Abstract
To inform the future use of hair cortisol measurement, we have investigated influences of potential confounding variables (natural hair colour, frequency of hair washes, age, sex, oral contraceptive (OC) use and smoking status) on hair cortisol levels. The main study sample comprised 360 participants (172 women) covering a wide range of ages (1–91 years; mean = 25.95). In addition, to more closely examine influences of natural hair colour and young age on hair cortisol levels, two additional samples comprising 69 participants with natural blond or dark brown hair (hair colour sample) as well as 28 young children and 34 adults (young age sample) were recruited. Results revealed a lack of an effect for natural hair colour, OC use, and smoking status on hair cortisol levels (all p's >0.10). No influence of frequency of hair washes was seen for proximal hair segments (p = 0.335) but for the third hair segment indicating lower cortisol content (p = 0.008). We found elevated hair cortisol levels in young children and older adults (p < 0.001). Finally, men showed higher hair cortisol levels than women (p = 0.002). The present data indicate that hair cortisol measurement provides a useful tool in stress-related psychobiological research when applied with the consideration of possible confounders including age and sex.
Keywords::
Introduction
The glucocorticoid hormone cortisol is a frequently assessed parameter in psychoneuroendocrine stress research. While in some of this research the primary focus lies in measuring dynamic changes in acute cortisol levels, research into the effects of chronic stress and its implications on health is often particularly interested in assessing long-term cortisol exposure. This, however, has been difficult, given that common measurement strategies only reflect acutely circulating cortisol levels (blood or saliva) or assess cortisol secretion over short time periods, usually not exceeding 24 h (urine). In this context, the analysis of cortisol in human hair is assumed to present a major methodological advancement, providing an easily obtainable retrospective index of cumulative cortisol exposure over extended periods of up to several months (Gow et al. Citation2010). Over the past decade, the validity of hair cortisol as an index of long-term cortisol secretion has been supported by research in both animals (Davenport et al. Citation2006; Accorsi et al. Citation2008) and human participants (Sauve et al. Citation2007; Kirschbaum et al. Citation2009; Stalder et al. Citation2010; Thomson et al. Citation2010; D'Anna-Hernandez et al. Citation2011; Manenschijn et al. Citation2011). In addition, several studies have now shown the utility of hair cortisol as a marker of chronic stress (Yamada et al. Citation2007; Kalra et al. Citation2007; Dettenborn et al. Citation2010; Fairbanks et al. Citation2011; Laudenslager et al. Citation2011), psychological disease (Dettenborn et al. Citation2011; Steudte et al. Citation2011a,Citationb) and health risk (Pereg et al. Citation2011). Finally, recent evidence showing considerable intra-individual stability in hair cortisol levels highlights the utility of this method for obtaining stable estimates of long-term cortisol secretion (Stalder et al. Citation2011).
While overall these studies suggest the great potential of hair cortisol analysis as an important new measure in psychobiological research, to date there is little available information on fundamental aspects that can influence hair cortisol concentrations. Specifically, few studies have examined influences of potential confounding variables on hair cortisol levels, which are the important prerequisites for adequate use of this method in future research. In most of this previous research, the influence of potential confounders was examined only secondary to another main research goal (Raul et al. Citation2004; Kirschbaum et al. Citation2009; Manenschijn et al. Citation2011). These studies were not designed to examine potential confounding influences directly. Additionally, while more comprehensive evidence regarding sociodemographic and health-related confounding influences is available from research using cortisol assessments in saliva or blood, a simple transfer of these findings to hair cortisol research seems inappropriate, since the nature of long-term cortisol assessment in hair differs substantially from these momentary cortisol measures. For example, acute cortisol assessments are strongly influenced by situational characteristics as well as by the degree of participant adherence (Kudielka et al. Citation2003), which may not be applicable to hair analyses. On the other hand, a number of hair-specific characteristics may influence the incorporation of cortisol into hair. Following we provide a brief review of previous research on a number of potential confounding influences on hair cortisol levels.
