Abstract
In this study, the effect of mirtazapine on rat kidneys versus ischemia-reperfusion (IR) damage was biochemically and histopathologically investigated. The results have shown that malondialdehyde (MDA) level of healthy rat group is 15.2 mol/g protein. The level of this substance was measured as 26.7 mol/g in only ischemia group. The MDA levels of IR and mirtazapine + renal ischemia-reperfusion (MRIR) groups were 39 ± 17.6 mol/g protein. While myeloperoxidase activity of healthy rat group was 20.2 u/g, the activities of only ischemia, IR, and MRIR groups were 28, 36.3, and 21 u/g, respectively. The glutathione levels were measured as 17.7, 12.8, 7.5, and 16.2 nmol/g in healthy, only ischemia, IR, and MRIR groups, respectively. Finally, glutathione S-transferase activities of healthy, only ischemia, IR, and MRIR groups were determined as 20, 13.8, 7.1, and 18.3 u/g, respectively. Histopathologically, while hemorrhage in interstitial area was observed in only ischemia group, significant tubular epithelial swelling, necrosis, and cast accumulation were seen in IR group. In MRIR group, only mild tubular epithelial swelling and mild hyaline cast accumulation were observed in kidney tissue. Consequently, it can be said that mirtazapine has a protective effect on IR-induced kidney damage.
INTRODUCTION
Kidney ischemia occurs in partial nephrectomy, organ transplantation, and in various urologic and vascular surgical interventions.Citation1 Ischemia is the oxygen deprivation in tissue depending on reduced or completely impeded arterial or venous blood flow into the tissue.Citation2 Long-term oxygen deprivation in our organs and tissues leads to serious cellular damage, even to death of those. Reperfusion is the restoration of blood flow to ischemic tissue. Free oxygen radicals (FOR) which settle in the tissue during reperfusion and released by polymorphonuclear leukocytes cause further tissue injury. This is called reperfusion tissue injury.Citation3 Hypoxanthine results from adenosine triphosphate in the tissue during ischemia.Citation4 On the other hand, during reperfusion hypoxanthine is converted into xanthine by xanthine oxidase, and at the same time superoxide radical results from molecular oxygen.Citation5,6 Superoxide radical and its breakdown products lead to cellular damage by lipid peroxidation.Citation7,8 This injury caused by oxidants is called oxidative stress.Citation9 Lipid peroxidation is considered as the key process in IR injury generated.Citation10 However, detrimental effects of these free radicals on lipids are reduced by antioxidant defense mechanisms continuously or eliminated completely. Antioxidants prevent the free radicals and cellular components from damage to which they may be exposed.Citation11 When these antioxidant defense mechanisms remain incapable, serious injuries occur in tissues. If oxidative damage plays a role at the beginning or in the pathology of the disease, antioxidant treatment may prevent the disease or delay the onset of disease.Citation12 Mirtazapine, which we shall try out in kidney ischemia-reperfusion (IR) injury is an antidepressant administered in the treatment of major depression. It has been demonstrated that mirtazapine prevents the oxidative stress-induced damage by preventing the increase in oxidant parameters and the suppression of antioxidants.Citation13 In literature surveys, no information and inventions have been recorded regarding the preventive effects of mirtazapine on kidney IR injury. Thus, the aim of our study is to investigate the biochemical and histopathological effects of mirtazapine on the injury created by IR in rat kidneys.
MATERIALS AND METHODS
Animals
In this study, a total of 40 Wistar albino male rats with varying weights between 220 and 230 were used, which were supplied from Ataturk University Practice and Research Center for Medical Experiments. Before this study, animals were kept and fed in groups at normal room temperature (22°C).
Chemical Substances
Of the chemical substances used for the experiments, thiopental sodium was provided by IE Ulagay, Istanbul, Turkey and mirtazapine was obtained from Organon Pharmaceuticals, NJ, USA.
