Abstract
Renal interstitial fibrosis (RIF) is the final common pathway for chronic kidney disease. Cell apoptosis is a critical detrimental event that leads to renal fibrosis. Paired box 2 (PAX2) plays a major role in the development of the kidney. This study was performed to investigate whether PAX2 was associated with cell apoptosis in the progression of RIF in unilateral ureteral obstruction (UUO) rats. Eighty Wistar male rats were divided into two groups randomly: sham operation group (SHO) and model group subjected to UUO (GU), n = 40, respectively. The model was established by left ureteral ligation. Renal tissues were collected 14 and 28 days after surgery. Protein expressions of PAX2, transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA), collagen-IV (Col-IV), fibronectin (FN), and caspase-3 were detected using immunohistochemical analysis; mRNA expression of PAX2 in renal tissue was detected by real-time reverse transcription polymerase chain reaction; and RIF index and cell apoptosis index in renal interstitium were also calculated. When compared with those in the SHO group, expressions of PAX2 (protein and mRNA) were markedly increased in the GU group (each p < 0.01). Protein expressions of TGF-β1, α-SMA, Col-IV, FN, and caspase-3 and RIF index and cell apoptosis index in the GU group were remarkably increased when compared with those in the SHO group (each p < 0.01). The protein expression of PAX2 was positively correlated with the protein expressions of TGF-β1, α-SMA, Col-IV, FN, and caspase-3 and with RIF index and cell apoptosis index (all p < 0.01). The apoptotic cell in our observation was mainly derived from renal tubular epithelial cells. In conclusion, the increased expression of PAX2 is associated with cell apoptosis in the progression of RIF in UUO rats, suggesting that PAX2 is a potentially therapeutic target for prevention of RIF.
Tian-Biao Zhou and Yuan-Han Qin wish it to be known that, in their opinion, they should be regarded as joint first authors.
INTRODUCTION
Renal interstitial fibrosis (RIF), associated with extensive accumulation of extracellular matrix (ECM),Citation1 is the final common pathway for chronic kidney disease, regardless of the etiology of the primary renal syndrome.Citation2 Moreover, interstitial fibrosis is the strongest morphologic predictor of clinical outcome and is most tightly linked to the progression of the disease.Citation2,3 Unilateral ureteral obstruction (UUO) is a well-characterized model of experimental obstructive nephropathy, culminating in renal tubular apoptosis and interstitial fibrosis.Citation4,5 Cell apoptosis, such as renal tubular epithelial cells (RTEC) apoptosis, is a critical detrimental event that leads to chronic kidney injury in association with renal fibrosis.Citation6
The gene paired box 2(PAX2) encodes for a transcription factor that is upregulated during nephrogenesis and becomes silenced in mature epithelium of the glomeruli, the proximal tubule, and the distal tubule.Citation7 PAX2 is essential for the development of the urogenital system, embryo, inner ear, neural tube, optic vesicle, optic cup, and optic tract.Citation8–10 PAX2 has been implicated as an oncogene in the carcinomas of the kidney, prostate, breast, ovary, and so on.Citation11
Cell apoptosis is a most important feature in the development of RIF. RTEC, a main cell in the renal interstitium and an important cell taking part in RIF, suffers from ischemic injury,Citation12,13 which can increase the production of reactive oxygen species (ROS), and undergoes epithelial–mesenchymal transition (EMT) in RIF induced by UUO.Citation14,15 Overexpression and deposition of ECM, such as collagen-IV (Col-IV) and fibronectin (FN), are the important characteristics of RIF. RTEC suffering from EMT plays a crucial role in the progression of RIF.Citation16,17 α-Smooth muscle actin (α-SMA), as a specific marker for EMT, takes part in the development and progression of RIF.Citation16,18 Of all the cytokines and growth factors involved, transforming growth factor (TGF)-β1 plays the most important role, and the increased expression of TGF-β1 is closely correlated with the development of RIF.Citation19,20 TGF-β1 is known to be one of the major mediators which leads to RIF by inducing the production of α-SMA and ECM (Col-IV and FN) in the renal interstitium.Citation21–23 So, TGF-β1, α-SMA, Col-IV, and FN are the important indicators for evaluating the grade of RIF lesion and the progression of RIF. Caspase-3 is a pivotal effector of the apoptosis machineryCitation24 and its activity is associated with cell apoptosis.Citation25,26 Cell apoptosis is most important to be associated with the development of RIF.Citation27–29
The overexpression of TGF-β1, α-SMA, Col-IV, FN, and caspase-3 is an important feature of RIF. Interestingly, overexpression of PAX2 is found in the renal tissue suffering from RIF.Citation30 Some studies have found that PAX2 is associated with the expression of TGF-β1, α-SMA, Col-IV, FN, and caspase-3.Citation31–34 PAX2 might be associated with the development of RIF.