Natural hair colour constitutes a possible characteristic that could affect cortisol incorporation into hair. Toxicology research suggests that the key factors influencing analyte incorporation into hair are melanin content of the hair as well as basicity and lipophilicity of the substance. More specifically, alkaline substances show high melanin binding and thus are incorporated at a higher rate into strongly pigmented hair (black, brown) than into less pigmented hair (blond, ginger) or non-pigmented hair (white; see Pragst and Balikova Citation2006). No such hair pigmentation-related effects were found for neutral compounds, such as carbamazepine (Rothe et al. Citation1997). Consistently, as glucocorticoids are neutral to acidic compounds, most studies have not found an influence of hair colour on cortisol concentrations in human hair (Raul et al. Citation2004; Sauve et al. Citation2007; Kirschbaum et al. Citation2009; Manenschijn et al. Citation2011). Interestingly, a recent study in dogs found that cortisol concentrations were related to the colour of their fur (Bennett and Hayssen Citation2010). To date, a systematic approach covering a wide range of hair colours is missing.
The impact of hair washing on hair cortisol concentrations is largely unknown. Lower alcohols, which are found in shampoo, are able to penetrate the hair shaft and may result in substances being extracted from the hair (Eser et al. Citation1997). Even though cortisol is hydrophobic, frequent hair washing and penetration of water into the hair shaft may result in the washout of hair cortisol. In line with this, a recent laboratory study on rhesus monkeys showed that repeated washing of hair with water or shampoo was associated with decreased hair cortisol levels (Hamel et al. Citation2011). Effects of hair washing may be responsible for the decline in cortisol concentrations from scalp-near hair segments to more distal hair segments, which have been reported by some (Kirschbaum et al. Citation2009; Dettenborn et al. Citation2010; Gao et al. Citation2010; Steudte et al. Citation2011b) but not all studies (Thomson et al. Citation2010; Dowlati et al. Citation2010; Manenschijn et al. Citation2011). Discrepancies among these studies may be related to differences in the laboratory methods being used or other currently unknown factors.
Age has repeatedly been reported to influence cortisol secretion, albeit with varying results. Importantly, most of this previous research has not covered the whole age range, but has focused on cortisol associations with age within specific periods of life. For example, in research on infants, young children and adolescents, mixed results have been reported with some studies demonstrating age-dependent alterations in salivary cortisol levels over the day indicating a possible influence of pubertal development on cortisol secretion (Kiess et al. Citation1995; Tornhage Citation2002; Groschl et al. Citation2003; Tornhage and Alfven Citation2006; Oskis et al. Citation2009) [however, these changes were not observed in other studies (Knutsson et al. Citation1997; Rosmalen et al. Citation2005)]. Besides young age, several studies have assessed cortisol levels in older adults, with most findings suggesting increased cortisol secretion with old age (Van Cauter et al. Citation1996; Deuschle et al. Citation1997; Laughlin and Barrett-Connor Citation2000; Touitou and Haus Citation2000; Ferrari et al. Citation2001; Seeman et al. Citation2001; Kudielka et al. Citation2004; Larsson et al. Citation2009). Interestingly, no associations with participant age have been observed in the existing research on hair cortisol levels (Raul et al. Citation2004; Dettenborn et al. Citation2010; Manenschijn et al. Citation2011) (results that may be due to the restricted age ranges and relatively small sample sizes of these studies).
Previous research into sex differences in cortisol secretion has also provided inconsistent results. This may depend on methodological factors as well as the particular age group being studied. Among children, findings of no sex differences (Vermeer and van Ijzendoorn Citation2006) as well as increased salivary cortisol levels in boys compared to girls (Ouellet-Morin et al. Citation2010) have been reported. Similarly, contradictory results have also emerged from studies in adolescents (Netherton et al. Citation2004; Rosmalen et al. Citation2005; Adam Citation2006). In the premenopausal adult age range, lower daytime cortisol levels in females compared to males (Netherton et al. Citation2004; Rosmalen et al. Citation2005; Adam Citation2006), no sex difference in a single afternoon cortisol sample (Seeman et al. Citation2001) and elevated morning cortisol levels in females compared to males (Larsson et al. Citation2009) have been reported. In postmenopausal individuals, evidence suggesting both higher (Laughlin and Barrett-Connor Citation2000; Gusenoff et al. Citation2001; Larsson et al. Citation2009) and lower (Seeman et al. Citation2001; Zhao et al. Citation2003) cortisol levels in women compared to men has been published. Finally, in a study covering three age groups (children, younger and elderly adults), elevated afternoon cortisol levels were found in elderly men compared to elderly women (Kudielka et al. Citation2004). In sum, previous research on the influence of sex on different measures of cortisol secretion has produced highly heterogeneous findings. Whether sex presents a substantial confounding influence on hair cortisol levels remains unclear; indeed, several studies have failed to show sex differences between males and females (Raul et al. Citation2004; Gao et al. Citation2010; Thomson et al. Citation2010; Manenschijn et al. Citation2011).
