Abstract
The B lymphocyte-induced maturation protein-1 (Blimp-1) is an important transcription factor for the maintenance of antigen-specific immune responses, and it is crucial in the development of systemic lupus erythematosus (SLE). This study aimed to investigate the role of Blimp-1 in the development of SLE and autoimmune-like symptoms. Lentivirus-mediated Blimp-1 siRNA was constructed and injected into MRL-Fas(lpr) lupus mice. The expression levels of Blimp-1, J-chain, C-myc, XBP-1 and BCMA in peripheral blood mononuclear cells (PMBCs) were determined by RT-PCR. Anti-dsDNA autoantibody levels were detected using ELISA. The expression levels of Blimp-1 in liver, kidney, spleen and lymph nodes of mice were also detected by Western blot. The 24-h urinary protein was monitored weekly. Our results demonstrated that in MRL-Fas(lpr) lupus mice, Blimp-1 was upregulated in PMBCs, liver, kidney, spleen and lymph nodes. Administration of Blimp-1 siRNA reduced the expression of Blimp-1 and the anti-dsDNA level by 78 and 28%, respectively, in the peripheral blood, and the expression of XBP-1, J-chain and BCMA was also decreased. Although the Blimp-1 level in liver showed no significant changes, the levels of Blimp-1 in kidney, spleen and lymph nodes were dramatically decreased by 95, 72 and 47%, respectively. Kidney diseases induced by SLE in lupus mice were mitigated, and urinary protein levels were significantly decreased. These results indicate that Blimp-1 plays an important role in promoting the progression of SLE. Therefore, Blimp-1 may provide a new therapeutic target in the treatment of SLE.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple systems and organs that seriously affects patients' quality of life. Currently, the primary clinical treatment for SLE uses hormones and non-specific immune inhibitors, such as glucocorticoid and cyclophosphamide. While these drugs widely inhibit immuno-competent cells and can effectively control the disease progression, their side effects are obvious and sometimes serious, such as infection and bone rarefaction. Autoantibody-producing B cells are considered to play a central role in the pathogenesis of SLE due to the nature of autoantibody production in SLE [Citation1]. In addition, due to the long survival time of autoreactive B lymphocytes, SLE is difficult to cure [Citation2]. Thus, the target for treatment of SLE is to inhibit B cell functions [Citation3–6].
In recent years, great progress has been made in B cell-targeted therapy [Citation7–9], which is only directed against the fissionable B-lymphocytes, not terminally differentiated cells, such as the plasma cells. However, because plasma cells are the direct source of pathogenic autoantibodies, this targeted therapy cannot eliminate the fundamental source of autoantibodies [Citation3]. Hence, investigating new targets for plasma cells is an important topic. Nevertheless, the mechanism by which plasma cell differentiation and antibody-production are maintained is not clear at present. B lymphocyte-induced maturation protein-1 (Blimp-1) is one of the master regulators that drive the terminal differentiation of B cells into antibody-secreting plasma cells [Citation10]. Blimp-1 is a transcription factor with five zinc finger structures and a molecular weight of 98 kDa. It is required for the differentiation of plasma cells, the secretion of immunoglobulins and the maintenance of long-lived plasma cells in bone marrow [Citation11–16].
Our previous study demonstrated that the number of plasma cells and the expression of Blimp-1 and BCMA were up-regulated in patients with active SLE [Citation10] and suggested that the increased number of plasma cells and overproduction of autoantibodies might be associated with high expression of Blimp-1 in SLE patients [Citation10]. We have also shown that Blimp-1 siRNA can reduce CD138 expression and recover CD19 expression in mouse B myeloma cells (J558L cells) (Supplementary Figure S1) and demonstrated that inhibition of Blimp-1 can induce reprogramming and dedifferentiation of plasma cells, which was in accord with the result of another research by Fujita et al. [Citation17]. Thus, Blimp-1 siRNA might be used to treat plasma cell-associated diseases. In this study, using lentivirus carrier-mediated Blimp-1 gene silencing technology, we investigated the changes of autoantibody production and lupus symptoms by inhibiting endogenous Blimp-1 in the spontaneous lupus mice model. This work provides novel information for understanding the roles of Blimp-1 in the development of SLE and as new therapeutic target for plasma cells in the treatment of SLE.
