Abstract
Background: Ethylene glycol (EG) exposure caused formation of calcium oxalate crystal that led to renal failure, which is associated with higher prevalence of hypertension. l-Arginine is known to have an antioxidant and nephro-protective potential. Objective: To evaluate the effect of l-arginine against EG-induced urolithiasis in uninephrectomized hypertensive rats. Material and methods: Uninephrectomized male Wistar rats (180–200 g) were used to induce urinary calculi through oral administration of EG (0.75%) in distilled water. Rats were treated with either distilled water (10 mg/kg, p.o.) or telmisartan (10 mg/kg, p.o.) or Cystone (500 mg/kg, p.o.) or l-arginine (250, 500, and 1000 mg/kg, p.o.) for 28 days. Various hemodynamic, biochemical, molecular, and histological parameters were assessed in kidney and heart. Results: Rats treated with l-arginine (500 and 1000 mg/kg) significantly restored altered relative organ weight, urine output, urine density, urinary pH, and water intake. EG-induced alterations in electrocardiographic (QRS interval, HR, and ST height) and hemodynamic (SBP, DBP, MABP, and LVEDP) abnormalities were significantly restored by l-arginine (500 and 1000 mg/kg) treatment. It also significantly restored alteration in serum and urine biochemical parameters induced by EG. The elevated oxido-nitrosative stress was also significantly decreased by l-arginine (500 and 1000 mg/kg) treatment. It also significantly down-regulated EG-induced up-regulated renal KIM-1, NGAL, eNOS, and iNOs mRNA expressions. Histological aberrations induced in the renal and cardiac tissues were also ameliorated by l-arginine treatment. Conclusion: l-Arginine exerts its nephro- and cardio-protective potential in EG-induced urolithiasis in uninephrectomized hypertensive rats via modulation of KIM-1, NGAL, eNOS, and iNOs mRNA expression.
Introduction
Nephrolithiasis or urolithiasis is a common disorder distributed worldwide that is estimated to occur in approximately 12% of the population, with a large recurrence rate of 70–80% in males and 47–60% females.Citation1 Urolithiasis denotes the presence of stones anywhere in the urinary tract, which includes kidneys and bladder.Citation2 The pathophysiological basis of the formation of kidney and bladder stones is entirely different. The majority of stones (up to 80%) which are found clinically and pre-clinicallyCitation3 are composed of mainly calcium oxalate.Citation4 It has been reported that high blood pressure is associated with abnormalities in calcium metabolism.Citation5 Cross-sectional epidemiological surveys described an association between hypertension and kidney stone disease.Citation6–8 However, a study carried out by researcher showed association between unilateral renal nephrectomy with hypertension.Citation9
Experimental animal models of urinary calculi with hypertension have been widely used to establish a direct relationship between urinary calcium and blood pressure.Citation6,Citation10 Increased urinary calcium concentration may be responsible for the formation of calcium stones.Citation11 Research on the pathogenesis of calcium oxalate stone formation has reported that renal epithelial cell injury initiates the stone formation.Citation12 Renal mass reduction or damage to one kidney, usually, induces morphological and functional hypertrophy, which increases filtered load and excessive urinary excretion of lithogenic substances in the remaining or alternative nephron units. This may cause the supersaturation of urine, leading to crystallization and urolithiasis as postulated by the hyperexcretion-crystallization hypothesis.Citation13,Citation14 Angiotensin-II plays an important role in this process and production of local angiotensin-II by activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes that have been identified as a major source of ROS generation. This elevated level of ROS causes the inflammatory response and accelerates oxidative stress.Citation15–18
Recent studies have been suggested various functions of angiotensin type 1 receptor blockers (ARBs) other than reducing blood pressure. Reports have revealed that the anti-inflammatory effect is related to the renoprotective and anti-atherosclerotic effects of ARBs.Citation19,Citation20 The suppression of angiotensin II activity by ARBs could protect the organs against inflammatory damage.
The medical management of urolithiasis mainly involves techniques like extracorporeal shock wave lithotripsy and percutaneous urolithotomy. However, the prevention of recurrence of stone formation is not assured. Moreover, these treatments cause undesirable side effects such as hemorrhage, hypertension, tubular necrosis and subsequent fibrosis of the kidney.Citation21,Citation22 Therefore, it is necessary to look for an alternative for the management of urolithiasis in uninephrectomized patient, which not only give relief but also have minimal side effect. Therefore, an isolated bioactive moiety from plant source is being sought.
l-Arginine is a semi-essential nitrogen-rich amino acid and a vital precursor for the synthesis of various endogenous molecules, including creatine, urea, polyamines, and nitric oxide (NO). NO is an important signaling molecule involved in neurotransmission, vasodilatation, immune regulation, and host defense.Citation23–25 l-Arginine has been reported to have many biologically important properties including anti-atherosclerotic, wound healing, gastro-protective,Citation26 and platelet aggregation inhibitory potential.Citation27 It is also known to have ameliorative effects against inflammatory bowel diseases,Citation28 intestinal ischemia-reperfusion injury,Citation29 and liver ischemia-reperfusion injury.Citation30 It has been reported that l-arginine plays a protective role in STZ-induced nephrotoxicity,Citation31 cisplatin-induced nephrotoxicity,Citation32 and cyclosporine-induced nephrotoxicity.Citation33 Administration of l-arginine showed significant effects against pulmonary hypertension in pre-clinicalCitation34 and clinical studies.Citation35,Citation36 It also enhances liver regeneration after hepatectomy.Citation37 However, very little is known about its possible attenuating effect in ethylene glycol (EG)-induced urolithiasis in uninephrectomized rats. Hence, the aim of this investigation was to evaluate the ameliorative effect of l-arginine against EG-induced urolithiasis in uninephrectomized hypertensive rats by assessing various hemodynamic, biochemical, molecular, and histological changes.
Materials and methods
Experimental animals
Adult male Wistar rats (180–200 g) were purchased from the National Institute of Biosciences, Pune (India). They were maintained at 24 ± 1 °C, with relative humidity of 45–55% and 12:12 h dark/Light cycle. The animals had free access to standard pellet chow (Pranav Agro-industries Ltd., Sangli, India) and water throughout the experimental protocol. All experiments were carried out between 09:00 and 17:00 h. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune. All the experiments were performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA).
Drugs, chemicals, and kits
l-Arginine was purchased from Sigma Chemical Co., (St Louis, MO). The standard drug telmisartan was provided by Lupin Chemicals Limited, Pune as a gift sample. 1,1′,3,3′-Tetraethoxypropane, crystalline beef liver catalase, reduced glutathione (GSH), 5,5′-dithiobis (2-nitrobenzoic acid), EG were purchased from S.D. Fine Chemicals, Mumbai, India. Total RNA Extraction kit and One-step RT-PCR kit was purchased from MP Biomedicals India Private Limited, India.
Ethylene glycol induced urolithiasis
Surgical preparations
For uninephrectomy, rats were anesthetized with ketamine (80 mg/kg body wt, i.p.). A small incision was made over the left flank area, and the left kidney was removed after careful ligation of the renal vessels and ureter. After surgery, all uninephrectomized rats were treated with soframycin twice a day for prophylaxis of wound infection for 4 days.