There was no study reporting the association of PAX2 with cell apoptosis in RIF rats. As those mentioned above, we drew a hypothesis that there was an association between PAX2 and cell apoptosis in RIF rats. This investigation was performed to explore whether PAX2 was associated with cell apoptosis in the progression of RIF in UUO rats.
MATERIALS AND METHODS
Animal Model
The Animal Care and Use Committee of Guangxi Medical University approved all protocols. Eighty Wistar male rats (6-weeks old) were purchased from the Experimental Animal Center of Guangxi Medical University, Nanning, China. The rats were randomly divided into two groups: sham operation group (SHO) and model group subjected to UUO (GU), n = 40, respectively. The ureter was ligated at approximately 1 cm below the renal hilum with a 3–0 silk suture. The abdominal wound was closed and the rats were returned to the cages. Control rats underwent abdominal incision and approximation with no ligation of the ureter.Citation35,36 Twenty rats in the two groups were killed 14 and 28 days after surgery, respectively, and their renal tissues were collected for histological and molecular biology determination.
Renal Morphology
After 10% neutral formaldehyde fixation, the renal tissues were dehydrated through a graded ethanol series and embedded in paraffin. Sections were prepared on a microtome and stained with Masson’s trichrome staining. Renal pathology was observed by light microscope; the severity of the renal lesion is given by the RIF index. Blue granular or linear deposits were interpreted as positive areas for collagen staining. Semi-quantitative evaluation was performed by computer-assisted image analysis (DMR + Q550, Leica Co., Wetzlar, Germany). The area of positive staining of fibrosis was measured at 400-fold original magnification in 20 fields (ignoring the fields containing glomerular parts) which were selected from coded sections for each rat at random.Citation37
Immunohistochemical Analysis of the Protein Expressions of PAX2, TGF-β1, α-SMA, Col-IV, FN, and Caspase-3
Renal tissue fixed with 4% buffered paraformaldehyde was embedded in paraffin and 4-μm-thick sections were stained. The positive area was measured quantitatively using a computer-aided manipulator (DMR + Q550, Leica Co.). For immunohistochemical analysis of PAX2, TGF-β1, α-SMA, Col- IV, FN, and caspase-3, the sections were deparaffinized, washed with phosphate buffer saline (PBS), and treated with 3% H2O2 in methanol for 10 min. All sections were then incubated with anti-PAX2 antibody (1:100) (ABSUN Co., Missouri City, TX, USA), anti-TGF-β1 antibody (1:100) (Zhongshan Co., Beijing, China), anti-α-SMA antibody (ready-to-use kit) (Shanghai Changdao Co., Inc., Shanghai, China), anti-Col-IV antibody (ready-to-use kit) (Bo Shide Co., Wuhan, China), anti-FN antibody (1:50) (Zhongshan Co.), and anti-caspase-3 antibody (1:200) (Thermo Fisher Scientific Co., Runcorn, UK), respectively. After incubation with second antibody immunoglobulin (Shanghai Changdao Co.), the sections were stained with diaminobenzidine (Maixin Bio Co., Fuzhou, China). The positive area of PAX2, TGF-β1, α-SMA, Col-IV, FN, or caspase-3 in renal tissue was measured. During evaluation of the interstitial areas, fields containing glomerular parts were ignored. All the evaluations were performed by two of the authors blinded to the experimental code.
Cell Apoptosis Assay
Cell apoptosis was examined by the TdT-mediated dUTP nick end labeling (TUNEL) assay (Roche Inc., Basel, Switzerland) as described previously.Citation38,39 Six slides from each kidney were evaluated for the percentage of apoptotic cells by using the TUNEL assay. Then 20 watch fields, which did not include the glomerular parts, were chosen at random under microscope on each section. Brown staining of cell nuclei was considered apoptotic cells. Positive brown cells and total cells were counted. The formula for apoptosis index as the indicator of apoptosis is as followsCitation38: cell apoptosis index = positive cells/total cells × 100%. The scores obtained by the two investigators were averaged.
