Abstract
Background. Adjuvant chemotherapy is established routine therapy for colon cancer (CC) patients with radically resected stage III and ‘high-risk’ stage II disease. The decision on recommending adjuvant chemotherapy, however, is based on data from older patient cohorts not reflecting improvements in pre-operative staging, surgery, and pathological examination. The aim is to review the current risk of recurrence in stage II and III patients and second, to estimate the relative importance of routinely assessed clinico-pathological variables.
Methods. The PubMed/MEDLINE and the Cochrane databases were systematically searched for randomized controlled studies and observational studies published after 1 January 2005 with patients included after January 1995 on prognosis in surgically treated stage II and III CC patients.
Results. Of 2596 studies identified, 37 met the inclusion criteria and 25 provided data for meta-analysis. The total patient sample size in the 25 studies reporting either disease-free (DFS) or recurrence-free survival was 15 559 in stage II and 18 425 in stage III. Five-year DFS for stage II patients operated without subsequent adjuvant chemotherapy was 81.4% [95% confidence interval (CI) 75.4–87.4; in studies with good/very good quality of reporting 82.7%, (95% CI 80.8–84.6)]. For stage II patients treated with adjuvant chemotherapy, the five-year DFS was 79.3% (95% CI 75.6–83.1). For stage III patients without chemotherapy, five-year DFS was 49.0% (95% CI 23.2–74.8) and for those treated with adjuvant chemotherapy, 63.6% (95% CI 59.3–67.9). The prognostic impact of commonly investigated clinico-pathological parameters, (pT-stage, pN-stage, differentiation, number of lymph nodes studied, MMR-status, and emergency surgery) were confirmed.
Conclusions. In this meta-analysis, studies with good quality of reporting show a five-year DFS of 82.7% for stage II CC without adjuvant chemotherapy, whereas the five-year DFS is 63.8% for stage III CC with adjuvant chemotherapy. Due to insufficient reporting on treatment quality the presented DFS is likely an under-estimation of what is achieved at high-quality centers today.
The risk of post-surgical recurrence for patients with stage II or III colon cancer (CC) is influenced by the quality of pre-operative staging, surgery, and pathological examination. The quality of these interventions has improved in recent years. The decision on recommending adjuvant chemotherapy, however, is still based on data from older patient cohorts not reflecting the improvements. Consequently, an updated analysis of recent studies with state-of-the-art patient care will be of great use to guide physicians and patients in their treatment decisions.
In 1990, a large randomized trial demonstrated that 12 months of adjuvant 5-fluorouracil (FU) with levamisol significantly improved overall and disease-free survival (DFS) in CC patients with metastatic involvement of regional lymph nodes (stage III) [Citation1]. Later studies established six months of adjuvant therapy with FU and leucovorin (LV) as standard of care [Citation2]. The positive results of the MOSAIC (Multi-center International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer) trial [Citation3] led to approval of oxaliplatin plus FU/LV (FOLFOX) for patients with stage III CC in 2004. Today, six months of combination therapy with FOLFOX or capecitabine plus oxaliplatin (CAPOX) is recommended for curatively resected stage III CC patients in virtually all clinical guidelines [Citation4–6], including the Nordic countries [Citation7–10].
In patients with stage II CC the role of adjuvant chemotherapy is less clear, partly due to the smaller number of stage II patients enrolled in randomized studies. A gain was shown in a pooled analysis of data from seven randomized studies demonstrating a significant benefit from FU-based adjuvant therapy across stages, but to a lesser degree in stage II than in stage III CC [Citation11]. A Cochrane-analysis demonstrated that different types of adjuvant chemotherapy reduce the risk of recurrence in completely resected stage II CC in the order of 20–25% and improve overall survival with 5–10% [Citation12]. The Adjuvant Colon Cancer Endpoints (ACCENT) group noted that the survival benefit after adjuvant FU-based chemotherapy is ‘primarily driven by stage III patients’, estimating an absolute 10% improvement in the eight-year survival rate in stage III, compared to 5% in stage II [Citation13].
Over time, the surgical techniques, pathological staging, pre-operative imaging, and the oncologic treatments have improved. When comparing patients enrolled in adjuvant trials before and after 1995, Shi et al. [Citation14] found data supporting stage-migration [Citation15] from stage II to stage III, probably due to improved lymph node staging after 1995, with lower recurrence rates in stage II as a consequence. A further consequence is that recurrence rates will be lower also in stage III (often denoted the Will-Rogers phenomenon). A recent population-based Danish study reported on similar signs of stage migration from stage II to stage III [Citation16]. Assignment of patients to adjuvant chemotherapy must be based on current data on recurrence risks for best estimates of the potential benefit.