General Procedure
Rats to be used in this study were assigned to four groups to undergo (1) renal ischemia (RI), (2) renal ischemia-reperfusion (RIR), (3) mirtazapine + renal ischemia-reperfusion (MRIR), and (4) sham surgery (SS). Surgical procedures were performed in sterile and suitable laboratory conditions by applying 25 mg/kg intraperitoneal thiopental sodium anesthesia.
Performing Study
Mirtazapine at a dose of 20 mg/kg was orally given to MRIR group (n = 10) via catheter 1 h before applying thiopental sodium anesthesia. Other three groups of rats also orally received distilled water as solvent. Following the injection of thiopental sodium, the rats were kept for an appropriate period of time until surgical intervention. Time period during which the animals remained in supine position is considered as the appropriate time for surgical intervention.
During this period, the left kidney was opened unilaterally from dorsal area in all the rats, and kidneys were reached. Then, clamps were attached to renal arteries and veins arriving at the kidney (SS group excluded), and ischemia was created for 1 h. Afterward, reperfusion was maintained in RIR and MRIR groups of rats for 6 h (by removing vascular clips). RI group was killed by high-dose anesthesia following ischemia, whereas RIR and MRIR groups were killed 6 h later following reperfusion in the same manner. Their right and left kidneys were removed, and examined biochemically and histopathologically. Biochemical and histopathological results obtained from MRIR group were compared with those of RI, RIR, and SS groups and evaluated.
Biochemical Analysis of Kidney Tissue
GSH, GST, MPO, and MDA analysis
After the macroscopic analysis, the glutathione (GSH), glutathione S-transferase (GST), myeloperoxidase (MPO), and malondialdehyde (MDA) enzyme activities and levels in rat kidney tissues were determined. For this purpose kidneys of rats were frozen at −80°C until before biochemical investigations. To prepare the tissue homogenates, kidney tissues were ground under liquid nitrogen using a mortar. The ground tissues (0.5 g each) were then treated with 4.5 mL of an appropriate buffer. The mixtures were homogenized on ice for 15 min by using an IKA Ultra-Turrax homogenizer, Staufen, Germany. Homogenates were filtered and centrifuged using a refrigerated centrifuge (Hettich, Tuttlingen, Germany) at 4°C. The supernatants were used for the determination of enzymatic activities. All assays were carried out in triplicate at room temperature.
Total GSH determination
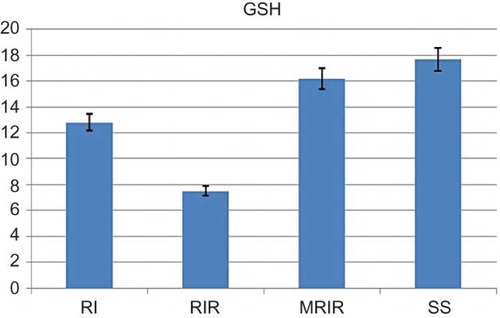
The amount of GSH in renal mucosa was measured according to the method of Sedlak and Lindsay.Citation14 The mucosal surface of the kidney was collected by scraping and was then weighed and homogenized in 2 mL of 50 mM Tris–HCl buffer containing 20 mM Ethylenediaminetetraacetic acid and 0.2 mM sucrose, pH 7.5. The homogenate was immediately precipitated with 0.1 mL of 25% trichloroacetic acid, and the precipitate was removed by centrifugation at 4064 × g for 40 min at 4°C. The supernatant was used to determine GSH using 5,5’-dithiobis (2-nitrobenzoic acid). The absorbance was measured at 412 nm using a spectrophotometer. The results of the GSH level in renal mucosa were expressed as nanomoles per milligram tissue (nmol/mg tissue).