Ethylene glycol induced urolithiasis in uninephrectomized rats
Rats were divided into following groups containing six rats in each.
Group I: Normal group (N): Non-nephrectomized rats received distilled water (10 mg/kg, p.o.) for 28 days.
Group II: Uninephrectomy control group (UC): Uninephrectomized rats received EG (0.75%) and distilled water (10 mg/kg, p.o.) for 28 days.
Group III: Telmisartan (10 mg/kg) group T (10): Uninephrectomized rats received EG (0.75 %) and Telmisartan (10 mg/kg, p.o.) for 28 days.
Group IV: Cystone (500 mg/kg) group C (500): Uninephrectomized rats received EG (0.75%) and Cystone (500 mg/kg, p.o.) for 28 days.
Group V: l-arginine (250 mg/kg) group LA (250): Uninephrectomized rats received EG (0.75%) and l-arginine (250 mg/kg, p.o.) for 28 days.
Group VI: l-arginine (500 mg/kg) group LA (500): Uninephrectomized rats received EG (0.75%) and l-arginine (500 mg/kg, p.o.) for 28 days.
Group VII: l-arginine (1000 mg/kg) group LA (1000): Uninephrectomized rats received EG (0.75%) and l-arginine (1000 mg/kg, p.o.) for 28 days.
Ethylene glycol-induced urolithiasis in uninephrectomized rats was induced in each group (except normal) by 0.75% of EG that was provided in their drinking water. The doses of l-arginine (250, 500, and 1000 mg/kg) were selected based upon the previous study.Citation38 Food intake, water intake, and urine output were recorded after placing individual animals in metabolic cages (Techniplast, Italy).
Invasive measurement of hemodynamic changes
Hemodynamic changes such as heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MABP), left ventricular end-diastolic pressure (LVEDP), and ventricular contractility assessment (dp/dt) were measured using a polyethylene cannula (PE 50) filled with heparinized saline (100 IU/mL) inserted into the right carotid artery. The cannula was connected to a transducer, and the signal was amplified by bioamplifier. Left ventricular systolic pressure was measured by means of a Millar micro-tip transducer catheter (Model SRP-320, Millar instrument, INC 320-7051, Houston, TX) inserted into the left ventricle via the right carotid artery and connected to a bioamplifier. The left ventricular functions like dP/dtmax, dP/dtmin and left ventricular end-diastolic pressure signals were obtained from primary signals (left ventricular systolic pressure and blood pressure) by means of an acquisition data system (AD Instruments Pvt. Ltd. with software LabChart 7.3; AD Instrument Pvt. Ltd, Gurgaon, India).Citation39
Renal function
Immediately after hemodynamic measurements blood was collected from the animals by ROP (retro-orbital puncture) and centrifuged at 7500 rpm for 15 min at 4 °C, the serum was transferred using a micropipette in Eppendorf tubes and stored at 4 °C till analyzed. Serum samples were assayed for BUN, uric acid, creatinine, sodium, LDH, and calcium which were measured using a spectrophotometer (UV–visible spectrophotometer, Jasco V-530, Tokyo, Japan) using commercially available reagent kits according to procedure provided by manufacturer (Accurex Biomedical Pvt. Ltd., Mumbai, India). Whereas urine samples were assayed for BUN, uric acid, creatinine, sodium, calcium, citrate, and albumin using commercially available reagent kits according to procedure provided by manufacturer (Accurex Biomedical Pvt. Ltd., Mumbai, India).
Endogenous antioxidant enzymes in kidney and heart
Animals were sacrificed; kidney and heart were isolated and weighed. Kidney and heart samples from two animals were arbitrarily selected for histopathology. The kidney and heart samples from remaining animals were cut into small pieces, placed in chilled 0.25 M sucrose solution, and blotted on a filter paper. The tissues were then homogenized in 10% chilled tris hydrochloride buffer (10 mM, pH 7.4) by tissue homogenizer (Remi Motors, Mumbai, India) and centrifuged at 7500 rpm for 15 min at 0 °C using Eppendorf 5810-R high-speed cooling centrifuge. The clear supernatant was employed to estimate superoxide dismutase (SOD), reduced glutathione (GSH), lipid peroxidation (MDA content), and nitric oxide (NO) as described previously.Citation18,Citation40–42
Determination of KIM-1, NGAL, eNOs, and iNOs by Reverse Transcriptase PCR in kidney
RNA isolation
The levels of mRNA were analyzed in kidney tissue using a reverse transcription (RT)-PCR as described previously.Citation43,Citation44
cDNA preparation
Single-stranded cDNA was synthesized from 5 µg of total cellular RNA using reverse transcriptase kit (MP Biomedicals India Private Limited, India) as described previously.Citation43 The primer sequence for Kidney Injury Molecule-1 (KIM-1), Neutrophil gelatinase-associated lipocalin (NGAL), endothelial nitric oxide synthase (eNOs), inducible nitric oxide synthase (iNOs), and β-actin are presented in Supplementary Material. Amplification of β-actin served as a control for sample loading and integrity. PCR products were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Size of amplicons was confirmed using a 100-bp ladder as a standard size marker. The amplicons were visualized, and images were captured using a gel documentation system (Alpha Innotech Inc., San Leandro, CA). Expression of all the genes was assessed by generating densitometry data for band intensities in different sets of experiments and was generated by analyzing the gel images on the Image J software, Version 1.33 (Wayne Rasband, NIH, Bethesda, MD) semi-quantitatively. The band intensities were compared with constitutively expressed β-actin. The intensity of mRNAs was standardized against that of the β-actin mRNA from each sample, and the results were expressed as PCR-product/β-actin mRNA ratio.
Histological evaluation of kidney and heart
At the end of experiment, kidney and heart were isolated and fixed for histopathological evaluation with 4% buffered paraformaldehyde solution and embedded in paraffin. Three to four micrometer thick paraffin sections were dewaxed and brought to the water through graded ethanol. Sections were stained with hematoxylin–eosin stain (H & E), then dehydrated through graded ethanol, cleared in xylene, and mounted with Distyrene Plasticizer Xylene. H & E stained sections were graded to verify morphological assessment of tissue damage. Photomicrographs were captured at 40 × magnification.
Statistical analysis
Data are expressed as mean ± standard error mean (SEM). Data analyses were performed using Graph Pad Prism 5.0 software (Graph Pad, San Diego, CA). Data of biochemical parameters were analyzed by one-way analysis of variance (ANOVA), and Dunnett’s tests were applied for post hoc analysis. Data of electrocardiographic parameters were analyzed by two-way ANOVA, and Bonferroni’s tests were applied for post hoc analysis. A value of p < 0.05 was considered to be statistically significant.
Results
Effect of l-arginine on body weight, relative organ weight, water intake, and urine output in EG-induced urolithiasis in uninephrectomized rats
There was significant (p < 0.001) decrease in the body weight, urine output, and water intake of UC rats as compared to the normal group. However, the administration of telmisartan (10 mg/kg) and Cystone (500 mg/kg) significantly (p < 0.001) increased the body weight, urine output, and water intake as compared to the UC group. l-Arginine (250, 500, and 1000 mg/kg) treatment significantly (p < 0.05, 0.01, and 0.001) and dose-dependently increased the body weight, urine output, and water intake as compared to the UC group ().