Oral contraceptives (OCs) affect the hypothalamic–pituitary–adrenal (HPA) system by increasing the adrenal production of cortisol (Goldzieher and Fotherby Citation1994). However, as ethinyl estradiol, present in all combined OC pills, is also reported to increase corticosteroid-binding globulin (CBG) levels (Bulbrook et al. Citation1973; Fujimoto et al. Citation1986), overall concentrations of free cortisol may not be changed. Hence, it is not surprising that results on cortisol secretion in OC-users compared to non-users have been mixed. Although total plasma cortisol levels during the baseline testing before a stress test were found to be higher in women using OCs than in women not using OCs or men, no differences in the cortisol response to psychosocial stress were found (Kirschbaum et al. Citation1999). Reinberg et al. (Citation1996) reported lower salivary cortisol levels throughout the day in OC-users compared to non-users, whereas another study found no differences in 12-h salivary cortisol values between female OC users, female non-OC users and men (Kirschbaum et al. Citation1999). In line with the latter findings, Bouma et al. (Citation2009) demonstrated no difference in morning salivary cortisol levels between OC-users and free-cycling girls, but OC-users showed a blunted response to awakening. Based on this literature and a lack of previous evidence on hair cortisol measures between OC-users and non-users, it remains unclear whether the use of OCs may affect hair cortisol concentrations.
Although cigarette smoking is known to stimulate the HPA axis (Wilkins et al. Citation1982; Mendelson et al. Citation2008; Xue et al. Citation2010), only slight changes in basal cortisol activity in habitual smokers have been reported (Rohleder and Kirschbaum Citation2006). Elevated salivary or serum cortisol concentrations in smokers have been demonstrated in studies examining the circadian cortisol profile (Steptoe and Ussher Citation2006), multiple measurements over the day (Kirschbaum et al. Citation1992; Baron et al. Citation1995), or single morning assessments (Friedman et al. Citation1987; del Arbol et al. Citation2000). On the other hand, no differences in 24-h urinary-free cortisol (Yeh and Barbieri Citation1989) or in salivary cortisol samples over a 12-h period (Kirschbaum et al. Citation1994) were seen between smokers and non-smokers. Hence, there is some evidence for a possible effect of cigarette smoking on basal HPA axis activity, indicating that this might also be a potentially important confounding influence on hair cortisol measurements.
Based on the literature summarized above, the aim of the present study was to investigate the influence of natural hair colour, frequency of hair washes, age, sex, OC use and smoking status on hair cortisol concentrations. To enable a careful examination of the influence of these variables, we conducted hair cortisol assessments in a large study sample covering a wide range of ages (main study sample). In addition, two further studies were carried out on smaller samples specifically tailored to allow a closer examination of hair cortisol associations with natural hair colour (hair colour sample) as well as of differences in hair cortisol concentrations between young children and adult controls (young age sample).
Materials and methods
Study participants
provides descriptive information on participants of the three study samples. In the main study, hair samples were obtained from 360 participants (n = 172 women) of all ages (1–91) resulting in 52 (14.4%) children (1–9 years of age), 25 (6.9%) adolescents (10–17 years of age), 252 (70%) younger adults (18–49 years of age) and 31 (8.6%) older or elderly participants (50–91 years of age). There was a wide range of natural hair colour with 33 (9.2%) light blond, 88 (24.4%) middle blond, 97 (26.9%) dark blond/light brown, 71 (19.7%) middle brown, 25 (6.9%) dark brown, 4 (1.1%) black, 9 (2.5%) red and 33 (9.2%) grey hair (includes white and coloured hair). To examine a possible effect of natural hair colour in more detail, additional 69 participants between 18 and 35 years of age with natural blond (n = 15 women, n = 18 men) or dark brown (n = 18 women, n = 18 men) hair were recruited (hair colour sample). To have a closer look at a possible age-effect on hair cortisol levels, we also compared 28 children (n = 18 girls) between 1 and 9 years of age (mean age ± SD: 3.6 ± 2.4) with 34 adults (n = 17 women) from 18 to 38 years of age (mean age ± SD: 24.21 ± 3.90) (young age sample). All participants were recruited among students of Dresden Technical University or among friends and families of the wider research team. Furthermore, some older participants were recruited in retirement homes. Exclusion criteria were chemical hair treatments (tinting, dyeing or permanent wave), known psychiatric conditions and pregnancy in women. Written informed consent was provided by all participants or by a parent for underaged participants. The study protocol was approved by the Ethics Committee of the Dresden Technical University Medical Clinic.
