Abstract
Diabetes mellitus, a disorder characterized by chronic hyperglycemia and excessive urine excretion, is associated with complications such as atherosclerosis, cardiac dysfunction, and nephropathy. Renal disease, which develops through a number of metabolic pathways in diabetes, is characterized by functional as well as structural abnormalities of the kidney. The most common cause of end-stage renal disease (ESRD) is diabetic nephropathy, which accounts for significant morbidity and mortality. Current conventional diabetes therapy using blood glucose-lowering medications has limitations in averting the development of renal diseases. The onset of diabetic nephropathy is associated with a progressive rate of decline in renal function, urinary albumin excretion, and glomerular filtration rate (GFR). Diabetes mellitus treatment should therefore aim to intervene on promoters of the decline in renal function in diabetes to avert adverse outcomes. Preventing the pathogenesis of nephropathy with therapeutic interventions based on specific alterations in kidney function represents a plausible approach. Cumulative evidence suggests that some herbal extracts with hypoglycaemic properties may have beneficial effects on some processes associated with a decline in renal function, as well as reduce the severity of nephropathy in diabetic experimental animals. On the other hand, some herbal extracts may be hazardous in diabetes, as reports indicate impairment of renal function. This review outlines current evidence supporting plant extracts with the potential of averting progressive renal diabetic complications as well as the nephrotoxicity of some herbal extracts.
INTRODUCTION
Diabetic nephropathy, the predominant cause of end-stage renal disease (ESRD) in Western countries, is a major cause of death and illness in diabetes.Citation[1,Citation2] The morbidity and mortality associated with diabetic nephropathy have placed in the forefront attempts to discover new therapies that may prevent the advent of diabetic nephropathy. The onset of nephropathy is associated with a progressive decline in renal function. Factors that denote the decline of renal function in diabetes include decreased glomerular filtration rate (GFR) and increased blood pressure and urinary albumin excretion.Citation[3,Citation4] The prevention of the occurrence and progression of diabetic nephropathy have become important clinical issues.Citation[2] To date, treatments for progressive diabetic nephropathy are antihypertensive agents, particularly inhibitors of the renin-angiotensin system (RAS), angiotensin-converting enzyme (ACE), angiotensin receptor antagonists, or their combination.Citation5-9 Although these treatments slow down the progression toward ESRD, clinical trials suggest that there is no effective treatment for diabetic nephropathy.Citation[2] However, a substantial body of evidence suggests that herbal extracts possess a range of important pharmacological properties that may retard the progressive decline in renal function in diabetes.Citation[10,Citation11] Studies suggest that plant extracts with hypoglycaemic properties alleviate kidney complications associated with clinical diabetes mellitus Citation12-15 and reduce the severity of nephropathy in experimental animals.Citation[16] Indeed, Chinese herbal extracts indicate therapeutic potential against diabetic nephropathy in terms of their effects on associated diabetic metabolic disorders.Citation[17] The mechanisms by which the plant extracts influence kidney function at molecular, physiological, and biochemical levels could be useful in the treatment of renal complications in diabetes. Anti-diabetic extracts of Sclerocarya birrea [(A. Rich) Hochst.] [Anachardiaceae], Persea americana (Miller) [Lauraceae], and Ficus thonningii (Blume) [Moraceae] have been reported to reverse the inability of the kidney to excrete Na+ in streptozotocin (STZ)-diabetic rats.Citation[18,Citation19] On the other hand, observations indicate that some anti-diabetic herbal extracts may impair kidney function, as indicated by an increased renal fluid and electrolyte retention and reduced GFR.Citation[20,Citation21] It is therefore possible to base diabetes treatment on specific alterations in kidney function to avert adverse outcomes. In this review, we discuss recent advances in the research on some plant extracts for pharmacological intervention in renal complications in diabetes and kidney-related injuries as well as the potential for nephrotoxicity from other plant extracts. The following sections discuss current evidence on therapeutic intervention with plant extracts and beneficial effects on the kidney in diabetes.