Table 1. Effect of l-arginine on body weight, urine output, water intake, urine density, relative organ weight, and urine pH in EG (ethylene glycol) induced urolithiasis in uninephrectomized rats.
The heart weight to body weight ratio and kidney weight to body weight ratio was increased significantly (p < 0.001) in the UC group as compared to the normal group. Administration of telmisartan (10 mg/kg) significantly (p < 0.001) reduced the heart weight to body weight ratio and significantly (p < 0.05) reduced kidney weight to body weight ratio as compared to UC control rats. On the other hand, cystone (500 mg/kg) administration significantly reduced (p < 0.001) the kidney weight to body weight ratio, however failed to produce any significant reduction in heart weight to body weight ratio as compared to UC control rats. l-Arginine (250, 500, and 1000 mg/kg) showed significant (p < 0.05, 0.01, and 0.001) and dose-dependent decrease in kidney weight to body weight ratio as compared to the UC group. When compared with the UC group, the heart weight to body weight ratio was significantly reduced (p < 0.001) by l-arginine (1000 mg/kg) treatment ().
Effect of l-arginine on urine density, urine dry weight, and urine pH in EG-induced urolithiasis in uninephrectomized rats
There was significant (p < 0.001) increase in the urine density and dry weight whereas significant (p < 0.001) decrease in the urine pH in the UC group as compared to the normal group. This increase in the urine density, dry weight, and decrease in urine pH was significantly restored (p < 0.001) by telmisartan (10 mg/kg) and cystone (500 mg/kg) treatment as compared to UC control rats. Administration of l-arginine (500 and 1000 mg/kg) significantly and dose dependently (p < 0.01 and 0.001) restored the EG-induced altered urine density, dry weight, and urine pH as compared to UC control rats ().
Effect of l-arginine on ECG parameters in EG-induced urolithiasis in uninephrectomized rats
Treatment with EG in the uninephrectomized rats resulted in the significant increase (p < 0.001) in heart rate, QRS interval, QTc interval, and ST height in UC group as compared to normal. However, administration of telmisartan (10 mg/kg) and cystone (500 mg/kg) significantly (p < 0.001) reduced the heart rate, QRS interval, and ST height as compared to the UC group. Treatment with l-arginine (250, 500, and 1000 mg/kg) significantly (p < 0.05, 0.01, and 0.001) and dose-dependently reduced the heart rate, QRS interval, and ST height as compared to UC rats ().
Table 2. Effects of l-arginine on electrocardiographic abnormalities in EG-induced urolithiasis in uninephrectomized rats.
Effect of l-arginine on hemodynamic parameters in EG-induced urolithiasis in uninephrectomized rats
The SBP, DBP, MABP, LVEDP, and max dp/dt were increased significantly (p < 0.001), and LV Contractility Index and min dp/dt were decreased significantly (p < 0.001) in UC group as compared to the normal group. Administration of telmisartan (10 mg/kg) significantly (p < 0.001) restored the alteration in SBP, DBP, MABP, LVEDP, LV Contractility Index, max dp/dt, and min dp/dt as compared to the UC group. When compared with UC control rats, cystone (500 mg/kg) treatment significantly (p < 0.01) decreased SBP, DBP, MABP, and LVEDP, however failed to produce any restoration in altered LVEDP, LV Contractility Index, max dp/dt, and min dp/dt. Administration of l-arginine (500 and 1000 mg/kg) significantly (p < 0.01 and 0.001) and dose dependently restored the EC-induced altered SBP, DBP, MABP, LVEDP, LV Contractility Index, max dp/dt, and min dp/dt as compared to UC rats ().
Table 3. Effect of l-arginine on hemodynamic parameters in EG-induced urolithiasis in uninephrectomized rats.
Effect of l-arginine on serum biochemical parameters in EG-induced urolithiasis in uninephrectomized rats
The serum BUN, uric acid, creatinine, sodium, LDH, and calcium levels were increased significantly (p < 0.001) in the UC group as compared to the normal group. Administration of telmisartan (10 mg/kg) significantly (p < 0.001) decreased BUN, uric acid, creatinine, sodium, LDH, and calcium level as compared to the UC group. Cystone (500 mg/kg) treatment decreased BUN, uric acid, creatinine, sodium, and calcium level significantly (p < 0.001) as compared to the UC group. When compared with the UC group, administration of l-arginine (500 and 1000 mg/kg) significantly and dose dependently (p < 0.01 and 0.001) decreased the serum BUN, uric acid, creatinine, sodium, LDH, and calcium levels ().
Table 4. Effect of l-arginine on serum and urine biochemical parameters in EG-induced urolithiasis in uninephrectomized rats.
Effect of l-arginine on urine biochemical parameters in EG-induced urolithiasis in uninephrectomized rats
BUN, uric acid, calcium, and creatinine level in urine of UC group was significantly (p < 0.001) increased whereas sodium, citrate, and albumin level in urine of UC group was significantly (p < 0.001) decreased as compared to the normal group. However, administration of telmisartan (10 mg/kg) significantly (p < 0.001) restored the BUN, uric acid, creatinine, calcium, citrate, and albumin as compared to the UC group. The increased level of BUN, uric acid, calcium, creatinine as well as decreased level of sodium, citrate, and albumin in urine after EG treatment was significantly (p < 0.001) restored by cystone (500 mg/kg) treatment. Administration of l-arginine (500 and 1000 mg/kg) significantly and dose dependently (p < 0.01 and 0.001) decreased the urinary BUN, uric acid, calcium, creatinine level whereas significantly (p < 0.05 and 0.01) increased the urinary sodium, citrate, and albumin level as compared to the UC group ().
Effect of l-arginine on renal oxido-nitrosative stress in EG-induced urolithiasis in uninephrectomized rats
There was significant (p < 0.001) decrease in renal SOD and GSH levels in UC as compared to the normal group. Administration of telmisartan (10 mg/kg) significantly (p < 0.001) increased the renal SOD and GSH whereas significantly (p < 0.001) decreased MDA and NO levels as compared to the UC group. While the administration of cystone (500 mg/kg) significantly (p < 0.05) increased renal SOD level, it significantly (p < 0.01 and 0.001) decreased renal MDA and NO levels as compared to UC control rats. Administration of l-arginine (500 and 1000 mg/kg) significantly (p < 0.01 and 0.001) and dose-dependently increased the activity of SOD and GSH, whereas the levels of MDA and NO were decreased significantly (p < 0.01 and 0.001) and dose-dependently as compared to the UC group ().
Figure 1. Effect of l-arginine on cardiac and renal SOD, GSH, MDA, and NO in EG-induced urolithiasis in uninephrectomized rats. Results are represented as mean ± SEM (n = 6) Data are analyzed by One-way ANOVA followed by post hoc Dunnett’s tests. ###p < 0.001 as compared with normal group. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with urolithiasis control group.