Table I. Sociodemographic and hair-related characteristics for participants of the three study samples.
Self-report measures and determination of natural hair colour
Information on relevant sociodemographic variables (sex, age, marital status, level of education), health-related variables (smoking status, OC use, medication intake, known psychiatric and somatic conditions) and haircare-related information (frequency of hair washes per week) were obtained using a self-developed questionnaire. For each hair segment (see below) of the main study sample and the hair colour sample, natural hair colour was defined by the study coordinator using a standardized formal colour chart with synthetic hair strands. Classes of 12 natural hair colours were identified, which were arranged according to darkness of hair in three groups (light, middle blond; dark blond, light brown; middle, dark brown). The study coordinator also determined hair structure (straight hair vs. curls).
Sample collection and preparation
Hair strands were cut as close as possible to the scalp from a posterior vertex position. Cortisol concentrations were determined from three 3-cm hair segments if possible. Due to different hair lengths, cortisol data were available from all participants (n = 360) for the first scalp-near hair segment, from n = 268 for the second segment and from n = 155 for the third segment. Based on an average hair growth rate of 1 cm per month (Wennig Citation2000), each 3-cm hair segment reflects hair grown over an approximate 3-month period. Hence, cumulative cortisol secretion over total periods of 3–9 months prior to hair sampling was examined for the main study sample. For the hair colour sample and the young age sample, cortisol levels were determined in the first scalp-near 3-cm hair segment only.
Wash and steroid extraction procedures followed the laboratory protocol described in Kirschbaum et al. (Citation2009) with 25 mg of powdered hair being used in the main study sample and the hair colour sample and 10 mg of powdered hair being used in the young age sample. The use of a smaller amount of hair (10 mg) in the latter sample (as also previously reported by our group in Stalder et al., in press) was done in order to further enhance participant's acceptance of the hair sampling procedure, which was considered particularly important in the sample of young children. For the analysis, each hair segment was washed for 3 min with 2.5 ml isopropanol in a 15-ml falcon tube on an overhead rotator. After drying for at least 12 h under a clean protected hood, hair segments were powdered using a Retsch ball mill (2 min at 30 Hz). An exact amount of 25 mg (or 10 mg) of powdered hair was weighed out and transferred into a 2-ml cryo vial (Eppendorf, Hamburg, Germany). For steroid extraction, 1.5 ml of pure methanol was added. The vials were then slowly rotated over a period of 24 h on an overhead rotator. After centrifugation in a microcentrifuge (10,000 rpm for 2 min), the clear supernatant was transferred into a new 2-ml cryo vial to let the alcohol evaporate at 60°C under a stream of nitrogen until the samples were completely dried. Finally, 0.4 ml of phosphate buffer (CALA, IBL-Hamburg, Germany) was added and the vials were vortexed. For samples from the hair colour sample, the steroid extraction was run twice to correct the initial problems with a dysfunctional phosphate buffer resulting in artificially raised absolute values. To resolve this problem, the phosphate buffer was evaporated under a stream of nitrogen, 1.5 ml of pure methanol was added again and subsequent steps of the above described procedure repeated. For all samples, cortisol determination was carried out using a commercially available immunoassay with chemiluminescence detection (CLIA, IBL-Hamburg, Germany). The intra- and inter-assay coefficients of variance for this assay are both below 8%.