The purpose of this study is, first, to define the risk of recurrence in recent, as proxy for improved care, patient cohorts, and second, to estimate the relative importance of routinely assessed clinico-pathological variables.
Methods
All authors agreed on a pre-specified protocol according to the guidelines of the Cochrane handbook for systematic reviews [Citation17,Citation18] before the literature review began.
The primary outcome measure was risk of recurrence for operated colon adenocarcinoma stage II and III patients. Secondary outcome measures were quantitative influence of commonly registered clinical and histopathological factors on the risk of recurrence.
Criteria for inclusion of studies
Randomized controlled studies and observational studies published after 1 January 2005 (with patients enrolled after January 1995) on prognosis in surgically treated CC stage II and III patients were identified. The date for patient enrollment was after initial screening of titles changed from 2000 to 1995 to include more studies and not to miss relevant studies. The outcome of interest was recurrence, defined as local, regional, or distant recurrence after surgery. Studies where colon and rectal tumors could not be distinguished, duplicates, or studies comprising 20 patients or less were excluded.
Search methods for identification of studies
An experienced healthcare librarian was consulted for the search strategy of PubMed/MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) databases. The PubMed/MEDLINE search was constructed of combining specific Medical Subject Heading (MeSH)-words with key words on the title relating to colon or colorectal cancer and recurrence or prognosis (Supplementary Appendix 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839). The Cochrane search was performed with word search using a closeness operator (Supplementary Appendix 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839). The reference lists of relevant reviews were screened for relevant studies. In addition, reference lists of relevant studies were screened and abstracts were hand-searched. There were no language restrictions; however, two studies in Japanese and one in Chinese were excluded due to lack of translation resources.
Data collection
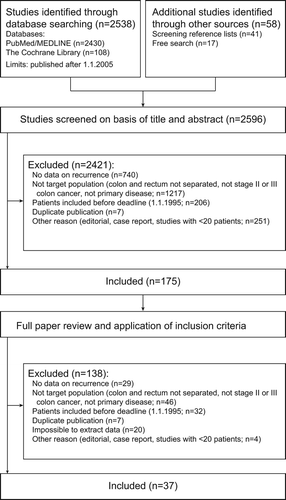
In the initial stage four researches (BEE, BG, CB, and TK) screened titles and subsequently selected abstracts in pairs according to the inclusion criteria. The potentially relevant full articles were then read (by BEE, CB, TH, and TK) in pairs to reach consensus on which articles to include. Disagreements about study eligibility were resolved by discussion. All authors agreed on included studies before data extraction began. Reasons for exclusion were documented and presented according to the PRISMA flow diagram () [Citation17,Citation18]. The following trial characteristics were extracted: time period and country, study design, sample size, intervention (including adjuvant treatment, if given), and outcome measure, including DFS or relapse-free survival (RFS), and univariable and multivariable hazard ratio (HR) for common clinico-pathological risk factors for recurrence. Information on the following risk factors for recurrence were collected [Citation5,Citation19]: pT-category, pN-category, number of lymph nodes examined, differentiation grade, vascular and neural invasion, perforation, obstruction, mismatch repair (MMR) status, pre-operative carcinoembrogenic antigen (CEA) level, and KRAS-mutation status.
Assessment of quality and risk of bias
The quality of reporting was assessed with a scoring tool, based on 5 subscales regarding: 1) reporting of the study results; 2) external validity; 3) bias in measurement and outcomes; 4) bias in the selection of study subjects; and 5) the power of the study [Citation20]. We used a simplified version with a maximum obtainable score of 28 [Citation21]. Studies were grouped qualitatively (≥ 20: very good; 15–19: good; 11–14: fair; ≤ 10: poor) [Citation22].
In order to address the quality of the medical care administered to the included patients, articles were searched for information on pre-operative staging, surgical procedures, patho-anatomical staging, and follow-up (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839).
