Abstract
We evaluated the effects of vitamins with antioxidant properties (a combination of vitamins C and E) and l-arginine treatment on renal failure in mice by measuring survival rate. The molecular changes were elucidated by determining endothelial tetrahydrobiopterin (BH4) levels and nitric oxide synthase (eNOS) mRNA expression in mice with renal ablation. Previous studies have shown that endothelial dysfunction in 5/6 nephrectomized mice is associated with decreased nitric oxide (NO) bioavailability and increased vascular superoxide production. WTC57 mice were divided into three groups: Group 1 was the sham-operated group (C); Group 2 was the 5/6 nephrectomized group (Nfx); and Group 3 was a group of 5/6 nephrectomized mice, treated with l-arginine and vitamins with antioxidant properties (NfxTx; 200 mg/kg l-arginine, 83 mg/kg vitamin C, and 46.6 mg/kg vitamin E). After 20 weeks of treatment, urinary protein excretion, blood pressure, BH4 and dihydrobiopterin (BH2) levels, eNOS mRNA, oxidative stress, and survival rate were determined. An increase in urinary protein excretion, blood pressure, and oxidative stress was prevented in the NfxTx group, but not in the Nfx group. BH4 and eNOS mRNA expression was increased by 32% and 78%, respectively, in the NfxTx group. Furthermore, the treatment increased the survival rate by 33%. Our results indicate that under normal conditions, NO appears to protect renal function. However, this NO-dependent protection is lost during kidney failure, probably due to increased reactive oxygen species synthesis. The treatment restores the viability of NO and prevents the BH4 oxidation. Therefore, this treatment may represent a therapeutic approach for the management of kidney disease.
INTRODUCTION
Nitric oxide (NO) is an important modulator of kidney function and can affect long-term blood pressure.Citation1 This is best evidenced by how blood pressure increases when renal NO production is inhibited.Citation2 Additionally, in the nephrectomy 5/6 rat model of chronic kidney disease (CKD), renal NO synthesis decreases in parallel with a decline in renal function.Citation3 Furthermore, various authors have reported that NO pathways are disturbed in models of acuteCitation4 and chronic renal failure;Citation5 recently we proposed that during the development of kidney failure after renal ablation, NO acts as antagonist to superoxide in renal tissues, suggesting that the loss of this mechanism promotes kidney damage.Citation6
In addition to a reduction in NO, CKD is accompanied by increased oxidative stress as a result of increased reactive oxygen species (ROS) production and decreased antioxidant enzyme capacity in the kidney. The major ROS is superoxide, and in the kidney, this is primarily generated by NADPH oxidase (nicotinamide adenine dinucleotide phosphate-oxidase).Citation6–8 Thus, renal damage due to kidney disease is a consequence of the loss of NO-dependent renal protection and the deleterious effects of ROS. In CKD, NO deficiency has many causes, including eNOS gene regulation and the inhibition of nitric oxide synthase (NOS) activity by endogenous inhibitors.Citation6,Citation9,Citation10 Previous studies have uncovered the beneficial effects of l-arginine treatment in several models of CKD,Citation6,Citation11–13 supporting the relevance of NO in kidney failure. However, the availability of cofactors, such as tetrahydrobiopterin (BH4), may also affect NOS activity. A decrease in the ratio of reduced BH4 to oxidized dihydrobiopterin (BH2) blunts NO production and contributes to kidney damage.Citation14,Citation15 Thus, increased ROS production during kidney failure may be due to decreased BH4 and reduced NOS activity. Moreover, because ROS and NO have counterbalancing activities and reciprocally reduce bioavailability, an imbalance in NO and ROS levels may shift the kidney into a state of superoxide dominance, causing CKD progression.Citation16
Therefore, treatment with antioxidants and l-arginine, which reduce oxidative stress and/or preserve NO bioavailability, has been considered for the prevention of CKD.Citation17–19 Thus, we hypothesized that the combined effects of vitamins with antioxidant properties (a combination of vitamins C and E) and l-arginine supplementation are mediated by a reduction in BH4 oxidation and increased eNOS activity. Thereby, l-arginine and vitamins restore NO production.
In this study, we evaluated the effects of vitamins with antioxidant properties and l-arginine treatment on pathophysiological and NO pathway changes during the development of chronic renal failure in mice with 5/6 nephrectomy.