Materials and methods
Mice
The SLE model, MRL-Fas(lpr) female mice, was obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). Mice aged at 3–18 weeks were used in the study. All the mice were housed under specific pathogen-free conditions and treated according to the Institution Guidelines for Laboratory Animal Research approved by the Institutional Animal Care and Use Committee. All mice were kept to 18 weeks old, and during this period, their mental state, behavior and appearance were observed. The superficial lymph nodes were also checked. At sacrifice, the kidneys, livers, spleens and lymph nodes were observed visually, and these tissues were collected. The expression of Blimp-1 was analyzed by hematoxylin and eosin (HE) staining, immunohistochemistry and Western blot.
Recombinant lentivirus-mediated Blimp-1 siRNA
Lentiviral vector has been reported to have more advantages over liposomes and two other viral vectors (adenovirus and retrovirus). Lentiviral vector has a long-term and stable expression in cells, and it can successfully infect terminally differentiated cells such as plasma cells. In addition, lentivirus can avoid interferon responses, which are easily induced by adenoviral vector. Moreover, lentivirus does not readily induce cellular and humoral immune responses of the host. Therefore, we chose lentiviral vector as the first choice for an RNAi vector in this study [Citation18,Citation19]. A pair of 19 oligonucleotides targeting the Blimp-1 cDNA sequence at the position 1550–1568 were designed and cloned into a siRNA-expressing lentiviral vector pLL3.7, which was kindly provided by Professor Parijs (Massachusetts Institute of Technology, Cambridge, MA) [Citation20]. The primer sequences were 5′-TGTTCCTGTTGCCACCGATCTTCAAGAGAGTACGG TGGCAACAGGAACTTTTTTC-3′ and (the complementary strand) 5′-TCGAGAAA AAAGTTCCTGTTGCCACCGTACTCTCTTGAAGTACGGTGGCAACAGGAACA-3′, with the Xho I cleavage site added to the 3′ end. The DNA fragment was cloned into the sites of Hpa I/Xho I in the pLL3.7 vector to generate the vector of Blimp-1 siRNA. To generate recombinant lentiviruses, 1.2 × 106 293T cells were co-transfected with 1.5 µg recombinant vectors (including pLP1, pLP2 and pLP/VSVG; Invitrogen, Carlsbad, CA) and 0.5 µg lentiviral expression vector pLL3.7 or Blimp-1 siRNA, respectively, by lipofectamine TM2000 (Invitrogen). The cells were cultured in DMEM complete medium (containing 1 mM sodium pyruvate and 0.1 mM non-essential amino acids) for 72 h. Their supernatants were harvested and centrifuged at 25 000 rpm for 2 h, and the pellets were collected and titrated for pure lentivirus. Viral products were desalted and stored at −80 °C in phosphate-buffered saline (PBS) containing 10% glycerol (v/v).
The lentiviral vector encoding Blimp-1 siRNA and empty lentiviral vector (pLL3.7 vector) with a titer of 1 × 109 pfu were injected intravenously into 15-week-old MRL-Fas(lpr) mice through tail vein once a week, for 3 weeks. Mice injected with Blimp-1 siRNA lentiviral vector are referred to as the study group (S group), and the pLL3.7 vector injection group was labeled the control group (C group). Specimens were harvested when the mice reached 18 weeks of age.