Real-Time Reverse Transcription Polymerase Chain Reaction to Detect PAX2 mRNA Expressions in Renal Tissue
The renal tissue was homogenized and total RNA was extracted with TRIzol (Beijing Tiangen Co., Beijing, China). The absorbance was measured using an ultraviolet spectrophotometer, and agarose gel electrophoresis confirmed that there had been no degradation of RNA by visualizing the 18S and 28S RNA bands under ultraviolet light.Citation40,41 Primers were designed according to primer design principles by Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA). The primers for PAX2 and internal control β-actin were as follows: F 5′-AAGCGACAGAACCCGACTATGT-3′ and R 5′-ACTCCTGTCCCTGCCCCAT-3′ for PAX2; F 5′-GCCCCTGAGGAGCACCCTGT-3′ and R 5′-ACGCTCGGTCAGGATCTTCA-3′ for β-actin. From the renal tissue of each rat, 1 μg total RNA was reverse transcribed into cDNA with an ExScript RT reagent kit (Takara Biotechnology Co., Dalian, China). PAX2 and β-actin were amplified with SYBR Premix Ex Taq (Beijing Tiangen Co.). Gene expression of β-actin was also measured in each sample and used as an internal control for loading and reverse transcription efficiency. The analysis for each sample was performed in triplicate. The average threshold cycle (Ct, the cycles of template amplification to the threshold) was worked out as the value of each sample. The data for fold change were analyzed using 2−ΔΔCt.Citation41,42 For example, the ΔΔCt for PAX2 mRNA expression in the GU group on the 14th day was as follows: ΔΔCtPAX2,14 day,GU group = (CtPAX2,14 day,GU group – Ctβ-actin,14 day,GU group) – (CtPAX2,14 day,SHO group – Ctβ-actin,14 day,SHO group), and the fold change for PAX2 mRNA expression in the GU group on the 14th day was .
Statistical Analysis
The data are shown as mean ± SD. Independent-samples t-test was performed to determine the differences between the SHO group and the GU group, and the Pearson’s correlation coefficient was used to determine the relationships between the indicators for detection. A value of p < 0.05 was considered as significant. Statistical analysis was performed using the Statistical Package for Social Studies SPSS version 13.0 (SPSS, Chicago, IL, USA).
RESULTS
Renal Morphology
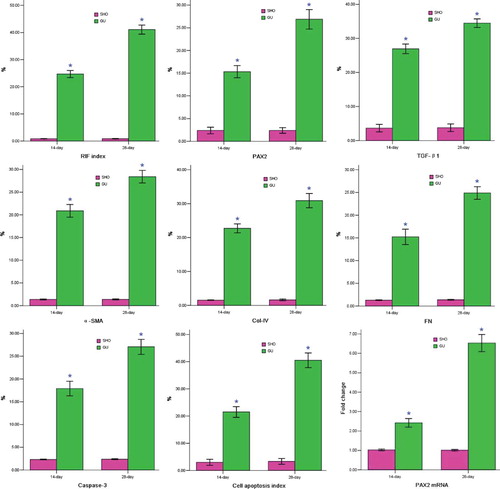
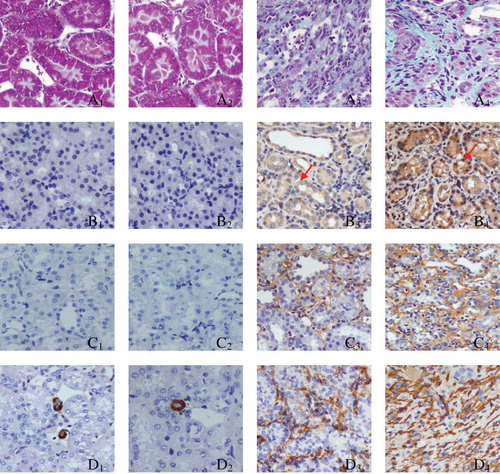
More collagen deposition, fibroblast proliferation, and diffused lymphocyte filtration in the renal interstitium of the GU group were observed when compared with those in the SHO group (). The RIF index in the GU was notably elevated when compared with that in the SHO (p < 0.01; ). In the GU group, RIF index on the 28th day was markedly increased compared with that of the 14th day (RIF index: 28th day/14th day = 1.75).
Figure 1. Statistical parameters in two groups.
Notes: *p < 0.01 compared with SHO. SHO, sham operation group; GU, model group subjected to unilateral ureteral obstruction; RIF, renal interstitial fibrosis; PAX2, paired box 2; TGF-β1, transforming growth factor-β1; α-SMA, α-smooth muscle actin; Col-IV, collagen-IV; FN, fibronectin; %, the percentage of positive area or apoptotic cell.