MATERIALS AND METHODS
Male WTC57 mice (Laboratories CINVESTAV-IPN, Mexico DF, Mexico) weighing 25–30 g were used. Animals received a balanced diet (Purina, St. Louis, MO, USA) and water ad libitum.
The Ethical Committee of the Centro de Investigation y de Estudios Avanzados del Instituto Politécnico Nacional (Internal Committee for the Care and Use of Laboratory Animals), which oversees the use of animals in scientific experiments, approved the protocols in this investigation.
Chronic kidney failure was induced in mice by nephrectomy of the right kidney and ablation of two-thirds of the left kidney (5/6 nephrectomy) as we have previously reported.Citation20 Mice from each strain were divided into three groups. Group 1 (n = 6) was the sham-operated group (C). Group 2 (n = 6) was the 5/6 nephrectomized group (Nfx). Group 3 (n = 6) was a group of 5/6 nephrectomized mice that were treated with a formulation containing antioxidants and l-arginine (NfxTx) (Merck, Naucalpan, México); the formulation consisted of 275.86 mg/mL l-arginine, 115.63 mg/mL vitamin C, and 63.05 mg/mL vitamin E (the latter two components are antioxidants). The treatment consisted of a daily oral administration of a formulation containing 200 mg/kg l-arginine, 83 mg/kg vitamin C, and 46.6 mg/kg vitamin E. The treatment began 24 h after either sham or renal ablation and continued for 20 weeks. At the end of the treatment period, mice were placed in metabolic cages to determine urinary protein excretion. Protein concentration was measured by the Bradford method.Citation21 A standard curve was prepared with bovine serum albumin, samples were diluted, and the absorbance was measured at 595 nm using a Beckman DU 650 spectrophotometer.
Blood Pressure Measurements
Twenty weeks after 5/6 nephrectomy, blood pressure was measured by carotid artery cannulation using a polyethylene catheter (I.D.011 “OD.024,” Clay Adams, NJ, USA) attached to a solid-state pressure transducer (DUO.18 WPI, Aston, UK). Blood pressure was measured for 15 min and recorded for further analysis.Citation6
Measurement of Biopterins
We analyzed the oxidized and reduced forms of biopterins in the serum by using an adaptation of the method described by Han et al. of capillary zone electrophoresis.Citation22
Briefly, the serum was deproteinized in methanol in 1:1 ratio, centrifuged at 15,000 × g for 15 min, and filtered with nitrocellulose membrane filters of 0.22 μm; the filtrate was diluted 1:10 with 0.1 M sodium hydroxide, placed on a step one cartridge Sep-Pak by Waters Classic NH2 (Milford, MA, USA) and analyzed directly in a system P/ACETM MDQ from Beckman Coulter (Brea, CA, USA). The capillary was preconditioned by passing a 1.0-M solution of sodium hydroxide for 30 min, then distilled water for 30 min, and finally the buffer was run (0.1 M Tris, 0.1M boric acid, and 2 mM EDTA at pH 8.75) for 30 min. The samples were injected under pressure to 0.5 psi/10 s hydrodynamics. The separation was carried out to 20 kV for 10 min at 445 nm. The migration time for BH4 was 4.342 ± 0.112 min and for BH2 was 3.329 ± 0.093 minutes. The capillary was washed between runs with 1.0 M NaOH for 2 min. The results are expressed in pmol/mL. Biopterin concentrations in the samples were determined by comparison with a standard curve.
Increasing concentrations of biopterin (2–2500 pmol/mL) in phosphate buffer (6 mM pH 7.4 ± 0.1) were used as standard curve. The solutions were run in the electrophoresis system under the same conditions described above. The variation in the migration time of the samples and standard curve was less than 0.32%.
RNA Isolation
Total RNA was extracted from the kidney using the TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. RNA concentration and purity were estimated by the OD 260/280 nm ratio, and RNA integrity was assessed by electrophoresis on a 1% agarose gel stained with ethidium bromide. RNA was stored at –70°C until further analysis.