Semi-quantitative reverse transcription PCR
Peripheral blood mononuclear cells (PMBCs) were collected from the blood. The total RNA of PMBCs was extracted from each group using TRIzol according to the manufacturers' protocols and reversely transcribed into cDNA using oligo (dT)18 primers and the avian myeloblastosis virus (AMV) reverse transcriptase (BioFlux, Hangzhou, China). The levels of Blimp-1 and BCMA mRNA transcripts were determined by PCR. The primer sequences are listed in and are selected as reported previously [Citation21]. The amplification reaction included 1X Taq buffer, 0.2 mM of each dNTP, 1.5 mM MgCl2, 10 pmol of each primer and 1 unit of Taq polymerease (Invitrogen), and the PCR program involved a denaturation step at 94 °C for 2 min followed by 30 cycles of 95 °C for 60 s, 58 °C for 60 s, and 72 °C for 60 s, and a final extension step at 72 °C for 10 min. The PCR products were separated by electrophoresis on 1.5% agarose gel and visualized with ethidium bromide staining. The initial RNA samples were used as the templates to determine potential contamination by genomic DNA.
Table 1. Primer sequences for PCR assays in this study.
Western blot
One hundred-milligram tissue samples of kidneys, liver, spleen and lymph nodes from mice were homogenized in 1 ml homogenization buffer including 50 µg/ml phenylmethylsulfonyl fluoride (PMSF) and 5 µg/ml leupeptin. Cells were frozen at −20 °C for 20 min, followed by high-speed centrifugation (12000 rpm, 20 min) at 4 °C. The supernatant was collected using an EP tube and stored at −80 °C. After quantification of protein concentrations in the supernatant, the lysates were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membrane was blocked with 2% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 for 2 h and probed with rabbit anti-Blimp-1 (1:200), XBP-1 (1:100), C-myc (1:400) (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-GAPDH monoclonal antibody (1:2000, Sigma, St. Louis, MO), respectively, at 4 °C overnight. After washing, the bound antibodies were detected by peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies and visualized using an enhanced chemiluminescence system (Santa Cruz Biotechnology). Protein expression level was determined by analyzing the optical density value obtained from Quantity One software (Bio-Rad, Hercules, CA) and was calculated from triplicate samples after normalization to GAPDH levels.
Detection of anti-dsDNA in mice
Anti-dsDNA antibodies in sera were detected from mice every 3 weeks in both the study and control groups using an ELISA kit (Alpha Diagnostic, San Antonio, TX), which detects IgG, IgM and IgA. Anti-dsDNA Ab levels were expressed as U/mL and were calculated using a positive standard serum.
Detection of 24-h urine protein in mice
Mice were housed in metabolic cages for the determination of 24-h urine protein concentrations. Coomassie blue staining was used to measure the 24-h urine protein levels in the MRL-Fas(lpr) mice after 15 and 18 weeks.
Immunohistochemical staining
Indirect immunohistochemical staining of tissue samples from kidneys, spleen and lymph nodes was performed according to the manufacturer's protocol. Rat anti-mouse Blimp-1 primary antibody (Santa Cruz Biotechnology) and goat anti-rat secondary antibody (BiyuntianInc, Nanjing, China) were used in this study.
HE staining of mouse tissues
After sacrifice, samples from kidneys, spleen and lymph nodes were fixed in 10% buffered formaldehyde and were embedded in paraffin. Tissue sections (4-µm thick) with HE staining were viewed and photographed with an Olympus microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Significant differences were analyzed using Student's t test. p < 0.05 was considered to indicate statistical significance.
Results
Blimp-1 siRNA reduced the expression of Blimp-1 in PMBCs and tissues
To examine the impact of Blimp-1 siRNA on Blimp-1 expression in MRL-Fas(lpr) mice, the Blimp-1 mRNA and protein expression levels were determined by RT-PCR and Western blot, respectively. Blimp-1 was highly expressed in PMBCs (), kidney, spleen, lymph nodes and liver of MRL-Fas(lpr) mice (). Interestingly, after administration of Blimp-1 siRNA for 21 days, the expression level of Blimp-1 mRNA in PMBCs declined by 78% (, right). No changes in Blimp-1 were detected in the liver; however, the Blimp-1 expression in kidney, spleen and lymph nodes declined by 95, 72 and 47%, respectively (). The results of immunohistochemical staining indicated that Blimp-1-positive cells (brown color) were mainly distributed in glomerular mesangial cells and tubular epithelial cells, and the number of Blimp-1 positive cells in glomerulus, renal tubular epithelium, spleen and lymph nodes in the Blimp-1 siRNA-treated group were significantly reduced compared to those in the non-treated control group (, p < 0.05), suggesting that the endogenous Blimp-1 level was significantly reduced following systemic injection of Blimp-1 siRNA.