KIDNEY FUNCTION CHANGES IN DIABETES MELLITUS
Sustained hyperglycaemia has been shown to be the major risk factor responsible for the development of a decline in kidney function in diabetic patientsCitation22–24 and experimental animals.Citation[20,Citation21,Citation25,Citation26] Common clinical manifestations of impaired kidney function in diabetes mellitus include hypertension, protenuria, declining GFR, as well as histological characteristics such as glomeruloscelorosis.
METABOLIC CHANGES AND MANAGEMENT IN DIABETES
Kidney pathophysiology in diabetes is precipitated by interactions between metabolic and haemodynamic factors. The changes may be as a result of the effects of excess glucose directly on kidney cells or indirectly through pathways that involve formation of oxidants and advanced glycation end products.Citation[22,Citation27,Citation28] Chronic hyperglycaemia accelerates the activation of the formation of advanced glycation end-products (AGEs), polyol pathway, and the protein kinase C pathway.Citation29-32 The metabolic factors are synergistically correlated with one another, and thus an effective treatment with widespread effects continues to be required. Therapeutic interference with the metabolic pathways involved in the development of diabetic nephropathy, however, preserves the structure of kidneys in experimental animals.Citation[33]
Advanced Glycation End Products (AGEs)
AGEs are a group of molecules formed from the reaction of reducing sugars with free amino groups of proteins, lipids, and nucleic acids. The interaction of AGE with its receptor (RAGE) activates several intracellular signaling pathways, such as mitogen-activated protein kinase, nuclear factor KB, and AP-1, and increases the production of cytokines.Citation[34] The effects are associated with increased production of growth factorsCitation[35] and reactive oxygen species (ROS).Citation[27,Citation36] The AGE-RAGE binding leads to albuminuria and mesangial expansion resulting in glomerular sclerosis.Citation[27,Citation28,Citation36–38 The events described lead to diabetic complications, which include retinopathy, nephropathy, and atherosclerosis. Therefore, the AGE-RAGE pathway is considered as a candidate molecular target for the prevention and treatment of diabetic nephropathy.Citation[39]
The accumulation of AGEs can be prevented by antioxidants like flavonoids or by preventing the glucose-dependent formation of intermediate products (Amadori, Schiff bases, or Milliard products). Indeed, blocking or deleting AGE's receptor (RAGE) in experimental animals reverses atherosclerosis.Citation[40] Amino guanidine and pyridoxamine, AGEs formation inhibitors, have renoprotective effects in diabetic animals.Citation[38,Citation41] Furthermore, the inhibition of AGEs effects can be achieved through breaking the AGEs cross links by drugs such as alagebrium or by inhibiting AGE signal transduction.Citation[38] Indeed, it has been reported that metformin, a plant-derived anti-diabetic drug, may be useful in the prevention of the development of AGEs.Citation[42] Extracts of Panax quinquefolium (Linnaeus) [Araliaceae], resveratrol, a phytoestrogen derived from Vitis vinifera (Linnaeus) [Vitaceae]; curcumin from Curcuma longa (Linnaeus) [Zingiberaceae]; and glycosides from Stelechocarpus cauliflorus (R.E. Fr) [Annonaceae] have also been reported to inhibit formation of AGEs or RAGE.Citation[28,Citation43-45
Polyol Pathway
