Abstract
Background: Radiotherapy (RT) for rectal cancer can have adverse effects on testicular function resulting in azoospermia and low testosterone levels. Variability of testicular dose (TD) due to differences in position of testes has been assessed with scrotal dosimeters and resulted in substantial variability of delivered TD. The aim of this study was to estimate planned and delivered TD using a treatment planning system (TPS).
Methods: In 101 men treated with RT for rectal cancer the cumulative mean TD (mTD) was calculated by TPS based on plan-computed tomography (CT) to evaluate the effect of different predictors on planned TD. The delivered TD was estimated by TPS based on repeated cone-beam CTs in 32 of 101 men to assess within-person variability of planned and delivered TD in a longitudinal analysis.
Results: The median planned mTD for short course RT was 0.57 Gy (range 0.06–14.37 Gy) and 0.81 Gy (range 0.36–10.80 Gy) for long course RT. The median planned mTD was similar to the median delivered mTD in the 32 men analysed over the entire course of RT (p=0.84). The mTD did not change significantly over time of planning and delivering RT. The variation in proximity between testes and planning target volume (PTV) was related to within-person variability of mTD in men on the 50th and 75th percentile of mTD and as expected the absolute difference between planned and delivered mTD increased with higher mTD.
Conclusion: Testicular doses calculated based on plan-CT are an accurate estimation of delivered TD based on repeated cone beam (CB)CT. The within-person variability of TD is related to variation in proximity between testes and PTV in men with moderate to high TD.
Current treatment of rectal cancer often includes preoperative radiotherapy (RT) to improve loco-regional control and decreases the relative risk of local recurrence by 50% to 70% [Citation1]. The reported acute and late adverse effects of RT include a negative impact on sexual and bowel function confining daily life of long-term survivors [Citation2,Citation3]. Cross-sectional and longitudinal studies also describe a negative impact of RT on Leydig cell function [Citation4–8]. According to a systematic review the average cumulative testicular dose (TD) during long course external beam RT varies between 1.24 Gy and 8.4 Gy, mainly depending on the distance between the testes and the target volume [Citation9]. These findings were confirmed among 83 men treated with long course RT (50.4 Gy) and concomitant chemotherapy [Citation10]. However, the cumulative TD did not correlate to longitudinal changes in androgen levels observed one year after RT. Longitudinal studies using scrotal dosimeters report substantial within-person variability of the TD for each fraction of RT [Citation5,Citation11]. The reason for this variability is not known. Differences in the position of the testes between each RT fraction or undetected dosimeter displacement may play a role.
To the best of our knowledge, the testes are usually not included as organ at risk in the treatment planning of RT for rectal cancer. The calculation of TDs with a treatment planning system (TPS) has theoretical advantages over measurements with scrotal dosimeters as the dose distribution of the testicular volume can be assessed and manual interference during placement of dosimeters is avoided. However, TD calculation with TPS based on the computed tomography (CT) for treatment planning does not account for the within-person variability of the delivered TD as the position of the testes may change between RT fractions.
The aim of this study is to calculate the planned TD and to evaluate longitudinal changes between planned and delivered TD during preoperative RT for rectal cancer using a CT-based TPS for dose calculation.
Material and methods
Men with rectal cancer stage I–III, planned for preoperative RT and rectal resection, were recruited prospectively during April 2010 to May 2014 at the colorectal units of a tertiary and a secondary referral centre in Stockholm, Sweden. Study participants were over 18 years of age, fluent in Swedish and had given informed consent (Clinical Trial ID NCT01216206). Patients, planned for local excision, with history of urogenital cancer or previous pelvic radiation for other diseases, were excluded. The Regional Ethical Review Board (Dnr 2009/1860-31/2) and the Radiation Protection Authority [K1180-2012 (2012_27)] approved the study.
