Abstract
Metabolic syndrome (MetSy) is associated with a high risk of cardiovascular complications. Arterial stiffness is an independent predictor of cardiovascular morbidity and mortality. This study investigated the effect of individual MetSy risk factors on central and peripheral parameters of aortic stiffness. In the Czech post-MONICA study, we measured aortic pulse-wave velocity (aPWV), lower extremity pulse-wave velocity (lePWV), augmentation index (AIx) and central augmentation pressure (cAP) in 936 subjects. Based on the definition of MetSy, we divided subjects according to number of risk factors. We used univariate and multivariate linear regression analysis to assess the association between number of risk factors and aPWV, lePWV, AIx and cAP. In analyses adjusted for age, gender, heart rate and mean arterial pressure, aPWV was higher in subjects with MetSy (MetSy+ group) than in those without (MetSy + group) (8.3 vs 7.7 m/s; p < 0.0001), but lePWV was not significantly different between the groups (11.0 vs 11.2 m/s; p = 0.2037). After adjustment for covariates, AIx in MetSy+ was lower than in MetSy− respondents (143.2 vs 146.8; p = 0.014). In adjusted analysis, aPWV rose with increasing number of MetSy risk factors (7.3 ± 0.1 vs 9.0 ± 0.1 m/s; p for trend < 0.0001). The number of MetSy risk factors did not affect lePWV (p = 0.11). AIx decreased with higher number of MetSy risk factors (148.3 vs 141.5; p = 0.020). This finding confirms the fact that PWV and AIx may have different associations with risk factors and AIx should not be used as an isolated parameter of arterial stiffness. The individual MetSy risk factors have only a small effect on lower extremity arterial stiffness.
Introduction
Metabolic syndrome (MetSy) as a cluster of cardiovascular risk factors is associated with a high risk of cardiovascular complications. MetSy is highly prevalent worldwide and its prevalence is growing. This trend seems to be in parallel with the rising prevalence of obesity. The prevalence of MetSy reached approximately 24% in the US adult population (Citation1), while in Europe the number is very similar at about 25% (Citation2)
Several studies have shown positive associations between aortic stiffness, measured by aortic pulse-wave velocity (aPWV), and MetSy (Citation3,Citation4). The mechanisms through which MetSy increases cardiovascular risk may also involve pathophysiological changes in the arterial wall, which lead to an increase in large artery stiffness (Citation5)
Arterial stiffness is a well-recognized independent predictor of cardiovascular morbidity and mortality (Citation6–9). European hypertension guidelines list aPWV as a marker of subclinical organ damage (Citation10). On the other hand, the relationship between lower extremity pulse-wave velocity (lePWV) and cardiovascular events is less clear, as is the knowledge about determinants of lePWV (Citation11). Several papers have demonstrated an association between increased cardiovascular risk and parameters of pressure-wave reflection, i.e. augmentation index (AIx) and augmentation pressure (Citation12). To our knowledge, no previous study has investigated the effect of the presence of individual MetSy risk factors on lePWV, AIx and central augmentation pressure (cAP).
Therefore, in the present study we aimed to compare the effect of individual MetSy risk factors on aPWV, peripheral pulse-wave velocity (represented by lePWV) and wave-reflection parameters, i.e. AIx and cAP, in subjects from a Czech general population.
Methods
Study population
The Czech post-MONICA (MONItoring trends and determinants in CArdiovascular disease) study is a population survey studying trends and determinants of cardiovascular risk factors in a 1% random sample of the Czech population in nine districts of the country. Methods of the Czech post-MONICA study are described elsewhere (Citation13). The present analysis included individuals examined in the city of Pilsen. The overall response rate in this district was 68.0%. From 1007 participants, we excluded 71 because either biochemistry data or some wave-reflection parameters were missing. Thus, the number of subjects analysed was 936. All individuals gave their informed consent before their inclusion in the study.