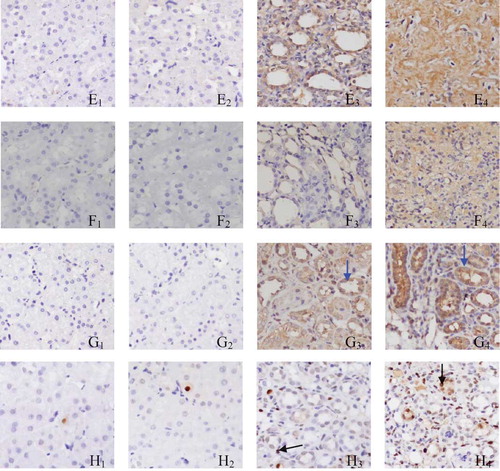
Protein Expression of PAX2, TGF-β1, α-SMA, Col-IV, FN, and Caspase-3
PAX2 staining in the GU group (B3 and B4; ) was more marked when compared with that in the SHO group (B1 and B2; ), especially in B4. Positive stainings for TGF-β1, α-SMA, Col-IV, and FN were strong in the area of ECM in the GU group than those in the SHO group, especially on the 28th day GU group. The staining for caspase-3 in the GU group (G3 and G4) was much notable when compared with that in the SHO group (G1 and G2), especially in H4. PAX2 and caspase-3 were also mainly located in the RTEC (). When compared with the SHO, in the GU group, the protein expressions of PAX2, TGF-β1, α-SMA, Col-IV, FN, and caspase-3 in the renal interstitium were significantly increased (all p < 0.01, and ). Those of the GU group on the 28th day were remarkably elevated when compared with those of the 14th day (PAX2: 28th day/14th day = 1.83; TGF-β1: 28th day/14th day = 1.30; α-SMA: 28th day/14th day = 1.33; Col-IV: 28th day/14th day = 1.41; FN: 28th day/14th day = 1.67; caspase-3: 28th day/14th day = 1.56).
Figure 2. Tissue parameters in two groups.
Notes: Masson staining for SHO group (A1, 14th day; A2, 28th day) and GU group (A3, 14th day; A4, 28th day). Representative samples of immunohistochemical staining for PAX2 (SHO, B1, 14th day, B2, 28th day; GU, B3, 14th day, B4, 28th day); TGF-β1 (SHO, C1, 14th day, C2, 28th day; GU, C3, 14th day, C4, 28th day); α-SMA (SHO, D1, 14th day, D2, 28th day; GU, D3, 14th day, D4, 28th day); Col-IV (SHO, E1, 14th day, E2, 28th day; GU, E3, 14th day, E4, 28th day); FN (SHO, F1, 14th day, F2, 28th day; GU, F3, 14th day, F4, 28th day); caspase-3 (SHO, G1, 14th day, G2, 28th day; GU, G3, 14th day, G4, 28th day); and cell apoptosis (SHO, H1, 14th day, H2, 28th day; GU, H3, 14th day, H4, 28th day) were observed in two groups. PAX2 and caspase-3 were also mainly located in the RTEC (red arrow for PAX2 and blue arrow for caspase-3), and the apoptotic cell in our observation was mainly derived from RTEC (black arrow). PAX2, paired box 2; TGF-β1, transforming growth factor-β1; α-SMA, α-smooth muscle actin; Col-IV, collagen-IV; FN, fibronectin; RTEC, renal tubular epithelial cells; SHO, sham operation group; GU, model group subjected to unilateral ureteral obstruction. Magnification ×400.
Cell Apoptosis
The staining for cell apoptosis was much significant in the renal interstitium of the GU group than that of the SHO group (), and the apoptosis index was significantly increased in the GU group when compared with that in the SHO (p < 0.01, ). In the GU group, the apoptosis index on the 28th day was notably increased than that on the 14th day (RIF index: 28th day/14th day = 1.90). Interestingly, the apoptotic cell in our observation was mainly derived from RTEC ().
mRNA Expression of PAX2
Renal tissue of the GU group showed consistently higher PAX2 mRNA expression when compared with that of the SHO (p < 0.01; ). When compared with that on the 14th day of the GU group, the mRNA expression of PAX2 on the 28th day of the GU group was markedly increased (PAX2 mRNA expression: 28th day/14th day = 2.70).
Correlation Analysis
PAX2 protein level was correlated positively with RIF index, TGF-β1, α-SMA, Col-IV, FN, caspase-3, or cell apoptosis index (r = 0.721, 0.792, 0.813, 0.885, 0.867, 0.843, 0.918; each p < 0.01).