Statistical analyses
The most sensitive endpoint for evaluating the need for adjuvant chemotherapy is risk of recurrence, or time to recurrence (TTR), but these are rarely reported [Citation23]. For the purpose of the review, time to recurrence was extracted as DFS or RFS. When using the definition by Punt et al. [Citation23], RFS is more sensitive as an endpoint estimating the need for adjuvant therapy than DFS, as it ignores second primary same cancers and other primary cancers. However, the definitions of DFS (and RFS) sometimes differ from the consensus statement [Citation23] or are not given. We use only the term DFS well aware that different studies did not consider exactly the same events. When both DFS and RFS were reported, the figure for DFS was used. We computed 95% confidence intervals (CI) and standard errors (SE) from the number of patients and rates using exact binomial CI. The studies were grouped according to stage (II or III) and adjuvant treatment given (yes, no, unclear). The category ‘adjuvant treatment unclear’ was used for studies where treatment was given only to a proportion of the patients or was not clearly described. The overall DFS and 95% CI were estimated by the random effects meta-analysis model. Meta-regression was used to analyze the effect of study quality on DFS. In a similar way available uni- and multivariable hazard ratios (HR) and CIs for important clinico-pathological variables were extracted. Significance levels and study population sizes were used to calculate missing CIs. The overall effect of each clinico-pathological variable was estimated with the random effects meta-analysis model (DerSimonian-Laird) including both three- and five-year HR values. A p-value of < 0.05 was considered statistically significant. All statistical analyses were done with STATA 11.0 software.
Results
Eligible studies
We identified 2596 potentially eligible studies that provided data on prognosis of patients operated for stage II or III CC. Of these, 37 met the inclusion criteria () [Citation11,Citation24–60]. Reasons for exclusion at each stage are presented in . We excluded 1263 otherwise eligible studies because they did not present stage-specific survival data, presented combined data on patients with colorectal cancers, or on recurrent or metastatic disease. Of the 37 included studies, 25 studies with survival data specific for either stage II or III CC were eligible for meta- analysis (, Supplementary Table II, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839). Eight studies provided univariable HR (, Supplementary Table III, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839) and 17 multivariable HR (Supplementary Tables IV and V, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839) for important clinico-pathological factors.
Table I. Main characteristics of eligible studies identifying risk of recurrence in patients with colon cancer stage II or III.
Table II. Five-year disease-free survival in colon cancer stratified according to stage and adjuvant treatment.
Table III. Univariable risk of recurrence for colon cancer stage II–III.
Study characteristics
Characteristics of the included 37 studies are presented in . The majority of the studies were published between 2009 and 2012. Eighty-four percent of the patients were operated on after the year 2000. Ten studies originated from European centers, six from North America, 11 from Asian countries, and three included patients from multiple countries. There were 16 single center studies. Five studies reported on clinical trials whereas seven studies were biomarker-based aiming for the identification of prognostic markers in selected cohorts of patients.
Eight studies presented data on the untreated course of resected stage II and III CC.
The total patient sample size in the 25 studies reporting DFS was 15 559 in stage II and 18 425 in stage III. The mean number of patients per study was 1722 (range 49–9645) in stage II, and 1036 (range 34–8616) in stage III. The total patient sample in the eight studies reporting univariable HRs for risk of recurrence was 3330. The mean number of patients per study was 370 (range 141–1404).
Disease-free survival at five years
Five-year DFS for stage II patients operated without subsequent adjuvant chemotherapy was 81.4% (95% CI 75.4–87.4), and for stage III 49.0% (95% CI 23.2–74.8) (). Considering stage II patients who were treated with adjuvant chemotherapy, five-year DFS was 79.3% (95% CI 75.6–83.1) and for stage III 63.6% (95% CI 59.3–67.9). For patients with unclear adjuvant status, five-year DFS was 81.1 (95% CI 77.3–84.8) for stage II patients and 58.7 (95% CI 50.0–67.5) for stage III. Reported original data of DFS-rates from the included studies are presented in Supplementary Table II (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839).
Influence by clinico-pathological variables
Results of a meta-analysis of the HRs for recurrence for clinico-pathological parameters are presented in , with the underlying original HRs in Supplementary Tables III and V to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839. A higher pT-stage indicates approximately a two-fold risk of recurrence (pT1-2 vs. pT3 and pT3 vs. pT4). The data extracted from studies only presenting multivariable analyses also support these findings (Supplementary Table IV to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839). Higher pN-stage (pN1 vs. pN2) increases the risk of tumor recurrence, whereas a higher number of sampled lymph nodes decrease the risk, as seen in analyses based on both uni-and multivariable data (, Supplementary Table IV to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839). Low histological differentiation also implies a higher risk of recurrence supported by both uni- and multivariable data.