GST activity
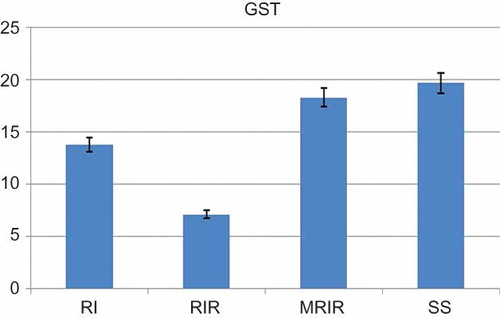
The total GST activity was determined as described by Habig and Jakoby.Citation15 Briefly, the enzyme activity was assayed spectrophotometrically at 340 nm in a 4 mL cuvette containing 0.1 M phosphate buffered saline (pH 6.5), 30 mM GSH, 30 mM 1-chloro-2,6-dinitrobenzene, and tissue homogenate.
MPO activity
MPO activity was measured according to the modified method of Bradley et al.Citation16 The homogenized samples were frozen and thawed three times and then centrifuged at 1500 × g for 10 min at 4°C. MPO activity in the supernatant was determined by adding 100 mL of the supernatant to 1.9 mL of 10 mmol/L phosphate buffer (pH 6.0) and 1 mL of 1.5 mmol/L o-dianisidine hydrochloride containing 0.0005% (wt/vol) hydrogen peroxide. The changes in absorbance at 450 nm of each sample were recorded on a UV–vis spectrophotometer. MPO activity in renal tissues was expressed as millimole per minute per milligram tissue (mmol/min/mg tissue).
Determination of lipid peroxidation or MDA formation
The concentrations of renal mucosal lipid peroxidation were determined by estimating MDA using the thiobarbituric acid test.Citation17 Briefly, the rat kidneys were promptly excised and rinsed with cold saline. To minimize the possibility of interference of hemoglobin with free radicals, any blood adhering to the mucosa was carefully removed. The corpus mucosa was scraped, weighed, and homogenized in 10 mL of 100 g/L potassium chloride. The homogenate (0.5 mL) was added to a solution containing 0.2 mL of 80 g/L sodium lauryl sulfate, 1.5 mL of 200 g/L acetic acid, 1.5 mL of 8 g/L 2-thiobarbiturate, and 0.3 mL distilled water. The mixture was incubated at 98°C for 1 h. Upon cooling, 5 mL of n-butanol:pyridine (15:l) was added. The mixture was vortexed for 1 min and centrifuged for 30 min at 3686 × g. The absorbance of the supernatant was measured at 532 nm. A standard curve was generated using 1,1,3,3-tetramethoxypropane. The recovery was over 90%. The results were expressed as nanomoles MDA per gram wet tissue (nmol/mg tissue).
BUN and Creatinine Analysis
Venous blood samples were collected in tubes without anticoagulant. Serum was separated by centrifugation after clotting and stored at −80°C until assayed. Creatinine and urea levels were determined by a colorimetric method employing a Cobas 8000 (Roche, Basel, Switzerland) spectrophotometric system. Blood urea nitrogen (BUN) levels were evaluated with the formula (BUN = UREA × 0.48).
In alkaline solution, creatinine forms a yellow-orange complex with picrate. The color intensity is directly proportional to the creatinine concentration and can be measured photometrically at 505 nm. Assays using rate-blanking minimize interference by bilirubin. Serum and plasma samples contain proteins which react non-specifically in the Jaffe method. Serum and plasma results must be reduced by 0.3 mg/dL (26 mol/L) to obtain accurate values. This correction causes a measurement error of ≤1% in urine specimens because these do not contain non-specific proteins.
Urea is hydrolyzed by urease to produce CO2 and ammonia as follows:
The ammonia formed then reacts with α-ketoglutarate and NADH in the presence of GLDH to yield L-glutamate and NAD+:
The decrease in absorbance due to the consumption of NADH is measured kinetically. The decrement in NADH complex activity is determined photometrically at 340 nm.
