Abstract
Objective. Previous studies have shown that smoking contributes importantly to short-term modulation of sympathetic nerve traffic. However, effect of smoking status on resting muscle sympathetic nerve activity (MSNA) in hypertension is unknown. Therefore, we tested the hypothesis that smoking is associated with chronic sympathetic activation in patients with essential hypertension. Methods. We measured MSNA, heart rate (HR) and blood pressure during undisturbed supine rest and in 30 hypertensive smokers (22 males, age 38±4 years, body mass index, BMI 27±1 kg/m2, mean±SEM). These measurements were compared with those obtained 38 non-smoking hypertensive patients matched for gender, age and BMI. All hypertensives underwent 24-h ambulatory blood pressure monitoring. Patients were newly diagnosed, never treated for hypertension and were free of any other known diseases. Results. In comparison with non-smokers, smokers had smaller office–daytime systolic blood pressure difference (6±2 vs 15±3 mmHg, respectively; p<0.01). Despite similar resting values, HR in smokers was greater than in non-smokers during both daytime (86±3 vs 77±2 beats/min, respectively; p< 0.001) and night-time (73±3 vs 66±2 beats/min, respectively; p<0.01). MSNA was elevated in smokers (36±3 bursts/min) compared with non-smokers (28±3 bursts/min; p<0.01). Similar results were obtained when MSNA was expressed as bursts/100 heart beats. Multiple linear regression analysis revealed that only age and smoking status were linked independently to MSNA (R2=0.42, p< 0.001). Conclusions. In patients with essential hypertension, smoking is independently associated with chronic increase in MSNA. These findings may have implications for our understanding of the mechanisms linking smoking to cardiovascular events.
Introduction
Smoking remains a major risk factor for cardiovascular morbidity and mortality (Citation1–3). Smoking-related cardiovascular events cannot be attributed solely to progression of atherosclerosis and other mechanisms are likely to be implicated. The cardiovascular responses to smoking represent a complex interplay between the hemodynamic factors, autonomic nervous system and multiple vasoactive mediators (Citation4). The sympathetic nervous system plays a key role in acute changes in blood pressure. Sympathetic activation may also contribute to chronic blood pressure elevation by effects on the kidney, on blood vessel structure and by resetting the baroreflex (Citation5–7). Previous studies have shown that smoking contributes importantly to short-term modulation of sympathetic nerve traffic (Citation8,Citation9). Acute cigarette smoking increases sympathetic nerve traffic to blood vessels, to skin and to the heart (Citation8). However, the effect of smoking status on resting muscle sympathetic nerve activity (MSNA) in hypertension is unknown. Therefore, we tested the hypothesis that smoking is associated with chronic sympathetic activation in patients with essential hypertension.
Materials and Methods
Subjects
We studied 68 hypertensive patients: 30 smokers (22 males) smoking 10–20 cigarettes/day for more than 2 years, and 38 non-smoking patients (30 males). Both groups had similar age (38±4 vs 42±3 years, respectively; mean±SEM), body mass index (BMI; 27±1 vs 27±1 kg/m2, respectively) and waist-to-hip ratio (0.92±0.03 vs 0.94±0.03, respectively).
The studies were approved by the Institutional Review Board on Human Investigation and written informed consent was obtained.
Measurements
Measurements of MSNA, blood pressure and HR were obtained in the morning or early afternoon. All smokers were asked to avoid smoking for at least 12 h prior to each study. Subjects were studied in the supine position. Multiunit postganglionic sympathetic nerve activity was recorded using tungsten microelectrodes (shaft diameter 200 µm, tapering to an uninsulated tip of 1–5 µm) inserted selectively into muscle fascicles of the peroneal nerve. A subcutaneous reference electrode was first inserted 2–3 cm away from the recording electrode, which was itself inserted into the nerve fascicle. The neural signals were amplified, filtered, rectified and integrated to obtain a voltage display of sympathetic nerve activity. MSNA was recorded during 10 min of undisturbed supine rest and expressed as bursts/min. Mean blood pressure was measured every minute with an Omron 705 IT sphygmomanometer. HR was measured by electrocardiography.