Perforation and/or obstruction indicating emergency surgery also increase the risk of recurrence supported by both uni- and multivariable data. Tumor invasion into neural structures indicates a higher risk of recurrence, however being statistically significant only in analysis of the multivariable data. Results regarding vascular invasion are similar, but also statistically significant in the univariable data. Deficient MMR-status nearly halves the risk of recurrence. Elevated pre-operative CEA-level is related to higher risk of recurrence, albeit being statistically significant only in the analyses of the multivariable data. A mutation in the KRAS-gene increased the risk of tumor recurrence according to multivariable, but not univariable data.
Quality assessment
No study reported all relevant aspects of the quality of care (). Of the 37 studies included in this review, none was scored as poor, 16 as fair, 17 as good, and four as very good according to the quality assessment score [Citation20,Citation21]. Five-year DFS in stage II patients in studies with fair quality of reporting was 75.4% (95% CI 68.3–82.5) and significantly lower than for studies with good/very good quality of reporting with DFS of 82.7% (95% CI 80.8–84.6) (). Comparing DFS in stage II patients by meta-regression we found a statistically significant difference between fair and good/very good quality studies (regression coefficient 0.067; 95% CI 0.037–0.1312, p = 0.039). In stage III patients having received adjuvant therapy the result was similar, but when the use of adjuvant therapy was unclear, the result was the opposite ().
Table IV. Five-year disease-free survival in colon cancer stratified according to disease stage, adjuvant treatment, and study quality1.
Discussion
Disease-free survival in stage II and III colon cancer patients
This systematic review and meta-analysis based on more than 15 000 patients with stage II CC operated in 1995 or later showed a five-year DFS of 81.4% (95% CI 75.4–87.4) for patients not treated with adjuvant chemotherapy. For stage II CC patients who received adjuvant treatment, the five-year DFS was 79.3% (95% CI 75.6–83.1). For all stage II CC patients from studies with good/very good quality of reporting [Citation20], the DFS was 82.7% (95% CI 80.8–84.6). In 8624 patients with stage III CC treated with adjuvant chemotherapy the five-year DFS was 63.6% (95% CI 59.3–67.9), whereas the natural course of the disease in 312 patients not receiving adjuvant chemotherapy led to a five-year DFS of 49.0% (95% CI 23.2–74.8). Stage II CC patients in studies where chemotherapy administration was not clearly described showed a five-year DFS of 81.1% (95% CI 77.3–84.8) and for stage III CC patients 58.7% (95% CI 50.0–67.5). All studies providing patients for the ‘unclear’ group are observational or retrospective [Citation26,Citation27,Citation37,Citation41,Citation45,Citation49,Citation56], and probably a significant proportion of patients in these series did not receive any chemotherapy. It is therefore remarkable that the five-year DFS for stage II CC patients in the studies with ‘unclear’ chemotherapy assignment seems no worse and actually slightly better than for adjuvantly treated patients. As expected, in stage III CC adjuvant treatment shows a trend towards a positive effect on DFS according to these data. Evaluation of the benefit from adjuvant therapy, however, was not the scope of this review.
In line with our results, Haller et al. [Citation61] found that five-year DFS for stage III CC patients treated with CAPOX was 66.1% and for those receiving bolus FU 59.8%. Shi et al. [Citation14] reported a five-year RFS for stage II CC of 80.5%, and for stage III 61.5% in the older trials in the ACCENT database, whereas patients in the newer trials had a five-year RFS of 84.7% for stage II and 61.7% for stage III. The outcome measure RFS ignores secondary primary same cancers, which are included in the events used to calculate DFS [Citation23]. The difference between DFS and RFS is larger in stage II than in stage III and becomes larger with longer follow-up. A difference of 10% between five-year DFS and five-year RFS was estimated in stage II CRC in a recent population-based Swedish study [Citation62]. The five-year DFS for stage II CC patients found in this systematic review is in agreement with the five-year RFS in the newer trials of the ACCENT database, whereas the five-year DFS of 63.6% in stage III CC patients demonstrated here appears better than what was shown in the newer trials of the ACCENT database [Citation14].
The endpoints TTR and RFS include fewer event categories than DFS and are thus more sensitive to the effects of adjuvant chemotherapy; however, DFS is more widely used and acknowledged in guidelines as the most appropriate primary endpoint for trials of adjuvant treatment [Citation23]. The results of this systematic review can therefore easily be compared to data in literature, even if DFS is not ideal to estimate the need for adjuvant therapy.