Histopathological Analysis
Kidneys were fixed in 10% neutral buffered formalin solution. Following the routine tissue follow-up, 4 m thick cross sections were acquired from prepared paraffin blocks. These cross sections were stained with hematoxylin and eosin and periodic acid-Schiff (PAS). They were analyzed under a light microscope (Olympus CX 51, Hamburg, Germany). Cortex, inner medulla, and especially outer medulla which are the most sensitive parts of kidney were evaluated with regard to ischemic injury. Cross sections of kidney were analyzed for tubular injury, tubular epithelial swelling, necrosis, tubular dilatation, intratubular cast formation (hyaline cast), and loss of brush border (microvillus).
Statistical Analysis
All data were subjected to one-way analysis of variance using Statistical Package for Social Sciences 18.0 (Armonk, NY, USA) software. Differences among groups were obtained using the least significant difference option and significance was declared at p < 0.05. The results are expressed as mean ± SEM.
RESULTS
Biochemical Findings
MDA, MPO, GSH, and GST results
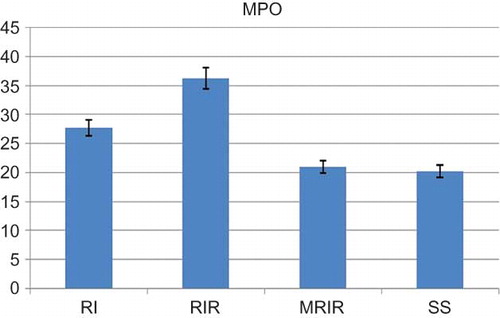
As indicated in , the MDA level in the renal tissue of SS group was 15.2 ± 1.42 mol/g protein, whereas this level was measured as 26.7 ± 1.62, 39 ± 1.92, and 17.7 ± 1.22 mol/g protein in RI, RIR, and MRIR groups, respectively. MPO activity in kidneys of SS group was 20.2 ± 1.85 u/g, whereas the same activity was measured as 27.7 ± 2.94, 36.3 ± 2.13, and 21 ± 1.50 u/g in RI, RIR, and MRIR groups, respectively (). GSH levels in SS, RI, RIR, and MRIR groups were found as 17.7 ± 1.54, 12.8 ± 1.45, 7.5 ± 0.76, and 16.2 ± 1.25 nmol/g protein, respectively (). GST activities in SS, RI, RIR, and MRIR groups were determined as 19.7 ± 2.20, 13.8 ± 0.95, 7.1 ± 0.91, and 18.3 ± 1.76 u/g, respectively ().
Figure 1. Malondialdehyde (MDA) level in the renal tissue of sham surgery (SS), renal ischemia (RI), renal ischemia-reperfusion (RIR), and mirtazapine + renal ischemia-reperfusion (MRIR) groups. The results are expressed as mean ± SEM.
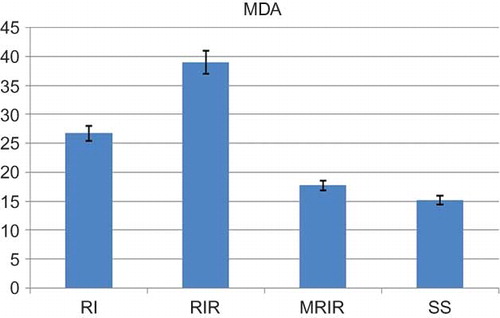
Figure 2. Myeloperoxidase (MPO) activities in kidneys of sham surgery (SS), renal ischemia (RI), renal ischemia-reperfusion (RIR), and mirtazapine + renal ischemia-reperfusion (MRIR) groups. The results are expressed as mean ± SEM.
BUN and creatinine results
In RI, RIR MRIR, and SS groups, BUN levels were 32.2 ± 2.33 (p > 0.05), 34.3 ± 1.89 (p > 0.05), 30.8 ± 2.88 (p > 0.05), and 29.0 ± 0.93 mg/dL, respectively, whereas serum creatinine levels were 0.65 ± 0.04 (p > 0.05), 0.67 ± 0.04 (p > 0.05), 0.62 ± 0.03 (p >0.05), and 0.59 ± 0.02 mg/dL, respectively.