Twenty-four-hour ambulatory blood pressure and HR monitoring was performed with the Spacelabs 90202 recorder (Spacelabs Inc., Redmond, WA). The recorders were programmed to obtain measurements every 15 min from 06:00 to 22:00 h, and every 20 min from 22:00 to 06:00 h. Subjects were asked to continue their regular activities during the recordings, and to go to bed not later than 23:00 h. The daytime period was defined as the interval from 08:00 to 22:00 h, and night-time as the interval from midnight to 06:00 h.
Results are expressed as means±SEM. Comparisons between the two groups were made by an unpaired Student's t-test. The determinants of MSNA were assessed by linear regression analysis, with age, BMI, waist-to-hip ratio, gender and systolic blood pressure (SBP) as independent variables. A p<0.05 was considered significant.
Results
Smokers and non-smokers were comparable for resting blood pressure and HR (). HR in smokers was greater than in non-smokers during both daytime and night-time (). In comparison with non-smokers, smokers had smaller office–daytime SBP difference ().
Table I. Blood pressure and heart rate in smokers and non-smokers.
MSNA was elevated in smokers compared with non-smokers (). Similar results were obtained when MSNA was expressed as bursts/100 heart beats (53±3 vs 44±4 bursts/100 heart beats, respectively; p= s0.04).
Figure 1. Muscle sympathetic nerve activity in hypertensive smokers and non-smoking hypertensives. Values are means±SEM.
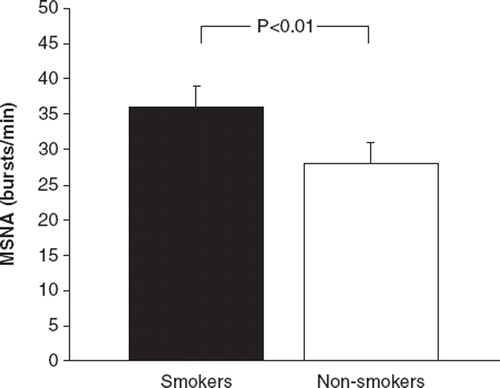
Analysis of pooled data revealed that MSNA correlated significantly with age (r=0.50, p<0.001), but not with BMI (r=0.08; NS), waist-to-hip ratio (r=0.14; NS) or SBP (r=0.21; NS). MSNA was similar in female and male patients (35±3 vs 32±2 bursts/min, respectively; p=NS). Stepwise multiple linear regression analysis revealed that only age and smoking status were linked independently to MSNA (R2=0.42, p< 0.001).
Discussion
This study provides direct evidence that smoking is associated with chronic sympathetic activation in hypertension. The link between smoking and increased MSNA is independent of age, BMI, waist-to-hip ratio, HR or blood pressure.
Despite the acute pressor effect of cigarette smoking, several earlier epidemiological studies failed to confirm an independent link between smoking and hypertension (Citation10). Therefore, smoking was traditionally not considered a risk factor for hypertension. The vast majority of these studies were based on office measurements in subjects abstaining from smoking. Also in our study, smokers and non-smokers had similar resting blood pressure. However, smokers had smaller office–daytime SBP difference. These findings are consistent with the results of other studies indicating that office measurement may underestimate pressor effects of smoking (Citation11,Citation12).
Sympathetic overactivity may play an important mechanistic role in the short- and long-term reduction of large artery compliance (Citation13), and conceivably contributes to the well-established association between arterial stiffening and cardiovascular risk (Citation14,Citation15). Prior studies have consistently shown that both acute and chronic cigarette smoking decreases arterial compliance (Citation16–19). The results of the current study indicate that the effects of smoking on arterial wall properties in habitual smokers may be mediated by chronic sympathetic activation.
The mechanisms underlying the link between smoking and chronic sympathetic activation are not clear, but several factors might be implicated. First, the sensitivity of the arterial baroreflex is markedly reduced by acute smoking (Citation20). Thus, increased sympathetic traffic might reflect the impairment of baroreflex function due to above-mentioned smoking-related reduced arterial distensibility and consequent loss of stretch receptor responsiveness. Second, nicotine increases peripheral chemoreceptor sensitivity, which may further enhance the sympathoexcitatory effects of smoking (Citation21,Citation22).
