Abstract
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease worldwide. The pathophysiologic mechanisms of diabetic nephropathy are incompletely understood but include overproduction of various growth factors and cytokines. Upregulation of vascular endothelial growth factor (VEGF) is a pathogenic event occurring in most forms of podocytopathy; however, the mechanisms that regulate this growth factor induction are not clearly identified. A2B receptors have been found to regulate VEGF expression under hypoxic environment in different tissues. One proposed hypothesis in mediating diabetic nephropathy is the modulation of VEGF-NO balance in renal tissue. We determined the role of adenosine A2B receptor in mediating VEGF overproduction and nitrite in diabetic nephropathy. The renal content of A2B receptors and VEGF was increased after 8 weeks of diabetes induction. The renal and plasma nitrite levels were also reduced in these animals. In vivo administration of A2B adenosine receptor antagonist (MRS1754) inhibited the renal over expression of VEGF and adverse renal function parameters. The antagonist administration also improved the kidney tissue nitrite levels. In conclusion, we demonstrated that VEGF induction via adenosine signaling might be the critical event in regulating VEGF-NO axis in diabetic nephropathy.
Introduction
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease. About one-third of the patients who develop diabetes eventually suffer from DN. The costs of DN are significantly higher than those from other diabetic complications because the patients are subjected to hemodialysis programs and renal transplant when failure occurs. Thus, the burden of DN on public health is enormous.Citation1 The pathomechanisms leading to these changes are not yet clearly understood and therefore, therapeutic approaches for relief of this disease are scarce or do not permit a favorable pharmacological intervention.
Vascular endothelial growth factor (VEGF-A) is one of the critical mediators in DN. It is constitutively expressed in podocytes, proximal tubular cells and medullary thick ascending limb cells in the juxtamedullary region of the normal kidney. Evidence is emerging that VEGF plays a critical role in maintaining renal homeostasis.Citation2,Citation3 An alteration in VEGF-A expression has been shown in a variety of renal diseases. Altered (increased or decreased) expression of VEGF leads to glomerular dysfunction and proteinuria. Both circulating and local VEGF-A levels are high in diabetes and the excessive VEGF-A has been shown to have a key role in mediating glomerular hypertrophy, proteinuria and retinopathy.Citation4–6 VEGF-A induced excessive angiogenesis has been extensively found in retinopathy, the same has been also seen in nephropathy.Citation7 The precise mechanism is unclear for contradictory status of VEGF-A in diabetic and non-diabetic kidney disease. Hypoxia has been found to stimulate VEGF-A expression. Adenosine is a critical mediator in physiological adaptation to hypoxia and contribute to diseases as diverse as inflammation and carcinogenesis.Citation8 Once liberated in the extracellular space, adenosine is either recycled or interacts with cell surface adenosine receptors. Presently, four subtypes of G protein-coupled receptors exist, designated A1, A2A, A2B or A3. A2B receptors are the lower affinity receptors and they have been found to induce angiogenesis.Citation9
The role of adenosine receptors in cellular dysfunction mediating progression of DN has been studied recently. Ex vivo exposure of rat kidney glomeruli to adenosine leads to an increase in VEGF-A content. Activation of A2B receptor subtype augments expression, and releases VEGF-A beyond basal levels in rat glomeruli.Citation10 Uncoupling of VEGF-nitric oxide (VEGF–NO) axis has been studied as the factor mediating DN.Citation11 Hypoxia has been found to play a critical role in pathogenesis of DN.Citation12 We thus hypothesize that hypoxic condition produced in hyperglycemic environment of DN may change the expression of adenosine receptors to angiogenic phenotype increasing levels of VEGF-A. The accelerated state of VEGF-A together with reduced endothelial NO bioavailability, in diabetic kidney; results in uncoupling of the VEGF–NO axis. The event consequently causes VEGF-A to produce diverse biologic effects that could contribute to DN. Herein, we examined the effects of A2B antagonist on VEGF–NO axis-mediated renal function in mice model of DN.