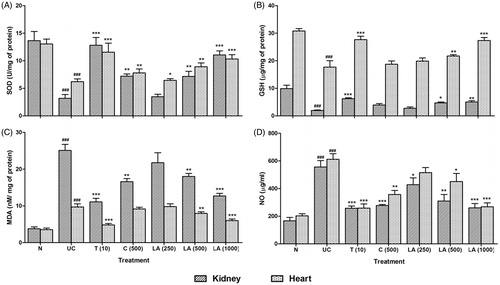
Effect of l-arginine on myocardial oxido-nitrosative stress in EG-induced urolithiasis in uninephrectomized rats
The SOD and GSH levels were reduced significantly (p < 0.001), whereas MDA and NO levels were significantly (p < 0.001) increased in the heart of UC control rats as compared to the normal group. Treatment with telmisartan (10 mg/kg) significantly (p < 0.001) increased SOD and GSH levels in heart and significantly (p < 0.001) decreased MDA and NO levels in heart as compared to the UC group. Cystone (500 mg/kg) treatment significantly increased (p < 0.01) myocardial SOD level whereas, there was significant (p < 0.01) decrease in myocardial NO level as compared to the UC control rats. When compared with the UC control group, l-arginine (500 and 1000 mg/kg) treatment significantly (p < 0.01 and 0.001) and dose-dependently increased SOD and GSH levels whereas decreased MDA and NO levels ().
Effect of l-arginine on renal KIM-1 and NGAL mRNA expression in EG-induced urolithiasis in uninephrectomized rats
There was significant (p < 0.001) up-regulation in the renal KIM-1 and NGAL mRNA expression of UC control rats as compared to normal rats. This up-regulation in the renal KIM-1 mRNA expression was significantly (p < 0.01) inhibited by the telmisartan (10 mg/kg) treatment as compared to UC control rats. However, telmisartan (10 mg/kg) failed to produce any significant down-regulation in the NGAL mRNA expression as compared to UC control rats. When compared with UC control rats, cystone (500 mg/kg) treatment did not show any down-regulation in KIM-1 and NGAL mRNA expression. Administration of l-arginine (500 and 1000 mg/kg) significantly (p < 0.05 and 0.01) and dose-dependently down-regulated EG-induced up-regulation in KIM-1 and NGAL mRNA expression as compared to UC control rats ().
Figure 2. Effect of l-arginine on renal KIM-1, NGAL, eNOs, and iNOs mRNA expression in EG-induced urolithiasis in uninephrectomized rats (A), quantitative representation of mRNA expression of KIM-1 (B), NGAL (C), eNOs (D), and iNOs (E). Results are represented as mean ± SEM (n = 4) Data are analyzed by One-way ANOVA followed by post hoc Dunnett’s tests. ###p < 0.001 as compared with normal group. *p < 0.05, **p < 0.01 as compared with urolithiasis control group.
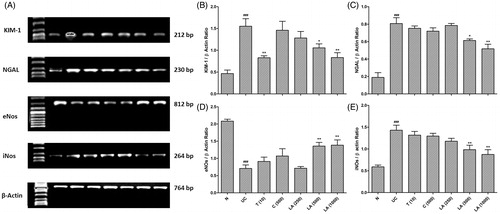
Effect of l-arginine on renal eNOs and iNOs mRNA expression in EG-induced urolithiasis in uninephrectomized rats
The renal eNOs and iNOs mRNA expression was significant (p < 0.001) down-regulated and up-regulated in the UC control rats as compared to normal rats respectively. Treatment with telmisartan (10 mg/kg) and cystone (500 mg/kg) failed to produce any significant restoration in the altered mRNA expression of eNOs and iNOs as compared to UC control rats. l-Arginine (500 and 1000 mg/kg) treatment significantly (p < 0.01) up-regulated eNOs mRNA expression and significantly (p < 0.01) down-regulated iNOs mRNA expression as compared to UC control rats ().
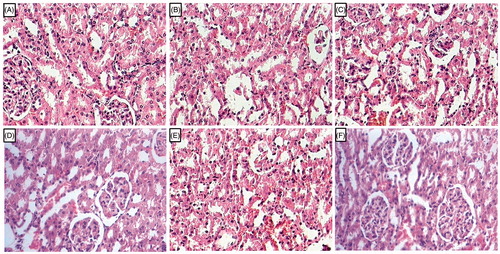
Effect of l-arginine on alteration in kidney histology in EG-induced urolithiasis in uninephrectomized rats
Kidney tissue from normal rats showed normal architecture without any changes like congestion, necrosis, and inflammatory infiltration. It revealed no calcifications in the kidneys. It showed the presence of intact glomerulus basement membrane and tubules (). Administration of EG showed the deposition of calcium oxalate crystal in the renal cortex and medulla of UC control rats. It also showed the renal damage with intrinsic lesions within the glomeruli and epithelium. Intracellular edema was also detected with the presence of intraluminal cell debris and inflammatory infiltration (). Telmisartan (10 mg/kg) and cystone (500 mg/kg) treated rats showed the reduction in the renal damage reflected by decreased calcium oxalate crystal deposition along with reduced inflammatory infiltration (). Structural restoration of the glomerulus basement membrane and tubules were observed in the rats treated with cystone (500 mg/kg; ). Renal tissue from l-arginine (500 mg/kg) treated rats showed the presence of few calcium oxalate crystal and it showed moderate intrinsic lesions within the glomeruli and epithelium (). Renal tissue from (1000 mg/kg) treated rats showed mild calcifications with a moderate number of inflammatory cells with reduced necrosis and edema. Thickens of glomerulus basement membrane was intact ().
Figure 3. Effect of l-arginine on alteration in kidney histology in EG-induced urolithiasis in uninephrectomized rats. Photomicrograph of sections of kidney of normal (A), urolithiasis control rats (B), Telmisartan (10 mg/kg) treated rats (C), Cystone (500 mg/kg) treated rats (D), l-arginine (500 mg/kg) treated rats (E), and l-arginine (1000 mg/kg)-treated rats (F). H & E staining at 100 × .
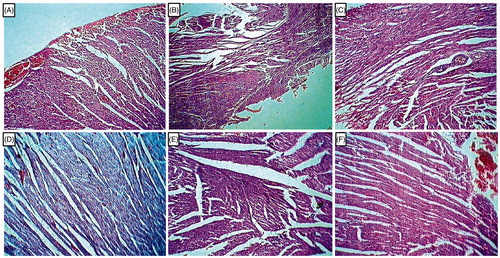
Effect of l-arginine on alteration in heart histology in EG-induced urolithiasis in uninephrectomized rats
Histological analysis of heart tissue from UC rats showed marked myocardial damage revealed by the features like inflammation, necrosis, nuclear pyknosis, cytoplasmic vacuolization, and cytoplasmic eosinophilia (). However, the normal group did not show any tissue damage in myocardial fibers (). Histological sections of hearts from telmisartan (10 mg/kg) treated rats showed markedly improved histological changes indicated by reduced myocardial damage, inflammation, and nuclear pyknosis (). However, myocardial tissue from cystone (500 mg/kg) as well as l-arginine (500 mg/kg) treated rats showed altered myocardial vasculature, reflected by the presence of vascular congestion, myocardial inflammation, and nuclear pyknosis (). Treatment with l-arginine (1000 mg/kg) remarkably improved the histological changes in the heart tissue reflected in reduced myocardial damage, diminished extent of inflammation and nuclear pyknosis ().