The activation of the polyol pathway (aldose reductase pathway) is involved in the pathogenesis of renal complications in diabetes mellitus via hyperglycaemia-induced glomerular and tubular cell damage.Citation[46] Hyperglycemia-induced renal polyol pathway activity occurs in tandem with oxidative changes, stimulation of the formation of AGEs, and protein kinase C (PKC) pathways. In addition, the pathway stimulates PKC-mediated glomerular prostaglandin production and loss of mesangial cell contractilityCitation[47] to possibly cause hyperfiltration and glomerular dysfunction in diabetes mellitus. Inhibition of aldose reductase with synthetic drugs such as zenarestat and sorbinil and plant extracts has been reported to prevent or alleviate in part some of the diabetic renal complications.Citation[27,Citation47,Citation48] Quercetin, silymarin, flavonoids, and puerarin isolated from plants inhibit aldose reductase activity.Citation[15] In addition, glycosides engeletin and astilbin isolated from Stelechocarpus cauliflorous [Annonaceae] leaves inhibit aldose reductase activity.Citation[45] Furthermore, rhizomes of Coptis chinensis (Franch) [Ranunculaceae]- and Phellodendron amurense (Ruprecht) [Rutaceae]-derived berberine inhibits aldose reductase activity with concomitant inhibition of oxidative stress.Citation[49]
Protein Kinase C
The activation of protein kinase C (PKC) and its isoforms has been implicated in the pathogenesis of diabetic nephropathy.Citation[50] The intracellular mechanism is the glucose-induced de novo synthesis of diacylglycerol, one of the intracellular activators of PKC isoformsCitation[27] implicated in the pathogenesis of diabetic nephropathy.Citation[27,Citation28,Citation51,Citation52] In addition to high glucose, several nephrotoxic factors such as inflammatory mediators, growth factors, hypoxia, and vasoactive hormones activate PKC-ß isoform,Citation[27,Citation50,Citation51,Citation53–56 which is highly expressed in various forms of glomerulonephritis.Citation[57] Thus, the inhibition of PKC system may provide a new therapeutic strategy to prevent complications of diabetes. Indeed, ruboxistaurin, a specific inhibitor of PKC-ß, has shown efficacy in the treatment of diabetic renal abnormalities both in diabetic patients and experimental animals.Citation[28,Citation50,Citation58,Citation59] Parthenolide, a plant-derived extract, has also been reported to avert a declining GFR and reduce proteinuria in experimental animals.Citation[60] Interestingly, PKC-modulating compounds are easily obtained from vegetables, berries, spices, tea, and wine, including quercetin.Citation61–64
Herbal-Related Nephrotoxicity
As herbal medicine becomes increasingly practiced worldwide, reports of adverse effects occasionally appear in literature, and questions are being asked about safety. Plants can contain pharmacologically useful and active compounds, but unsurprisingly, they can contain toxic substances. Indeed, current evidence also indicates that certain herbal extracts and plant products ameliorate the deterioration of kidney function in diabetes mellitus.Citation[65] Against this background is the observation that the Hypoxis hemerocallidea extract (i.e., the corm, popularly known as the “African Potato”), besides possessing anti-diabetic properties, impairs kidney function, as indicated by its increasing renal fluid and electrolyte retention and reducing GFR in experimental animals.Citation[66]
EXTRACTION AND ISOLATION OF BIOACTIVE PLANT COMPOUNDS
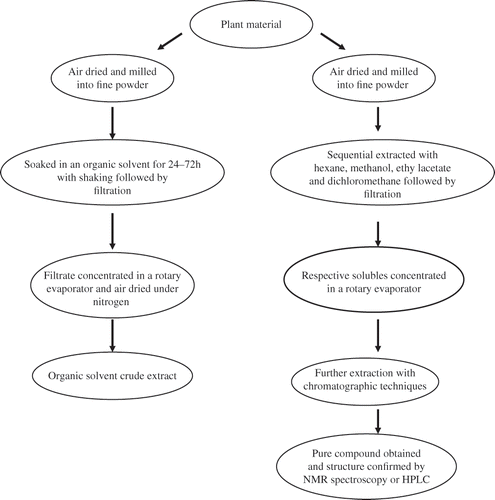
The chemical constituents of medicinal plant extracts, which determine the therapeutic activity, can be classified into major groups, such as essential oils, alkaloids, acids, steroids, tannins, and saponins. Each one of these classes of chemicals has a preferred effective method of extraction. Hence, the techniques for the extraction and analysis of medicinal plants play an important role in ensuring and providing high-quality herbal products. The techniques include solid-phase microextraction, supercritical-fluid extraction, pressurized-liquid extraction, solid-phase extraction, and surfactant-mediated extraction.Citation67–69 The most commonly used sample-preparation techniques for the extraction, clean up, and concentration of analytes from medicinal plants or herbal materials are depicted in .