Materials and methods
Chemicals and reagents
Streptozotocin and nitrate reductase were purchased from Sigma Chemical Co., Milwaukee, WI. An enzyme linked immunosorbent assay (ELISA) kit for the estimation of VEGF-A was purchased from RayBiotech Inc., Norcross, GA. MRS1754 were purchased from Abcam plc.UK. DAN (2,3-diaminonaphthalene), sodium nitrite and nicotinamide adenine dinucleotide phosphate (NADP) were purchased from Himedia Laboratories, Mumbai, India. Kits for the estimation of plasma creatinine and blood urea nitrogen (BUN) were purchased from Crest Biosystem, Goa, India.
Animal model and experimental protocol
All animal experiments were conducted in accordance with the guidelines of CPCSEA, India. Male C57BL/6 mice were a kind gift from Zydus Research Centre, Ahmedabad (body weight: 22 ± 2 g). Animals were maintained at a room temperature in a light (12 h light/12 h dark)-controlled environment with access to food and water ad libitum. One week after the acclimatization animals were randomly assigned to different treatment as depicted in .
Table 1. Allocation of treatment to animals.
Induction of diabetes
The low dose streptozotocin (STZ) protocol described by Animal Models of Diabetic Complications Consortium (AMDCC) was followed for induction of diabetes.Citation13 In brief, mice were fasted prior to injection for 4 h. A single intraperitoneal STZ (50 mg/kg) injection was administered to each mouse for 5 days consecutively (n = 12). Mice were supplied with 10% sucrose water to avoid sudden hypoglycemia post-injection. A normal control group of mice were injected with 400 µL vehicle (sodium citrate buffer). Body weight and blood glucose (SD CHECK™ GOLD Blood Glucose Meter, SD Biosensor, Korea) were monitored 1 week after STZ injection and every week thereafter. Mice with blood glucose levels of >300 mg/dL were considered diabetic. Following 8 weeks of diabetes induction (n = 6), the mice were treated with the antagonist of A2B adenosine receptor, MRS1754 (1 mg/kg, i.p.) for two weeks. The animals in the antagonist control group were administered MRS1754 (1 mg/mL) for two weeks (n = 6).
Collection of urine, blood and tissue samples
Mice were housed in metabolic cages for collection of 24 h urine samples. Blood was drawn from retro orbital tract. The setting up of cages and collecting samples were carried out between 15:00 and 16:00 to avoid food derived creatinine interference. At the end of the treatment all the animals were sacrificed and whole kidneys were removed. The kidneys were weighed and frozen in liquid nitrogen in RNA later™ for isolation of total RNA. The samples were stored at −80 °C until used for biochemical analysis.
Renal function parameters
Plasma and urine creatinine (modified Jaffe's kinetic method), BUN (GLDH kinetic method) were measured by commercially available kits following manufacturers' instructions. Urine albumin (Bromocresol green method using mouse albumin as standard) was measured to calculate urinary albumin excretion (UAE) from 24 h urine samples. Creatinine clearance was calculated according to the U/P × V principle for the matching plasma and urine samples.Citation14
Quantitative VEGF-A determinations
Kidney tissues were washed with cold phosphate buffered saline (PBS) and homogenized (10%w/v) in ice bath. The homogenate was then centrifuged at 20,000 rpm at 4 °C. The supernatant was stored at −80 °C till further analysis. VEGF-A protein was measured in plasma and homogenate by ELISA following the manufacturer’s protocols (RayBiotech Inc., Norcross, GA).
Quantitative NO determinations
As an indicator of nitric oxide bioavailability, nitrite and nitrate were estimated in urine and kidney homogenate by spectrofluorometric analysis. The method was adopted from the literature and modified slightly.Citation15 Briefly, 10 µL of nitrate reductase (0.5 U/mL) was added to 100 µL of sample, after 4 h; 20 µL of DAN (0.05 mg/mL), 130 µL HCl (1.5 N) were added. After 10 min, the reaction was stopped by 130 µL of NaOH (2 N). The resultant solution was diluted to 2 mL and the emission scan was recorded by a spectrofluorometer (LS 55 Fluorescence spectrometer, Perkin Elmer) exciting at 360 nm and reading at 415 nm. Sodium nitrite was used as reference standard.