The relation between the smoking-related arterial stiffness and the chronic sympathetic activation is likely to represent a positive feedback mechanism. The sympathetic system might contribute to impairment of large artery function through promotion of vascular muscle growth and associated fibrosis. In turn, reduced arterial distensibility and impaired baroreflex function facilitate further activation of the sympathetic nervous system. The resetting of baroreflex by both sympathetic overactivity and structural changes might facilitate target organ damage. Taken together, these pathophysiological considerations provide compelling precedent for the concept of interactive contribution of smoking-related sympathetic overactivity and arterial stiffness to cardiovascular risk. Smoking-related chronic sympathetic activation might help to explain the results of the LIFE study showing that both composite and stroke risks were lower with losartan than atenolol in never-smokers, but not in current-smokers (Citation23).
In conclusion, smoking is associated with chronic sympathetic activation in hypertension. These findings may have implications for our understanding of the mechanisms linking smoking to cardiovascular events.
Acknowledgements
The Authors are supported by Foundation for Polish Science TEAM/2008-2/5 and MISTRZ 8/2008 grants, and by European Union LSHM-CT-2006-037093 InGenious grant.
Declaration of interest: The authors report no conflict of interest.
References
- Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–497.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case–control study. Lancet. 2004;364: 937–952.
- Kannel W, Higgins M. Smoking and hypertension as predictors of cardiovascular risk in population studies. J Hypertens. 1990;8 Suppl 5:S3–S5.
- Szczęch R, Hering D, Narkiewicz K. Smoking and cardiovascular risk: New mechanisms and further evidence for a ‘guilty’ verdict. J Hypertens. 2004;22:31–34.
- Mark AL. The sympathetic nervous system in hypertension: A potential long-term regulator of arterial pressure. J Hypertens. 1996;14 Suppl 5:S159–S165.
- Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: Translation from pathophysiology into clinical practice. Acta Physiol Scand 2003;177:275–284.
- Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: Achievements and perspectives. Hypertension. 2009;54:690–697.
- Narkiewicz K, van de Borne PJH, Hausberg M, Cooley RL, Winniford MD, Somers VK. Cigarette smoking increases sympathetic outflow. Circulation. 1998;98:528–534.
- Hering D, Somers VK, Kara T, Kucharska W, Jurak P, Bieniaszewski L, Narkiewicz K. Sympathetic neural responses to smoking are age dependent. J Hypertens. 2006;24:691–695.
- Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Press. 2005;14:69–71.
- Mann SJ, James GD, Wang RS, Pickering TG. Elevation of ambulatory SBP in hypertensive smokers. JAMA. 1991;265:2226–2228.
- Narkiewicz K, Maraglino G, Biasion T, Rosii G, Sanzuol F, Palatini P. Interactive effects of cigarettes and coffee on daytime systolic blood pressure in patients with mild essential hypertension. J Hypertens. 1995;13:965–970.
- Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, . An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010 (in press).
- Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, . Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34: 1203–1206.
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–15.
- Failla M, Grappiolo A, Carugo S, Calchera I, Giannattasio C, Mancia G. Effects of cigarette smoking on carotid and radial arterial distensibility. J Hypertens. 1997;15: 1659–1664.
- Mahmud A, Feely J. Effects of smoking on arterial stiffness and pulse pressure amplification. Hypertension 2003;41:183–187.
- Kool MJ, Hoeks AP, Struijker Boudier HA, Reneman RS, Van Bortel LM. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol. 1993;22:1881–1886.
- Liang YL, Shiel LM, Teede H, Kotsopoulos D, McNeil J, Cameron JD, . Effects of blood pressure, smoking and their interaction on carotid artery structure and function. Hypertension 2001;37:6–11.
- Mancia G, Groppelli A, Di Rienzo M, Castiglioni P, Parati G. Smoking impairs baroreflex sensitivity in humans. Am J Physiol. 1997;273:H1555–H1560.
- Argacha JF, Xhaët O, Gujic M, Adamopoulos D, Beloka S, Dreyfuss C, . Nicotine increases chemoreflex sensitivity to hypoxia in non-smokers. J Hypertens. 2008;26:284–294.
- Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol. 2008;35:458–463.
- Reims HM, Oparil S, Kjeldsen SE, Devereux RB, Julius S, Brady WE, .; LIFE Study Group. Losartan benefits over atenolol in non-smoking hypertensive patients with left ventricular hypertrophy: The LIFE study. Blood Press. 2004;13:376–384.
