Abstract
Hypertension and hypothyreoidism are frequent side effects of VEGFR-inhibitors. We investigated whether hypertension or hypothyreoidism diagnosed during sunitinib treatment is associated with treatment efficacy. Material and methods. Sixty-four consecutive patients with metastatic renal cell cancer (RCC) were treated with sunitinib in a single center. Hypertension was defined as persistent blood pressure >150/100 mmHg or blood pressure requiring intensification of pre-existing anti-hypertensive medication. Hypothyreoidism was defined as elevation of TSH levels and clinical symptoms requiring hormone replacement therapy (≥Gr. II hypothyreoidim). Results. Twenty-four (38%) patients developed hypertension and 12 (19%) hypothyreoidism. The dose of sunitinib administered was not significantly associated with hypertension or hypothyreoidism. There was no correlation between hypertension and hypothyreoidism. Hypertension was associated with frequent tumor response to sunitinib, a long time to disease progression and long overall survival (p= 0.001, 0.0003 and 0.001, respectively). In a multivariate analysis, hypertension was an independent predictor of progression-free survival (hazard ratio, 0.21; 95% CI 0.076 to 0.59, p=0.0030). There were no statistically significant differences in the frequency of ≥ grade 3 adverse events between patients with or without hypertension. Conclusion. Sunitinib-associated hypertension may be a strong predictive marker for treatment efficacy in metastatic RCC.
Few predictive factors for efficacy of vascular endothelial growth factor (VEGF) pathway-targeting therapies have been identified. Hypertension is a common side effect associated with VEGFR inhibitors, and it has been proposed as a predictor of antitumor efficacy in metastatic renal cell carcinoma (RCC) [Citation1–5].
Sunitinib is an inhibitor of several tyrosine kinase receptors including VEGFR, PDGFR, c-KIT, and RET. The pathogenesis of sunitinib-related hypertension is likely multifactorial. Antagonism of VEGF decreases bioavailability of nitric oxide, a potent vasodilator, leading to constriction of vasculature and reduction in renal excretion of sodium ions, which may lead to an increase of the blood pressure. Anti-angiogenic therapy-related hypertension may also be caused by vascular rarefaction resulting from the reduced endothelial function [Citation6].
There are currently paucity of data available regarding hypertension that develops during sunitinib treatment, treatment response and survival. The available data, which is mostly retrospective, suggest that patients with RCC who develop hypertension during sunitinib might benefit more from treatment than those who do not develop hypertension [Citation1,Citation3,Citation5]. Since it is important to confirm these findings, we analyzed a series of consecutive patients diagnosed with advanced RCC and treated with sunitinib monotherapy for the presence treatment-associated hypertension and outcome.
Material and methods
Patients and treatment
Sixty-four consecutive patients diagnosed with progressive metastatic RCC and treated with single-agent sunitinib at the Department of Oncology, Helsinki University Central Hospital, Helsinki, Finland, within the time period from October 2006 to December 2008 were identified from the hospital registry and included in the study. Sunitinib was administered either 50 mg four weeks on / two weeks off (n= 21; 33%), 37.5 mg (n = 27; 42%), or 25 mg (n=16; 25%) continuously [Citation7]. None of the patients had received prior sorafenib, eight patients (13%) had received prior bevacizumab and 31 (48%) prior cytokine therapy.
Data collected from the hospital case records included patient demographic features, treatments given, adverse events, hospitalizations and outcome data. Data cut-off date was set at June 1, 2009 to provide a median follow-up time of longer than 10.2 months at the time of data analysis. This exploratory study was approved by an Institutional Review Board.
Assessment or response and adverse effects
Response to treatment was assessed by physical examination and computed tomography (or MRI) every 12 weeks (two cycles). All imaging data were evaluated by radiologists with experience in tumor response evaluation, which was assessed according to the RECIST criteria [Citation8]. Responses were confirmed after two cycles of further treatment with sunitinib. Adverse effects were collected from hospital records, and reported according to common terminology criteria for adverse events (CTCAE) version 3.0.
Assessment of blood pressure and thyroid function
Blood pressure was measured on days 1 and 28, and day 1 of each sunitinib cycle, respectively. We categorized patients according to their blood pressure into two groups, Group A and Group B. Group A consisted either of patients whose blood pressure was higher than 150/100 mmHg and who started medication for hypertension during sunitinib treatment or of patients who had one or more antihypertensive drugs before initiation of sunitinib treatment and who needed intensification of antihypertensive therapy during therapy (either dose increase or addition of a new antihypertensive agent). The rest of the patients, who had blood pressure 150/100 mmHg or less throughout sunitinib therapy formed Group B.
Thyroid function was assessed at six week intervals by measuring serum TSH and T4 levels. Management of thyroid dysfunction was initiated whenever patient was symptomatic and measured TSH was above the upper limit of normal (grade II hypothyroidism or greater).