Real time quantitative PCR for VEGF-A and A2B mRNA
RNeasy Mini Kit (Qiagen, Valencia, CA) was used for the RNA extraction according to the manufacturer’s instructions. Reverse transcription of total RNA to cDNA was performed with the Verso cDNA Synthesis Kit (Thermo Scientific, ABgene and Surrey, UK) in a DNA Thermal cycler (Perkin-Elmer Applied Biosystems, Foster City, CA) with random hexamers as primers. The quality of DNA and total RNA was checked by bioanalyzer (2100 Bioanalyzer Instrument, Agilent Technologies, Santa Clara, CA). The real-time PCR was performed (7500 Fast, Applied Biosystems, Grand Island, NY) using the Quanti Tect SYBR Green PCR Kit (Qiagen, Valencia, CA), with the cDNA synthesized above as template in a reaction; following manufacturer`s instructions. Specific primers () used for the mouse adenosine A2B receptor (gene bank accession number GI:145966718) and VEGF-A were adopted from the earlier literature.Citation16,Citation17 The gene for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as reference. Fold change in gene expression was calculated for each gene. Melt curves were performed upon completion of the cycles to ensure that the non-specific products were absent.
Table 2. Sequences of primer used in real time PCR.
Histochemistry and assessment of glomerulosclerosis
Kidney tissue was fixed in formalin, embedded in paraffin, sectioned and stained with Masson trichrome reagent. One hundred glomeruli were randomly selected for determination of glomerulosclerosis. Glomeruli that exhibited adhesion of the capillary tuft to Bowman’s capsule, capillary obliteration, mesangial expansion or segmental tuft sclerosis were defined as glomerulosclerotic. The extent of glomerular damage was expressed as the percentage of glomeruli that exhibited sclerosis. The degree of glomerulosclerosis was graded from 1 to 4 points according to the percentage of effected glomeruli (1 ≤ 10%, 2 ≤ 10–20%, 3 ≥ 20–50%, and 4 ≥ 50%). Blind analysis was done on all sections by one observer.
Statistical analysis
Data are presented as the mean ± standard error of mean (SEM). Statistical analysis was performed using Systat13 (syatat Inc., San Jose, CA). For comparisons of continuous variables, a test of normality was performed (Shapiro–Wilk test) prior to assessing statistical significance using either a t-test (parametric) or a Fligner-Wolfe test (nonparametric) when comparing two groups. Association between the expressions of both genes and, VEGF-A and NO levels, were analyzed by Pearson’s correlation coefficient.
Results
General parameters
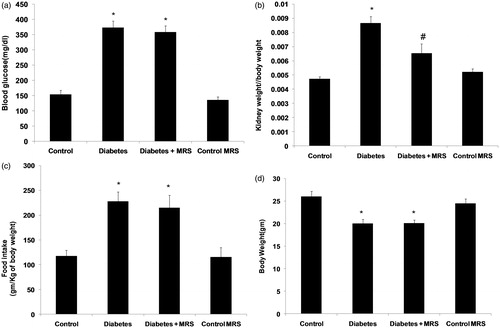
Following 10 weeks of diabetes induction, plasma glucose concentration (), kidney hypertrophy index (), and food intake () were significantly increased compared with non-diabetic control animals (p < 0.05). Similarly a significant decrease in body weight (p < 0.05, ) was observed in animals treated with streptozotocin. The treatment with A2B adenosine receptor antagonist did not alter the increased levels of plasma glucose, food intake and body weight significantly. The treatment with antagonist significantly recovered kidney hypertrophy index in diabetic animals (p < 0.05). However, antagonist administration had no effect on all above parameters when administered to vehicle treated animals (MRS control group).
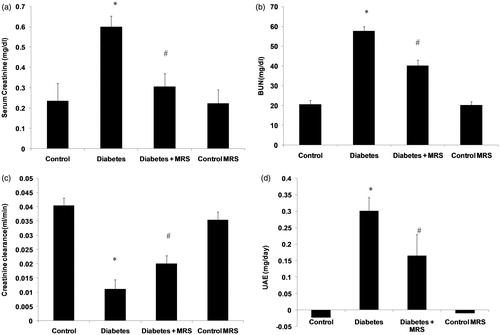
Renal function parameters
Serum creatinine (), blood urea nitrogen () and urinary protein excretion () were increased in animals after 10 weeks of diabetes (p < 0.05). Similarly, creatinine clearance was lower in diabetic animals compared with non-diabetic control animals (, p < 0.05). Urine albumin levels in control and control MRS animals were below the detection limit of the method employed for analysis. The treatment with A2B adenosine receptor antagonist recovered blood urea nitrogen, serum creatinine, creatinine clearance and urinary protein excretion in diabetic mice (p < 0.05). Moreover, no change in renal functions was found in vehicle treated animals (MRS control group) after two weeks of antagonist treatment.