Figure 1. The Blimp-1 mRNA expression in PMBCs of mice at 3 weeks after administration of the lentivirus Blimp-1 siRNA (study group) or PLL3.7 (control group). PMBCs of the mice (8 mice in each group) were collected, and mRNA expression of Blimp-1 detected by RT-PCR. C: control group, S: study group.
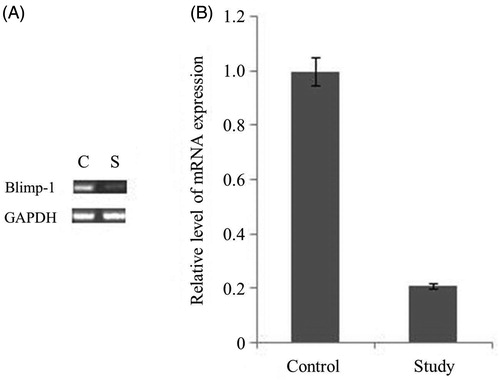
Figure 2. The expression levels of Blimp-1 protein in the kidney, liver, lymph nodes and spleen in the experimental groups. (A) 15-week-old MRL-Fas(lpr) mice received an intravenous tail vein injection of lentivirus vector. After 21 days, the mice were sacrificed, and the Blimp-1 expression in kidney, spleen, lymph node and liver was analyzed by Western blot. (B) Blimp-1 expression was analyzed by semi-quantitative Western blot by using GAPDH for normalization. *Compared with controls, p < 0.05. C: control group, S: study group. The results are representative of three individual experiments.
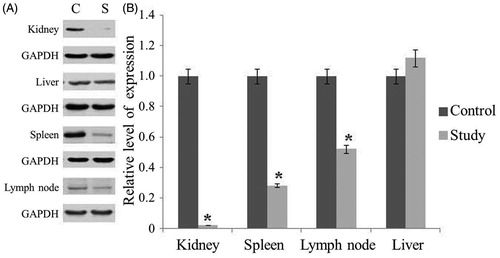
Figure 3. Immunohistochemical staining of Blimp-1. C: control group, S: study group. The numbers of Blimp-1 positive cells (marked by black arrows) in glomerulus, renal tubular epithelium, spleen, and lymph nodes of the control group were obviously greater than those in the Blimp-1 siRNA-treated group. Blimp-1 expression of blood, kidney, spleen and lymph node was decreased significantly following Blimp-1 siRNA administration.

Blimp-1 siRNA reduced the level of anti-dsDNA Ab in lupus mice
The level of anti-dsDNA Abs in serum of MRL-Fas(lpr) mice was analyzed every 3 weeks to explore whether Blimp-1 could affect the production of anti-dsDNA Ab. As shown in , the level of anti-dsDNA Ab increased gradually with the age of mice. At 15 weeks of age, the study group was injected with Blimp-1 siRNA, and the control group was injected with pLL3.7 vector only. While the anti-dsDNA Ab level continued to increase in control group, the level of anti-dsDNA Ab in the study group remained unchanged. When mice were sacrificed at 18 weeks of age, the anti-dsDNA Ab level in the study group was significantly lower than that of the control group (p < 0.05, ). These results suggest that inhibition of endogenous Blimp-1 could prevent anti-dsDNA Ab production.
Figure 4. The level of anti-dsDNA Ab in serum of the 18-week-old MRL-Fas(lpr) mice. At 15 weeks old, MRL-Fas(lpr) mice (8 mice per group) received an intravenous tail vein injection of lentivirus (1×109 pfu) or controls. The anti-dsDNA Ab concentrations in mouse serum were determined at 3-week intervals. Similar results were obtained in three individual experiments. *p < 0.05 compared with control.