Figure 4. Effect of l-arginine on alteration in heart histology in EG-induced urolithiasis in uninephrectomized rats. Photomicrograph of sections of heart of normal (A), urolithiasis control rats (B), Telmisartan (10 mg/kg) treated rats (C), Cystone (500 mg/kg) treated rats (D), l-arginine (500 mg/kg treated rats (E), and l-arginine (1000 mg/kg) treated rats (F). H & E staining at 100×.
Discussion
Urinary stone or calculi is the third prevalent disease of the urinary system that affects approximately 12% of the population worldwide and need serious medical attention throughout lifetime of patient.Citation45 It has been reported that approximately 80% of urinary calculi are comprised of calcium oxalate and calcium phosphate which causes urinary obstruction, hydronephrosis, infection, and hemorrhage in the urinary tract system.Citation46 Nowadays, extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy, and rigid and flexible urethroscopy are the newly developed methods that have been employed for the effective and safe removal of urinary calculi. However, 50–70% of the stones will recur after treatment if urolithiasis patients neglect the preventive measures.Citation47 Moreover, this surgical treatment may cause serious side effect including reduction in renal plasma flow and glomerular filtration rate along with morphological changes in the treated kidney. In some patients treatment may exhibit severe renal damage in the ipsilateral kidney and therefore need to require surgical removal later. If urolithiasis patients, who have undergone uninephrectomy or whose unilateral kidney has lost its function, continue to be exposed to dietary and/or other lithogenic risk factors, the remnant kidney may carry a higher risk of stone formation and is therefore more prone to further damage. Moreover, urolithiasis has been reported to have a higher prevalence of hypertension and proteinuria.Citation48 Hence, this study was undertaken to evaluate anti-urolithiasis and antihypertensive the effect of l-arginine in uninephrectomized-hypertensive laboratory rats.
In order to maintain homeostasis of body fluid composition, there was induction of compensatory hyperfunction of the remaining kidney in uninephrectomized animals. Hyperfiltration and hyperexcretion occurred to minimize the reduced filtration rate and solute as well as water excretion in uninephrectomy condition.Citation49,Citation50 However, this compensatory hyperfunction by remnant nephron unit may lead to its pathological aberrations followed by glomerular dysfunction. Moreover, elevated urinary excretion of lithogenic substances in the remaining nephron units leads to earlier hypercalciuria followed by renal hypertension as postulated by the hypertension-crystallization hypothesis.Citation13,Citation14 In this investigation, administration of lithogenic substances such as EG in the uninephrectomized rats showed impaired renal function with hypercalciuria and renal hypertension.
EG is rapidly absorbed and metabolized in the liver by alcohol dehydrogenase/aldehyde dehydrogenase to glycolic acid.Citation51 EG has in itself a low toxicity, but in vivo it is broken down to four organic acids viz: glycoaldehyde, glycolic acid, glyoxylic acid, and oxalic acid. Glycolic acid is oxidized to glyoxylic acid, which in turn further oxidized to oxalic acid by glycolate oxidase or lactate dehydrogenase.Citation52 These metabolites are toxic to cells that cause depression in central nervous system along with cardiopulmonary, as well as renal failure.Citation53 High intake of EG ( >2500 mg/kg body weight), particularly when given as an oral bolus, cause the saturation dependent accumulation of glycolic acid in the plasma. Therefore, glycolate oxidase (GO) is one of the rate-limiting reaction in the metabolism of EG. Hence, EG has been chosen as an appropriate material for the induction of urolithiasis in uninephrectomized rats as previously developed models.Citation54,Citation55
It has been documented that uninephrectomy alone unable to induced renal dysfunction as well as urolithiasis or crystal deposition in the remaining kidney.Citation55 However, administration of lithogenic substance such as EG in uninephrectomized condition caused significant renal damage and calcium oxalate crystal deposition in the remnant kidney. Hence, hypercalciuria is considered as the important hallmark of stone formation.Citation50,Citation56 Feeding with EG significantly elevated the wet and dry kidney weight in uninephrectomized rats that are directly associated with urinary calculi formation and calcium oxalate crystal deposition. The severity crystal deposition and stone formation is directly associated with renal calcium content.Citation57,Citation58 In this investigation, EG administration in uninephrectomized condition caused significant hypercalciuria reflected by the increased wet and dry kidney weight which is in accordance with the findings of previous investigators.Citation57,Citation58 However, administration of l-arginine significantly decreased renal wet and dry weight, indicating its inhibitory potential against calcium oxalate crystal deposition.
Administration of EG produced type I distal renal tubular acidosis leads to nephrolithiasis and nephrocalcinosis conditions.Citation59,Citation60 These conditions are associated with the elevated urinary pH, urine density, and calcium excretion as well as decreased excretion of sodium, citrate, and albumin in urine reflected the EG-induced renal damage in uninephrectomized rats.Citation55 Citrate has been reported to have negatively charged inhibitors of urinary calcium and decreased level of urinary citrate indicated the hypercalciuria. Moreover, uric acid is documented to have an inhibitory effect against the calcium oxalate solubility, and the elevated level of urinary uric acid reflected the increased calcium oxalate crystal deposition.Citation61 It has been documented that citrate form complex with calcium in the urine thereby reducing the concentration of calcium oxalate and preventing its agglomeration.Citation62 However, l-arginine treatment significantly restored the altered levels of ions, uric acid, and citrate thus prevented the risk of stone formation.
It has been reported that EG caused deterioration of renal function due to stone formation resulted in obstruction of urine outflow thus decreases glomerular filtration rate. This in turn leads to accumulation of nitrogenous waste products BUN, uric acid, and creatinine in serum. The elevated level of nitrogenous waste product in serum has been implicated as the hallmark of renal damage clinically.Citation63 In this study, EG administration produced significant nephrotoxicities indicated by significant elevation in these nitrogenous waste products in serum whereas l-arginine treatment significantly attenuated these elevated nitrogenous waste products level, thus reflecting its nephro-protective potential. Result of this study is in line with the findings of previous investigators where l-arginine significantly restored the altered level of BUN and creatinine in renal failure rats.Citation32,Citation33,Citation64
Lactate dehydrogenase (LDH), a bifunctional enzyme of the glycolytic pathway is considered as a most sensitive indicator of acute kidney injury. Rise in LDH level is associated with loss of proximal tubular cell brush border due to renal epithelial cellular necrosis by intoxicant. It is also played a vital role in oxidation of glycolic acid (an EG metabolite) to oxalic acid that induced toxicity in renal plasma membrane.Citation53,Citation65 The elevated level of LDH suggests that EG induces nephrotoxicity and its restoration by l-arginine indicates its nephro-protective potential.