Figure 2. Effect of A2B adenosine receptor antagonist (MRS1754) on the renal functions in control and diabetic animals. (a) Serum creatinine; (b) Blood urea nitrogen; (c) Creatinine clearance; (d) Urinary albumin excretion. Results shown are the means ( ± SEM). Notes: *p < 0.05; #p < 0.05 versus Diabetes group; n = 6.
Change in VEGF-A and A2B adenosine receptor gene expression
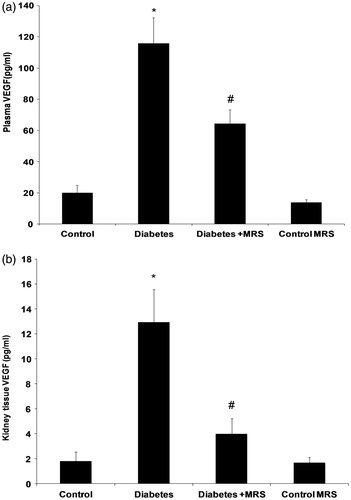
To evaluate the impact of diabetes on expression level of VEGF-A and A2B receptors in mice kidney, we examined mRNA levels in kidneys of normal and STZ-induced diabetic animals. The changes in mRNA level were evaluated based on results from real-time PCR performed on cDNA transcribed from RNA isolated from whole kidney. There was a significant increase in the mRNA level of receptor in diabetic kidney, 10 weeks after diabetes induction (, p < 0.05). In diabetic animals, the level of A2B receptor and VEGF-A mRNA were raised 11- and 12–fold, respectively. Administration of MRS1754 for two weeks blocked the renal increase in VEGF-A expression. Additionally, when antagonist was administered to vehicle treated animals (MRS control group); a partial but not significant decrease in VEGF-A expression was observed. The expression of A2B receptor and VEGF-A genes was positively correlated (, r = 0.575).
Figure 3. Real-time RT-PCR analysis of (a) A2B adenosine receptors and (b) VEGF mRNA level in kidney. RNA was isolated reverse-transcribed to cDNA, as described. The cDNA was then subjected to real-time PCR using specific primers and expression levels calculated and normalized to an internal control (GAPDH). (c) Correlation between expressions of both the genes (Pearson’s correlation). Notes: Data are means (±SEM). *p < 0.05 versus control group; #p < 0.05 versus diabetes group. n = 5.
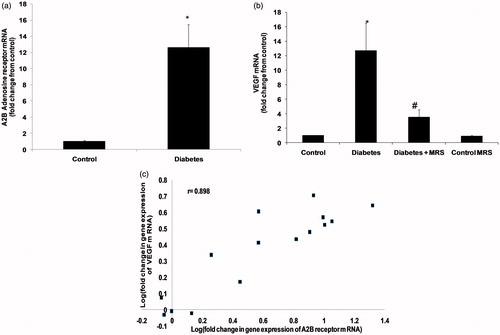
VEGF-A protein levels
VEGF-A protein measurement by ELISA supported results obtained by gene expression study (p < 0.05, ). Comparison of VEGF-A protein level in kidneys of normal and diabetic mice indicated significantly increased level of this mRNA in diabetic kidney. A two-week treatment with MRS1754 blocked the increase in VEGF-A. However, antagonist administration had no effect on VEGF-A levels when administered to vehicle treated animals (MRS control group).