MDA and GSH levels and MPO and GST activities detected in contralateral kidneys of groups that underwent MRIR, RIR, and RI were provided in (contralateral kidneys of the group received mirtazapine that underwent RIR—MRIRCL; contralateral kidneys of the group that underwent RIR—RIRCL; and contralateral kidneys of the group that underwent RI only—RICL).
Table 1. The MDA and GSH levels and MPO and GST activities detected in contralateral kidneys of RICL, RIRCL, and MRIRCL groups.
Histopathological Findings
Renal tissue of SS group
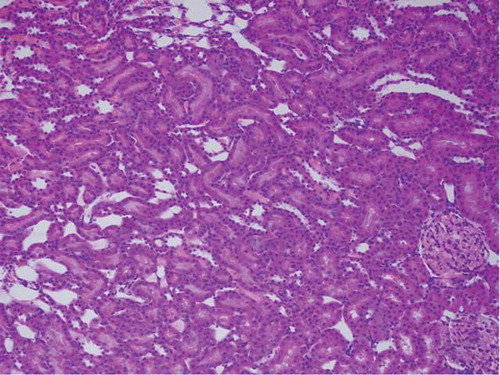
Renal tissue of SS group was evaluated as a normal histological tissue ().
Figure 6. The histopathological examination of the renal tissue of renal ischemia (RI) group. Hemorrhage (arrow), but no tubular injury, was observed in interstitial area.
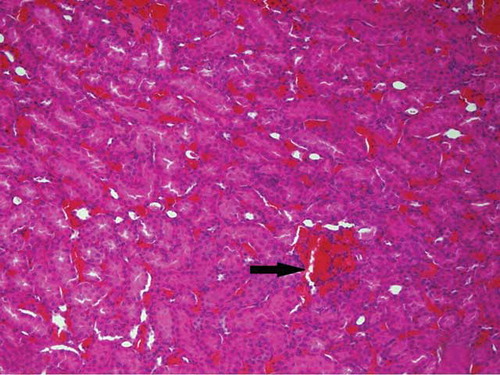
Figure 7. The histopathological examination of the renal tissue of renal ischemia-reperfusion (RIR) group. Remarkable tubular epithelial swelling (A, Arrow-1), tubular epithelial necrosis (A, Arrow-2), hyaline cast accumulation in dilated tubules (A, Arrow-3), and loss of microvillus (B, Arrow-1) were observed.
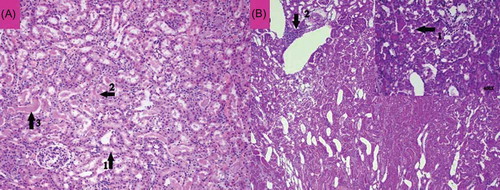
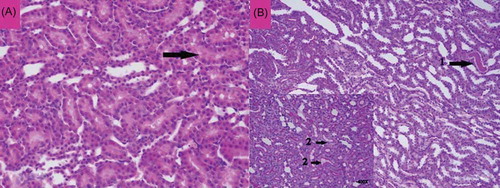
Figure 8. The histopathological examination of the renal tissue of mirtazapine + renal ischemia-reperfusion (MRIR) group. Mild swelling in tubular epithelial cells (A, Arrow) and mild hyaline cast accumulation (B, Arrow-1) were observed. Microvilli structures in proximal tubules were observed to be preserved (B, Arrow-2).
Renal tissue of RI group
Hemorrhage (arrow), but no tubular injury, was observed in interstitial area of the renal tissue of RI group ().