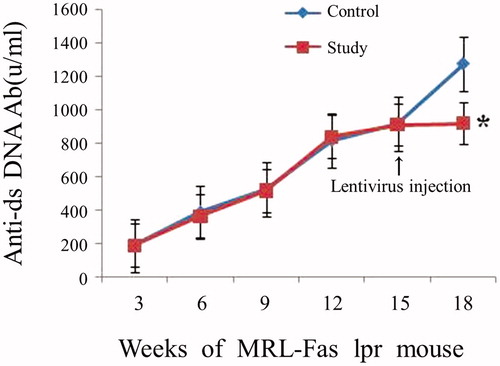
Figure 5. The effect of Blimp-1 inhibition on the expression of BCMA, XBP-1, J-chain, and C-myc. Western blot and RT-PCR were performed 3 weeks after administration of the lentivirus Blimp-1 siRNA (study group) or PLL3.7 (control group). (A) Peripheral blood of the mice (8 mice per group) was collected, and mRNA expression of Blimp-1, BCMA, XBP-1, J-chain, and C-myc were detected by RT-PCR. (B) Spleen cells of mice were collected, and protein expression of XBP-1 and C-myc were detected by Western blot. Similar results were obtained from three individual experiments. C: control group, S: study group.
Impact of down-regulation of Blimp-1 on the levels of BCMA, XBP-1, J-chain and C-myc
To explore how Blimp-1 regulates the production of auto-antibody levels, we analyzed the changes in RNA expression of XBP-1, BCMA, C-myc and J-chain, which are downstream mediators of Blimp-1 and are also necessary for the production of antibodies as reported in other studies [Citation21–26]. By RT-PCR, we found that following Blimp-1 siRNA injection, the expression of BCMA, XBP-1 and J-chain in the peripheral blood decreased by 92, 86 and 70%, respectively, in MRL-Fas(lpr) mice compared to levels in the control group. By contrast, the expression of C-myc was increased about 65%. Similar to mRNA levels, by Western blot, we also found that administration of Blimp-1 siRNA reduced the protein expression of XBP-1 and increased the level of C-myc (). These results suggest that the inhibition of endogenous Blimp-1 down-regulates the expression of BCMA, XBP-1 and J-chain and up-regulates C-myc mRNA.
Inhibition of endogenous Blimp-1 reduced the level of 24-h urinary protein in SLE mice
To determine whether administration of Blimp-1 siRNA could improve renal function and decrease urine protein concentration, we used metabolic cages to collect and measure 24-h urinary protein from 15- and 18-week-old SLE mice. The results demonstrated that after 3 weeks of Blimp-1 siRNA injection, the level of 24-h urinary protein was significantly lower in the study group than in the control group (p < 0.05; ).
Table 2. The 24-h urinary protein concentration in experimental groups.
Blimp-1 siRNA attenuated renal pathological changes in SLE mice
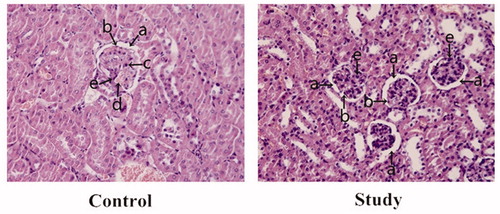
The renal pathological appearance was also examined. In the control group, the numbers of glomerular mesangial cells and endothelial cells were increased. In addition, the glomerular capillary was dilated and congested with mild lymphocytic infiltration, which further narrowed the capsular space. However, after Blimp-1 siRNA administration, the glomerular pathological changes were mitigated. In addition, the numbers of mesangial cells and endothelial cells as well as the capsular space were similar to those of normal controls, and no significant inflammatory changes were found in the glomerulus (). These pathological results suggest that Blimp-1 siRNA can mitigate the renal inflammatory changes.