DISCUSSION
PAX2, a nuclear transcription factor, was recently observed in the renal interstitium. Normal expression of PAX2 is necessary for the regular development of renal tissue and kidney cells.Citation43–45 Some reports found that the expression of PAX2 was closely associated with some kidney diseases. Li et al.Citation46 conducted a study in rats with obstructive nephropathy and found that PAX2 was re-expressed in the renal tubules, which might participate in the pathogenesis of renal tubular damage and RIF. The result of Li et al. was similar to the result in our investigation. Benetti et al.Citation47 performed a study in a 13-year-old boy suffering from familial juvenile hyperuricemic nephropathy, and renal biopsy in their investigation showed that PAX2-positive immunostaining was notably seen at the corticomedullary junction and most of the glomeruli featuring an enlargement of Bowman space (glomerular cysts), with mild interstitial fibrosis, inflammatory infiltrate, and focal tubular atrophy at the cortical level. Mure et al.Citation48 performed a study in 26 fetal lambs that underwent surgical UUO and found that PAX2 protein was highly expressed in the nephrogenic zone, decreasing progressively to being markedly decreased in control lamb kidneys. Murer et al.Citation30 conducted an investigation in 17 biopsies of juvenile nephronophthisis and observed that the failure of PAX2 repression was involved in interstitial fibrosis and cysts formation. The results from those four studies mentioned above might draw a conclusion that the increased expression of PAX2 took part in the progression of RIF. Furthermore, ROS could induce the PAX2 expression,Citation49,50 and overexpression of PAX2 was found in the renal tissue affected by RIF.Citation30 PAX2 was associated with the development of RIF. In conclusion, the increased PAX2 might involve in the progression of RIF.
In this investigation, we found that the PAX2 expression (mRNA or protein) in the SHO group was markedly attenuated when compared with that in the GU group in this investigation. RIF index, protein expressions of TGF-β1, α-SMA, Col-IV, FN, and caspase-3, and cell apoptosis index were markedly increased in the GU group when compared with that in the SHO group, especially on the 28th day. We speculated that the mechanism was as follows: The generation of ROS was increased in the renal tissue of UUO rats.Citation51,52 The increased ROS might upregulate the gene expression of PAX2,Citation49,50 which regulated the expression of TGF-β1.Citation53,54 The disorder of TGF-β1 might induce the expressions of α-SMA, Col-IV, and FN,Citation21–23 and the increased TGF-β1 upregulated the expression of caspase-3.Citation55–57 The overexpression of caspase-3 was associated with cell apoptosis.Citation25,26 So, the overaccumulation of ECM was observed and indexes of RIF and cell apoptosis were increased. Interestingly, the apoptotic cell in our observation was mainly derived from RTEC. We speculated that PAX2 might be associated with the cell apoptosis in UUO rats, especially with RTEC apoptosis.
In our study, we found that the increased PAX2 was associated with RIF. It was consistent with the conclusion of Li et al.Citation46 There was rare report to investigate whether PAX2 was associated with cell apoptosis in the progression of RIF. In our study, we found that the PAX2 was a gene which could induce cell apoptosis in UUO rats, especially for RTEC apoptosis, and PAX2 might be an apoptosis-induced gene in our investigation. The role of PAX2 for accelerating the cell apoptosis was never reported. However, some investigations found that PAX2 could play an important role for suppressing cell apoptosis. Dziarmaga et al.Citation58 performed a study in renal tissue and cells and found that PAX2 activated neuronal apoptosis inhibitory protein (Naip) gene transcription (sevenfold) in vitro and Naip transcript level was increased fourfold in HEK293 cells stably transfected with PAX2. Naip mRNA was significantly reduced in heterozygous PAX2 mutant mice. These observations suggested that the powerful effects of PAX2 on renal branching morphogenesis and final nephron number might be mediated by activation of Naip which then suppressed apoptosis in ureteric bud (UB) cells. Hueber et al.Citation59 conducted an investigation and found that human embryonic kidney 293 cells transfected with a PAX2 expression vector and exposed to cisplatin exhibited a higher caspase-3 cleavage when compared with control cells. Conversely, murine-collecting duct cells stably transfected with PAX2 antisense cDNA had twofold increase in cisplatin-induced apoptosis. These findings suggested that PAX2 conferred resistance to cisplatin-induced apoptosis in non-transformed kidney cells and fetal kidney explants. Hueber et al.Citation59 also observed that renal cell carcinoma cells stably transfected with shRNAs targeted against the PAX2 homeodomain were three- to sixfold more susceptible to cisplatin-induced caspase-3 activation than control renal cell carcinoma cell lines. They drew a conclusion that PAX2 suppressed cisplatin-induced apoptosis in cultured renal cell carcinoma cells. Cai et al.Citation60 found that PAX2-specific short interfering RNA increased high NaCl concentration-induced activation of caspase-3 and apoptotic bodies in mouse inner medullary collecting cells, and they drew a conclusion that PAX2 protected renal medullary cells from high NaCl concentration-induced apoptosis. Mansour et al.Citation61 reported that astrocytes lost PAX2 expression with aging, and the expression of active caspase 3 and the number of cell apoptosis were increased throughout adult life of Wistar rats. The conclusions for the association of PAX2 with cell apoptosis from the studies mentioned above were different from ours.
