported lost a soccer match live on TV. She felt as if someone was striking on her head with a hammer.
The patient initially felt like “Were these headaches caused by psychological shock?” She also developed nausea and become concerned, then she visited the medical facility. However, the subsequent examination and a head CT were both normal. Having been told that she might have a cold, the patient was sent to his home with an analgesic medication. Despite he took the medication for two days, her headache still remained.
Patient data ➂ (Physical findings and Laboratory data)
Vital signs: JCS-0, GCS (E4, V5, M6), BP 156/82 mmHg, Pulse 95 beats/min, BT 36.4°C.
Physical findings: HEENT (head, eyes, ears, nose, throat). Chest, Abdomen within normal limit; motor impairment is unremarkable in all extremities; no aphasia and dysarthria, cerebellar sign; no nuchal rigidity and Kernig sign; no great occipital tenderness; no eye pain; pupil are 2 mm bilaterally, prompt to light reflex.
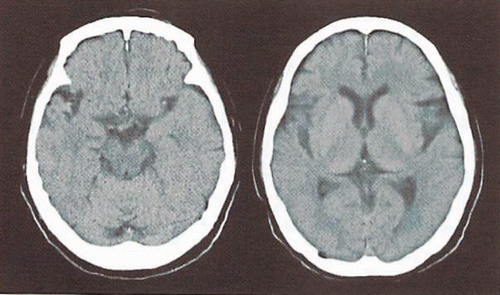
Chest X-ray: No apparent abnormalities. EKG: No apparent abnormalities; Head CT ().
Case 3
A 32-year-old female with “right upper quadrant abdominal pain.”
Patient data ➀ (History)
HPI: The patient, a 32-year-old female, developed abdominal pain in her left lower quadrant starting two weeks ago followed by increased vaginal discharge. Four days later, the patient went seen by a nearby obstetrician/gynecologist but nothing was appreciated. Five days before, the patient developed a piercing pain from the right hypochondriac region to the right flank. The patient had loose stool but had no nausea or vomiting. Pain did not change with food intake, but pain aggravated during deep breathing and movement. Two days ago, the patient also developed a fever of 38°C. Pain in the right upper quadrant increased and she called ambulance and was brought to this hospital. The patient drank occasionally and smoked a pack a day.
PMH: No remarkable history.
SH: The patient's mother had hypertension and cholelithiasis; the patient had no known food or drug allergies, had not traveled abroad, and had no pets.
Patient data ➁ (Physical findings)
Vital signs: BP 126/80 mmHg, Pulse 80 beats/min regular, BT 37.8°C, RR 16/min.
Physical findings: No anemia, no icterus; no abnormalities in his neck. Heart: No rub/murmur/gallop. Lung: clear to auscultation bilaterally. Abdomen: soft and flat, no tenderness, tenderness from the right hypochondriac region to the right flank, but no peritoneal sign, liver percussion tenderness was remarkable, right costovertebral angle (CVA) tenderness positive with radiation to the right flank, Murphy sign positive; no edema on extremities.
Patient data ➂ (Laboratory data)
Blood test: WBC 9800/µL, RBC 410 × 104/µL, Hb 13.5 g/dL, Ht 39.9%, Plt 37.8 × 104/µL, T-P 7.9 g/dL, Alb 3.9 g/dL, AST 24 IU/L, ALT 31 IU/L, LDH 303, ALP 233 IU/L, GGT 14 IU/L, Amy 28 IU/L, CK 44 IU/L, LDL-C 188 mg/dL, TG 69 mg/dL, UN 7 mg/dL, Cr 0.63 mg/dL, BS 92 mg/dL, Na 140 mEq/L, K 4.0 mEq/L, Cl 103 mEq/L, CRP 11.0 mg/dL.
Urinalysis: Gravity 1.015, pH 6.0, protein±, glucose negative, blood±, acetone negative, bilirubin negative, urobilinogen±, RBC 3∼5/F, WBC 3∼5/F.