The research protocol included the administration of a standardized questionnaire to obtain information on each subject's medical history, smoking and use of medications. Blood pressure was measured in triplicate in the right arm with the subject in the sitting position after at least 5 min at rest. Standard mercury sphygmomanometers and correctly sized cuffs were used. The participant's right arm was supported at heart level. The maximum inflation level was determined before the actual measurement. Blood pressure values were recorded to the nearest 2 mmHg. The mean value of the last two readings was used for further analysis. Mean arterial pressure (MAP) derived from office blood pressure measurement was calculated as diastolic blood pressure (DBP) plus one-third of pulse pressure. Furthermore, blood samples were obtained for biochemical analyses. Diabetes was defined as fasting plasma glucose ≥ 7.0 mmol/l or the use of oral antidiabetic drugs and/or insulin. Height and body weight were determined for all participants. Body mass index (BMI) was calculated as body weight/height² (kg/m²). The waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest, standing, at the end of gentle expiration.
The MetSy was defined on the basis of the National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines (Citation14), modified according to the harmonized definition for the Czech population, which is based on the presence of three or more of the following characteristics: (i) arterial hypertension or antihypertensive drug treatment (blood pressure ≥ 130/≥ 85 mmHg); (ii) pathological waist circumference (≥ 102 cm in men, ≥ 88 cm in women); (iii) serum triglycerides ≥ 1.7 mmol/l or lipid-lowering drug treatment; (iv) high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/l in men, < 1.3 mmol/l in women; (v) fasting glycaemia ≥ 5.6 mmol/l or impaired fasting glucose or the presence of type 2 diabetes mellitus.
Arterial measurement
Large artery properties were measured using the SphygmoCor device (AtCor Medical, West Ryde, NSW, Australia) in the recumbent position as reported previously (Citation11). The aPWV and lePWV were assessed according to recommendations (Citation15). Consecutive registrations of the pulse waves are electrocardiographically gated and, thus, the time shift (Dt) between the foot of wave at the first and second sites can be calculated. The distance between the two sites was measured on the body surface. To determine aPWV, we measured the distance from the jugular fossa to the pulsation of the femoral artery in the groin and subtracted the distance from the jugular fossa to the carotid pulsation to obtain the travelled distance (D). The distance between the femoral artery and dorsal pedal/posterior tibial arteries was measured to calculate lePWV. PWV was calculated as D (m)/Dt (s) (Citation16).
Pulse pressure (PP) was defined as the difference between systolic and diastolic blood pressure (PP = SBP− DBP). The cAP and central AIx were measured at the carotid artery. cAP was calculated as the difference between the second and first systolic peaks of the aortic pressure waveform (P2 − P1, where P1 is an inflection point of the reflected pressure wave, and P2 is a point of maximal-systolic blood pressure enhanced by the return of wave reflections) (Citation17). The aortic AIx was defined as P2/P1, i.e. the ratio of pulse pressures measured at the peaks of secondary and primary waves, expressed as a percentage.
Statistical methods
For database management and statistical analyses, we used the SAS software, version 9.3 (SAS Institute, Cary, NC, USA). Data are presented as mean ± SD or proportions.
A Student's t test and chi-squared test were used to compare differences between subjects with and without MetSy. To explore the effect of MetSy factors, we divided subjects into five groups according to the number of MetSy risk factors. We used univariate and multivariate linear regression analyses to assess associations between the number of risk factors and aPWV, lePWV, AIx and cAP. Furthermore, we investigated how much variance in aPWV can be explained by the individual MetSy risk factors.
Results
Characteristics of participants
Out of 936 participants, 334 subjects (35.7%) fulfilled the definition of MetSy. The clinical characteristics of the study cohort according to the presence or absence of MetSy are given in . The subjects with MetSy (MetSy+), compared to those without Metsy (MetSy−), were older, had higher blood pressure, glycaemia and triglycerides (TAG; p for all < 0.0001). MetSy+ subjects were more frequently men, were obese or had a pathological waist circumference, and more often used antihypertensive, antidiabetic or lipid-lowering medication (p for all ≤ 0.0047).