Our estimate of DFS could be higher than in daily clinical practice due to a selection bias of healthier patients into trials. Furthermore, our meta-analysis includes data from two large studies that use RFS as endpoint [Citation11,Citation51], thereby ‘boosting’ DFS. However, several aspects imply that the DFS presented here are lower than can be expected for patients treated according to guidelines today. No study recruited patients later than 2008. More recent cohorts, e.g. with data from quality registries after quality assurance of all important parts of the care, may show the full extent of the benefits of state-of-the-art treatment with patients adequately staged, operated by specialized surgeons, the surgical specimen investigated according to modern standards [Citation63], and the patients adequately followed up. It was impossible to evaluate the quality of care in a meaningful way since few studies reported on more than single aspects of intervention and patient care. We applied a validated scoring tool [Citation20] in order to grade the quality of the reporting of studies. Patients with stage II disease from studies with good/very good quality of reporting [Citation20] had significantly better DFS of 82.7% (95% CI 80.8–84.6) compared to patients from studies with fair quality with DFS of 75.4% (95% CI 68.3–82.5). The category good/very good is dominated by randomized controlled trials, likely ensuring better quality of diagnostic work-up and follow-up, but not necessarily surgical intervention and pathological examination. However, they mostly include a selected patient cohort and selection bias could explain the better survival in studies with good/very good quality of reporting. It is not easy to explain, however, why this is not seen in stage III patients.
Taken together, there are several reasons to regard the presented DFS results as an under-estimation of what should be possible to achieve today. Based on the results from studies with good/very good quality of reporting, current treatment of CC can be improved. Still, the five-year DFS of 82.7% for stage II in the group of studies with the best quality of reporting is a valuable starting point for calculations of the current benefit of adjuvant treatment in curatively resected CC without lymph node metastases. Furthermore, the recent introduction of multidisciplinary treatment decisions will hopefully improve the overall outcome as well.
Prognostic value of clinical and histopathological factors
Survival varies widely for patients within the same disease stage [Citation64], and identification of those that benefit from adjuvant chemotherapy cannot be done without an individual risk assessment. In this systematic review, data from patients treated after 1995 confirm the prognostic impact of a number of commonly investigated parameters. The overall more favorable prognosis for patients with tumors harboring a deficient MMR-system is comparable to recent reports [Citation65–67], although one study suggests that the prognostic value of the MMR-status is limited to proximal tumors only [Citation68]. Emergency surgery due to obstruction or perforation is associated with a high degree of surgical morbidity and mortality, and, as demonstrated here, with an increased risk of recurrence (HR = 1.97). Presence of mutated KRAS had no prognostic impact (HR = 1.04) in our data. This could be due to a small sample size; a prognostic impact of KRAS mutations, if present, is probably not as large as for the clinico-pathological parameters, with HRs around 1.40 found in the literature [Citation65,Citation68,Citation69].
For the meta-analysis of the impact of prognostic factors we pooled data across stages. This assumption of stage-independent prognostic value may not be valid in all cases, as reported for the prognostic importance of MMR in stage II versus III in one study [Citation68]. For the rest of the markers, well documented differences between the prognostic importance in stage II and III are missing, and the assumption of stage independence is probably valid.
The initial search identified almost 2600 studies for screening. It seems likely that all key publications were identified. There were no language restrictions, and the included studies represent patients from North American, European, and Asian populations. The number of studies finally included in this review is limited, reflecting strict inclusion criteria in accordance with the aim of the study. The strict inclusion criteria, however, could exclude potentially informative studies. The large multicenter randomized QUASAR trial, comparing surgery alone with surgery and adjuvant chemotherapy, initiated patient inclusion in May 1994 prior to our (extended) time limit of this review [Citation67]. The QUASAR study would have substantially increased the number of patients. The absolute recurrence risk of 17% in the surgery-only arm fits well with the good/high quality studies included in our meta-analysis (DFS of 82.7%, ). The HRs for risk factors of recurrence reported from the QUASAR study are not fundamentally different, and the overall conclusions would likely not have changed.