Renal tissue of RIR group
In the renal tissue of RIR group, remarkable tubular epithelial swelling (A, Arrow-1), tubular epithelial necrosis (A, Arrow-2), and hyaline cast accumulation in dilated tubules (A, Arrow-3) were present, especially in outer medulla (outer stripe). Loss of microvillus (B, Arrow-1) and mononuclear inflammatory cells in interstitial area were recorded during PAS staining.
Renal tissue of MRIR group
Mild swelling of tubular epithelial cells (A, Arrow) and mild hyaline cast accumulation (B, Arrow-1) were noted in the renal tissue of MRIR group. Microvilli structures in proximal tubules were observed to be preserved (B, Arrow-2). No necrosis of tubular epithelial cells and no inflammation in interstitial area were recorded.
Renal tissue of MRIRCL group
No pathological findings such as tubular epithelial swelling, necrosis, hyaline cast accumulation, and inflammation were recorded in MRIRCL group ().
Renal tissue of RIRCL group
Mild hemorrhage in interstitial area (, Arrow-1) and tubular hyaline cast accumulation (, Arrow-2) were observed in RIRCL group, and microvilli structures in proximal tubular epithelial cells appeared to be preserved (, Arrow-3). No inflammation was noted.
Renal tissue of RICL group
No pathological findings were recorded in RICL group ().
DISCUSSION
In this study, the biochemical and histopathological effects of mirtazapine on the oxidative stress (injury) which was created by IR in rat kidneys were investigated. Our biochemical and histopathological study results revealed that renal injury caused by ischemia became stronger with reperfusion. It was demonstrated that improving blood flow in ischemic tissue both accelerated and intensified the injury.Citation18 Aguilar et al.Citation19 reported that ischemic-reperfusion grade of injury in tissues depended on the balance between FOR and antioxidant system.
Figure 9. The histopathological examination of the renal tissue of mirtazapine + renal ischemia-reperfusion contralateral kidney (MRIRCL) group.
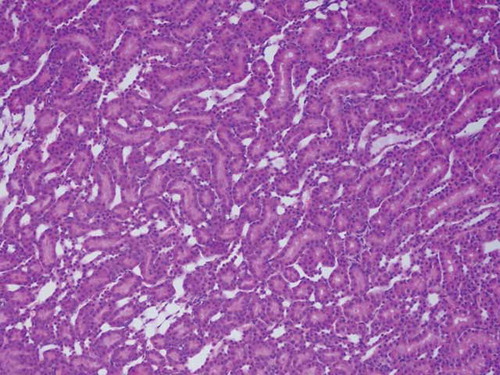
Figure 10. The histopathological examination of the renal tissue of renal ischemia-reperfusion contralateral kidney (RIRCL) group. Mild hemorrhage in interstitial area (Arrow-1) and tubular hyaline cast accumulation (Arrow-2) were observed. Microvilli structures in proximal tubular epithelial cells appeared to be preserved (Arrow-3).
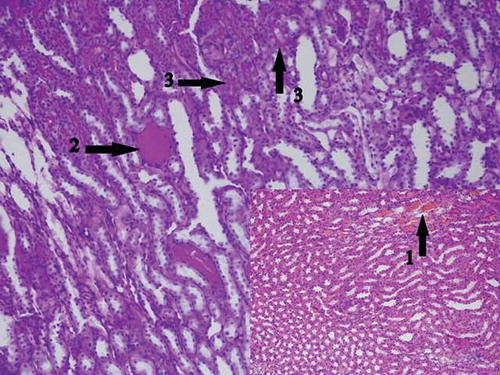
Figure 11. The histopathological examination of the renal tissue of renal ischemia contralateral kidney (RICL) group.