We have used organic solvents to extract bioactive compounds from plants such as Syzygium cordatum and Ficus thonningii.Citation[70] Investigations have also been extensively conducted with triterpenoids, xanthones, polysaccharides, or flavonoids isolated from plants.Citation[13] An overview of some effects of the renal effects of some hypoglycaemia-inducing plant species obtained from current literature is given in .
Table 1 Partial survey of medicinal plants/plant extracts that influence renal function/structure
CONCLUSION
Literature evidence suggests that some plant extracts contain pharmacologically active compounds, which may have beneficial roles in slowing progressive renal disease. Furthermore, there are clearly many points in diabetes at which therapeutic approaches with plant-derived extracts could be tried to provide renoprotection. Targeting multiple options of altered kidney function in diabetes may be more appropriate to retard the development of diabetic nephropathy.
ACKNOWLEDGMENTS
The authors report no conflicts of interest.
REFERENCES
- Selby JV, FitzSimmons SC, Newman JM, Katz PP, Sepe S, Showstack J. The natural history and epidemiology of diabetic nephropathy. Implications for prevention and control. JAMA 1990;263(14):1954–1960.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.
- Rossing P. Promotion, prediction and prevention of progression of nephropathy in type 1 diabetes mellitus. Diabet Med. 1998;15:900–919.
- Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999;55(1):1–28.
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355 (9200):253–259.
- Mogensen CE, Neldam S, Tikkanen I, Randomized controlled trial of dual blockade of rennin-angiotensin system in patients with hypertension, microalbuminiria and non-insulin dependent diabetes in Candesartan and Lisinopril Microalbuminuria (CALM) study. BMJ. 2000;321 (7274): 1440–1444.
- Yusuf S, Dagenais G, Pogue J, Bosh J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk populations. The Heart Outcomes Prevention Study Investigators. N Engl J Med. 2000;342:154–160.
- Brenner BM, Cooper ME, de Zeeuw D, RENAAL Study Investigators. N Engl J Med. 2001;345(12):861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860.
- Nakagawa T, Yokozawa T, Terasawa K, Nakanishi K. Therapeutic usefulness of Keishi-bukuryo-gan for diabetic nephropathy. J Pharm Pharmacol. 2003;55(2):219–227.
- Yokozawa T, Kim HY, Yamabe N. Amelioration of diabetic nephropathy by dried Rehmannie radix (Di Huang) extract. Am J Chin Med. 2004;32(6):829–839.
- Marles R, Farnsworth N. Plants as sources of antidiabetic agents. In: Wagner H, Farnsworth NR ( eds.). Economic and medicinal plant research. Vol. 6. Academic Press Ltd., London, UK; 1994:149–187.
- Wang TG, Ng TB. Natural products with hypoglycemic, hypotensive, hypocholestrolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65(25):2663–2677.
- Sharma SB, Nasir A, Prabhu KM. Murthy PS, Dev G. Hypoglycaemic and hypolipidaemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol. 2003;85(2–3):201–206.
- Li W, Dai RJ, Antihyperglycaemic effect of Cephalotaxus sinensis leaves and GLUT-4 translocation facilitating activity of its flavonoid constituents. Biol Pharm Bull. 2007;30(6):1123–1129.
- Kim YY, Kang KM, Chung SH. Long-term administration of Sopungsungiwon (SP) prevents diabetic nephropathy in Zucker diabetic fatty rats. Arch Pharmaceut Res. 2002; 25(6):917–922.