It was difficult to understand why the role of PAX2 for cell apoptosis was different in different cells and tissues. We evaluated the results from our experiment repeatedly and found that the data from our investigation were believable and the results were stable. First, the expression of PAX2 was elevated. The result was consistent with that of Li et al.Citation46 Furthermore, the cell apoptosis in UUO model was increased, especially for the RTEC. It was also consistent with other studies.Citation46,51,62,63 We might draw a stable conclusion that the increased expression of PAX2 was associated with cell apoptosis, especially with RTEC apoptosis, but the decreased expression of PAX2 did not take part in the progression of RIF. However, the exact mechanism is complicated and not well elucidated at the moment and more studies are needed in the future.
In conclusion, overexpression of PAX2 was associated with the increased cell apoptosis in the progression of RIF in UUO rats, although the detailed mechanisms are not fully understood. So, how to downregulate the PAX2 expression is very important for the prevention of RIF, and PAX2 might be a potential therapeutic target for the prevention of cell injury in the progression of RIF. This observation might offer some new insights to prevent the progression of RIF. However, cell cultures, such as RTEC, and inhibition of signaling pathway for PAX2 need to be explored for its detailed mechanism.
ACKNOWLEDGMENTS
This study was supported by the Nature Science Foundation of China (no. 81060061), the Natural Science Foundation of the Guangxi Zhuang Autonomous Region (no. 0832121), and the Health Department of Guangxi Zhuang Autonomous Region (no. 200917). The authors gratefully acknowledge the most helpful comments on this article received from Professor Liang Rong, Department of Pediatric-Neonatology, Baylor College of Medicine, Houston, TX, USA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
Tian-Biao Zhou and Yuan-Han Qin wish it to be known that, in their opinion, they should be regarded as joint first authors.
REFERENCES
- Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167.
- Terashima H, Kato M, Yasumo H, Tsuchida H, Mizuno M, Sada T. A sensitive short-term evaluation of antifibrotic effects using newly established type I collagen reporter transgenic rats. Am J Physiol Renal Physiol. 2010;299:792–801.
- Barnes JL, Glass WN. Renal interstitial fibrosis: A critical evaluation of the origin of myofibroblasts. Contrib Nephrol. 2011;169:73–93.
- Correa-Costa M, Semedo P, Monteiro AP, . Induction of heme oxygenase-1 can halt and even reverse renal tubule-interstitial fibrosis. PLoS One. 2010;5:e14298.
- Morisada N, Nomura M, Nishii H, . Complete disruption of all nitric oxide synthase genes causes markedly accelerated renal lesion formation following unilateral ureteral obstruction in mice in vivo. J Pharmacol Sci. 2010;114:379–389.
- Okamura DM, Pasichnyk K, Lopez-Guisa JM, . Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol. 2011;300:245–253.
- Luu VD, Boysen G, Struckmann K, . Loss of VHL and hypoxia provokes PAX2 up-regulation in clear cell renal cell carcinoma. Clin Cancer Res. 2009;15:3297–3304.
- Sehgal R, Sheibani N, Rhodes SJ, Belecky AT. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol. 2009;332:429–443.
- Busse A, Rietz A, Schwartz S, Thiel E, Keilholz U. An intron 9 containing splice variant of PAX2. J Transl Med. 2009;7:36.
- Warchol ME, Richardson GP. Expression of the Pax2 transcription factor is associated with vestibular phenotype in the avian inner ear. Dev Neurobiol. 2009;69:191–202.
- Bose SK, Gibson W, Bullard RS, Donald CD. PAX2 oncogene negatively regulates the expression of the host defense peptide human beta defensin-1 in prostate cancer. Mol Immunol. 2009;46:1140–1148.
- Meng L, Qu L, Tang J, Cai SQ, Wang H, Li X. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vascul Pharmacol. 2007;47:174–183.
- Kim DS, Kim B, Tahk H, . Transglutaminase 2 gene ablation protects against renal ischemic injury by blocking constant NF-kappaB activation. Biochem Biophys Res Commun. 2010;403:479–484.
- Kaneyama T, Kobayashi S, Aoyagi D, Ehara T. Tranilast modulates fibrosis, epithelial-mesenchymal transition and peritubular capillary injury in unilateral ureteral obstruction rats. Pathology 2010;42:564–573.
- Cheng J, Truong LD, Wu X, Kuhl D, Lang F, Du J. Serum- and glucocorticoid-regulated kinase 1 is upregulated following unilateral ureteral obstruction causing epithelial-mesenchymal transition. Kidney Int. 2010;78:668–678.