Statistical analysis
Kolmogorov–Smirnov tests revealed that hair cortisol data were not normally distributed. Log transformations effectively reduced the skewness statistic and hence log-transformed values were used in all inferential statistics. For descriptive purposes, information on mean values and standard deviations are presented in original units (pg/mg). In the main study sample, seven participants with high outlying values (three standard deviations above the mean) across all segments were detected. No clear causes based on current knowledge were evident for the high cortisol concentrations of these individuals (mean age: 36.14; four females), and hence their data were excluded from our analysis. Analogously, three outliers were detected and excluded in the hair colour sample and the young age sample.
A two-way repeated-measures ANOVA with hair segment as a within-subject factor was used for testing segment differences in hair cortisol levels. Associations with continuous variables (darkness of natural hair colour, frequency of hair washes) were examined using Spearman correlations. Associations between hair cortisol levels and participant age were examined using regression analyses, exploring both linear as well as theory-based nonlinear associations. Group comparisons for categorical variables (sex, smoking status, medication intake and OC use) were conducted using univariate ANOVAs, with respective results being confirmed using ANCOVAs controlling for potential confounding variables.
Results
Segment effect
Analyses in 155 participants from the main study sample for whom data of all three hair segments were available revealed decreasing cortisol content from the segment closest to the scalp to more distal hair segments (F (2,153) = 66.040, p < 0.001). This decrease was 16.32% from the first scalp-near segment (mean = 15.07) to the second segment (mean = 12.61) and 12.29% from the second to the third segment (mean = 11.06). Pairwise comparisons indicated significant mean differences between all segments (all p's < 0.001).
Natural hair colour
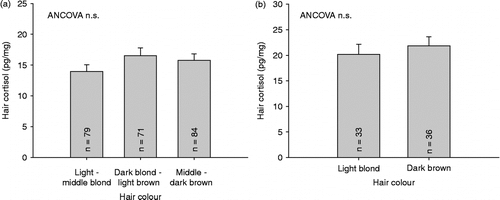
Arranging main study participants' natural hair colour in three groups according to darkness of hair (carried out on 18–49-year-old participants, controlling for sex, see below) indicated that hair cortisol levels did not differ between the three groups, although a non-significant trend was found (F (2,232) = 2.538, p = 0.081; see ). Additionally, the eight red and three black hair samples showed comparable descriptive cortisol values (red hair: mean = 12.69; black hair: mean = 12.08). Confirmatory analyses in the hair colour sample also revealed no differences in hair cortisol concentrations between individuals with light blond and dark brown hair (p = 0.524; see ).
Hair washes
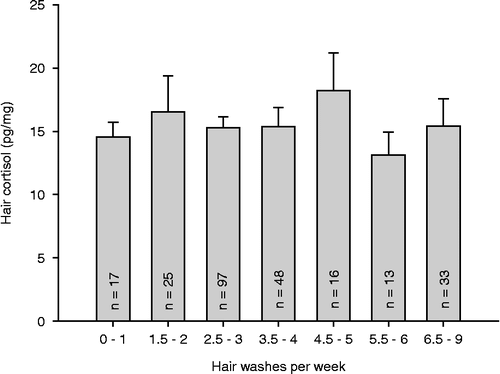
illustrates cortisol levels of the first hair segment depending on the self-reported frequency of hair washes per week (carried out on 18–49-year-old participants). No association between frequency of hair washes and hair cortisol levels was found for the first segment (r = − 0.061, p = 0.335) or for the second segment (n = 184, r = − 0.102, p = 0.168). In the third segment, cortisol levels were negatively correlated with the frequency of hair washes (n = 113, r = − 0.248, p = 0.008).
Curls/waves
There was no influence of curls or waves on hair cortisol levels (curls: p = 0.468; waves: p = 0.633; carried out on 18–49-year-old participants, controlling for sex).
Age
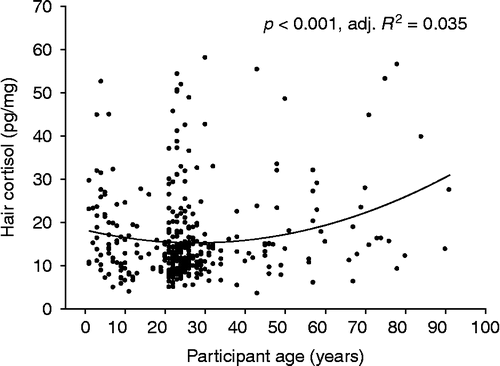
A positive linear relationship between participant age and hair cortisol levels was found in analyses conducted across the whole age range (p = 0.030, adj. R 2 = 0.010). In addition, nonlinear regression analysis revealed a quadratic relationship between the two variables (p < 0.001, adj. R 2 = 0.035, see ).