- Yamabe N, Yokozawa T. Activity of the Chinese prescription Hachimi-jio-gan against renal damage in the Otsuka Long Evans Tokushima fatty rat: A model of human type 2 diabetes mellitus. J Pharm Pharmacol. 2006;58(4):535–545.
- Musabayane CT, Gondwe M, Kamadyaapa DR, Chuturgoon AA, Ojewole JAO. Effects of Ficus thonningii (Blume) [Moraceae] stem-bark ethanolic extract on blood glucose, cardiovascular and kidney functions of rats, and on kidney cell lines of the proximal (LLC-PK1) and distal tubules (MDBK). Ren Fail. 2007;29(4):389–397.
- Gondwe M, Kamadyaapa DR, Tufts MA, Chuturgoon AA, Musabayane CT. Sclerocarya birrea [(A. Rich.) Hochst.] [Anacardiaceae] stem-bark ethanolic extract (SBE) modulates blood glucose, glomerular filtration rate (GFR) and mean arterial blood pressure (MAP) of STZ-induced diabetic rat. Phytomedicine. 2008;15:699–709.
- Bwititi P, Musabayane CT, Nhachi, CFB. Effects of Opuntia megacantha on blood glucose and kidney function in streptozotocin diabetic rats. J Ethnopharmacol. 2000;69(3):247–252.
- Musabayane CT, Ndhlovu CE, Balment RJ. Renal fluid and electrolyte handling in streptozotocin-diabetic rats. Ren Fail. 1995;17(2):107–116.
- Rabkin R. Diabetic nephropathy. Clin Cornerstone. 2003;5(2):1–11.
- Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: A trigger for impaired glucose metabolism. J Am Soc Nephrol. 2007;18:2226–2232.
- Bloomgarden ZT. Diabetic nephropathy. Diabetes Care. 2008;31(4):823–827.
- Oh YK, Joo KW, Lee JW, Jeon US, Lim CS, Han JS, Knepper MA, Na KY. Altered renal sodium transporter expression in an animal model of type 2 diabetes mellitus. J Korean Med Sci. 2007;22(6):1034–1041.
- Kumar GS, Shetty AK, Salimath PV. Modulatory effect of bitter gourd (Momordica charantia LINN.) on alterations in kidney heparan sulfate in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2008;115(2):276–283.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820.
- Sheetz MJ, King GL. Molecular understanding of hyperglycaemia’s adverse effects for diabetes complications. JAMA. 2002;288(20):2579–2588.
- Cooper ME, Gilbert RE, Epstein M. Pathophysiology of diabetic nephropathy. Metabolism. 1998;47(12 Suppl. 1):3–6.
- Brands MW, Fitzgerald SM, Hewitt WH, Hailman AE. Decreased cardiac output at the onset of diabetes: Renal mechanisms and peripheral vasoconstriction. Am J Physiol Endo Metab. 2000;278(5):E917–E924.
- Lehmann R, Schleicher ED. Molecular mechanism of diabetic nephropathy. Clin Chim Acta. 2000;297(1–2):135–144.
- Rao NK, Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. Seeds in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2006;6:17.
- Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozotocin-induced diabetic rat. Diabetes. 1991;40(10): 1328–1334.
- Yeh CH, Sturgis L, Haidacher J, Requirement for p38 and p44/p42 mitogen- activated protein kinase in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50(6):1495–1504.
- Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M. Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int. 2003;63(2):464–473.
- Yonekura H, Yamamoto Y, Sakurai S, Watanabe T, Yamamoto H. Roles of the receptor for advanced glycation end products in diabetes-induced vascular injury. J Pharmacol Sci. 2005;97(3):305–311.
- DeGroot J. (2004). The AGE of the matrix: Chemistry, consequence and cure. Curr Opin Pharmacol. 4(3):301–305.
- Hartog JWL, Voors AA, Bakker SJL, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. Eur J Heart Fail. 2007;9(12):1146–1155.
- Fan Q, Kobayashi M, Yamashita M, Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dial Transplant. 2004;19(12): 3012–3021.