Figure 6. The renal pathological changes in the experimental groups. (a) capsular space; (b) endothelial cells; (c) lymphocytes; (d) capillary; (e) mesangial cells. In the control group, the numbers of glomerular mesangial and endothelial cells were increased compared to the study group, and glomerular capillary was dilated and congested with mild lymphocytic infiltration, which further narrowed the capsular space. After Blimp-1 expression was inhibited by Blimp-1 siRNA (study group), the glomerular pathological changes in the study group were mitigated. The numbers of mesangial and endothelial cells and the capsular space tended to be normal, and there were no significant inflammatory changes found in glomerulus.
Discussion
We previously found that Blimp-1 expression is upregulated in SLE patients and in the SLE mouse model [Citation10]. To explore the roles of Blimp-1 during SLE progression, in the present study, we investigated the effects of inhibition of endogenous Blimp-1 by lentivirus-mediated RNAi method. This inhibition significantly reduced the levels of Blimp-1 in the kidney, spleen and lymph nodes. In addition, the renal pathological changes were obviously alleviated, and the level of serum anti-dsDNA dropped significantly compared to that in non-treated controls. These results indicate that inhibition of Blimp-1 may provide a novel strategy for the treatment of SLE.
SLE is a type of systemic autoimmune disease characterized by increased pathogenic autoantibodies, which can often lead to injury to tissues and damage to organs in the form of immune complexes [Citation27–29]. SLE is characterized by B-cell hyperactivity that, at least in part, results from polymorphisms in genes encoding B-cell signaling components that increases disease susceptibility. Thus, B cells appear to play a central role in the immuno-pathogenesis of SLE. Therefore, therapeutic strategies targeting B cells have been a focus for SLE treatment. With the recent availability of biologic agents that can deplete B cells or block their function in vivo, it has become possible to target B cells therapeutically, such as CD20 monoclonal antibody and BAFF inhibitor [Citation4]. However, when only targeting B lymphocytes in the treatment of SLE, it is difficult to avoid recurrence of the disease [Citation30]. Studies have revealed that the reasons for recurrence of SLE are CD20 and BAFF, which disappear during terminal differentiation of B cells into plasma cells. In other words, the CD20 monoclonal antibody or BAFF inhibitor cannot remove plasma cells. In addition, 40% of plasma cells secreted autoantibodies that have longevity, and these autoantibodies can reactivate potential pathological immune responses [Citation31,Citation32]. Random clinical trials have also confirmed that treatment of lupus by rituximub is ineffective. Thus, in order to alleviate the symptoms of SLE for a long time, plasma cells (including the long-lived plasma cells) and their functions should be inhibited [Citation8].
Plasma cells are unique antibody-producing cells that play an important role in immune responses. Dysregulation of plasma cell differentiation and survival will result in various autoimmune diseases, such as SLE, and rheumatoid arthritis [Citation10,Citation33]. A variety of transcription factors, such as Pax-5, Bcl-6, XBP-1 and Blimp-1, play critical roles during the differentiation, survival and antibody secretion of plasma cells. Several lines of evidences have demonstrated that increased expression of Blimp-1, BCMA and XBP-1, and steady absence of Pax-5 are necessary for the survival of plasma cells [Citation22–25]. Blimp-1 expression is regulated by Pax-5, and Blimp-1 acts upstream of BCMA and XBP-1 [Citation21,Citation26]. The results from the present study show that inhibition of endogenous Blimp-1 in a spontaneous lupus mice model reduces levels of BCMA, XBP-1 and J-chain as well as the level of autoantibodies. As a result, lupus nephritis symptoms were alleviated and the autoimmune symptoms were also mitigated. It is also likely that Blimp-1 regulates the expression of BCMA and XBP-1 through C-myc pathways and then affects the secretion of J-chain and Ig, influencing autoantibody production.