Perspectives: Risk stratification in curatively resected stage II–III colon cancer
With a five-year DFS of 82.7% for stage II, as seen in the studies with good/very good quality of reporting, it is our best estimate that the recurrence risk after five years in pT3N0 without any risk factor is (at the most) 10% in hospitals with high quality of the care. Adding one risk factor could increase the risk to about 15% for poor differentiation or few sampled lymph nodes, or up to 20% for vascular invasion, pT4, vessel or neural invasion, obstruction, or N1. Since independent prognostic importance has been reported in the multivariable analyses in several of the studies, as well as in an older study not included [Citation70], two or more risk factors increase the risk further, although not said precisely by how much. Gertler et al. [Citation70] report that five-year RFS decreased from 91% in stage II with no risk factor to 88% with one, 82% with two and 75% with three risk factors, although the latter estimate was based on very few individuals. Even simple calculations, however, indicate that the recurrence risk in pT4N0M0 (stage II) must be roughly the same as in stage III disease (pT3N1) without additional risk factors, and that pT4N0M0 disease with additional risk factors can carry a much higher recurrence risk than pT3N1M0 without other risk factors. Should all these patients then receive the same treatment with a doublet of chemotherapy [Citation4–9]? 5-FU-based monotherapy represents a less toxic alternative for low- (and moderate-?) risk patients where the absolute gain from the added therapy is small. The current evidence does not identify any of the commonly assessed clinical and histopathological features as individually powerful enough for further risk stratification in high-risk stage II and stage III CC. A weighted contribution of the individual markers, as seen in a prognostic index, may provide a more clinically relevant stratification, but such attempts have not been done. The prognostic value of new, additional molecular markers, however, has been explored in hundreds of studies. In this perspective, it is striking that it was difficult to find recent reports on the prognostic value of standard clinico-pathological variables, the backbone of all routine care. The knowledge-base for this systematic review would have been much larger if the prognostic value of commonly assessed factors had been presented in the many publications where new markers are explored.
Conclusions
In conclusion, this systematic review and meta- analysis shows a five-year DFS of slightly above 80% for stage II CC, regardless of post-operative treatment, and a five-year DFS of 64% for stage III CC treated with adjuvant chemotherapy. The impact of commonly investigated clinical and histopathological factors on these survival rates was confirmed. The effects of ongoing efforts towards optimized patient management on recurrence rates should be further evaluated in prospective preferably population-based studies, and the results published.
Supplementary material available online
Supplementary Appendix 1 and 2, Tables I–V to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.975839.
ionc_a_975839_sm8195.pdf
Download PDF (43 KB)Acknowledgments
Professor Jaakko Kaprio, Department of Public Health, University of Helsinki, Finland is acknowledged for valuable help with statistics. For help with screening a study in Czech, Dr. Martin Plišek, Department of Internal Medicine, Rumburk, Czech Republic, is acknowledged. This work was financially supported by Finska Läkaresällskapet (CB and TK), Kurt och Doris Palander Foundation (TK), and the Swedish Cancer Society (BG). The Acta Oncologica Foundation took the initiative to the work and supported an initial meeting.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8.
- O’Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ, Jr, Erlichman C, Shepherd L, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol 1998;16: 295–300.
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479–516.
- Benson AB, 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Localized colon cancer, version 3. 2013: Featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw 2013;11:519–28.
- Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(Suppl 6):vi64–72.
- Danish Colorectal Cancer Group. National guidelines for diagnostic and treatment of colorectal cancer. 2013. [cited 2014 Jan 3]. Available from: http://www.dccg.dk/
- Österlund P, Lepistö A, Järvinen HJ. Koolonkarsinooma. Lääkätieteellinen aikakauskirja duodecim 2009;125:619–28.
- Helsedirektoratet. Vonen B, Grønlie Guren M, Edna T, Rønning Dørum LM, Klemp M, editors. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av tykk- og endetarmskreft. 2013. [cited 2014 Feb 19]. Available from: http://www.helsedirektoratet.no/publikasjoner.
- Colorectal cancer. Swedish National Care Programme 2008. Regional Cancer Centre, Umea 2008. 2008. [cited 2014 Mar 17]. Available from: http://www.cancercentrum.se/sv/Vardprogram/Kolorektalcancer/.
- Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 2004;22:1797–806.
- Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev 2008;(3):CD005390.
- Sargent D, Sobrero A, Grothey A, O’Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872–7.
- Shi Q, Andre T, Grothey A, Yothers G, Hamilton SR, Bot BM, et al. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: Evidence of stage migration from the ACCENT database. J Clin Oncol 2013;31:3656–63.
- Sjo OH, Merok MA, Svindland A, Nesbakken A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis Colon Rectum 2012;55:307–15.