It has been reported that hypertension is associated with urolithiasis.Citation66 The reports of previous experimental studies have suggested that hypertension occurred in the altered acid–base state.Citation67,Citation68 In this investigation, alteration in the acid–base state occurred after EG administration in uninephrectomized rats along with alteration in hemodynamic and ECG parameters. Treatment with l-arginine significantly restored the altered levels of hemodynamic and ECG parameters. The result of this study corroborates the findings of previous investigators.Citation69,Citation70
Crystal-induced renal epithelial injury to a significant degree mediated by oxidative stress was reflected by increased lipid peroxidation and cellular damage in renal tissue.Citation30 In renal epithelial cells, toxic responses generated due to a higher concentration of oxalate leads to altered membrane surface properties, disruption of mitochondrial function, and the formation of ROS. The elevated concentration of MDA in UC group is indicative of oxidative tissue damage, as lipid peroxides and peroxidation products accumulate and are usually excreted in the urine.Citation71–73 Elevated oxidative stress caused depletion of enzymes that played an important role in the electron transport chain. Moreover, oxidative stress can elicit inflammation, enhance vasoconstrictors accrual, and endorse tubular cell apoptosis and necrosis.Citation74–77 This higher level of ROS diminishes the copper–zinc–SOD enzyme motion alongside GSH performance in cells, which causes consequential hammering of cell integrity and necrosis.Citation78,Citation79 Concrete with this notion, we found that UC control rats drastically lessened SOD, GSH levels, and elevated MDA content. Activity of SOD and GSH enzymes increased in l-arginine treated groups which confirmed that l-arginine has potent anti-oxidant potential which prevents the cardiac and renal oxidative damage. It has been documented that l-arginine possesses potent free radical scavenging property.Citation33,Citation38 The restoration in the decreased level of SOD and GSH further confirms the anti-oxidative potential of l-arginine in cardiac and renal tissue against EG-induced oxidative damage which is in line with the finding of previous workers.Citation33,Citation38
Nitric oxide (NO) has been implicated in an array of disorders including diabetes, cardiomyopathy, nephropathy, neuropathy, arthritis, etc.Citation80–84 Nitrite is hallmark of endogenous nitric oxide production, and their levels serve as the gold standard reflecting the increased oxidative stress resulting in cellular dysfunction.Citation85–87 It has been documented that administration of the substance with NO scavenging or NOS (nitric oxide synthase) inhibitory potential prevented EG-induced toxicity via inhibition of renal microvascular constriction to improve hemodynamic abnormality.Citation88 In the present investigation, UC control rats showed elevated NO levels after EG administration whereas l-arginine treatment decreased NO level in both renal and cardiac tissues resulting in the inhibition of renal microvascular constriction which might help in improvement of electrocardiographic and hemodynamic abnormalities.
Clinically, acute kidney injury (AKI) is an important issue for patients during critical care in the intensive care unit (ICU).Citation89,Citation90 In AKI, the mortality rates remained high due to paucity in the sensitive and specific biomarkers of renal cell injury.Citation89,Citation91 However, recently developed techniques have identified the two sensitive and specific biomarkers for early AKI, namely, kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL).Citation92,Citation93 It has been reported that the sensitivity for NGAL to predict AKI is 0.815.Citation94 NGAL has also been known as human neutrophil lipocalin (HNL) and widely expressed by renal tubular epithelium. Renal epithelial injury and inflammation leads to rapidly induction in the expression of NGAL in nephron. KIM-1 is a transmembrane tubular protein with an immunoglobulin and mucin domain, rapidly expressed in the proximal tubule after AKI.Citation95 KIM-1 played a vital role in phagocytosis within proximal tubules. Up-regulated expressions of NGAL and KIM-1 in kidney are hallmark of AKI as it is detectable before a rise in serum creatinine or urinary N-acetyl-glucosaminidase concentrations. In this investigation, elevated ROS after EG administration leads to AKI reflected by the up-regulated KIM-1 and NGAL mRNA expression in renal tissue of UC control rats. However, treatment with l-arginine showed significant down-regulation in the renal mRNA expression of KIM-1 and NGAL.
It has been reported that eNOs played a vital role in the management of hypertension.Citation96,Citation97 Elevated level of eNOs caused release of moderate NO that is beneficial for the vasodilator action. Whereas, high NO levels generated by iNOS resulted in the production of peroxynitrite anion via interacting with superoxide anion. This peroxynitrite induces leukocyte adhesion, infiltration of inflammatory cell, and cellular dysfunction.Citation98 It has been documented that l-arginine is served as a precursor for NO synthesis, and the conversation is catalyzed by nitric oxide synthase (NOs).Citation99 This synthesized NO was released by endothelial NOs (eNOs) from the blood vessels of endothelium resulting in vasodilation via cyclic guanosine monophosphate (cGMP).Citation100,Citation101 In this investigation, oral administration of l-arginine significantly improved hemodynamic changes via amelioration of EG-induced decreased endothelial nitric oxide synthase (eNOS) activity. The result of this study is in line with the findings of previous workers where l-arginine has been shown to increase eNOS expression in pulmonary endothelial cells.Citation38,Citation102
Currently, ARBs (Losartan, valsartan, telmisartan, and olmesartan) are used worldwide to treat hypertension and proteinuria. Several studies have confirmed the inhibitory effect of ARBs by suppressing oxidative stress and tubule-interstitial injury through inhibition of osteopontin expression.Citation103 In this study, administration of telmisartan and cystone showed amelioration in the EG-induced urolithiasis in uninephrectomized hypertensive rats via modulation of ROS; however, it failed to produce any significant down-regulation in the KIM-1 and NGAL mRNA expression. In conclusion, l-arginine showed the nephro-protective and cardioprotective property against EG-induced urolithiasis in uninephrectomized hypertensive rats via modulation of KIM-1, NGAL, eNOs, and iNOs mRNA expression.