Change in nitric oxide levels
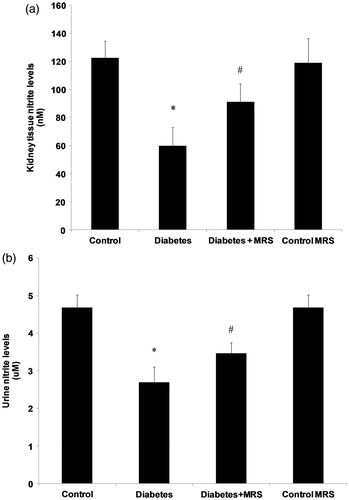
STZ treatment significantly decreased urinary excretion of nitrite (). Plasma availability () and kidney concentration () of nitrite were also reduced when compared with the control levels. The administration of MRS1754 for two weeks blocked the renal decrease in nitrite (p < 0.05).
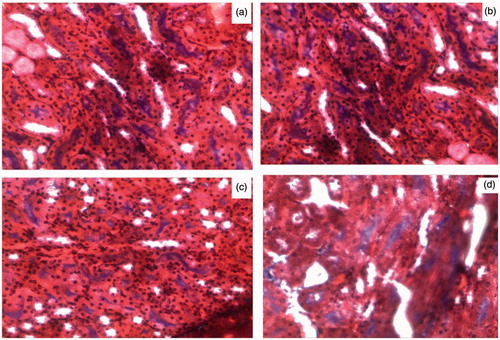
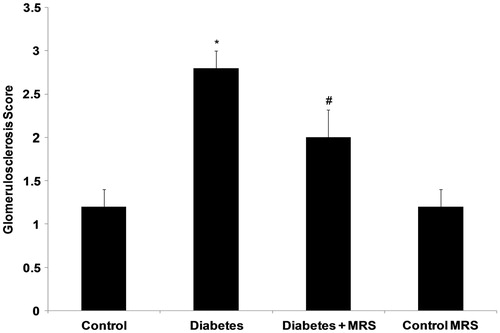
Glomerulosclerosis
shows histochemical staining of representative kidney sections from each group, and depicts the quantification for glomerulosclerosis derived from the analysis of the kidney sections. Glomerulosclerosis was significantly increased in diabetic mice, involving at least 20–50% of glomeruli, p < 0.05 versus all other groups (). Treatment with MRS1754 prevented the increase in glomerulosclerosis observed in diabetic mice. The images of the characteristic features of DN can be viewed online in the supplementary section of article.
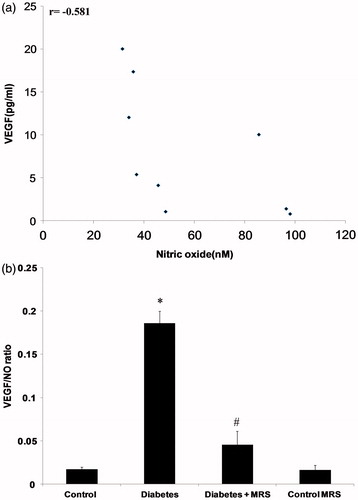
Relative VEGF-A level
shows the negative correlation (r = −0.581) between VEGF-A and nitric oxide in diabetes in kidney tissue homogenate. The relative concentration (VEGF/NO ratio) of VEGF-A was found to evaluate the impact of A2B inhibition on uncoupling state of VEGF–NO axis. The relative level of VEGF-A was significantly increased after 10 weeks of diabetes induction (). The treatment with MRS1754 improved the VEGF/NO ratio.
Discussion
The present study demonstrates that the A2B receptor has a pathogenic effect in early glomerular dysfunctions observed in STZ-induced diabetic mice and; it can be blocked by using a selective antagonist in vivo. Eight weeks after induction of diabetes in mice, marked albuminuria associated with a reduction of renal function; and early histological changes of diabetic nephropathy are established. Administration of A2B receptor antagonist led to a marked reversal of albuminuria along with a restoration of creatinine clearance and BUN. Blood glucose and food intake were the same in control and antagonist treated groups; thus, these factors are unlikely to explain the difference in proteinuria observed. These alterations mediated by A2B receptors correlate with an increased expression of this receptor in mice diabetic kidney. Results obtained are comparable to the earlier in-vivo and in-vitro studies.Citation18,Citation19 The differential expression of adenosine receptor was first described by Pawelczyk et al.Citation20 The alteration turns off the inhibition on VEGF-A release accompanied by an increase in the production of this growth factor release in diabetic state.