Table I. Clinical characteristics of the study cohort according to the presence (MetSy+) or absence (MetSy−) of metabolic syndrome.
The two groups had similar ratios of smokers and non-smokers. In unadjusted analysis, both aPWV (9.0 vs 7.3 m/s; p < 0.0001) and lePWV (11.4 vs 10.9 m/s; p < 0.0001) were higher in MetSy+ than in MetSy− subjects (). However, after adjustment for age, gender, heart rate and MAP, the difference remained significant only for aPWV (8.3 vs 7.7 m/s; p < 0.0001), but not for lePWV (11.0 vs 11.2 m/s; p = 0.2037). In unadjusted analysis, central AIx was higher in the MetSy+ group (147.5 vs 145.8; p = 0.33) than in MetSy−. After adjustment for covariates, AIx in MetSy+ was lower than in MetSy− respondents (143.2 vs 146.8; p = 0.014). Finally, cAP was significantly higher in the MetSy+ group (16.2 vs 14.0; p < 0.0001), but this difference did not hold after adjustment for age, gender and heart rate (14.8 vs 14.6; p = 0.68).
Distribution of individual risk factors
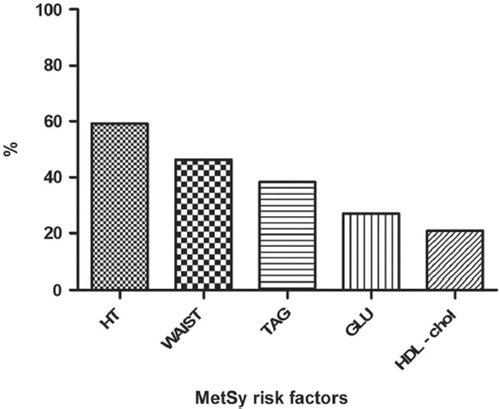
shows the distribution of individual MetSy risk factors in the whole study cohort. The most frequent risk factor was arterial hypertension (59.2%), followed by pathological waist circumference (46.4%), while the least frequent risk factor was a low level of HDL-C (20.9%).
Figure 1. Distribution of individual metabolic syndrome (MetSy) risk factors. HT, arterial hypertension; WAIST, pathological waist (waist circumference ≥ 102 cm in men, ≥ 88 cm in women); TAG, triglycerides; GLU, glycaemia; HDL-chol, high-density lipoprotein cholesterol.
The clinical characteristics of the study cohort according to the number of MetSy risk factors are presented in . gives arterial parameters according to the number of MetSy risk factors. After adjustment for age, gender, heart rate and MAP, the aPWV rose with increasing number of MetSy risk factors (7.3 ± 0.1 m/s in subjects with no risk factor vs 9.0 ± 0.1 m/s in subjects with at least four risk factors; p for trend ˂ 0.0001) (). The number of MetSy risk factors did not affect lePWV (p = 0.11) (). The AIx decreased with higher number of MetSy risk factors (148.3 ± 1.5 vs 141.5 ± 1.6%; p = 0.020) (). For cAP, we observed a curvilinear relationship (p = 0.025) ().
Figure 2. Association between number of metabolic syndrome (MetSy) risk factors, pulse-wave velocity and parameters of wave reflection. RFs, risk factors; PWV-ao, aortic pulse-wave velocity; PWV-le, lower extremity pulse-wave velocity; cAIx, central augmentation index; cAP, central augmentation pressure.
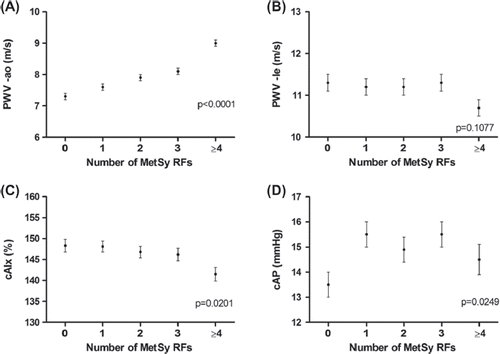
Table II. Clinical characteristics of the study cohort according to number of metabolic syndrome (MetSy) risk factors.