Preoperative radiotherapy for rectal cancer
Preoperative RT was given according to the recommendation of the multidisciplinary team conference as short course RT with a prescribed dose of 25 Gy (5 × 5 Gy) or as long course RT with a prescribed dose of 50 Gy (25 × 2 Gy)/50.4 Gy (28 × 1.8 Gy). One participant had a prescribed dose of 64.4 Gy (28 × 2.3 Gy). The oncologists delineated the gross tumour volume (GTV) and the clinical target volume (CTV) on plan-CT using the TPS Eclipse (Varian, Palo Alto, CA, USA). The CTV covered, apart from the primary tumour and the mesorectum, lymph nodes outside the mesorectum at risk of containing cancer cells [Citation12]. The pelvic floor was included to the CTV in men planned for extralevator abdominoperineal excision. The planning target volume (PTV) was defined by addition of an isotropic margin around the CTV (6 mm for short course RT and 9 mm for long course RT) to account for organ movement and set up errors. External beam RT was delivered to the undressed patient in supine position without specific gonadal protection using a four-field conformal technique with 15 MV or 18 MV photons (Varian). Two study participants had volumetric modulated arc therapy (6 MV).
Calculation of the cumulative testicular dose
The testicular tissue was delineated on CT as one volume excluding other scrotal structures such as skin, soft tissue or epididymis (Supplementary Figure 1, available online at http://www.informahealthcare.com). The Analytical Anisotropic Algorithm (AAA 11.0.31) of the Eclipse system was used to calculate the physical TD based on dose-volume histograms of the testicular volume. The cumulative TD was reported as mean TD (mTD), minimum TD and maximum TD. The shortest distance between the centre of the testicular volume and the lower end of PTV/beam (distance testes-PTV) in the sagittal view was also registered. Beam models are developed to achieve high accuracy for the high dose region within the geometrical boundaries of the beam. In this study the low dose region outside the geometrical beam was of particular importance. Analysis of the beam commissioning data indicated that the applied beam model (Eclipse AAA 11.0.31) underestimated the doses outside the geometrical boundaries in average by 15%. Thus all TDs were adjusted by the factor 1.15 to correct for this systematic underestimation of the calculated doses.
Planned testicular dose
The planned TD was calculated for all study participants based on the plan-CT according to the method described above. This resulted in a single estimate of the cumulative TD, which allowed for statistical model building and cross-sectional analysis of between-person variability and predictors of the planned TD.
Estimate of delivered testicular dose
Men treated with short course RT between August 2012 and May 2014, had repeated cone beam CTs (CBCT) to estimate the delivered TD. The CBCTs were acquired in treatment position immediately after each fraction. As described above the cumulative delivered TD was calculated for each CBCT with TPS. The within-person variability of the TD during planning and delivering of RT was assessed in a longitudinal analysis.
Patient setup verification is the primary purpose of CBCT technology. CBCT images can also be used for dose calculation [Citation13]. After calibration the expected accuracy of CBCT-based dose calculation in soft tissue is within a few percentages compared to CT-based dose calculation, which own measurements confirmed [Citation14,Citation15]. Under the assumption of negligible systematic differences between TD calculation based on plan-CT or CBCT and a small set up error, the observed within-person variability of the TD is due to differences in the position of the testes. The image quality of the CBCT was inferior compared to the plan-CT complicating discrimination between testicular tissue and other scrotal structures. We assumed that the individual size of the testes remained stable during the observation period (planning/delivering of short course RT). The size of delineated testicular volume on repeated CBCT was matched with the size of delineated testicular volume on the corresponding plan-CT to avoid within-person variation of TD due to different sizes of testicular volume over time. Owing to the limitation of the scan range of CBCT (16 cm) in cranio-caudal direction and the treatment planning isocentre in relation to the testes position, the treatment couch had to be moved 10–13 cm towards the gantry in cranial direction to scan the entire testes. Thus only part of the pelvic region was included on CBCT scans. The limited field of view of CBCT did not cover the complete PTV and resulted in a loss of photon scattering from the irradiated volume not included on CBCT. The effect of the equivalent missing irradiated volume on the respective plan-CT was estimated to compensate for this loss of photon scattering. These individualised factors were registered for each CBCT and ranged from 1.1 to 1.6.
Statistical analysis
The distribution of mTD was skewed and the relationship of the distance between the centre of the testicular volume to the PTV was hyperbolic. The distribution of mTD was compared between groups of patients with non-parametric tests (Wilcoxon rank-sum and Kruskal-Wallis). We estimated quantile regression models for the 25th, 50th and 75th percentile of the inverse mTD as a quadratic function of the distance testes-PTV.