Reverse Transcription and Real-Time PCR Assays
cDNA was obtained by RT-PCR using the SuperscriptTM III First-Strand Synthesis System (Invitrogen) according to manufacturer's instructions. Briefly, two reaction mixtures were prepared: mix 1 – 2 μg total RNA, 5 μM oligo dT, 1 mM of each deoxyribonucleotide triphosphate, and Diethylpyrocarbonate (DEPC)-treated water in a total volume of 10 μL; mix 2 – 1X RT buffer, 5 mM MgCl2, 10 mM Dithiothreitol (DTT), 40 U RNaseOUTTM, and 200 U SuperScripTM III reverse transcriptase (RT). Mix 1 was incubated at 65°C for 5 min and then placed on ice. Mix 2 was added to mix 1, and the mixture was incubated at 50°C for 50 min. The RT reaction was quenched by heating at 85°C for 5 min. RNase H (1 μL) was then added to degrade the RNA. Real-time PCR reactions were performed in a 96-well plate on a 7500 Sequence Detector (Applied Biosystems, Maxico) using the TaqMan® Universal PCR Master Mix (Perkin–Elmer, Foster City, CA, USA). Primers and the fluorogenic probe were designed using the Primer Express software (Perkin–Elmer Life Sciences). The sequences for primers and the probe were forward primer, 5′-CATCTGTAACCACATTAAATACGCAACA-3′; reverse primer, 5′- GCTGTTCCAGATCCGGAAGTC-3′; and TaqMan® probe, carboxyfluorescein (FAM) 5′-ATGGCTGAACGAAGATT-3′. The PCR reaction was performed in a total volume of 25 μL containing 400 nM of each oligonucleotide, 200 mM TaqMan® probe, and 3 μL of cDNA. The reaction was initiated with successive incubations at 50°C for 2 min and 95°C for 10 min. Forty PCR cycles were performed, which consisted of a heating step at 95°C for 15 s and an annealing/extension step at 60°C for 1 min. The expression of the ribosomal 18S gene was measured as an endogenous control using a fluorogenic probe labeled with VICTM dye (Applied Biosystems). Negative controls were included in the same plate. The relative expression of eNOS mRNA was calculated by the ΔCt method described by Livak and Schmittgen, 2001.Citation23
Oxidative Fluorescent Microphotography
Dihydroethidium (DHE), an oxidative fluorescence dye, was used to evaluate in situ production of superoxide as previously described.Citation24 Briefly, unfixed frozen kidney samples were cut into 10-μm thick sections and placed on glass slides. DHE (10 μM, Molecular Probes D1168, CA, USA) was applied to each tissue section and the sections were then coverslipped. DHE is cell permeable, and in the presence of superoxide, it is oxidized to form ethidium and intercalates into the DNA.Citation25 Ethidium is excited at 488 nm and has an emission spectrum of 610 nm. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. Images were obtained using a laser-scanning confocal imaging system (Olympus America Inc. FV-300, CA, USA). The appearance of red fluorescence was indicative of ROS generation.
Survival
Mouse survival was determined during the 20-week time course.
Statistics
Data are expressed as the mean ± SEM. Analysis of variance was performed to determine significant differences between groups. For multiple comparisons among groups, the Tukey test was used. These statistical analyses were performed by using Sigma Stat software. Survival data were analyzed by Kaplan–Meier analysis (SPSS). For all comparisons, differences were considered significant at p < 0.05.
RESULTS
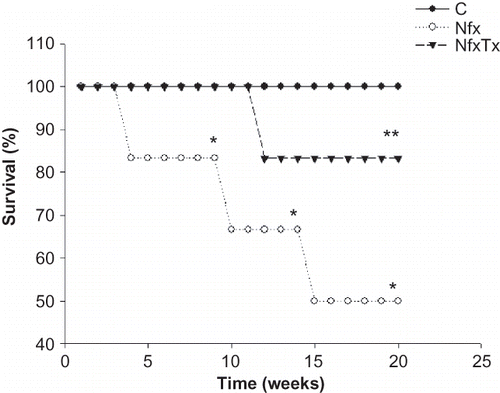
Similar to our previous report, 20 weeks post nephrectomy, the 5/6 nephrectomized mice had a significant elevation of urinary protein excretion, blood pressure, and DHE staining (superoxide production) ( and 2). Twenty-week chronic treatment as diet supplementation with a combination of l-arginine and vitamins C and E prevented these changes in the excretion of urinary protein, blood pressure, and superoxide production by 52%, 22%, and 33%, respectively ( and 2). Additionally, evaluation of NO pathway in 5/6 nephrectomized mice showed reduced BH4 levels, whereas, BH2 levels were elevated and substantial decrease in eNOS mRNA expression was present in the nephrectomized mice ( and ). Maintenance of kidney function and the prevention of tissue damage by l-arginine/vitamins C and E treatment were associated with restoration of the NO metabolic pathway. Accordingly, the BH4 concentration was recovered by 32%, BH2 production was inhibited by 36%, and eNOS mRNA expression increased by 78% ( and ). Finally, survival rate of the 5/6 nephrectomized mice at the end of the 20-week time course was half of that of the sham-operated mice and increased by 33% after l-arginine/vitamins C and E treatment ().