Our study also demonstrated overexpression of Blimp-1 in PMBCs, liver, kidney, spleen and lymph nodes of MRL-Fas(lpr) lupus mice, and administration of Blimp-1 siRNA significantly reduced Blimp-1 expression in these organs. Therefore, this study provides evidence of the correlation between Blimp-1 expression and lupus symptoms in these organs. Zhou et al. recently reported that Blimp-1 siRNA can inhibit B-cell differentiation and prevent the development of lupus in mice [Citation34]. We further confirmed these results in MRL-Fas(lpr) lupus mice. Moreover, we analyzed Blimp-1 expression in different tissues and observed the pathological, functional changes in SLE mice. The results indicate that inhibition Blimp-1 can improve symptoms of SLE and may become a new treatment for SLE. The improvement of symptoms may be related to the decrease in autoantibody levels, alleviation of kidney disease changes and reduction of urine protein. It should be noted that because Blimp-1 plays an important role in maintaining normal immune responses, and our purpose was to use Blimp-1 siRNA to inhibit rather than clear the expression of Blimp-1 in various vital organs, the Blimp-1 expression levels in spleen, lymph node and liver did not seem to be highly depleted, specifically with no significant changes in the liver (p > 0.05). In spleen and lymph nodes, we hypothesize that the higher number of lymphocytes can prevent the level of Blimp-1 from being depleted too much. Regarding liver, this may be the result of the method using to inject Blimp-1 siRNA. Because the plasma cells reside in bone marrow, we did not successfully obtain sufficient bone marrow specimens, and thus, it is hard to evaluate the data. In addition, because the life span of female MRL-Fas(lpr) mice is about 17 weeks, the mice were sacrificed at 18 weeks old to observe pathological changes, resulting in only one time point and the inability to collect survival data in the present study. Furthermore, we also took steps to avoid potential off-target effects, such as using a lentiviral vector rather than liposomes to infect mice [Citation19], avoiding the siRNA seed region matching the 3′-untranslated region (UTR) of off-target genes [Citation35] and applying BLAST similarity searches against sequence databases to identify potential off-target genes [Citation36].
In conclusion, our results suggest that Blimp-1 may be a new target for plasma cells in the treatment of SLE. This not only lays a foundation for further constructing a specific immune inhibitor screening model and investigating new drugs in the treatment of SLE, but also provides a new strategy for the treatment of other autoimmune diseases and drug design for plasma cells.
Supplementary material available online
Supplementary Figure 1.
Acknowledgements
We thank Medjaden Bioscience Limited for assistance in the preparation of this manuscript.
Declaration of interest
The authors have no financial conflicts of interest. This work was supported by a grant from the National Natural Science Foundation of China (81101199).
References
- Sanz, I., and F. E. H. Lee. 2010. B cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6: 326–337
- Chatenoud, L. 2006. Immune therapies of autoimmune diseases: are we approaching a real cure? Curr. Opin. Immunol. 18: 710–717
- Levesque, M. C., and E. W. S. Clair. 2008. B cell-directed therapies for autoimmune disease and correlates of disease response and relapse. J. Allergy Clin. Immun. 121: 13–21
- Tieng, A. T., and E. Peeva. 2008. B-cell-directed therapies in systemic lupus erythematosus. Semin. Arthritis Rheu. 38: 218–227
- Ding, C., S. Foote, and G. Jones. 2008. B-cell-targeted therapy for systemic lupus erythematosus – an update. Biodrugs 22: 239–249
- Marino, E., and S. T. Grey. 2012. B cells as effectors and regulators of autoimmunity. Autoimmunity 45: 377–387
- Stohl, W., and R. J. Looney. 2006. B cell depletion therapy in systemic rheumatic diseases: different strokes for different folks? Clin. Immunol. 121: 1–12
- Sutter, J. A., J. Kwan-Morley, J. Dunham, et al. 2008. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin. Immunol. 126: 282–290