FOR result in cellular injuries in glomeruli, tubules, and interstitial structures of kidneys through lipid peroxidation.Citation20 It was detected that MDA, a product of lipid peroxidation, showed an increase within a short period of 1 h following reperfusion.Citation21 In our study, it was observed that MDA level significantly increased compared with SS group 1 h later following ischemia, and thereafter showed further increase with reperfusion. MDA is the final product of lipid peroxidation, which increases due to oxidation of unsaturated fatty acids and leads to further degradation of cells.Citation22,23 This literature findings that show that MDA levels in injured tissues increase remain consistent with our study results. Ischemic period is specified minimally as 30 min for renal ischemic injury to be revealed.Citation24 We created ischemia for 60 min to more clearly reveal the injury. There are also studies showing that remarkable injuries occur within this period (60 min) in the renal tissue where ischemia was created.Citation25 In the literature, injuries were created by ischemia (30, 60, 90 min) and by reperfusion (2, 24, 72, 120 h) at various intervals.Citation26 We specified the reperfusion period in our study as 6 h. It was observed that more remarkable injuries occurred in RIR group compared with SS and RI groups within this period. Studies demonstrated that the grade of reperfusion injury created within 6 h is similar to that created in the long term (24 h).Citation27 The fact that MDA level in MRIR group is lower compared with RI and RIR groups shows that mirtazapine protects the renal tissue from oxidative stress created by ischemia and IR.
Moreover, the level of MPO, an oxidative injury product, was found higher in renal tissues of RI and RIR rat groups. MPO is released from activated neutrophils. When tissue damages because of various diseases, neutrophils are activated. Reperfusion also activates neutrophils. Activated neutrophils release not only MPO but also several FOR.Citation28 It is asserted that neutrophils are major agents responsible for reperfusion injury.Citation29,30 The fact that MPO activity in MRIR group is almost similar to that of SS group reveals that mirtazapine creates a nephroprotective effect on MPO as well.
Oxidant–antioxidant systems are in a sensitive equilibrium in healthy tissues. If this equilibrium is not maintained, cellular damage occurs. Our study results showed that oxidant–antioxidant equilibrium was in favor of oxidants in RI and RIR groups, whereas the same equilibrium was recorded to be in favor of antioxidants in MRIR group. GSH and GST consumptions in RI and RIR groups were substantially sustained by mirtazapine. GSH is an endogenous antioxidant protecting cells against oxidant injury.Citation31 In addition to cell membrane lipids, antioxidants also protect molecules such as proteins, nucleic acids, and carbohydrates against oxidative injury.Citation32 GSH generates a cellular protective effect by interacting directly with toxic radicals such as hydrogen peroxide (H2O2), hydroxyl (·OH), and superoxide (O2).Citation33 It is also reported in studies conducted a very long time ago that GSH level is reduced during reperfusion injury following ischemia.Citation34
In our study, blood creatinine and BUN levels of rats in SS group and those of which underwent RI, RIR, and MRIR were investigated as well. However, the difference between creatinine and BUN levels in all groups (SS, RI, MRIR) was found to be statistically insignificant. The reason for that may be considered as the unilateral application of IR in rats and the elimination of creatinine from healthy kidneys (that never underwent ischemia). Our reason for the application of unilateral IR was to assess the IR injury that occurred in various unilateral urological surgical interventions.
No significant difference was observed in only ischemia, IR, and mirtazapine + IR rat groups in the name of oxidant–antioxidant balance. Mild hemorrhage and hyaline cast accumulation were seen only in contralateral kidneys of IR group.
In this study, the results of oxidant–antioxidant parameters used in the evaluation of tissue injury are supported by our results regarding histopathological findings. That is to say, MDA and MPO levels in the renal tissue were lower compared with the RIR group. Histopathological findings in RI group, where less oxidants were produced, were found to be less in number and milder. On the other hand, histopathological findings in RIR group (tubular epithelial swelling, necrosis, loss of microvilli, and inflammatory cells), where oxidant parameters were remarkably increased and antioxidants levels were reduced, were more in number and more severe. Frega et al.Citation35 reported that the injury in ischemic kidney that underwent reperfusion was more compared with the injury created by ischemia only. Again in RIR group, it was observed that the structural changes were intensified in tubular epithelial cells. The findings of this study are also compatible with literature findings.Citation36 In conclusion, it was revealed that the oxidative injury created by ischemia in the renal tissue became stronger with reperfusion and that the generated IR injury was prevented by mirtazapine. This favorable effect of mirtazapine may be clinically beneficial in preventing RIR injury.