Figure 1. Effects of chronic l-arginine and vitamin supplementation on kidney failure. Urinary protein excretion (panel A) and blood pressure (panel B) were evaluated in sham-operated mice (C), 5/6 nephrectomized mice (Nfx), and treated 5/6 nephrectomized mice (NfxTx). Blood pressure and urinary protein excretion were measured 20 weeks after the 5/6 nephrectomy was performed. Each bar represents the mean ± SEM. For the 5/6 nephrectomized mice with and without treatment, the mean represents the mice that survived at the end of the 20-week period.
Note: * and ** denote significance at p < 0.05 in comparison between sham versus nephrectomized mice and nephrectomized versus treated nephrectomized mice, respectively.
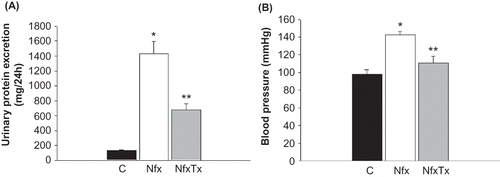
Figure 2. Effects of chronic l-arginine and vitamin supplementation on superoxide generation in 5/6 nephrectomized mice. Superoxide () production was evaluated by confocal fluorescence microscopy 20 weeks after 5/6 nephrectomy was performed in sham-operated mice (C), 5/6 nephrectomized mice (Nfx), and treated 5/6 nephrectomized mice (NfxTx). The red fluorescence in panel A denotes the presence of
. Fluorescence is expressed as % fluorescence. Each bar represents the mean ± SEM. For the 5/6 nephrectomized mice with and without treatment, the mean represents the mice that survived at the end of the 20-week period.
Note: * and ** denote significance at p < 0.05 in comparison between sham versus nephrectomized mice and nephrectomized versus treated nephrectomized mice, respectively.
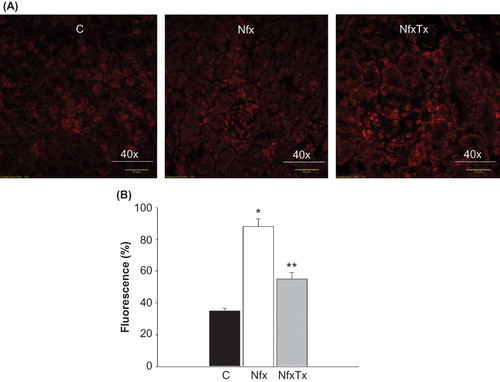
Figure 3. Effects of chronic l-arginine and vitamin supplementation on tetrahydrobiopterin synthesis in 5/6 nephrectomized mice. Plasma levels of BH4 (panel A) and BH2 (panel B) were measured by capillary electrophoresis in sham-operated mice (C), 5/6 nephrectomized mice (Nfx), and treated 5/6 nephrectomized mice 20 weeks after 5/6 nephrectomy (NfxTx) was performed. Each bar represents the mean ± SEM. For the sham mice and the 5/6 nephrectomized mice with and without treatment, the mean represents the mice that survived at the end of the 20-week period.
Note: * and ** denote significance at p < 0.05 in comparison between sham versus nephrectomized mice and nephrectomized versus treated nephrectomized mice, respectively.