- Tang R, Yang C, Tao JL, . Epithelial-mesenchymal transdifferentiation of renal tubular epithelial cells induced by urinary proteins requires the activation of PKC-alpha and betaI isozymes. Cell Biol Int. 2011;35:953–959.
- Wang QL, Tao YY, Yuan JL, Shen L, Liu CH. Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010;11:31.
- Minz RW, Bakshi A, Chhabra S, Joshi K, Sakhuja V. Role of myofibroblasts and collagen type IV in patients of IgA nephropathy as markers of renal dysfunction. Indian J Nephrol. 2010;20:34–39.
- Ma FY, Tesch GH, Ozols E, Xie M, Schneider MD, Nikolic-Paterson DJ. TGF- beta1 activated kinase-1 (TAK1) regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol. 2011;300:F1410–F1421.
- Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956.
- Djamali A, Vidyasagar A, Yagci G, Huang LJ, Reese S. Mycophenolic acid may delay allograft fibrosis by inhibiting transforming growth factor-beta1-induced activation of Nox-2 through the nuclear factor-kappaB pathway. Transplantation. 2010;90:387–393.
- Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol. 2004;165:2033–2043.
- Kelley R, Werdin ES, Bruce AT, . Tubular cell-enriched subpopulation of primary renal cells improves survival and augments kidney function in rodent model of chronic kidney disease. Am J Physiol Renal Physiol. 2010;299:1026–1039.
- Mohamed SA, Misfeld M, Hanke T, . Inhibition of caspase-3 differentially affects vascular smooth muscle cell apoptosis in the concave versus convex aortic sites in ascending aneurysms with a bicuspid aortic valve. Ann Anat. 2010;192:145–150.
- Cai H, Gu Y, Sun Q, Zeng J, Dong N, Zhao G. Effect of hematoporphyrin monomethyl ether-mediated photodynamic therapy on hypertrophic scar fibroblasts. Photodermatol Photoimmunol Photomed. 2011;27:90–96.
- Yue Z, She RP, Bao HH, . Necrosis and apoptosis of renal tubular epithelial cells in rats exposed to 3-methyl-4-nitrophenol. Environ Toxicol. doi:10.1002/tox.20688.
- He W, Wang Y, Zhang MZ, . Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120:1056–1068.
- Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–1778.
- Mandache E, Gherghiceanu M, Serafinceanu C, Penescu M, Mircescu G. Myofibroblast involvement in tubular basement membrane remodeling in type II diabetic nephropathy. Rom J Morphol Embryol. 2011;52:75–79.
- Murer L, Caridi G, Della VM, . Expression of nuclear transcription factor PAX2 in renal biopsies of juvenile nephronophthisis. Nephron 2002;91:588–593.
- Lindoso RS, Verdoorn KS, Einicker-Lamas M. Renal recovery after injury: The role of Pax-2. Nephrol Dial Transplant. 2009;24:2628–2633.
- Huang B, Pi L, Chen C, . WT1 and Pax2 re-expression is required for epithelial-mesenchymal transition in 5/6 nephrectomized rats and cultured kidney tubular epithelial cells. Cells Tissues Organs. doi:10.1159/000327530.
- Lozanoff S, Johnston J, Ma W, Jourdan-Le SC. Immunohistochemical localization of Pax2 and associated proteins in the developing kidney of mice with renal hypoplasia. J Histochem Cytochem. 2001;49:1081–1097.
- Hueber PA, Iglesias D, Chu LL, Eccles M, Goodyer P. In vivo validation of PAX2 as a target for renal cancer therapy. Cancer Lett. 2008;265:148–155.
- Xie P, Sun L, Nayak B, . C/EBP-beta modulates transcription of tubulointerstitial nephritis antigen in obstructive uropathy. J Am Soc Nephrol. 2009;20:807–819.
- Zhang D, Sun L, Xian W, . Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest. 2010;90:436–447.
- Zhou TB, Qin YH, Zhou C, . Less expression of prohibition is associated with the increased caspase-3 expression and cell apoptosis in renal interstitial fibrosis rats. Nephrology (Carlton). doi:10.1111/j.1440-1797.2011.01522.x.
- Hu X, Zhou X, He B, . Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur J Pharmacol. 2010;638:84–89.
- Ha T, Hua F, Liu X, . Lipopolysaccharide-induced myocardial protection against ischemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–553.
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. apoE expression in glomerulus and correlation with glomerulosclerosis induced by adriamycin in rats. Ren Fail. 2011;33:348–354.
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. All-trans retinoic acid regulates the expression of apolipoprotein E in rats with glomerulosclerosis induced by adriamycin. Exp Mol Pathol. 2011;90:287–294.