Declaration of interest: None of the authors in this study had conflicts of interest, sources of financial support, corporate involvement, patent holdings, and so on.
REFERENCES
- Conesa EL, Valero F, Nadal JC, . N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:730–737.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72:65–83.
- Eltzschig HK, Collard CD. Vascular ischemia and reperfusion injury. Br Med Bull. 2004;70:71–86.
- Miller WL, Thomas RA, Berne RM, Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978;43:390–397.
- Waud WR, Rajagopalan KV. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976;172:354–364.
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970;245:4053–4057.
- Fridovich I. The biology of oxygen radicals. Science 1978;201:875–880.
- Kellogg EW, 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975;250:8812–8817.
- Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004;430:97–103.
- Tappel AL. Lipid peroxidation damage to cell components. Fed Proc. 1973;32:1870–1874.
- Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–186.
- Scheibmeir HD, Christensen K, Whitaker SH, Jegaethesan J, Clancy R, Pierce JD. A review of free radicals and antioxidants for critical care nurses. Intensive Crit Care Nurs. 2005;21:24–28.
- Bilici M, Ozturk C, Dursun H, . Protective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parameters. Dig Dis Sci. 2009;54:1868–1875.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205.
- Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405.
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Willgoss DA, Zhang B, Gobe GC, Kadkhodaee M, Endre ZH. Repetitive brief ischemia: Intermittent reperfusion during ischemia ameliorates the extent of injury in the perfused kidney. Ren Fail. 2003;25:379–395.
- Aguilar A, Alvarez-Vijande R, Capdevila S, Alcoberro J, Alcaraz A. Antioxidant patterns (superoxide dismutase, glutathione reductase, and glutathione peroxidase) in kidneys from non-heart-beating-donors: Experimental study. Transplant Proc. 2007;39:249–252.
- Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412–426.
- Rabl H, Khoschsorur G, Colombo T, . A multivitamin infusion prevents lipid peroxidation and improves transplantation performance. Kidney Int. 1993;43:912–917.
- Sener G, Sener E, Sehirli O, . Ginkgo biloba extract ameliorates ischemia reperfusion-induced renal injury in rats. Pharmacol Res. 2005;52:216–222.
- Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15.
- Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005;95:377–383.
- Paller MS, Hebbel RP. Ethane production as a measure of lipid peroxidation after renal ischemia. Am J Physiol. 1986;251:839–843.
- Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK. Kidney ischemia-reperfusion: Modulation of antioxidant defenses. Mol Cell Biochem. 2000;205:1–11.
- Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1–7.
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295.
- Beuther E. Glutathione in Red Blood Cell Metabolism. A Manual of Biochemical Methods. 2nd ed. New York: Grune Strutton; 1975:112–114.
- Siegers CP, Riemann D, Thies E, Younes M. Glutathione and GSH-dependent enzymes in the gastrointestinal mucosa of the rat. Cancer Lett. 1988;40:71–76.
- Reiter RJ, Melchiorri D, Sewerynek E, . A review of the evidence supporting melatonin’s role as an antioxidant. J Pineal Res. 1995;18:1–11.
- Rangan U, Bulkley GB. Prospects for treatment of free radical-mediated tissue injury. Br Med Bull. 1993;49:700–718.
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760.
- Paller MS. Renal work, glutathione and susceptibility to free radical-mediated postischemic injury. Kidney Int. 1988;33:843–849.
- Frega NS, DiBona DR, Guertler B, Leaf A. Ischemic renal injury. Kidney Int Suppl. 1976;6:17–25.
- Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998;18:490–497.