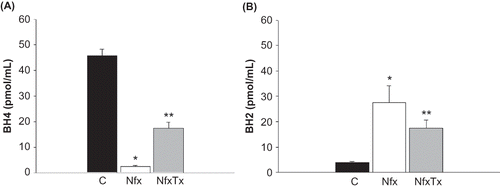
Figure 4. Effects of chronic l-arginine and vitamin supplementation on eNOS gene expression in 5/6 nephrectomized mice. eNOS mRNA expression was detected by real-time PCR analysis 20 weeks after 5/6 nephrectomy was performed in sham-operated mice (C), 5/6 nephrectomized mice (Nfx), and treated 5/6 nephrectomized mice (NfxTx). Each bar represents the mean ± SEM. For the sham mice and the 5/6 nephrectomized mice with and without treatment, the mean represents the number of mice that survived at the end of the 20-week period.
Note: * and ** denote significance at p < 0.05 in comparison between sham versus nephrectomized mice and nephrectomized versus treated nephrectomized mice, respectively.
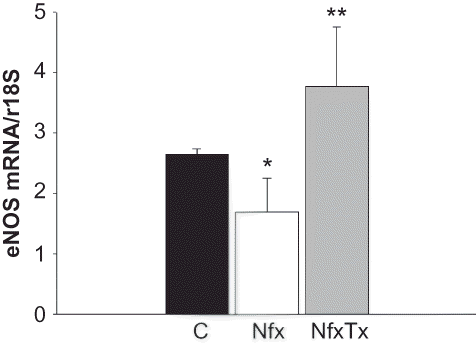
Figure 5. Effects of l-arginine and vitamin supplementation on survival rate after renal ablation. Survival rate was calculated as the percent of mice alive divided by the number of dead animals at each time point of sham-operated mice (C), 5/6 nephrectomized mice (Nfx), and treated 5/6 nephrectomized mice (NfxTx). Each group started with six mice and finished with six, three, and five mice for sham-operated mice, 5/6 nephrectomized mice, and treated 5/6 nephrectomized mice, respectively.
Note: * and ** denote significance at p < 0.05 in comparison between sham versus nephrectomized mice and nephrectomized versus treated nephrectomized mice, respectively.
DISCUSSION
Using a common animal model of kidney damage, we monitored several parameters of renal function over a period of 20 weeks. In addition, we evaluated NO pathway and oxidative stress, two mechanisms that may contribute to kidney damage. The effects of l-arginine/vitamins with antioxidant properties diet supplementation were measured during renal damage.
The remnant kidney model of CKD results in end-stage renal failure, which is characterized by proteinuria, hypertension, increased oxidative stress, decreased NO production, and death. In this study, we provide evidence that the deleterious effects of CKD were prevented by the administration of a combination of l-arginine, vitamins E and C, resulting in decreased oxidative stress and preserving NO bioavailability.
Hypertension and proteinuria are important risk factors for kidney disease.Citation20,Citation26,Citation27Our data show that treatment with a combination of l-arginine, vitamins C and E decreased the amount of proteinuria and hypertension. This supports previous reports that demonstrate that l-arginine or mixture of antioxidants (l-carnitine, catechin, and vitamins E and C) decrease inflammatory cytokine production, kidney damage, blood pressure, and dyslipidemia in a rat model of chronic renal failure.Citation17 Pharmacological renoprotection is produced by antihypertensive and antiproteinuric drugs. Recent clinical evidence shows that the renoprotective efficacy is dependent on the ability to maintain blood pressure at a low to normal range.Citation20,Citation26 Moreover, proper blood pressure maintenance can improve 5-year kidney survival by 35% in patients with CKD.Citation9 Thus, to better control blood pressure during renal failure, the hypertensive effects of the disease must be reduced.
There are several antihypertensive therapies that can be used to lower blood pressure during kidney disease. However, our data, in addition to previous reports, suggest that an impaired NO pathway is responsible for the hypertension in this model. Therefore, we evaluated the effect of l-arginine, which stimulates NO synthesis, and vitamins C and E, which prevent oxidation of NO cofactors, on the development of renal damage. Our results clearly demonstrate that l-arginine/vitamins with antioxidant properties diet supplementation prevented renal failure in 5/6 nephrectomized mice by reducing hypertension and proteinuria and increasing 33% survival rate.