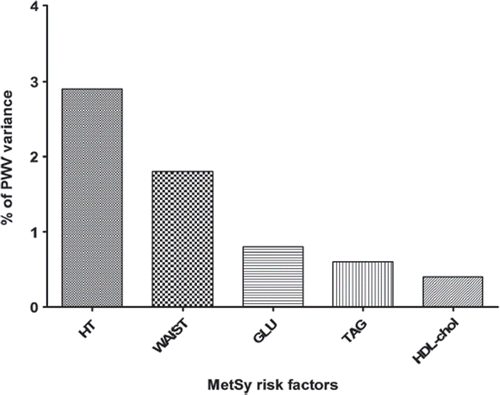
In the next step, we investigated which MetSy risk factor is the most significant determinant of aPWV. The highest proportion of PWV variance was explained by the presence of arterial hypertension (2.9%), the next one was pathological waist circumference (1.8%), while the remaining factors played only a minor role ().
Figure 3. Proportion of aortic pulse-wave velocity (PWV) variance explained by individual metabolic syndrome (MetSy) risk factors. HT, arterial hypertension; WAIST, pathological waist (waist circumference ≥ 102 cm in men, ≥ 88 cm in women); GLU, glycaemia; TAG, triglycerides; HDL-chol, high-density lipoprotein cholesterol.
The main results were not altered even after additional adjustment for lipid-lowering drugs and antidiabetes treatment (data not shown).
Discussion
The main finding of this study is that aPWV increased linearly with rising numbers of individual MetSy risk factors in a general population. On the other hand, the peripheral PWV was not related to MetSy risk factors. After adjustment for confounders, central AIx decreased with a high number of MetSy risk factors. To our knowledge, no previous study has investigated the impact of MetSy risk factors on several parameters of wave reflection. The existence of a strong association between the presence of MetSy and arterial stiffness has been shown in many cross-sectional papers. Safar et al., in a longitudinal study (Citation18), observed accelerated aortic ageing (expressed by increased progression of aPWV) in subjects with present MetSy compared to subjects without MetSy. Moreover, Schillaci et al. reported that the presence of MetSy and number of MetSy risk factors increased cardiovascular risk independently of several traditional cardiovascular risk factors (Citation19). In our cross-sectional study, we compared patients with no risk factors up to at least four MetSy risk factors. The increase in aortic stiffness was significantly more expressed with growing number of MetSy risk factors. Of the MetSy risk factors, the parameter with the strongest effect on aortic stiffness was arterial hypertension. The second highest effect on aortic stiffness came from pathological waist circumference. This finding is in agreement with Wohlfahrt et al., who demonstrated that central obesity parameters are more closely associated with higher aPWV than BMI (Citation20).
On the other hand, the lowest proportion of aPWV variance was explained by HDL-C level. This fact can be partially explained by the frequent use of lipid-lowering drug treatment in the MetSy+ group (42%, of whom 82% used statins). Indeed, several studies have demonstrated the beneficial effects of statin therapy on HDL-C. Barter et al. demonstrated that the rising HDL-C level achieved by statin therapy was totally independent of the reduction in low-density lipoprotein cholesterol (LDL-C). Moreover, it has been found that baseline concentrations of HDL-C, plasma TAG level and the presence of diabetes are powerful, independent predictors of statin-induced elevations of HDL-C (Citation21). Lowering the LDL-C level and increasing the HDL-C level may improve artery wall properties, so cholesterol-lowering therapy may reduce large artery stiffness (Citation22). Matsuo et al. described a positive vascular (antioxidant and anti-inflammatory) effect of statins independent of any cholesterol-lowering effect; therefore, statins may also be useful as antiarteriosclerotic agents (Citation23). Alternatively, the lowest effect of HDL-C on aortic stiffness can be explained by the fact that cholesterol level may not be a strong predictor of arterial stiffness. Indeed, recent understanding is that HDL-C is merely a cardiovascular risk marker and not causally related to coronary heart disease (Citation24).