Figure 3. Quadratic relationship between hair cortisol levels in the first scalp-near hair segment and participant age (n = 360; main study sample).
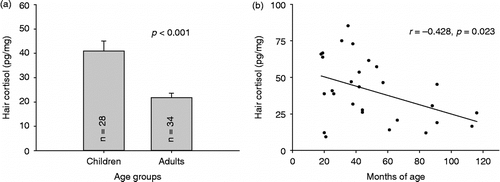
Results of the young age sample also showed higher hair cortisol levels in children compared to adults (F (1,60) = 21.119, p < 0.001; ηp 2 = 0.260). Mean hair cortisol concentrations in children were more than twofold higher than the respective values in adults (see ). shows a significant negative correlation between age and hair cortisol, indicating that the younger the child the higher the hair cortisol content (age in months: r = − 0.428, p = 0.023).
Figure 4. Cortisol concentrations in the first scalp-near hair segment of participants of the young age sample. Shown are (a) mean ( ± SEM) values of young children (1–9 years) and adults (18–38 years) and (b) the association between hair cortisol levels and age (in months) among young children (n = 28).
Sex
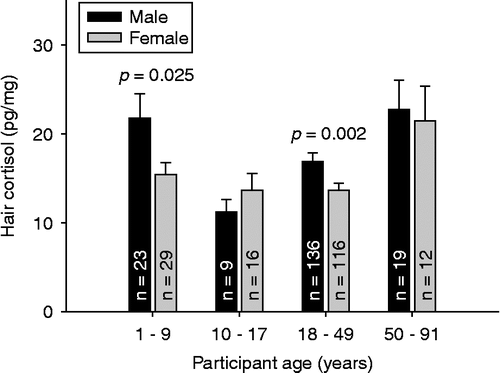
In the main study sample, higher hair cortisol levels were found in male compared with female participants in the adult age range of 18–49 years (F (1,251) = 9.573, p = 0.002; ηp 2 = 0.037) as well as in children aged 1–9 years (F (1,51) = 5.304, p = 0.025; ηp 2 = 0.078). No sex differences were found in adolescents between 10 and 17 years of age (F (1,24) = 0.837, p = 0.370) or among the elderly (50–91 years of age) (F (1,30) = 0.064, p = 0.803). illustrates sex differences in hair cortisol levels for different age groups. Analyses in the hair colour sample also revealed higher cortisol levels in men (n = 36) compared with women (n = 33) between 18 and 38 years of age (hair colour sample: F (1,68) = 5.652, p = 0.020; ηp 2 = 0.078). The young age sample with 28 children (10 vs. 18 males and females, respectively) revealed ηp 2 of 0.085 (p = 0.131).
Overall medication intake, OC use and smoking
Analyses in 18–49-year-old participants, controlling for sex, indicated no influences of overall medication intake (p = 0.610), use of OCs (among females only: p = 0.110) or smoking status (p = 0.836) on hair cortisol levels. The finding of no influence of medication intake on hair cortisol levels was also confirmed in the elderly age group (F (1,28) = 0.245, p = 0.625).
Discussion
The present study set out to examine potential hair-related and sociodemographic confounding influences on hair cortisol levels. Our results indicate that hair cortisol concentrations are robust to influences of natural hair colour, general medication intake, use of OCs and smoking status. Similarly, more frequent washing of hair was only found to be associated with reduced cortisol levels in the third most distal hair segment, but not in the first two most proximal segments. On the other hand, the current results revealed a quadratic relationship with participant age, indicating that hair cortisol levels were increased in young children and older adults. In addition, men showed higher hair cortisol levels compared to women.
Our result of no effect of hair colour on hair cortisol concentrations is in line with previous research, indicating that neutral or acidic compounds are incorporated independently of the melanin content and, thus, the darkness of the hair (Nakahara et al. Citation1995; Gygi et al. Citation1997; Borges et al. Citation2001; Raul et al. Citation2004; Sauve et al. Citation2007; Kirschbaum et al. Citation2009). Similarly, the current finding corresponds to previous evidence from smaller studies showing no association between natural hair colour and hair cortisol levels (Raul et al. Citation2004; Sauve et al. Citation2007; Kirschbaum et al. Citation2009). On the other hand, these results are opposed to recent evidence from a study in dogs reporting decreased cortisol levels in dark compared to lighter fur (Bennett and Hayssen Citation2010). However, the hair structure and growth pattern of dogs' fur is different from human hair, which may have contributed to the inconsistent result in this study.