- Lykke J, Roikjaer O, Jess P, Danish Colorectal Cancer Group. The relation between lymph node status and survival in Stage I–III colon cancer: Results from a prospective nationwide cohort study. Colorectal Dis 2013;15:559–65.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews and interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009;62:e1–34.
- Benson III BA, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines) colon cancer. 2013. [cited 2013 Dec 17]. Available from: http://www.nccn.org.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52: 377–84.
- Kennedy DA, Stern SJ, Matok I, Moretti ME, Sarkar M, Adams-Webber T, et al. Folate intake, MTHFR polymorphisms, and the risk of colorectal cancer: A systematic review and meta-analysis. J Cancer Epidemiol 2012;2012:952508.
- Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: A systematic review. J Gen Intern Med 2012; 27:1033–46.
- Punt CJ, Buyse M, Kohne CH, Hohenberger P, Labianca R, Schmoll HJ, et al. Endpoints in adjuvant treatment trials: A systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst 2007;99:998–1003.
- Andre T, Quinaux E, Louvet C, Colin P, Gamelin E, Bouche O, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: Final results of GERCOR C96.1. J Clin Oncol 2007;25:3732–8.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
- Belt EJ, Brosens RP, Delis-van Diemen PM, Bril H, Tijssen M, van Essen DF, et al. Cell cycle proteins predict recurrence in stage II and III colon cancer. Ann Surg Oncol 2012;19(Suppl 3):S682–92.
- Chin CC, Wang JY, Changchien CR, Huang WS, Tang R. Carcinoma obstruction of the proximal colon cancer and long-term prognosis – obstruction is a predictor of worse outcome in TNM stage II tumor. Int J Colorectal Dis 2010;25:817–22.
- Cho YB, Yun SH, Hong JS, Yun HR, Lee WS, Lee WY, et al. Carcinoma obstruction of the left colon and long-term prognosis. Hepatogastroenterology 2008;55:1288–92.
- Coco C, Zannoni GF, Caredda E, Sioletic S, Boninsegna A, Migaldi M, et al. Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients. J Exp Clin Cancer Res 2012;31:71.
- Derwinger K, Carlsson G, Gustavsson B. A study of lymph node ratio as a prognostic marker in colon cancer. Eur J Surg Oncol 2008;34:771–5.
- Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis 2010;25: 1427–33.
- Faerden AE, Sjo OH, Bukholm IR, Andersen SN, Svindland A, Nesbakken A, et al. Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Dis Colon Rectum 2011;54:200–6.
- Farina-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VE, Rutten HJ, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol 2010;21:2396–402.
- Fernandez-Cebrian JM, Nevado Santos M, VorwaldKuborn P, Pardo de Lama M, Martin-Cavanna J, Pacheco Martinez P, et al. Can the clinical outcome in stage II colon carcinomas be predicted by determination of molecular marker expression? Clin Transl Oncol 2007;9:663–70.
- Galizia G, Orditura M, Ferraraccio F, Castellano P, Pinto M, Zamboli A, et al. The lymph node ratio is a powerful prognostic factor of node-positive colon cancers undergoing potentially curative surgery. World J Surg 2009;33:2704–13.
- Giraldez MD, Lozano JJ, Cuatrecasas M, Alonso-Espinaco V, Maurel J, Marmol M, et al. Gene-expression signature of tumor recurrence in patients with stage II and III colon cancer treated with 5'fluoruracil-based adjuvant chemotherapy. Int J Cancer 2013;132:1090–7.
- Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol 2010;101: 396–400.
- Huh JW, Kim HR, Kim YJ. Prognostic value of perineural invasion in patients with stage II colorectal cancer. Ann Surg Oncol 2010;17:2066–72.
- Huh JW, Kim CH, Kim HR, Kim YJ. Factors predicting oncologic outcomes in patients with fewer than 12 lymph nodes retrieved after curative resection for colon cancer. J Surg Oncol 2012;105:125–9.
- Kandemir EG, Mayadagli A, Karagoz B, Bilgi O, Turken O, Yaylaci M. Prognostic significance of thrombocytosis in node-negative colon cancer. J Int Med Res 2005; 33:228–35.
- Kang J, Hur H, Min BS, Kim NK, Lee KY. Prognostic impact of inferior mesenteric artery lymph node metastasis in colorectal cancer. Ann Surg Oncol 2011;18:704–10.