Some studies suggest that vascular disorders in CKD rats may be partially due to NOS uncoupling caused by a BH4 deficiency and the accumulation of endogenous inhibitors of NOS and l-arginine uptake resulted in a decrease in NO production and an increase of superoxide production.Citation10,Citation29 Our results clearly demonstrate that these effects were totally or partially decreased by the l-arginine and vitamins with antioxidant properties diet supplementation in 5/6 nephrectomized mice. These results are in agreement with previous in vivo studies of l-arginine, vitamins C, and E or BH4 supplementation.Citation8,Citation16,Citation28–32
The mechanism underlying l-arginine/antioxidant-dependent kidney protection may be associated with increased NO synthesis.Citation33 The attenuation of NO production in diabetic kidneys may be due to a reduction in eNOS mRNA and protein expression or to a ROS-mediated breakdown of NO.Citation34 Furthermore, superoxide reacts with NO to form peroxynitrite, which oxidizes BH4 and uncouples eNOS activity, resulting in reduced NO synthesis.34 Our results show that decreased eNOS mRNA and BH4 were associated with increased ROS and BH2 accumulation in renal tissue. This supports the relevance of the NO pathway to maintain renal function. Moreover, 5/6 nephrectomized mice that were fed a diet supplement containing a combination of l-arginine and vitamins C and E were protected from renal damage. This was associated with the prevention of ROS production and increased BH4 and mRNA eNOS levels supporting the hypothesis that ROS can affect NO synthesis by increasing NO breakdown. The diet supplement used in our studies restored NO metabolism by inhibiting ROS-mediated NOS breakdown. This is probably due to a direct interaction of vitamins C and E with ROS. Moreover l-arginine may be an effective adjuvant to vitamins C and E diet supplementation to promote NO synthesis. In this study, we did not test vitamins E and C or l-arginine as single elements on the diet. However, several laboratories have demonstrated the beneficial effects of these compounds as single element in the diet supplement on the renal function or NO pathway.Citation8,Citation11,Citation31 Furthermore, previous results from our laboratory have shown that the use of l-arginine and vitamins E and C as the single component of the diet supplementation resulted in increased NO synthesis and prevention of ROS effects in diabetic rats.Citation19 However, these effects were potentiated when the l-arginine and vitamins were combined. This consensus supports our hypothesis that the restoration of NO metabolism is best achieved when ROS generation is prevented and NOS is stimulated during kidney failure.
In conclusion, our results indicate that under normal conditions, NO protects kidney function. However, NO-dependent protection is lost during the kidney disease, probably due to the impact of ROS on NO synthesis, which therefore results in renal damage in 5/6 nephrectomized mice. l-Arginine and antioxidant diet supplementation restores kidney protection by stimulating NO synthesis and reducing BH4 oxidation. This treatment regimen may represent a therapeutic approach for the prevention of kidney damage.
Acknowledgments
Monica G. Arellano-Mendoza, Hilda Vargas-Robles, and Leonardo Del Valle-Mondragon designed the research; Monica G. Arellano-Mendoza, Hilda Vargas-Robles, and Bruno Escalante conducted the research; Monica G. Arellano-Mendoza and Amelia Rios analyzed data; and Monica G. Arellano-Mendoza and Bruno Escalante wrote the article and had primary responsibility for final content. All authors read and approved the final manuscript. We thank Fabiola Castorena for technical assistance. This project was supported by the Mexican Council of Science and Technology (CONACYT) as PhD Research Grant 81359 to Bruno Escalante.
We also thank Merck, México, for the donation of a new drug formulation containing a combination of antioxidants and l-arginine.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Persson PB. Nitric oxide in the kidney. Am J Physiol regul Integr Comp Physiol. 2002;283:R1005–R1007.
- Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW, Jr. Effects of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physyiol. 1994;266:H1918–H1926.
- Aiello S, Noris M, Todeschini M, Renal and systemic nitric oxide in rats with renal mass reduction. Kidney Int. 1997;52:171–181.
- Conger J, Robinnette J, Villar A. Increased nitric oxide synthase activity despite lack of response to endothelium-dependent vasodilatation in post ischemic acute renal failure in rats. J Clin Invest. 1995;96:631–638.
- Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol. 2008;294:F1–F9.
- Arellano Mendoza MG, Castillo Henkel C, Medina Santillan R Kidney damage after renal ablation is worsened in endothelial nitric oxide synthase (-/-) mice and improved by combined administration of l-Arginine and antioxidants. Nephrology. 2008;13:218–227.