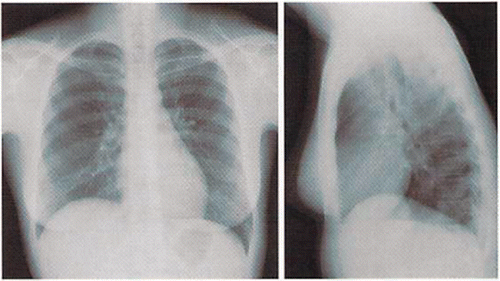
Chest-X ray: No abnormality in both lungs, no pleural effusion. No free air below the diaphragm ().
Abdominal X-ray: No remarkable abnormality in colon including dilatation or niveau formation. Clear bilateral abdominal wall muscle, no evidence of cholelithiasis, appendix stone, or urolithiasis.
Patient data ➃ (Laboratory data)
Abdominal ultrasonography
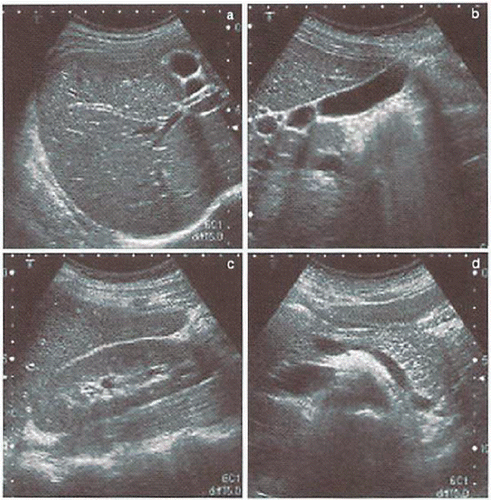
No abnormality in the liver, no stone and wall thickness in the gallbladder. No remarkable abnormalities in pancreas, spleen, and both kidneys ().
Case 4
A 40-year-old male with “lower back pain.”
Patient data ➀ (History)
HPI: The patient, a 40-year-old male, was originally healthy. At about 5:00 PM on the day during his work, the patient developed lower back pain. Pain was located in the center and on the left of the lower back. The pain was a dull pain. Around 6:00 PM, he began feeling discomfort in his lower abdomen; the patient required an ambulance and arrived at this hospital at 7:00 PM.
PMH: Six months before, the patient had lower back pain and was treated by a nearby orthopedic surgeon with oral medication.
SH: Nothing appreciated in the patient's family history. He did not drink but smoked one pack a day for 20 years.
Patient data ➁ (Interview)
The patient's chief complaint was lower back pain. He had previously undergone health checkups done at his workplace, but these examinations revealed no serious medical conditions such as hypertension or diabetes. Six months before, the patient had lower back pain and was seen by a nearby orthopedic surgeon. At that time, the pain was alleviated by oral medication for about a month. At about 5:00 PM on the day in question, the patient suddenly developed lower back pain while working. The patient had dull pain in the center and on the left of the lower back. Numbness in the lower extremities was not noted. There was no urination following pain. There was no cold sweating, but pain increased over time. As of about 6:00 PM, the patient began feeling discomfort in his lower abdomen; the patient called an ambulance and arrived at this hospital at 7:00 PM.
Patient data ➂ (Physical findings)
Vital signs: BP 158/106 mmHg, Pulse 90 beats/min regular, BT 36.8°C, RR 16/min.
Physical findings: Heart: No rub/murmur/gallop. Lung: Clear to auscultation bilaterally. Abdomen: Soft and flat, mild tenderness at bilateral lower quadrants; left CVA tenderness existed; no lower leg edema.
Patient data ➃ (Laboratory data)
Blood test: WBC 9600/µL, RBC 456 × 104/µL, Hb 14.7 g/dL, Ht 43.7%, CRP 1.2 mg/dL, Na 138 mEq/L, K 3.9 mEq/L, Cl 96 mEq/L, UN 21 mg/dL, Cr 0.92 mg/dL, AST 41 IU/L, ALT 52 IU/L, LDH 198 IU/L, CK 87 IU/L, LDL-C 165 mg/dL, BS 154 mg/dL.
Urinalysis: Glucose negative; protein negative, blood±.