Where Q1/mTD(p | x) indicates the conditional p-quantile of the inverse mTD (1/mTD) given covariates. We exploited the invariance property of the quantiles and estimated the quantiles for mTD by inverting the estimates from the above model [Citation16]. Data were analysed with Stata version 13 (StataCorp LP, College Station, TX, USA)
Results
During April 2010 and May 2014, 115 men with rectal cancer stage I–III were included. Preoperative RT was given to 101 of 115 men. shows characteristics of the study participants treated with RT and surgery. The majority had short course RT (n = 76) and 25 men had long course RT with prescribed doses of 50.4 Gy (n = 22), 50 Gy (n = 2) and 64.4 Gy (n = 1). Twelve individuals treated with short course RT (25 Gy) had preoperative chemotherapy after RT according to the experimental arm of the “Rectal Cancer And Pre-operative Induction Therapy Followed by Dedicated Operation” (RAPIDO) trial [Citation17]. The 23 men with prescribed doses of 50.4 Gy or 64.4 Gy had concomitant chemotherapy.
Table I. Characteristics of the study participants treated with preoperative radiotherapy and surgery.
Cross-sectional analysis of the planned testicular dose
The median planned mTD for short course RT was 0.57 Gy (range 0.06–14.37 Gy) and 0.81 Gy (range 0.36–10.80 Gy) for long course RT (). The stratification by three levels of distance testes-PTV resulted in significant differences of the planned mTD for both types of RT (p < 0.001). Increasing distance of the tumour from the anal verge, exclusion of the anal canal from the PTV and surgery with preservation of the pelvic floor (i.e. low anterior resection, intersphincteric abdominoperineal excision or Hartmanńs procedure) were associated with significantly lower planned mTD in men treated with short course RT (p < 0.001). These factors had a similar effect on the planned mTD in men treated with long course RT without fulfilling the criteria of statistical significance. The regression analysis adjusted for the type of RT showed that the four stratification factors mentioned above were significant predictors in the univariate analysis (Supplementary , available online at http://www.informahealthcare.com). The distance between testes-PTV remained a significant predictor in the full model for the 25th and 75th percentile. The predicted effect of the distance testes-PTV on the planned mTD is shown in Supplementary Figure 2 (available online at http://www.informahealthcare.com). The hyperbolic nature of this relation resulted in an exponential increase of the planned mTD in men with the testes closer than 5 cm to the PTV. Body mass index and weight were not associated with the planned mTD.
Table II. Stratified analysis of the planned testicular dose.
Longitudinal analysis of the planned and the delivered testicular dose
The delivered mTD could be assessed on 127 CBCTs acquired from 32 men treated with short course RT. Incomplete scanning of the testicular volume (n = 21) and logistic difficulties (n = 12) were the reasons for missing CBCT observations. The spaghetti plot shows the longitudinal assessment of the mTD on planning CT and repeated CBCTs (). The median planned and in average delivered mTD was similar (0.52 Gy vs. 0.51 Gy; p=0.84). The graphical analysis showed no systematic difference between mTD assessed on planning CT and CBCT (). illustrates the estimated 25th, 50th, 75th and 90th percentile of the absolute difference between planned and delivered mTD that has to be expected for a given planned mTD. For example, the 50th and 90th percentile of this difference was smaller than 0.5 and 1.0 Gy respectively for men with a planned mTD below 1.0 Gy. The longitudinal regression analysis confirmed that the time-point of TD assessment (planning CT or CBCT no. 1–5) had no significant effect on mTD (). However, differences in the position of the testes during planning and delivering of RT, estimated by the distance testes-PTV, had a heterogenous effect on the within-person variability of mTD. The effect of the distance testes-PTV was not significant for the within-person variability in men with low mTD (25th percentile) but had a significant effect in men with moderate and high mTD (50th and 75th percentile). This corresponded to the graphical analysis of as the within-person variability of mTD increased in men with higher mTD.
Figure 1. Spaghetti plot of planned and delivered testicular dose for 32 men treated with short course radiotherapy (25 Gy). CT = computed tomography, CBCT = cone beam computed tomography.