- Zhou TB, Qin YH, Ou C, . All-trans retinoic acid can regulate the expressions of gelatinases and apolipoprotein E in glomerulosclerosis rats. Vasc Pharmacol. 2011;55:169–177.
- Dressler GR. Patterning and early cell lineage decisions in the developing kidney: The role of Pax genes. Pediatr Nephrol. 2011;26:1387–1394.
- Negrisolo S, Benetti E, Centi S, . PAX2 gene mutations in pediatric and young adult transplant recipients: Kidney and urinary tract malformations without ocular anomalies. Clin Genet. 2011;80:581–585.
- Raca G, Jackson CA, Kucinskas L, . Array comparative genomic hybridization analysis in patients with anophthalmia, microphthalmia, and coloboma. Genet Med. 2011;13:437–442.
- Li L, Wu Y, Zhang W. PAX2 re-expression in renal tubular epithelial cells and correlation with renal interstitial fibrosis of rats with obstructive nephropathy. Ren Fail. 2010;32:603–611.
- Benetti E, Caridi G, Vella MD, . Immature renal structures associated with a novel UMOD sequence variant. Am J Kidney Dis. 2009;53:327–331.
- Mure PY, Gelas T, Dijoud F, . Complete unilateral ureteral obstruction in the fetal lamb. Part II: Long-term outcomes of renal tissue development. J Urol. 2006;175:1548–1558.
- Zhang SL, Chen YW, Tran S, Chenier I, Hebert MJ, Ingelfinger JR. Reactive oxygen species in the presence of high glucose alter ureteric bud morphogenesis. J Am Soc Nephrol. 2007;18:2105–2115.
- Chen YW, Liu F, Tran S, . Reactive oxygen species and nuclear factor-kappa B pathway mediate high glucose-induced Pax-2 gene expression in mouse embryonic mesenchymal epithelial cells and kidney explants. Kidney Int. 2006;70:1607–1615.
- Yeh CH, Chiang HS, Lai TY, Chien CT. Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn. 2011;30:472–479.
- Meng L, Qu L, Tang J, Cai SQ, Wang H, Li X. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vascul Pharmacol. 2007;47:174–183.
- Fujita H, Hida M, Kanemoto K, Fukuda K, Nagata M, Awazu M. Cyclic stretch induces proliferation and TGF-beta1-mediated apoptosis via p38 and ERK in ureteric bud cells. Am J Physiol Renal Physiol. 2010;299:648–655.
- Liu S, Cieslinski DA, Funke AJ, Humes HD. Transforming growth factor-beta 1 regulates the expression of Pax-2, a developmental control gene, in renal tubule cells. Exp Nephrol. 1997;5:295–300.
- Huang S, Zhang F, Miao L, . Lentiviral-mediated Smad4 RNAi induced anti-proliferation by p16 up-regulation and apoptosis by caspase 3 down-regulation in hepatoma SMMC-7721 cells. Oncol Rep. 2008;20:1053–1059.
- van der Heide LP, van Dinther M, Moustakas A, ten Dijke P. TGFbeta activates mitogen- and stress-activated protein kinase-1 (MSK1) to attenuate cell death. J Biol Chem. 2011;286:5003–5011.
- Zheng X, Boerboom D, Carriere PD. Transforming growth factor-beta1 inhibits luteinization and promotes apoptosis in bovine granulosa cells. Reproduction 2009;137:969–977.
- Dziarmaga A, Hueber PA, Iglesias D, . Neuronal apoptosis inhibitory protein is expressed in developing kidney and is regulated by PAX2. Am J Physiol Renal Physiol. 2006;291:913–920.
- Hueber PA, Iglesias D, Chu LL, Eccles M, Goodyer P. In vivo validation of PAX2 as a target for renal cancer therapy. Cancer Lett. 2008;265:148–155.
- Cai Q, Dmitrieva NI, Ferraris JD, Brooks HL, van Balkom BW, Burg M. Pax expression occurs in renal medullary epithelial cells in vivo and in cell culture, is osmoregulated, and promotes osmotic tolerance. Proc Natl Acad Sci USA. 2005;102:503–508.
- Mansour H, Chamberlain CG, Weible MN, Hughes S, Chu Y, Chan-Ling T. Aging-related changes in astrocytes in the rat retina: Imbalance between cell proliferation and cell death reduces astrocyte availability. Aging Cell 2008;7:526–540.
- Chevalier RL, Forbes MS, Thornhill BA. Formation of atubular glomeruli in the developing kidney following chronic urinary tract obstruction. Pediatr Nephrol. 2011;26:1381–1385.
- Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–1778.