Case 5
A 94-year-old female with one week history of “abdominal distention.”
Patient data ➀ (History)
HPI: A 94-year-old female experienced abdominal distention starting one week prior to the visit. She had not passed gas or stool for five days. She also denied abdominal pain but admitted that she began vomiting up small bits of food residue five days ago. Since that day, vomiting became more frequent and finally she visited a doctor. She was referred to this hospital for further testing and treatment, transported here by ambulance. The patient denied loose stool or blood in the stool.
PMH: The patient was diagnosed with colon cancer 29 years earlier and underwent surgery (details such as the stage of cancer were not known). Abnormal findings on plain chest films were noted several years ago but she did not do further investigation that time. She had been constipated for a long time. She had given birth three times. She has no known drug allergies.
Review of systems: The patient was found to have no subjective symptoms related to ENT, eyes, skin neuropsychiatric status, reproductive, metabolic, and central nervous system.
Patient data ➁ (Interview)
She had not had raw or heavy food prior to the abdominal symptom. Her defecation was two to three times a day until his bowel movement stopped. Her stool was not too hard in general. She denied any spontaneous pain in her abdomen.
Patient data ➂ (Physical findings)
Stature 145 cm, Body weight 40 kg.
Vital signs: BP 186/108 mmHg, Pulse 86 beats/min regular, BT 36.7°C, RR 18/min, SpO2 96%(ambient air).
Physical findings: Alert and oriented, no anemia, no icterus. Dryness of oral mucosa, no thyroid enlargement, no apparent jugular venous distention, no lymph node was palpated in her neck, axillae, or inguinal region. Heart: No rub/murmur/gallop. Lung: Almost clear to auscultation bilaterally, with decreased breath sounds. Abdomen: Soft and distended, no tenderness, no bowel sound heard, tenderness at left medial thigh when flexed or extended of the left hip, no edema on extremities.
Patient data ➃ (Laboratory data)
Blood test showed evidences of mild inflammation: WBC 8300/µL, CRP 2.7 mg/dL.
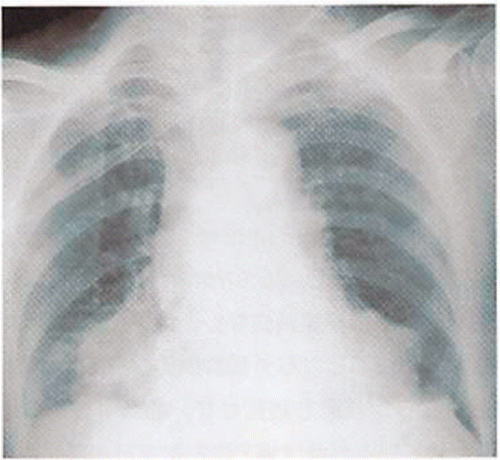
Chest X-ray: Abnormal shadow in the right lower lobe ().
Abdominal X-ray (supine): Dilatation of the small intestine, no dilatation of the colon (a collection of contrast media, which was injected at the former physician, from bilateral ureters to the bladder).
Abdominal CT: Extensive dilatation of the small intestine ().