Statistical analyses
Frequency tables were analyzed with the χ2 test. Time to progression was calculated from the date of sunitinib initiation to the date of documented cancer progression or to death, whenever death preceded cancer progression. Patients without disease progression were censored at the time of last follow-up. Overall survival was calculated from the start of sunitinib therapy to the date of death from any cause. Life-tables were computed according to the Kaplan-Meier method. Survival curves were compared with the log-rank test. The influence of covariables on time to disease progression was assessed using a Cox's proportional hazard model. Besides hypertension, the following covariables entered into the model: pre-treatment blood haemoglobin level (tested normal vs. low), pre-treatment serum calcium level (normal vs. elevated), the World Health Organization (WHO) performance status (0 vs. ≥1) and the time from the initial diagnosis to the onset of metastatic disease (≤12 months vs. >12 months). All p-values are two-sided.
Results
Patient population and treatment administered
The characteristics of the 64 study participants are presented in . The median age was 64 (range, 44 to 81) and the median follow-up time of the patients alive was 11.7 months (range, 2.1–29.7). Most (n=59, 92%) of the patients had RCC with clear cell histology, and all but six patients had undergone nephrectomy prior to initiation of sunitinib treatment.
Table I. Patient characteristics.
The median duration of sunitinib treatment was 6.2 months (range, 0.3 to 29.7), time to progression 10.2 months (95% CI, 5.1 to 18.0), and overall survival 17.4 months (95% CI, 11.6 to NA). Nineteen (29%) patients had partial response (PR), 37 (58%) stabilized disease (SD) and eight (13%) progressive disease (PD) as their best response. Eighteen (28%) patients discontinued sunitinib treatment due to an adverse event without confirmation of disease progression. There were no treatment-related deaths.
Effect of hypertension on outcome
A total of 24 (38%) patients were diagnosed with persistent hypertension >150/100 mmHg or required intensification of medication due to worsening of hypertension (Group A), whereas the rest of the patients (Group B, n= 40, 62%) had blood pressure <150/100 mmHg during sunitinib treatment and required neither initiation nor intensification of antihypertensive medication. There were no statistically significant differences in the frequency of the most important prognostic factors of RCC between Groups A and B (prior nephrectomy, pre-treatment blood haemoglobin and serum calcium levels, the WHO performance status, the time from the initial diagnosis to the diagnosis of metastatic disease, or the number of organ sites affected; p>0.05 for all comparisons). Treatment-related development of hypertension was not either associated with age.
The mean blood pressure at the time of initiation of hypertension medication was 157.0/100.6 mmHg (). The median time to initiation of antihypertensive treatment or medication intensification was 28 days as calculated from the start of sunitinib treatment (range, 10 to 80 days). There was no correlation between the sunitinib dose and the frequency of hypertension, since 38% of the patients treated with a daily dose of 50 mg and 37% of those treated with less than 50 mg per day required initiation or intensification of antihypertensive medication (p=0.66). There were no statistically significant differences in the frequency of grade 3 adverse events between Groups A and B (including haemorrhage, proteinuria, cerebrovascular adverse events; p>0.05 for all comparisons).
Table II. Systolic and diastolic blood pressure among patients requiring treatment or sunitinib-related hypertension.
Table III. Cox regression analysis of progression-free survival.
The median duration of sunitinib treatment was 8.5 months in Group A and 4.1 months in Group B (p =0.0023). Twelve (50%) patients had PR as best response in Group A, whereas in Group B only five (13%) patients had PR as the best response (p=0.0010). Furthermore, significantly more patients in Group B (n=27) than in Group A (n=2) had PD as the best response (p<0.0001).
Development of hypertension was also associated with longer time to disease progression and overall survival as compared with no hypertension (log-rank test; p=0.0003 and p= 0.0001, respectively). The median time to disease progression was not reached in Group A versus 4.7 months in Group B (95% CI, 3.3 to 6.5; ). Similarly, overall survival was not reached in Group A and 9.5 months in Group B ().
Figure 1. Time to progression (Panel A) and overall survival (B) by hypertension treatment during sunitinib therapy.
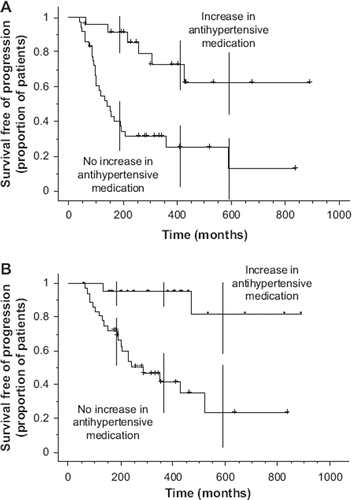
Twelve patients (19%) experienced symptoms of hypothyroidism and had elevated TSH levels during sunitinib treatment. The median time to the onset of hypothyroidism was 6.2 months (range, 1.8 to 12.5 months). The median TSH level at the time of thyroid hormone replacement therapy was 10.2 mU/l (range, 4.97 to 40.5 mU/l). There was no correlation between sunitinib dose and diagnosis of hypothyroidism (p =0.51). Similarly, there was no statistically significant correlation between patients requiring initiation or intensification of hypertension medication and patients who developed Gr. II or more severe hypothyroidism (p= 0.42). Interestingly, among patients with treatment-related hypothyroidism requiring thyroid hormone replacement therapy, six (50%) patients had PR as their best response and only one patient (8%) had PD as the best response. Since patients who responded to sunitinib had more time to develop hypothyroidism than those who progressed and hypothyroidism developed relatively late, a comparison of such groups in a Kaplan-Mayer analysis was not considered feasible.