Supplementary material available online
irnf_a_1011967_sm7768.docx
Download MS Word (13 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Charles L, Smith M, Guay D. Nephrolithiasis Pharmacotherapy – A Pathophysiological Approach. 3rd ed. Appleton and Langue 1999;1033–1036
- Dole S, Kandhare AD, Ghosh P, Gosavi TP, Bodhankar SL. The health outcome after homeopathic treatment in cases of BPH and LUTS: a prospective clinical study. J Pharm Biomed Sci. 2012;22:1–6
- Christina A, Packia Lakshmi M, Nagarajan M, Kurian S. Modulatory effect of Cyclea peltata Lam. on stone formation induced by ethylene glycol treatment in rats. Methods Find Exp Clin Pharmacol. 2002;24:77–79
- Trinchieri A, Castelnuovo C, Lizzano R, Zanetti G. Calcium stone disease: A multiform reality. Urol Res. 2005;33:194–198
- Gotoh M, Mizuno K, Ono Y, Takahashi M. High blood pressure, bone-mineral loss and insulin resistance in women. Hypertens Res. 2005;28:565–570
- Cappuccio FP, Kalaitzidis R, Duneclift S, Eastwood JB. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 1999;13:169–177
- Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens 1998;11:46–53
- Shivakumar V, Kandhare A, Rajmane A, et al. Estimation of the long-term cardiovascular events using ukpds risk engine in metabolic syndrome patients. Indian J Pharm Sci. 2014;76:174–178
- Kiprov D, Colvin R, McCluskey R. Focal and segmental glomerulosclerosis and porteinuria associated with unilateral renal agenesis. Lab Invest. 1982;46:275–281
- Kandhare AD, Raygude KS, Ghosh P, Gosavi TP, Bodhankar SL. Patentability of Animal Models: India and the Globe. Int J Pharm Biol Arch. 2011;2:1024–1032
- Cirillo M, Laurenzi M, Panarelli W, Stamler J. Urinary sodium to potassium ratio and urinary stone disease. The Gubbio Population Study Research Group. Kidney Int. 1994;46:1133–1139
- Yoshioka I, Tsujihata M, Akanae W, Nonomura N, Okuyama A. Angiotensin type-1 receptor blocker candesartan inhibits calcium oxalate crystal deposition in ethylene glycol-treated rat kidneys. Urology. 2011;77:1007.e9–1007.e14
- Vermeulen C, Ellis J, Hsu T-C. Experimental observations on the pathogenesis of urinary calculi. J Urol. 1966;95:681–690
- Robertson W, Peacock M, Nordin B. Calcium crystalluria in recurrent renal-stone formers. Lancet. 1969;294:21–24
- Yoshioka I, Tsujihata M, Akanae W, Nonomura N, Okuyama A. Angiotensin type-1 receptor blocker candesartan inhibits calcium oxalate crystal deposition in ethylene glycol-treated rat kidneys. Urology. 2011;77:1007.e9–1007
- Adil M, Visnagri A, Kumar VS, Kandhare AD, Ghosh P. Protective effect of naringin on sodium arsenite induced testicular toxicity via modulation of biochemical perturbations in experimental rats. Pharmacologia. 2014;5:222–234
- Kandhare A, Raygude K, Ghosh P, Bodhankar S. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int J Green Pharm. 2011;5:236–243
- Kandhare AD, Bodhankar SL, Singh V, Mohan V, Thakurdesai PA. Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol. 2013;3:23–30
- Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD (P) H oxidase expression by angiotensin II in human endothelial cells protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851
- Watanabe D, Tanabe A, Naruse M, Morikawa S, Ezaki T, Takano K. Renoprotective effects of an angiotensin II receptor blocker in experimental model rats with hypertension and metabolic disorders. Hypertens Res. 2009;32:807–815
- Krambeck AE, Rule AD, Li X, Bergstralh EJ, Gettman MT, Lieske JC. Shock wave lithotripsy is not predictive of hypertension among community stone formers at long-term followup. J Urol. 2011;185:164–169
- Ghosh P, Kandhare AD, Raygude KS, et al. Determination of the long term diabetes related complications and cardiovascular events using UKPDS risk engine and UKPDS outcomes model in a representative western Indian population. Asian Pac J Trop Dis. 2012;2:S642–S650
- Bhalsinge RR, Worlikar PS, Tilak AV, Bodhankar SL, Ghosh P, Kandhare AD. Effect of nicotine on brain GABA levels in depressed rats. Am J Pharm Tech Res. 2012;2:556–563
- Kandhare AD, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Protective effect of Phyllanthus amarus by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: Role of phyllanthin and hypophyllanthin. Apollo Med. 2013;10:87–97
- Kandhare AD, Kumar VS, Adil M, Rajmane AR, Ghosh P, Bodhankar SL. Investigation of gastro protective activity of Xanthium strumarium L. by modulation of cellular and biochemical marker. Orient Pharm Exp Med. 2012;12:287–299
- Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I. Dietary L-arginine supplementation reduces Methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol. 2012;12:41
- Cooke JP, Singer A, Tsao P, Zera P, Rowan R, Billingham M. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–1172
- Xekouki P, Papandreou I, Aroni K, et al. The effect of L-Arginine in experimental colitis. Ann Gastroenterol 2004;17:270–275
- Cao B, Li N, Wang Y, Li J-S. Protective effect of L-arginine preconditioning on ischemia and reperfusion injury associated with rat small bowel transplantation. World J Gastroenterol. 2005;11:2994–2997
- Acquaviva R, Lanteri R, Destri GL, et al. Beneficial effects of rutin and L-arginine coadministration in a rat model of liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G664–G670
- Reyes AA, Karl IE, Kissane J, Klahr S. L-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J Am Soc Nephrol. 1993;4:1039–1045
- Mansour MA, Al-Shabanah OA, El-Khashef HA. L-arginine ameliorates kidney function and urinary bladder sensitivity in experimentally-induced renal dysfunction in rats. J Biochem Mol Biol 2003;36:373–378
- Mansour M, Hesham Daba M, Gado A, Al-Rikabi A, Al-Majed A. Protective effect of L-arginine against nephrotoxicity induced by cyclosporine in normal rats. Pharmacol Res. 2002;45:441–446
- Sasaki S, Asano M, Ukai T, et al. Nitric oxide formation and plasma l-arginine levels in pulmonary hypertensive rats. Respir Med. 2004;98:205–212
- Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation. 1995;92:1539–1545
- Nagaya N, Uematsu M, Oya H, et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med. 2001;163:887–891
- Kurokawa T, An J, Tsunekawa K, et al. Effect of L-arginine supplement on liver regeneration after partial hepatectomy in rats. World J Surg Oncol. 2012;10:99–104
- Ahmed LA, Obaid AAZ, Zaki HF, Agha AM. Naringenin adds to the protective effect of L-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation and nitric oxide. Eur J Pharm Sci. 2014;62:161–170
- Visnagri A, Kandhare AD, Ghosh P, Bodhankar SL. Endothelin receptor blocker bosentan inhibits hypertensive cardiac fibrosis in pressure overload-induced cardiac hypertrophy in rats. Cardiovasc Endocrinol. 2013;2:85–97
- Kandhare AD, Raygude KS, Shiva Kumar V, et al. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed Aging Pathol. 2012;2:173–186
- Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219C:101–112
- Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol induced neuropathic pain. Fundam Clin Pharmacol. 2013;27:603–622
- Kandhare AD, Shivakumar V, Rajmane A, Ghosh P, Bodhankar SL. Evaluation of the neuroprotective effect of chrysin via modulation of endogenous biomarkers in a rat model of spinal cord injury. J Nat Med. 2014;68:586–603
- Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014;52:814–828
- Ajayi L, Jaeger P, Robertson W, Unwin R. Renal stone disease. Medicine. 2007;35:415–419
- Prien EL, Prien Jr EL. Composition and structure of urinary stone. Am J Med. 1968;45:654–672