- Kjaer-Frifeldt S, Hansen TF, Nielsen BS, Joergensen S, Lindebjerg J, Soerensen FB, et al. The prognostic importance of miR-21 in stage II colon cancer: A population-based study. Br J Cancer 2012;107:1169–74.
- Kumar S, Chang EY, Frankhouse J, Dorsey PB, Lee RG, Johnson N. Combination of microsatellite instability and lymphocytic infiltrate as a prognostic indicator for adjuvant therapy in colon cancer. Arch Surg 2009;144:835–40.
- Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol 2007; 14:1712–7.
- Mroczkowski P, Schmidt U, Sahm M, Gastinger I, Lippert H, Kube R. Prognostic factors assessed for 15,096 patients with colon cancer in stages I and II. World J Surg 2012;36:1693–8.
- Niedzwiecki D, Bertagnolli MM, Warren RS, Compton CC, Kemeny NE, Benson AB, 3rd, et al. Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: Results from CALGB 9581. J Clin Oncol 201;29:3146–52.
- Venook AP, Niedzwiecki D, Lopatin M, Ye X, Lee M, Friedman PN, et al. Biologic determinants of tumor recurrence in stage II colon cancer: Validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol 2013;31:1775–81.
- Oh SY, Kim YB, Suh KW, Paek OJ, Moon HY. Prognostic impact of fascin-1 expression is more significant in advanced colorectal cancer. J Surg Res 2012;172:102–8.
- Park SJ, Lee KY, Kim SY. Clinical significance of lymph node micrometastasis in stage I and II colon cancer. Cancer Res Treat 2008;40:75–80.
- Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 2007;25:3456–61.
- Sargent DJ, Resnick MB, Meyers MO, Goldar-Najafi A, Clancy T, Gill S, et al. Evaluation of guanylyl cyclase C lymph node status for colon cancer staging and prognosis. Ann Surg Oncol 2011;18:3261–70.
- Tanaka M, Chang P, Li Y, Li D, Overman M, Maru DM, et al. Association of CHFR promoter methylation with disease recurrence in locally advanced colon cancer. Clin Cancer Res 2011;17:4531–40.
- Twelves C, Wong A, Nowacki MP, AbtM, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
- Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117–25.
- Roth AD, Delorenzi M, Tejpar S, Yan P, Klingbiel D, Fiocca R, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst 2012;104:1635–46.
- Xing X, Peng L, Qu L, Ren T, Dong B, Su X, et al. Prognostic value of PRL-3 overexpression in early stages of colonic cancer. Histopathology 2009;54:309–18.
- Yan DW, Li DW, Yang YX, Xia J, Wang XL, Zhou CZ, et al. Ubiquitin D is correlated with colon cancer progression and predicts recurrence for stage II–III disease after curative surgery. Br J Cancer 2010;103:961–9.
- Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
- Zaanan A, Flejou JF, Emile JF, Des GG, Cuilliere-Dartigues P, Malka D, et al. Defective mismatch repair status as a prognostic biomarker of disease-free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res 2011;17:7470–8.
- Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
- Birgisson H, Wallin U, Holmberg L, Glimelius B. Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival. BMC Cancer 2011;11:438.
- Quirke P, West NP, Nagtegaal ID. EURECCA consensus conference highlights about colorectal cancer clinical management: The pathologists expert review. Virchows Arch 2014;464:129–34.
- Nitsche U, Maak M, Schuster T, Kunzli B, Langer R, Slotta-Huspenina J, et al. Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective. Ann Surg 2011;254:793–800; discussion 800–1.
- Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–70.
- Bertagnolli MM, Redston M, Compton CC, Niedzwiecki D, Mayer RJ, Goldberg RM, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: Prospective evaluation of biomarkers for stages II and III colon cancer – a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153–62.
- Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611–9.
- Sinicrope FA, Mahoney MR, Smyrk TC, Thibodeau SN, Warren RS, Bertagnolli MM, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31:3664–72.
- Russo A, Bazan V, Agnese V, Rodolico V, Gebbia N. Prognostic and predictive factors in colorectal cancer: Kirsten Ras in CRC (RASCAL) and TP53CRC collaborative studies. Ann Oncol 2005;16(Suppl 4):iv44–9.
- Gertler R, Rosenberg R, Schuster T, Friess H. Defining a high-risk subgroup with colon cancer stages I and II for possible adjuvant therapy. Eur J Cancer 2009;45: 2992–9.