We performed a Cox multivariate analysis to find out whether development or worsening of hypertension was an independent predictive factor for treatment efficacy using the progression-free survival as an endpoint. In this analysis, sunitinib-treatment related hypertension was an independent predictive factor for progression-free survival (hazard ratio, 0.21; 95% CI 0.076 to 0.59; p=0.0030).
Discussion
In line with earlier findings [Citation1,Citation3,Citation5], the present results suggest that development or worsening of hypertension is a predictive factor for response to sunitinib and favorable outcome in metastatic RCC. We found a surprisingly strong association (), and hypertension was an independent predictive factor for progression-free survival in a multivariate survival analysis. Although the present series was retrospective in nature, it was based on consecutive patients. To our knowledge, the present series is the first one to address the association between hypertension and hypothyreosis among patients treated with an antiangiogenic agent. We found no association between hypertension and hypothyreosis, but found the time of onset of hypertension and hypothyreosis to be different. Since hypothyreosis was usually diagnosed relatively late among patients being treated with sunitinib, it is likely not a useful predictive factor for response, whereas hypertension usually occurs early and may, therefore, be used as a potential tool to identify patients who are most likely to benefit from sunitinib treatment. However, it is noteworthy that in our analysis, about 25% of the patients with no development or worsening of hypertension, had ≥ 12 month TTP which is longer than the median TTP of metastatic RCC patients being treated with sunitinib.
Despite intriguing recent results that IL-8 has an important role in the resistance of RCC to sunitinib [Citation9], thus far no predictive biomarkers that reliably predict patient outcome during VEGF-signaling inhibiting antiangiogenic agent therapy have been identified despite intensive research. The most extensively studied candidates include tumor expression of VEGF and VEGFR, the phosphorylation status of VEGFR, serum levels of soluble VEGF receptors 1 to 3, serum circulating endothelial cells and their precursors, and genetic polymorphisms of VEGF and VEGFR-2. Jubb et al. evaluated also tumor expression of VEGF-A, thrombospondin-2 and tumor mean vessel density, but found none of these factors to be associated with the clinical benefit among cancer patients treated with chemotherapy with or without bevacizumab [Citation10]. In another study mutations of k-ras, b-raf and TP53 were not associated with survival in a patient population treated with chemotherapy with or without bevacizumab [Citation11].
A recent report found that hypertension during antiangiogenic treatment is associated with certain VEGF single nucleotide polymorphisms, suggesting that such polymorphisms might serve as predictive factors for efficacy of antiangiogenic treatment [Citation12]. Yet, these provocative findings need to be confirmed in other series. Sunitinib treatment may be associated with cardiotoxicity, which may be exacerbated by hypertension [Citation13]. This emphasizes the need to monitor and manage hypertension during sunitinib treatment.
We conclude that the present data provides further support to the hypothesis that hypertension is a predictive factor for treatment response to sunitinib and favorable survival in patients treated for advanced RCC. The present retrospective results suggest that the difference in outcome is substantial between patients who develop sunitinib-associated hypertension and those who do not. Prospective studies clearly warranted to confirm this hypothesis, as may also be studies where the sunitinib dose is tailored based on changes in the blood pressure.
Declaration of interest: P. Bono has received speaker fees from pfizer. The authors alone are responsible for the content and writing of the paper.
References
- Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol 2007;18:1117.
- Rini BI, Schiller JH, Fruehauf J, Cohen EE, Tarazi JC, Rosbrook B, . Association of diastolic blood pressure ≥ 90 mmHg with overall survival in patients treated with axitinib. J Clin Oncol 2008;26(18S):3543.
- Ravaud A, Sire M. Arterial hypertension and clinical benefit of sunitinib, sorafenib and bevacizumab in first and second-line treatment of metastatic renal cell cancer. Ann Oncol 2009;20:966–7.
- Bono P, Elfving H, Utriainen T, Osterlund P, Saarto T, Alanko T, . Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol 2009;20:393–4.
- Rini BI, Cohen DP, Lu D, Chen I, Hariharan S, Gore ME. Hypertension (HTN) as a biomarker of efficacy in patients (pts) with metastatic renal cell carcinoma (mRCC) treated with sunitinib. ASCO GU meeting 2010; Abstr 312.
- van Heeckeren WJ, Ortiz J, Cooney MM, Remick SC. Hypertension, proteinuria, and antagonism of vascular endothelial growth factor signaling: Clinical toxicity, therapeutic target, or novel biomarker? J Clin Oncol 2007;25: 2993–5.
- Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, . Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol 2009;27:4068–75.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L. Guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, . Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 2010;70: 1063–71.
- Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 2006;24:217–27.
- Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, . Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst 2005;97:981–9.
- Schneider BP, Radovich M, Miller KD. The role of vascular endothelial growth factor genetic variability in cancer. Clin Cancer Res 2009;15:5297–302.
- Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, . Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370: 2011–9.