The mechanism by which A2B receptor antagonist mediate protection in STZ-induced diabetic nephropathy is not known. The pathomechanism leading to diabetic nephropathy involves release of diverse growth factors, increased VEGF-A being the most often involved. VEGF-A has been found to act in autocrine manner to induce proteinuria caused by podocytopathy. A2B receptors have been investigated for attenuating kidney injury in diabetes directly through effects on signaling pathways inducing VEGF-A. A2B receptor antagonist was demonstrated to block the increased release of TGF-β from diabetic glomeruli in vitro and the myofibroblast transdifferentiation of mesangial cells in vivo.Citation21 In these studies, intervention of VEGF-A induction was a remarkable event. Therefore, interception of both the TGF-β and VEGF-A signaling via A2B receptors may give new insights into drug discovery. We also correlate the increase in low affinity A2B receptor with an increase in VEGF-A expression.
Several studies have shown that the VEGF-A expression in renal podocytes increased upon exposure to high glucose concentration and it is intracellular mediated by PKC-alpha and ERK1/2 signalling molecules.Citation22 Our results also support increased expression of VEGF-A in renal tissue in diabetic state. The in vitro studies also showed reversal of increased VEGF-A in the presence of MRS1754.Citation10,Citation23 In agreement with this evidence, our study in vivo implies A2B receptors could be a transducer of hyperglycemic conditions to mediate VEGF-A increase in diabetic mielu.
A low renal VEGF-A was observed in many types of experimental kidney diseases, and administration of VEGF-A was found to be protective.Citation24 A contradiction in terms occurs in diabetes, wherein renal VEGF-A levels are high and a detrimental effect of VEGF-A on kidney disease has been shown.Citation4,Citation5 Normally, VEGF-A stimulates endothelial nitric oxide release; thus, elevated NO and VEGF-A act in coordination with each other as a trophic factor for vascular endothelium. The surplus NO, derived from the endothelial cell; acts as an inhibitory factor that prevents excess endothelial cell proliferation, vascular smooth muscle cell proliferation and macrophage infiltration. In diabetic state, where NO bioavailability is reduced; high levels of VEGF-A lead to excessive endothelial cell proliferation, stimulation of macrophage chemotaxis and vascular smooth muscle cell activation.Citation25 Consistent with this, in our study, we could find a reduced state of nitric oxide in plasma, urine and renal tissue homogenate in diabetic mice. These events are consistent with the postulate that oxidative stress promotes NO degradation in the renal cortex during the early stage of diabetes mellitus.Citation26,Citation27 It is interesting to note that reversal in albuminuria is greater in diabetic mice than the normal control mice treated with antagonist alone; suggesting that the diabetic conditions modulate expression of adenosine receptors toward angiogenic phenotype; which are otherwise low-affinity receptors. The elevated receptor expression induces renal VEGF-A level (as indicated by positive correlation between the receptor and VEGF-A) which would be added to hyperglycemia-induced low NO availability, and becomes a critical event triggering uncoupled state of VEGF-A–NO axis. In consistent with this hypothesis, we could find reversal of VEGF-A–NO axis in diabetic animals after 2 weeks of antagonist administration. A2B antagonist may also directly modulate NO which is indicated by increase in NO levels; 2 weeks after MRS1754 treatment.
The current literature evidence indicates Angiotensin II (Ang II) as contributing factor to induce VEGF-A expression in diabetic glomerular tissue. Intra-renal renin and angiotensinogen levels are increased in diabetic animals. High glucose has been demonstrated to induce renin and Ang II production in glomerular cells.Citation28,Citation29 A2BAR antagonist blocked the progression of renal fibrosis generated in the ADA−/− animals; in mice infused with Ang II or subjected to unilateral ureteral obstruction. These studies suggest a common pathogenic pathway involving adenosine signaling in chronic kidney disease.Citation30 Recently, notch I signaling have also been proved to increase VEGF-A and podocytopathy.Citation31 Therefore, it remained to be revealed if A2B receptors also interrelate activation of the notch I signaling. Our major contribution is to demonstrate that this pathogenic pathway occurs early in the progression of the diabetic renal disease, and supports the option of A2BAR as a pharmacological target intervention in DN.