- Mimic-Oka J, Simic T, Djukanovic L. Alteration in plasma antioxidant capacity in various degree of chronic renal failure. Clin Nephrol. 1999;51:233–241.
- Tain YL, Freshour G, Dikalova A, Griendling K, Baylis, C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol Renal Physiol. 2007;292:F1404–F1410.
- Hasdan G, Benchetrit S, Rashid G, Green J, Bernheim J, Rathaus M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61:586–590.
- Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress and progression of chronic renal failure. Semin Nephrol. 2004;24:354–365.
- Katoh T, Takohashi K, Klahr S, Reyes AA, Badr KF. Dietary supplementation with l-arginine ameliorates glomerular hypertension in rats with subtotal nephrectomy. J Am Soc Nephrol. 1994;4:1690–1694.
- Reyes AA, Karl IE, Kissane J, Klahr S. L-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J Am Soc Nephrol. 1993;4: 1039–1045.
- De Nicola L, Thomson SC, Wead LM, Brown MR, Gabbai FB. Arginine feeding modifies cyclosporine nephrotoxicity in rats. J Clin Invest. 1993;92:1859–1865.
- Kashihara N, Satoh M. Molecular pathogenesis of chronic kidney disease. Nippon Rinsho. 2008;66:1671–1677.
- Sucher R, Gehwolf P, Oberhuber R, Tetrahydrobiopterin protects the kidney from ischemia reperfusion injury. Kidney Int. 2010;77:681–689. Epub 2010 Feb 17.
- Yamamizu K, Shinozozaki K, Ayajiki K, Gemba M, Okamura T. Oral administration of both tetrahydrobiopterin and l-arginine prevents endothelial dysfunction in rats with chronic renal failure. J Cardiovscular Pharmacol. 2007;49:131–139.
- Korish AA. Oxidative stress and nitric oxide deficiency in inflammation of chronic renal failure. Possible preventive role of l-arginine and multiple antioxidants. Saudi Med J. 2009;30: 1150–1157.
- Wuhl E, Schaefer F. Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol. 2008;23:705–716.
- Coronel I, Arellano-Mendoza MG, Del Valle-Mondragon L, et al. l-arginine and antioxidant diet supplementation partially restores nitric oxide dependent regulation of phenylephrine renal vasoconstriction in diabetic rats. J Ren Nutr. 2010;20: 158–168.
- Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent injury and hypertension. J Am Soc Nephrol. 1994;4:2023–2031.
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Ann Biochem. 1976;72:248–254.
- Han F, Huynh BH, Shi H, Lin B, Ma Y. Pteridine analysis in urine by capillary electrophoresis using laser-induced fluorescence detection. Anal Chem. 1999;71:1265–1269.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–408.
- Miller FJ, Jr, Gulterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305.
- Rothe G, Valet G. Flow cytometric analysis of respiratory bursa activity in phagocytes whit hydroethidium and 2′,7′dichlorofluorescein. J Leukoc Biol. 1995;55:253–258.
- Van Dokkum RP, Jacob HJ, Provoost AP. Genetic differences define severity of renal damage after l-NAME induced hypertension in rats. J Am Soc Neprhol. 1998;9:363–371.
- Robertso JL, Goldschmidt M, Kronfeld DS, Tomaszewski JE, Hill GS, Bovee KC. Long-term renal responses to high dietary protein in dogs with 75% nephrectomy. Kidney Int. 1986; 29:511–519.
- Wuhl E, Schaefer F. Can we slow the progression of chronic kidney disease? Curr Opin Pediatr. 2010;22:170–175.
- Ashab I, Peer G, Blum M, Oral administration of l-arginine and captopril in rats prevents chronic renal failure by nitric oxide production. Kidney Int. 1995;47:1515–1521.
- Huang A, Vita JA, Venema RC, Keaney JF, Jr. Ascorbic acid enhances endothelial nitric oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275: 17399–17406.
- Baker TA, Milstien S, Katsic ZS. Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J Cardiovasc Pharmacol. 2001;37:333–338.
- Okon EB, Chung AWY, Rauniyar P, Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423.
- Guzik TJ, Mussa S, Gastaldi D, Mechanism of increased vascular superoxide production in human diabetes mellitus: role of NADPH oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662.
- Settergreen M, Bohn F, Malmstrom RE, Channon KM, Pemow J. l-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis. 2009;204:73–78.