Although previous hair cortisol studies have looked at a possible association between hair cortisol levels and age, this is the first study that has examined such associations across a wider age range. We found higher hair cortisol levels in children compared to adults, an effect that was seen in both the results of our main study sample and the additional sample of very young children and comparison adults. Analyses carried out within the children subgroup of the additional young age sample also tentatively suggest that hair cortisol concentrations may be particularly high in very young children and decrease during their subsequent development. Previous cortisol research using salivary cortisol measurements has produced mixed results with regard to the influence of age. However, few of these studies have investigated cortisol among the very young, which may be due to difficulties with specimen collection, e.g. problems with obtaining a sufficient amount of saliva (Clements et al. Citation2007). As hair analysis is easy to conduct, not restricted to sampling at specific times of the day and does not interfere with the child's daily routine, it appears to be an ideal method for obtaining both ethologically and methodologically valid estimates of long-term cortisol secretion in this age group. More studies using hair analyses to explore cumulative cortisol release in babies, toddlers and young children are needed in order to corroborate the present findings in this age group. If these results of increased hair cortisol levels among the very young can be confirmed, future studies should use this method to better understand the role of cortisol release in this developmental context.
In addition, our results suggest a curvilinear relationship between hair cortisol levels and age, not only suggesting increased hair cortisol levels among the very young but also among the elderly. While this finding is in line with the majority of previous research on salivary and plasma cortisol levels (Van Cauter et al. Citation1996; Deuschle et al. Citation1997; Laughlin and Barrett-Connor Citation2000; Touitou and Haus Citation2000; Ferrari et al. Citation2001; Seeman et al. Citation2001; Kudielka et al. Citation2004; Larsson et al. Citation2009), the number of participants in the old and very old age range was relatively small in the present study. Hence, these results will need to be confirmed by future research including a larger number of elderly individuals. Similarly, it has been reported that the rate of hair growth may be reduced in some older individuals depending on the pigmentation of hair (Van Neste Citation2004). To the best of our knowledge, the influence of hair growth rate on cortisol incorporation into hair has not been examined yet, and hence, it cannot be excluded that this may have affected respective findings. Future research may thus be best advised to also assess participants' hair growth rates in order to corroborate the present findings of elevated hair cortisol levels in elderly individuals.
We found increased hair cortisol levels among male compared to female adult participants. A similar effect for children was seen in our main study sample and in the present subsamples, albeit with too little power to result in statistical significance. No such sex effect was seen for adolescent and elderly participants. Interestingly, the finding of increased hair cortisol concentrations in male adults was remarkably consistent across the three present samples, which is surprising given that this effect had not been observed in previous hair cortisol research (Raul et al. Citation2004; Gao et al. Citation2010; Thomson et al. Citation2010; Manenschijn et al. Citation2011). The fact that the present findings are at variance with previous hair cortisol data in a way echoes previous research on salivary cortisol, which has also produced highly inconsistent results regarding sex differences (Seeman et al. Citation2001; Kudielka et al. Citation2004; Adam Citation2006). Nevertheless, this is the first large-scale study looking at sex effects on hair cortisol levels, suggesting that there may be an influence with male participants tending to have higher hair cortisol levels. Thus, future studies need to consider participant sex as a possible confounder in hair cortisol research using mixed samples.
No effect of OC use on hair cortisol levels was seen in the present results. This may seem surprising as previous work suggests that OCs affect the HPA axis by increasing the adrenal production of cortisol (Goldzieher and Fotherby Citation1994). However, as ethinyl estradiol also increases CBG levels (Bulbrook et al. Citation1973; Fujimoto et al. Citation1986), CBG–cortisol binding may be increased, resulting in no net change in overall free cortisol concentrations. Hence, it may well be that for cumulative cortisol levels in hair—which are most likely to reflect the free, unbound fraction of cortisol in blood, although this still needs to be experimentally shown—the aforementioned effects of OCs on total adrenal cortisol production and protein-binding may balance each other out, thus resulting in comparable (free) hair cortisol concentrations in OC users and non-users. Clearly, further research, also examining the precise mechanisms of cortisol incorporation into hair, will be needed to allow a firmer interpretation of this finding.