In patients with cancer treated with bevacizumab, a humanized monoclonal anti-VEGF-A antibody, thrombotic microangiopathy developed as a complication.Citation32 Administration of A2B antagonist may inhibit pathological change in VEGF, mediated by hypoxic diabetic environment instead of the widespread non-specific inhibition which might have resulted into adverse effects. The correlation between the activities of these low-affinity receptors with increased ligand availability have already been observed in diabetic kidney in experimental animals.Citation30 In accordance with these findings, elevated adenosine levels in plasma of clinically manifested DN patients were also found.Citation33 Hence, the measurement of adenosine levels in plasma and urine or expression of these receptors in biopsy specimen could represent a novel specific marker for early diagnosis or patient at risk for development of DN. It has been described that A2B receptor blockade reverses insulin resistance in type II diabetes animal models and points toward another therapeutic potential.Citation34 A2B receptor blockers useful in humans such as CVT-6883 are being developed in phase 1 trial,Citation35 may be a novel alternative for the management of DN patients.
Our studies do not exclude the possibility that A2B receptor antagonist induce a favorable intraglomerular hemodynamic effect to reduce proteinuria. It is possible that A2B receptor antagonist mitigate proteinuria through direct effects by blocking vasoactive inflammatory mediators. Additional studies are necessary to address this issue.
In summary, our study demonstrates that administration of selective A2B receptor antagonist attenuates renal dysfunction in DN. We believe that the renal tissue-protective effect of A2B receptor antagonist is mediated primarily by inhibiting induction of VEGF-A; thereby, preventing distraction of VEGF–NO axis in diabetic kidney. We conclude that A2B receptor antagonist represent a novel therapeutic option for the treatment of diabetic kidney disease and for potentially other diabetic complications; where VEGF-A is the culprit.
Declaration of interest
The authors state no conflict of interest.
Supplementary material available online
Supplementary Figures I–IV
Supplementary Material
Download PDF (950.8 KB)Acknowledgments
The authors thank Dr. C. J. Joshi (Department of Animal Biotechnology, Anand Agricultural University, Anand, Gujarat, India) for providing facility to carry out gene expression studies and ELISA experiments. They also thank Ms. Pankti Desai (Department of Pharmaceutical Chemistry and Analysis, Ramanbhai Patel College of Pharmacy) for assistance in spectrofluorometric experiments. Authors are also thankful to Dr D. J. Godasara (Department of Veterinary Pathology, Anand Agricultural University, Anand, Gujarat, India) for assistance in histopathological studies.
References
- Peter Rossing MDD. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diabetes Reports. 2006;6(6):479–483
- Futrakul N, Butthep P, Laohareungpanya N, Chaisuriya P, Ratanabanangkoon K. A defective angiogenesis in chronic kidney disease. Renal Fail. 2008;30(2):215–217
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65(6):2003–2017
- Tsuchida K, Makita Z, Yamagishi S, et al. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999;42(5):579–588
- De Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12(5):993–1000
- Ho C, Hsu Y-C, Tseng C-C, Wang F-S, Lin CL, Wang JY. Simvastatin alleviates diabetes-induced VEGF-mediated nephropathy via the modulation of Ras signaling pathway. Renal Fail. 2008;30(5):557–565
- Xu L, Kanasaki K, Kitada M, Koya D. Diabetic angiopathy and angiogenic defects. Fibrogen Tissue Repair. 2012;5:13. doi: 10.1186/1755-1536-5-13
- Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20(13):2242–2250
- Feoktistov I, Goldstein AE, Ryzhov S, et al. Differential expression of adenosine receptors in human endothelial cells role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90(5):531–538