- Ihara Y, Egashira, K, Nakano K, Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J Mol Cell Cardiol. 2007;43(4):455–464.
- Lassila M, Seah KK, Allen T, Accelerated nephropathy in diabetic apolipoprotein E-knockout mouse: Role of advanced glycation. J Am Soc Nephrol. 2004;15(8):2125–2138.
- Tanaka Y, Iwamoto H, Onuma T, Kawamori R. Inhibitory effect of metformin on formation of advanced glycation end products. Curr Therapeutic Res. 1997;58(10): 693–697.
- Rahbar S, Figarola JL. Novel inhibitors of advanced glycation end-products. Arch Biochem Biophys. 2003;419(1):63–79.
- Kim HY, Kang KS, Yamabe N, Nagai R, Yokozawa T. Protective effect of heat-processed American ginseng against diabetic renal damage in rats. J Agri Food Chem. 2007; 55(21):8491–8497.
- Wirasathien L, Pengsuparp T, Suttisri R. Ueda H, Moriyasu M, Kawanishi K. Inhibitors of aldose reductase and advanced glycation end-products formation from the leaves of Stelechocarpus cauliflorus R.E. Fr. Phytomedicine. 2007;14(7–8):546–550.
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4):612–628.
- Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000; 77:S3–S12.
- Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038.
- Wei-ha L, Zi-qing H, Hong N, Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin Medical J. 2008;121(8):706–712.
- Evcimen ND, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007;55:408–510.
- Aiello LP. The potential role of PKC in diabetic retinopathy and macular oedema. Surv Ophthalmol. 2002;47 (Suppl. 2):S263–S269.
- Khan ZA, Chakrabarti S. Cellular signalling and potential new treatment targets in diabetic retinopathy. Exp Diabetes Res. 2007;2007:31867.
- Ganz MB, Saksa B, Saxena R, Sedor JR. PDGF and IL-1 induce and activate specific protein kinase C isoforms in mesangial cells. Am J Physiol. 1996;40:F108–F113.
- Feng W, Ding W, Qian Q, Chai Z. Use of the enriched stable isotope Cr-50 as a tracer to study the metabolism of chromium (III) in normal and diabetic rats. Biol Trace Elem Res. 1998;63(2):129–138.
- Fujita H, Omori S, Ishikura K, Hida M, Awazu M. ERK and p38 mediate high-glucose induced hypertrophy and TGF-beta expression in renal tubular cells. Am J Physiol Renal Physiol. 2004;286(1):F120–F126.
- Noh H, King GL. The role of protein kinase C in diabetic nephropathy. Kidney Int Suppl. 2007;106:S49–S53.
- Ganz MB, Hawkins K, Reilly RF. High glucose induces the activity and expression of Na+/H+ exchange in glomerular mesangial cells. Am J Physiol Renal Physiol. 2000;278: F91–F96.
- Ishi H, Jirousek MR, Koya D, Amelioration of vascular dysfunctions in diabetic rats by oral PKC ß inhibitor. Science. 1996;272(5562):728–731.
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 2005;28(11):2686–2690.
- Lôpez-Franco O, Suzuki Y, Sanju8n G, Nuclear factor KB inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161(4):1497–1505.
- Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. J Agri Food Chem. 1999;47(6):2274–2279.
- Arts IC, van de Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J Agric Food Chem. 2000;48(5):1746–1751.
- Varadkar P, Dubey P, Krishna M, Verma, N. Modulation of radiation-induced protein kinase C activity by phenolics. J Radiol Pro. 2001;21(4):361–370.
- Kandaswami C, Lee LT, Lee LP, The antitumor activities of flavinoids. In Vivo. 2005;19(5):895–909.
- Grover JK, Rathi SS, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin-induced diabetic mice. J Ethnopharmacol. 2001; 76(3):233–238.