- Hesse A, Miersch W-D. Special aspects of stone composition and aetiology of different types of urinary calculi. Int Urol Nephrol. 1989;21:257–267
- Hakim RM, Goldszer RC, Brenner BM. Hypertension and proteinuria: Long-term sequelae of uninephrectomy in humans. Kidney Int. 1984;25:930–936
- Katz A, Epstein F. Relation of glomerular filtration rate and sodium reabsorption to kidney size in compensatory renal hypertrophy. Yale J Biol Med. 1967;40:222–230
- Kaufman JM, Siegel NJ, Hayslett JP. Functional and hemodynamic adaptation to progressive renal ablation. Circ Res. 1975;36:286–293
- Thangarathinam N, Jayshree N, Metha AV, Ramanathan L. Effect of polyherbal formulation on ethylene glycol induced urolithiasis. Int J Pharm Pharm Sci. 2013;5:994–997
- Chavada KS, Fadadu KN, Patel KV, Patel KG, Gandhi TR. Effect of flavanoid rich fraction of Citrus medica Linn.(rutacea) on ethylene glycol induced urolithiasis in rats. J Drug Deliv Ther 2012;2:109–116
- Leth PM, Gregersen M. Ethylene glycol poisoning. Foren Sci Int. 2005;155:179–184
- Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J Ethnopharmacol. 2009;122:106–116
- Lee YH, Huang WC, Chang LS, Chen MT, Huang JK. Uninephrectomy enhances urolithiasis in ethylene glycol treated rats. Kidney Int. 1992;42:292–299
- Pabico RC, McKenna BA, Freeman RB. Renal function before and after unilateral nephrectomy in renal donors. Kidney Int. 1975;8:166–175
- Hayslett JP. Functional adaptation to reduction in renal mass. Physiol Rev. 1979;59:137–164
- Halabe A, Wong N, Sutton R. The effect of verapamil and thiazide in the prevention of renal stone formation. Urol Res. 1990;18:155–158
- Pyrah L, Hodgkinson A. Nephrocalcinosis. Br J Urol. 1960;32:361–373
- Preminger G, Sakhaee K, Skurla C, Pak C. Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis. J Urol. 1985;134:20–23
- Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl.(Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2012;144:160–170
- Kok D, Papapoulos S, Bijvoet O. Excessive crystal agglomeration with low citrate excretion in recurrent stone-formers. Lancet. 1986;327:1056–1058
- Grover PK, Resnick MI. Evidence for the presence of abnormal proteins in the urine of recurrent stone formers. J Urol. 1995;153:1716–1721
- Ashab I, Peer G, Blum M, et al. Oral administration of L-arginine and captopril in rats prevents chronic renal failure by nitric oxide production. Kidney Int. 1995;47:1515–1521
- Leth PM. Botulism in greenland. Forensic Sci Int. 2014;238:1–2
- Taylor EN, Mount DB, Forman JP, Curhan GC. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780–789
- Lucas P, Lacour B, McCarron D, Drueke T. Disturbance of acid-base balance in the young spontaneously hypertensive rat. Clin Sci (Lond). 1987;73:211–215
- Lucas P, Lacour B, Comte L, McCarron D, Drüeke T. Abnormal parameters of acid-base balance in genetic hypertension. Kidney Int Suppl. 1988;25:S19–S22
- Morikawa E, Moskowitz MA, Huang Z, Yoshida T, Irikura K, Dalkara T. L-arginine infusion promotes nitric oxide-dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke. 1994;25:429–435
- Bartunek J, Dempsey S, Weinberg EO, et al. Chronic l-arginine treatment increases cardiac cyclic guanosine 5′-monophosphate in rats with aortic stenosis: Effects on left ventricular mass and beta-adrenergic contractile reserve. J Am Coll Cardiol. 1998;32:528–535
- Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337–351
- Kandhare AD, Raygude KS, Ghosh P, et al. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed. 2012;5:337–344
- Visnagri A, Kandhare AD, Shiva Kumar V, et al. Elucidation of ameliorative effect of Co-enzyme Q10 in streptozotocin-induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol. 2012;2:157–172
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. 2012;511:18–22
- Raygude KS, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant effect of fisetin by modulation of endogenous biomarkers. Biomed Prevent Nutr. 2012;2:215–222
- Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012;20:331–341
- Goswami S, Kandhare A, Zanwar AA, et al. Oral l-glutamine administration attenuated cutaneous wound healing in Wistar rats. Int Wound J. 2014. [Epub ahead of print]. DOI: 10.1111/iwj.12246
- Kamble H, Kandhare AD, Bodhankar S, Mohan V, Thakurdesai P. Effect of low molecular weight galactomannans from fenugreek seeds on animal models of diabetes mellitus. Biomed Aging Pathol. 2013;3:145–151
- Gosavi TP, Ghosh P, Kandhare AD, et al. Therapeutic effect of H. pylori nosode, a homeopathic preparation in healing of chronic H. pylori infected ulcers in laboratory animals. Asian Pac J Trop Dis. 2012;2:S603–S611
- Gosavi TP, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant activity of Argentum metallicum, a homeopathic preparation. Pharma Lett. 2012;4:626–637
- Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132–145
- Patil MVK, Kandhare AD, Bhise SD. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund's complete adjuvant induced arthritis. Biomed Aging Pathol. 2012;2:6–15
- Patil MVK, Kandhare AD, Bhise SD. Effect of aqueous extract of Cucumis sativus Linn. fruit in ulcerative colitis in laboratory animals. Asian Pac J Trop Biomed. 2012;2:S962–S969
- Patil M, Kandhare A, Bhise S. Pharmacological evaluation of ameliorative effect of aqueous extract of Cucumis sativus L. fruit formulation on wound healing in Wistar rats. Chron Young Scientists. 2011;2:207–213
- Patil M, Kandhare A, Bhise S. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci. 2012;4:337–343
- Patil MVK, Kandhare AD, Bhise SD. Pharmacological evaluation of ethanolic extract of Daucus carota Linn root formulated cream on wound healing using excision and incision wound model. Asian Pac J Trop Biomed. 2012;2:S646–S655
- Ilbey Y, Ozbek E, Simsek A, Cekmen M, Somay A, Tasci AI. Pyrrolidine dithiocarbamate treatment prevents ethylene glycol-induced urolithiasis through inhibition of NF-kB and p38-MAPK signaling pathways in rat kidney. Arch Ital Urol Androl. 2010;82:87–94
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818
- Parmar A, Langenberg C, Wan L, May CN, Bellomo R, Bagshaw SM. Epidemiology of septic acute kidney injury. Curr Drug Targets. 2009;10:1169–1178
- Matejovic M, Chvojka J, Radej J, et al. Sepsis and acute kidney injury are bidirectional. Contrib Nephrol. 2011;174:78–88
- Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024
- Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol. 2011;49:720–728
- Mitani Y, Maruyama K, Sakurai M. Prolonged administration of l-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation 1997;96:689–697
- Fagan JM, Rex SE, Hayes-Licitra SA, Waxman L. L-arginine reduces right heart hypertrophy in hypoxia-induced pulmonary hypertension. Biochem Biophys Res Commun. 1999;254:100–103
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624
- Palmer RM, Ashton D, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666
- Busse R, Mülsch A, Fleming I, Hecker M. Mechanisms of nitric oxide release from the vascular endothelium. Monogr Am Heart Assoc. 1993;87:V18–V25
- Nadaud S, Philippe M, Arnal J-F, Michel J-B, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857–863
- Tan X, Sun WD, Li JC, et al. L-arginine prevents reduced expression of endothelial nitric oxide synthase (NOS) in pulmonary arterioles of broilers exposed to cool temperatures. Vet J. 2007;173:151–157
- Umekawa T, Hatanaka Y, Kurita T, Khan SR. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol. 2004;15:635–644