- Valladares D, Quezada C, Montecinos P, et al. Adenosine A2B receptor mediates an increase on VEGF-A production in rat kidney glomeruli. Biochem Biophys Res Commun. 2008;366(1):180–185
- Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol-Renal Physiol. 2007;292(6):F1665–F1672
- Palm F, Nordquist L, Wilcox CS, Hansell P. Oxidative Stress and Hypoxia in the Pathogenesis of Diabetic Nephropathy. Studies on Renal Disorders. Springer; 2011:559–586
- Wu KK, Huan Y. Streptozotocin-induced Diabetic Models in Mice and Rats. Current Protocols in Pharmacology. John Wiley and Sons; 2008:5.47.1–5.47.14
- Eisner C, Faulhaber-Walter R, Wang Y, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2009;77(6):519–526
- Rao AM, Dogan A, Hatcher J, Dempsey R. Fluorometric assay of nitrite and nitrate in brain tissue after traumatic brain injury and cerebral ischemia. Brain Res. 1998;793(1):265–270
- Kreckler LM, Wan TC, Ge Z-D, Auchampach JA. Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Therapeut. 2006;317(1):172–180
- Liu J, Jha P, Lyzogubov VV, Tytarenko RG, Bora NS, Bora PS. Relationship between complement membrane attack complex, chemokine (CC motif) ligand 2 (CCL2) and vascular endothelial growth factor in mouse model of laser-induced choroidal neovascularization. J Biol Chem. 2011;286(23):20991–21001
- Cárdenas A, Toledo C, Oyarzún C, et al. Adenosine A2B receptor-mediated VEGF induction promotes diabetic glomerulopathy. Laboratory Invest. 2013;93:135–144
- Valladares D, Quezada C, Montecinos P, et al. Adenosine A2B receptor mediates an increase on VEGF-A production in rat kidney glomeruli. Biochem Biophys Res Commun. 2008;366(1):180–185
- Pawelczyk T, Grden M, Rzepko R, Sakowicz M, Szutowicz A. Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am J Pathol. 2005;167(2):315–325
- Roa H, Gajardo C, Troncoso E, et al. Adenosine mediates transforming growth factor-beta 1 release in kidney glomeruli of diabetic rats. FEBS Lett. 2009;583(19):3192–3198
- Hoshi S, Nomoto K-I, Kuromitsu J, Tomari S, Nagata M. High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem Biophys Res Commun. 2002;290(1):177–184
- Karczewska J, Piwkowska A, Rogacka D, Stępiński J, Angielski S, Jankowski M. Purinergic modulation of glucose uptake into cultured rat podocytes: Effect of diabetic milieu. Biochem Biophys Res Commun. 2011;404(2):723–727
- Kang D-H, Kim Y-G, Andoh TF, et al. Post-cyclosporine-mediated hypertension and nephropathy: amelioration by vascular endothelial growth factor. Am J Physiol-Renal Physiol. 2001;280(4):F727–F736
- Nakagawa T, Sato W, Sautin YY, et al. Uncoupling of vascular endothelial growth factor with nitric oxide as a mechanism for diabetic vasculopathy. J Am Soc Nephrol. 2006;17(3):736–745
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9
- Xia Z, Nagareddy PR, Guo Z, Zhang W, McNeill JH. Antioxidant N-acetylcysteine restores systemic nitric oxide availability and corrects depressions in arterial blood pressure and heart rate in diabetic rats. Free Radical Res. 2006;40(2):175–184
- Singh R, Singh AK, Alavi N, Leehey DJ. Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol. 2003;14(4):873–880
- Vidotti D, Casarini D, Cristovam P, Leite C, Schor N, Boim M. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol-Renal Physiol. 2004;286(6):F1039–F1045
- Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011;22(5):890–901
- Lin C-L, Wang F-S, Hsu Y-C, et al. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes. 2010;59(8):1915–1925
- Schraermeyer U, Julien S. Formation of immune complexes and thrombotic microangiopathy after intravitreal injection of bevacizumab in the primate eye. Graefes Arch Clin Exp Ophthalmol. 2012;250(9):1303–1313
- Xia J-F, Liang Q-L, Hu P, Wang Y-M, Li P, Luo G-A. Correlations of six related purine metabolites and diabetic nephropathy in Chinese type 2 diabetic patients. Clin Biochem. 2009;42(3):215–220
- Figler RA, Wang G, Srinivasan S, et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60(2):669–679
- Kalla RV, Zablocki J. Progress in the discovery of selective, high affinity A2B adenosine receptor antagonists as clinical candidates. Purinergic Signal. 2009;5(1):21–29