Appendix 2
Appendix 3
List 2
Differential Diagnosis list (frequency order)
Q1: Epigastralgia 
Gastritis
Costochondritis
Intercostal neuralgia
Gastroesophageal reflux disease
Somatization disorder
Premature ventricular contraction
Constipation
Peptic ulcer disease
Esophageal hiatal hernia
Acute coronary syndrome•☆
Upper gastrointestinal perforation•
Appendicitis•☆
Pulmonary thromboembolism•
Gastric neoplasm•
Pancreatitis•
Pericarditis•☆
Myocarditis•☆
Cardiac tamponade•
Aortic dissection•☆
Rupture of abdominal aortic aneurysm•
Gastric spasm☆
Pancreatic tumor
Esophageal foreign body
Hypertrophic obstructive cardiomyopathy•
Gastric anisakidosis•☆
Diabetic ketoacidosis•☆
Adrenal insufficiency•☆
Rectus abdominis hematoma•
Superior mesenteric artery thrombosis•
Superior mesenteric artery syndrome☆
Esophageal spasm☆
Esophageal rupture•
Esophageal achalasia
Familial Mediterranean fever☆
Q2: Headache 
Migraine
Tension type headache
Exertional headache
Upper respiratory infection
Heat illness
Dental caries☆
Cervical spondylosis
Head trauma
Temporomandibular arthrosis☆
Sinusitis☆
Meningitis•
Encephalitis•
Subdural hematoma•☆
Epidural hematoma•
Systemic infection•☆
Subarachnoid hemorrhage•☆
Acute angle closure graucoma•☆
Pituitary apoplexy•☆
Systemic lupus erythematosis•
Great occipital neuralgia
Brain tumor•
Brain abscess•
CO intoxication
Cluster headache
Carotid-cavernous fistura
Cerebral venous thrombosis☆
Giant cell arteritis•☆
Medication overuse headache
Trigeminal neuralgia
Pheochromocytoma☆
Somatization disorder
Pseudotumor cerebri☆
Low cerebrospinal pressure
Tolosa Hunt syndrome
Calcific tendinitis of longus coli☆
Q3: Right upper quadrant abdominal pain 
Cholelithiasis
Constipation
Rib fracture
Peptic ulcer disease
Trauma•
Cholecystitis•
Infective colitis
Hepatitis
Urolithiasis
Pyelonephritis
Pneumonia
Pleuritis
Fitz-Hugh–Curtis syndrome•☆
Pancreatitis•
Cholangitis•
Budd–Chiari syndrome☆
Empyema
Rupture of ectopic pregnancy•☆
Subphrenic abscess•☆
Renal infarction•☆
Perirenal abscess•☆
Inflammatory bowel disease☆
Superior mesenteric artery thrombosis•☆
Pulmonary thromboembolism•☆
Malaria•☆
Diverticulosis☆
Colon tumor
Small intestine neoplasm☆
Hepatic tumor
Endometriosis☆
Echinococcosis•☆
Q4: Low back pain 
Nonspecific back pain
Osteoporotic compression fracture
Rib fracture☆
Urolithiasis
Renal infarction•☆
Pancreatitis•☆
Pyelonephritis
Trauma
Cholelithiasis
Peptic ulcer disease
Herniated disk
Iliopsoas muscle abscess•☆
Rupture of abdominal aortic aneurysm•☆
Aortic dissection•☆
Reactive arthritis☆
Perirenal abscess
Spinal epidural abscess•
Septic discitis•
Spinal abscess•
Spondylitis•
Osteomyelitis•☆
Spinal epidural abscess•☆
Spondylolisthesis
Prostate carcinoma•☆
Varicella zoster infection
Spinal infarction•☆
Multiple myeloma•☆
Pancreatic carcinoma
Pancreatic pseudocyst
Paget's disease
Retroperitoneal fibrosis☆
Urinary diversion
Horseshoe kidney
Wandering kidney
Q5: Abdominal distention 
Constipation
Obesity
Intestinal gas
Aerophagia
Urinary retention•☆
Gastrointestinal perforation•☆
Hypothyroidism☆
Obturator hernia•☆
Femoral hernia•☆
Postoperative adhesive intestinal ileus
Strangulation of the intestine•☆
Abdominal incisional hernia☆
Hypokalemia
Hepatic encephalopathy
Right heart failure•☆
Giant abdominal aortic aneurysm•☆
Peritonitis carcinomatosa•
Surgical pneumoperitoneum
Gastric atony☆
Ogilvie's syndrome☆
Ovarian carcinoma•
Ovarian hemorrhage
Uremia•
Hepatocellular carcinoma•
Pancreatitis•☆
Endometrial carcinoma•
Cervical carcinoma•
Superior mesenteric artery thrombosis•☆
Superior mesenteric artery syndrome☆
Giant fibroid of the uterus
Mitral stenosis•☆
Budd–Chiari syndrome•☆
Emphysematous pyelonephritis•☆
Emphysematous cholecystitis•☆
Disseminated strongyloidiasis•