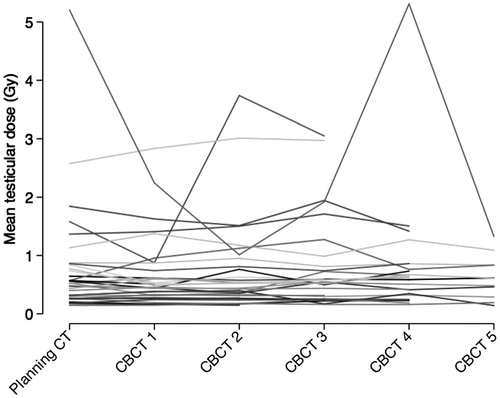
Figure 2. Scatter plot of planned and in average delivered testicular dose for 32 men treated with short course radiotherapy (25 Gy).
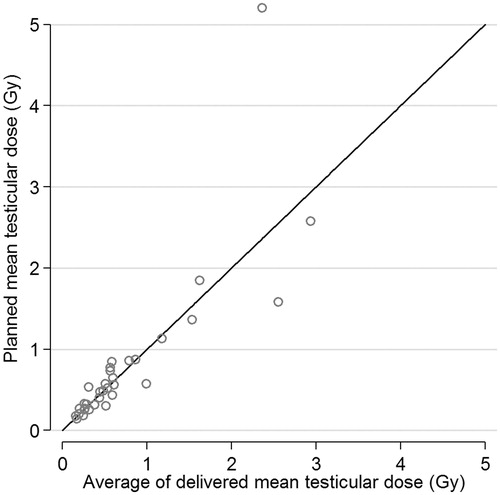
Figure 3. Estimated 25th, 50th, 75th and 90th percentiles of the absolute difference between planned and delivered mean testicular dose for a given planned mean testicular dose. TD = testicular dose.
Table III. Longitudinal quantile regression analysis with the inverse of the mean testicular dose based on planning and repeated cone beam CT as outcome.
Discussion
We assessed the testicular radiation exposure with the TPS in 101 men treated with preoperative RT for rectal cancer. The planned mTD relied mainly on the proximity of the testes to the lower end of the PTV. The differences between the mTD assessed on plan-CT and repeated CBCTs were statistically not significant and small for men with a planned mTD below 1 Gy. The variation in the position of the testes between the fractions of RT had a significant effect on the within-person variability of mTD in men with moderate to high mTD, which is not surprising due to the rapid dose fall at the field edge.
The dose distribution of the testicular volume is substantial and cannot be detected by dosimeters placed on the scrotal skin. Testicular dose measurement with thermoluminescent dosimeters (TLD) is an alternative to dose calculation by TPS. However, an accurate measurement of dose to a superficial organ at photon energies of 15–18 MV is not trivial in the clinical setting of this study due to the build-up at these energies. The TPS enables to calculate dose-volume histograms and to evaluate different dose measures for the volume of interest. The mean dose may be compared in volumes that have different dose-distributions for each fraction. Under the assumption of identical radiation sensitivity throughout the testicular volume, the mean dose is a relevant dose measure to relate to clinical effects of the testicular irradiation. Apart of the conceptual advantages of dose calculation on TPS over dose measurement with scrotal dosimeters, the risk of cremaster reflex induction and undetected dislocation of the dosimeter is absent [Citation11]. The disadvantage is an additional TD of 0.1–0.2 Gy from five CBCTs [Citation18]. All study participants were therefore offered pre-treatment cryopreservation of sperms and follow-up of androgen levels during two years after RT to detect individuals with indication for testosterone replacement.
The average planned mTD corresponds to 5.0% of the prescribed dose during short course RT and 3.5% of the prescribed dose during long course RT. This is a low proportion compared to the testicular exposure reported with 3–17% of the prescribed dose in a systematic review that summarises TDs during RT for rectal cancer assessed with scrotal dosimeters, TPS or equations based on phantom measurements [Citation9]. The difference between short and long course RT in the proportion of prescribed dose in average absorbed by the testes is most likely due to the skewed distribution of mTD. The proportion of prescribed dose in median absorbed by the testes is 2.3% for 25 Gy and 2.0% for 50.4 Gy and probably the result of some inhomogeneity between both groups. The treatment in prone position on a double-hole belly board seems not to reduce TD as the average TD was 7.7% of the prescribed dose calculated with TPS [Citation10]. This study had a comparable proportion of low rectal cancer (0.46 vs. 0.41), defined by a tumour within 6 cm from the anal verge, and used similar criteria to define the target volume.