- Musabayane CT, Xozwa K, Ojewole JAO. Effects of Hypoxis hemerocallidea (Fisch. & C.A. Mey.) [Hypoxidaceae] corm (African Potato) aqueous extract on renal electrolyte and fluid handling in the rat. Renal Failure. 2005;27(5):763–770.
- Ong ES. Extraction methods and chemical standardization of botanicals and herbal preparations. J Chromatogr B. 2004; 812:23–33.
- Wang X, Guan S, Liu R, HPLC determination of four triterpenoids in rat urine after administration of total triterpenoids from Ganoderma lucidum. J Pharm Biomed Anal. 2007;43:1185–1190.
- Cheng X, Shin YG, Levine BS, Smith AC, Tomaszewsk JE, van Breemen RB. Qualitative analysis of betulininc acid in mouse, rat and dog plasma using electrospray liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2089–2092.
- Musabayane CT, Mahlalela N, Shode FO, Ojewole JAO. Effects of Syzygium cordatum (Hochst.)[Myrtaceae] leaf extract on plasma glucose and hepatic glycogen in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;97(3): 485–490.
- Kapoor R, Srivastava S, Kakkar P. (2009). Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ Toxicol Pharmacol. 2009;27(1):62–69.
- Yokozawa T, Yamabe N, Ki HY, Protective effects of morroniside isolated from Corni fructus against renal damage in streptozotocin-induced diabetic rats. Biol Pharm Bull. 2008;31(7):1422–1428.
- Ugochukwu NH, Cobourne MK. Modification of renal oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with extracts from Gongronema latifolium leaves. Clin Chim Acta. 2003;336(1–2):78–81.
- Alam MM, Javedi K, Jafri MA. Effect of Rheum emodi (Revandi Hindi) on renal functions in rats. J Ethnopharmacol. 2005;96:121–125.
- Sato S, Yamate J, Hori Y, Hatai A, Nozawa M, Sagai M. Protective effect of polyphenol-containing azuki bean (Vigna angularis) seed coats on the renal cortex in streptozotocin-induced diabetic rats. J Nutr Biochem. 2005;16(9): 547–553.
- Musabayane CT, Munjeri O, Mdege ND. Effects of Helichrysum ceres on renal function and blood pressure in the rat. Renal Failure. 2003;25(1):5–14.
- Mapanga RF, Tufts MA, Shode FO, Musabayane CT. Renal effects of plant-oleanolic acid in streptozotocin-induced diabetic rats. Renal Failure. 2009;31(6): 481–491.
- Gondwe M, Kamadyaapa DR, Tufts MA, Chuturgoon AA, Ojewole JAO, Musabayane CT. Effects of Persea americana (Mill) [Lauraceae] [“Avocado”] ethanolic extract on blood glucose and kidney function in streptozotocin-induced diabetic rats and on kidney cell lines of the proximal (LLC-PK1) and distal tubules (MDBK). Methods Find Expl Clin Pharmacol. 2008; 30(1):25–35.
- Afzal M, Khan NA, Ghufran A, Iqbal A, Inamuddin M. Diuretic and nephroprotective effect of Jawarish Zarooni Sada—a polyherbal unani formulation. J Ethnopharmacol. 2004;91:219–223.
- Yamabe N, Kang KS, Goto E, Tanaka T, Yokozawa T. Beneficial effect of Corni fructus, a constituent of hachimi-jio-gan on advanced glycation end product-mediated renal injury in streptozotocin-treated diabetic rats. Biol Pharm Bull. 2007;30(3):520–526.
- Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-κβ. Nutrition. 2009; 25(9):964–972.
- Al-Qattan K, Thomson M, Ali M. Garlic (Allium sativum) and ginger (Zingiber officinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. Eur e-J Clin Nutr Metab. 2008;3:e62–e71.
- Bwititi PT, Machakaire T, Nhachi CFB, Musabayane CT. Effects of Opuntia megacantha leaves extract on renal electrolyte and fluid handling in streptozotocin (STZ)-diabetic rats. Renal Failure. 2001;23(2):49–58.