In contrast to aortic stiffness, we did not observe any association between the number of MetSy risk factors and peripheral arterial stiffness. This may be due to the different histological structure of these arteries. Indeed, we have previously shown different impacts of several classic cardiovascular risk factors on aPWV and lePWV (Citation11).
The negative association observed between AIx and number of MetSy risk factors, despite the fact that the analysis was adjusted for gender and heart rate, may seem to be illogical as both factors influence wave reflections. Nevertheless, this can be partially explained by the increasing pulse pressure and thus increasing denominator in the equation used for the calculation of AIx. This finding confirms that PWV and AIx may have different associations with risk factors and AIx should not be used as an isolated parameter of arterial stiffness.
Our results have to be interpreted within the context of the study's limitations. The main limitation is the cross-sectional nature of this study, meaning that the question of cause and consequence cannot be answered on the basis of our data. On the other hand, the sample size was relatively large, which allowed us to compare the proportional representation of individual risk factors and their influence on the aortic stiffness.
In conclusion, in the present study we investigated the impact of individual MetSy risk factors on aortic stiffness. Arterial hypertension had the highest effect, while HDL-C had the smallest effect. Moreover, our results suggest that MetSy risk factors have only a small effect on lower extremity arterial stiffness.
Funding
This study was supported by the Charles University Research Fund (project no. P36), by project ED2.1.00/03.0076 from the European Regional Development Fund, and by unrestricted research grants from Krka Czech Republic s.r.o. and Servier Czech Republic s.r.o.
Declaration of interest: None of the authors has a conflict of interest with regard to the data presented in this paper. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an Abstract.
References
- Ford SE, Giles WH. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care. 2003;26:575–81.
- Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36.
- Scuteri A, Cunha PG, Rosei EA, Badariere J, Bekaert S, Cockcroft JR, et al.; MARE Consortium. Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis. 2014;233:654–60.
- Ferreira I, Boreham CA, Twisk JW, Gallagher AM, Young IS, Murray LJ, Stehouwer CD.Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: the Northern Ireland Young Hearts Project. J Hypertens. 2007;25: 1009–20.
- Stehouwer CDA, Henry RMA, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–39.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
- Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6.
- Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–60.
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–46.
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. J Hypertens. 2013;31: 1281–357.
- Wohlfahrt P, Krajčoviechová A, Seidlerová J, Galovcová M, Bruthans J, Filipovský J, et al. Lower-extremity arterial stiffness vs aortic stiffness in the general population. Hypertens Res. 2013;36:718–24.
- Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–5.
- Cífková R, Škodová Z, Lánská V, Adámková V, Novozámská E, Jozífová M, et al. Prevalence, awareness, treatment, and control of hypertension in the Czech Republic. Results of two nationwide cross-sectional surveys in 1997/1998 and 2000/2001, Czech Post-MONICA Study. J Hum Hypertens. 2004;18:571–9.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome. Circulation. 2009;120:1640–5.
- Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al.; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8.
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–84.
- Protogerou AD, Blacher J, Aslangul E, Le Jeunne C, Lekakis J, Mavrikakis M, Safar ME. Gender influence on metabolic syndrome's effects on arterial stiffness and pressure wave reflections in treated hypertensive subjects. Atherosclerosis. 2007;193:151–8.
- Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol. 2006; 47:72–5.
- Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C, Mannarino E. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004;43:1817–22.
- Wohlfahrt P, Somers VK, Cifkova R, Filipovsky J, Seidlerova J, Krajcoviechova A, et al. Relationship between measures of central and general adiposity with aortic stiffness in the general population. Atherosclerosis. 2014;235:625–31.
- Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ.Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER database. J Lipid Res. 2010;51:1546–53.
- Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002; 39:1020–5.
- Matsuo T, Iwade K, Hirata N, Yamashita M, Ikegami H, Tanaka N, et al. Improvement of arterial stiffness by the antioxidant and anti-inflammatory effects of short-term statin therapy in patients with hypercholesterolemia. Heart Vessels. 2005;20:8–12.
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80.