- Garaud, J. C., J. N. Schickel, G. Blaison, et al. B cell signature during inactive systemic lupus is heterogeneous: toward a biological dissection of lupus. Plos One 6: e23900
- Luo, J., X. Niu, H. Liu, et al. 2013. Up-regulation of transcription factor Blimp1 in systemic lupus erythematosus. Mol. Immunol. 56: 574–582
- Ochiai, K., A. Muto, H. Tanaka, et al. 2008. Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int.l Immunol. 20: 453–460
- John, S. A., J. L. Clements, L. M. Russell, and L. A. Garrett-Sinha. 2008. Ets-1 regulates plasma cell differentiation by interfering with the activity of the transcription factor Blimp-1. J. Biol. Chem. 283: 951–962
- Hamel, K. M., V. M. Liarski, and M. R. Clark. 2012. Germinal center B-cells. Autoimmunity 45: 333–347
- Diehl, S. A., H. Schmidlin, M. Nagasawa, et al. 2008. STAT3-mediated up-regulation of BLIMP1 is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J. Immunol. 180: 4805–4815
- Fairfax, K. A., L. M. Corcoran, C. Pridans, et al. 2007. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J. Immunol. 178: 4104–4111
- Shapiro-Shelef, M., K. I. Lin, L. J. McHeyzer-Williams, et al. 2003. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19: 607–620
- Fujita, N., D. L. Jaye, C. Geigerman, et al. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 119: 75–86
- Kafri, T., U. Blomer, D. A. Peterson, et al. 1997. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet. 17: 314–317
- Klinghoffer, R. A., J. Magnus, J. Schelter, M. et al. 2010. Reduced seed region-based off-target activity with lentivirus-mediated RNAi. RNA. 16: 879–884
- Rubinson, D. A., C. P. Dillon, A. V. Kwiatkowski, et al. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33: 401–406
- Deng, S., T. Yuan, X. Cheng, et al. 2010. B-lymphocyte-induced maturation protein1 up-regulates the expression of B-cell maturation antigen in mouse plasma cells. Mol. Biol. Rep. 37: 3747–3755
- Soro, P. G., A. P. Morales, M. J. Martinez, et al. 1999. Differential involvement of the transcription factor Blimp-1 in T cell-independent and -dependent B cell differentiation to plasma cells. J. Immunol. 163: 611–617
- Tunyaplin, C., A. L. Shaffer, C. D. Angelin-Duclos, et al. 2004. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J. Immunol. 173: 1158–1165
- Shaffer, A. L., K. I. Lin, T. C. Kuo, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17: 51–62
- Sciammas, R., and M. M. Davis. 2004. Modular nature of Blimp-1 in the regulation of gene expression during B cell maturation. J. Immunol. 172: 5427–5440
- Shaffer, A. L., M. Shapiro-Shelef, N. N. Iwakoshi, et al. 2004. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21: 81–93
- Radic, M., M. Herrmann, J. van der Vlag, and O. P. Rekvig. 2011. Regulatory and pathogenetic mechanisms of autoantibodies in SLE. Autoimmunity 44: 349–356
- Wong, C. K., P. T. Wong, L. S. Tam, E. K. Li, et al. 2009. Activation profile of intracellular mitogen-activated protein kinases in peripheral lymphocytes of patients with systemic lupus erythematosus. J. Clin. Immunol. 29: 738–746
- Mason, J. A., and D. Bossingham. 2009. The clinical characterisation of systemic lupus erythematosus in a Far North Queensland Indigenous kindred. Lupus. 18: 144–148
- Kallies, A., and S. L. Nutt. 2007. Terminal differentiation of lymphocytes depends on Blimp-1. Curr. Opin. Immunol. 19: 156–162
- Tarlinton, D. M., and P. D. Hodgkin. 2004. Targeting plasma cells in autoimmune diseases. J. Exp. Med. 199: 1451–1454
- Shapiro-Shelef, M., K. I. Lin, D. Savitsky, et al. 2005. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J. Exp. Med. 202: 1471–1476
- Shapiro-Shelef, M., and K. Calame. 2005. Regulation of plasma-cell development. Nat. Rev. Immunol. 5: 230–242
- Zhou, Z., A. Li, Z. Wang, et al. 2013. Blimp-1 siRNA inhibits B cell differentiation and prevents the development of lupus in mice. Human Immunol. 74: 297–301
- Birmingham, A., E. M. Anderson, A. Reynolds, et al. 2006. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods. 3: 199–204
- Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 26: 199–213