The large range of the planned mTD results in TDs that might be of concern for testicular function in selected individuals. The threshold doses inducing temporary or permanent damages to Sertoli or Leydig cells are not defined for preoperative RT of rectal cancer. Historical studies in men irradiated for Hodgkin’s lymphoma and testicular seminoma report a high risk of permanent azoospermia if TD exceeds 1.2–1.4 Gy during a fractionated RT regimen [Citation19,Citation20]. The radiation tolerance of Leydig cells seems to be higher but a negative impact on Leydig cell function has to be expected in selected individuals treated with multimodal therapy for rectal cancer [Citation9]. So far a dose-response relationship between the TD and the longitudinal changes in serum testosterone levels has not been established [Citation10].
According to the cross-sectional analysis of planned mTD surrogate parameters can be used to identify men with low mTD without calculation of dose-volume histograms. The planned mTD is below 1.0 Gy if the distance testes-PTV is larger than 5 cm. Individuals with rectal cancer in the upper third (11–15 cm from the anal verge) have a planned mTD below 2.0 Gy. The relation of the anal canal to the PTV and the type of surgery seem not to be appropriate to identify men with planned mTD below 2.0 Gy. The mentioned threshold values 1.0 and 2.0 Gy are arbitrary as the threshold values of transient or permanent damage of spermatogenesis and Leydig cell function in RT for rectal cancer are not known. It might be of clinical use to realise that the testes are an organ at risk and should be included in treatment planning at least in individuals with the testes within 5 cm from the lower end of PTV. Data in the study are sparse for planned TD larger than 1 Gy but the variation in position of the testes has a significant impact on within-person variability in individuals with median to high TD. This might be a target group with particular benefit of gonadal protection [Citation21,Citation22].
This observational study has limitations with a potential impact on internal and external validity. The presented cumulative TDs are higher than in the general population with rectal cancer due to an increased frequency of low rectal cancer in the study group. The subgroup analysed with repeated CBCTs was recruited consecutively without further restrictions than given informed consent and prescribed dose of 25 Gy. We have reduced measurement bias due to systematic differences between dose calculation on plan-CT and CBCT. Phantom measurements indicated a relation between patient size (thickness along the beam) and TD [Citation23]. Body mass index and weight had no confounding effect on TD in our data. The regression analyses are adjusted for the effect of the type of RT as TDs derived from short or long course RT are not directly comparable. The statistical analyses appropriately allowed for the marked skewness of the distribution of TD. The number of men treated with long course RT was small (n = 25) and might be a reason that the predictors of the planned TD did not reach statistical significance.
Conclusion
Testicular doses calculated based on plan-CT result in an accurate estimation of the delivered TD based on repeated CBCT during preoperative RT for rectal cancer. The distance between testes and the PTV is the most important predictor of the TD and can be used to identify individuals with low TD if the TD is not calculated from the planning CT. The within-person variability of the delivered TD is related to differences in the position of the testes in men with moderate to high TD during short course RT and should be considered during positioning of the patient in the radiation suite. The hyperbolic distribution of the TD should be respected in further studies evaluating a dose-response relationship between TD and testicular function.
Supplementary material available online Supplementary Figures 1 and 2, Table 1
Suppl.zip
Download Zip (4.3 MB)Acknowledgements
The Regional Agreement on Medical Training and Clinical Research (ALF) between the Stockholm Community Council and Karolinska Institutet, the Stockholm Cancer Society and the Swedish Cancer Society provided financial support. The Cancer League of Graubünden (Switzerland) also supported the study. We thank all our colleagues at the colorectal units of Ersta Hospital and Karolinska University Hospital for the support during enrolment. We thank also our colleagues at the Department of Oncology, Karolinska University Hospital, for the acquisition of the cone beam CTs.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World J Gastroenterol 2013;19:8489–501.
- Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol 2007;46:504–16.
- Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: Development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–8.
- Dueland S, Guren MG, Olsen DR, Poulsen JP, Magne Tveit K. Radiation therapy induced changes in male sex hormone levels in rectal cancer patients. Radiother Oncol 2003;68:249–53.
- Hermann RM, Henkel K, Christiansen H, Vorwerk H, Hille A, Hess CF, et al. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother Oncol 2005;75:83–8.
- Yau I, Vuong T, Garant A, Ducruet T, Doran P, Faria S, et al. Risk of hypogonadism from scatter radiation during pelvic radiation in male patients with rectal cancer. Int J Radiat Oncol Biol Phys 2009;74:1481–6.
- Yoon FH, Perera F, Fisher B, Stitt L. Alterations in hormone levels after adjuvant chemoradiation in male rectal cancer patients. Int J Radiat Oncol Biol Phys 2009;74:1186–90.
- Bruheim K, Svartberg J, Carlsen E, Dueland S, Haug E, Skovlund E, et al. Radiotherapy for rectal cancer is associated with reduced serum testosterone and increased FSH and LH. Int J Radiat Oncol Biol Phys 2008;70:722–7.
- Buchli C, Martling A, Arver S, Holm T. Testicular function after radiotherapy for rectal cancer – a review. J Sex Med 2011;8:3220–6.
- Hennies S, Wolff HA, Jung K, Rave-Frank M, Gaedcke J, Ghadimi M, et al. Testicular radiation dose after multimodal curative therapy for locally advanced rectal cancer. Influence on hormone levels, quality of life, and sexual functioning. Strahlenther Onkol 2012;188:926–32.
- Piroth MD, Hensley F, Wannenmacher M, Zierhut D. [Male gonadal dose in adjuvant 3-d-pelvic irradiation after anterior resection of rectal cancer. Influence to fertility]. Strahlenther Onkol 2003;179:754–9.
- Glimelius B, Tiret E, Cervantes A, Arnold D, Group EGW. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(Suppl 6):vi81–8.
- Yoo S, Yin FF. Dosimetric feasibility of cone-beam CT-based treatment planning compared to CT-based treatment planning. Int J Radiat Oncol Biol Phys 2006;66:1553–61.
- Sriram P. A study on evaluation of kV-CBCT-image-based treatment planning using anthropomorphic phantom. J Med Biol Eng 2011;31:429–435.
- Guan H, Dong H. Dose calculation accuracy using cone-beam CT (CBCT) for pelvic adaptive radiotherapy. Phys Med Biol 2009;54:6239–50.
- Bottai M, Cai B, McKeown RE. Logistic quantile regression for bounded outcomes. Stat Med 2010;29:309–17.
- Nilsson PJ, van Etten B, Hospers GA, Pahlman L, van de Velde CJ, Beets-Tan RG, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer – the RAPIDO trial. BMC Cancer 2013;13:279.
- Palm A, Nilsson E, Herrnsdorf L. Absorbed dose and dose rate using the Varian OBI 1.3 and 1.4 CBCT system. J Appl Clin Med Phys 2010;11:229–240.
- Hahn EW, Feingold SM, Nisce L. Aspermia and recovery of spermatogenesis in cancer patients following incidental gonadal irradiation during treatment: A progress report. Radiology 1976;119:223–5.
- Centola GM, Keller JW, Henzler M, Rubin P. Effect of low-dose testicular irradiation on sperm count and fertility in patients with testicular seminoma. J Androl 1994;15:608–13.
- Mazonakis M, Damilakis J, Varveris H, Gourtsouiannis N. Radiation dose to testes and risk of infertility from radiotherapy for rectal cancer. Oncol Rep 2006;15:729–33.
- Hood RC, Wu QJ, McMahon R, Czito B, Willett C. IMRT treatment of anal cancer with a scrotal shield. Med Dosim 2012;37:432–5.
- Budgell GJ, Cowan RA, Hounsell AR. Prediction of scattered dose to the testes in abdominopelvic radiotherapy. Clin Oncol 2001;13:120–5.