The present finding of a decline in cortisol levels from scalp-near to distal hair segments is in line with some (Kirschbaum et al. Citation2009; Dettenborn et al. Citation2010; Gao et al. Citation2010; Steudte et al. Citation2011b) but not all (Thomson et al. Citation2010; Manenschijn et al. Citation2011) previous work. Interestingly, Manenschijn and colleagues point out that those studies without a washout effect did not wash their samples with isopropanol before laboratory analysis. They further argue that more distal hair segments may be more damaged by outer influences (e.g. UV irradiation, cosmetic products, frequent washing), and thus facilitate washout of cortisol by isopropanol. While recent data from our laboratory indicate that a decline in cortisol concentrations from proximal to distal hair segments may still be seen even when washing with isopropanol is omitted (unpublished observation), it is still conceivable that the level of hair damage may interact with external influences, resulting in an increased washout of cortisol in more distal hair segments. The present result of an influence of the number of hair washes on cortisol content in distal but not in scalp-near hair segments is in line with this reasoning. Given that a decline in hair cortisol concentrations in more distal hair segments is not always observed, it remains to be tested whether the present finding of an influence of hair washing on cortisol concentrations in more distal hair segments also extends to results obtained using different laboratory methods.
The present research has limitations that need to be acknowledged. While we have tried to comprehensively assess the variables under investigation in the present study (natural hair colour, hair washes, age, sex, OC use and smoking), we were unable to thoroughly capture other variables assumed to also influence cortisol release, such as perceived stress (Kalra et al. Citation2007), abdominal obesity (Manenschijn et al. Citation2011) or mood status (Dettenborn et al., Citation2011), among others. Hence, the present findings should be confirmed by future research also controlling for these variables. Similarly, as the recruitment for this study took place among students, friends and family of the wider research team, it is conceivable that the present results may specifically apply to a relatively healthy and well-educated population of individuals. While it is considered unlikely that this may have a strong influence on most of the examined characteristics (e.g. associations with sex or natural hair colour), future research may benefit from corroborating the present result in a more representative study sample.
In sum, the present research presents the first large-scale study to systematically investigate possible confounders of hair cortisol measurements. Based on its results, participant age and sex, as well as frequency of hair washing (for more distal hair segments), have been identified as important variables to be considered as potential confounding influences in future research. Other factors under study here, including natural hair colour, OC use, smoking status and number of hair washes (in scalp-near hair segments), do not seem to have important effects on hair cortisol levels. Altogether, this relative robustness to a range of potential confounding influences and the ease and non-invasiveness of hair sampling highlight the particular usefulness of this method.
Acknowledgements
We thank the participants for their valuable time and effort to the study. Funding for this study was provided by the German Research Foundation (DFG, DE 1162/3-1). The DFG had no further role in study design, collection, analysis, interpretation of data, writing of this report and in the decision to submit this paper for publication.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Gamberoni M, Tamanini C, Seren E. 2008. Cortisol determination in hair and faeces from domestic cats and dogs. Gen Comp Endocrinol. 155:398–402.
- Adam EK. 2006. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 31:664–679.
- Baron JA, Comi RJ, Cryns V, Brinck-Johnsen T, Mercer NG. 1995. The effect of cigarette smoking on adrenal cortical hormones. J Pharmacol Exp Ther. 272:151–155.
- Bennett A, Hayssen V. 2010. Measuring cortisol in hair and saliva from dogs: Coat color and pigment differences. Domest Anim Endocrinol. 39:171–180.
- Borges CR, Wilkins DG, Rollins DE. 2001. Amphetamine and N-acetylamphetamine incorporation into hair: An investigation of the potential role of drug basicity in hair color bias. J Anal Toxicol. 25:221–227.
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. 2009. Adolescents' cortisol responses to awakening and social stress: Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 34:884–893.
- Bulbrook RD, Herian M, Tong D, Hayward JL, Swain MC, Wang DY. 1973. Effect of steroidal contraceptives on levels of plasma androgen sulphates and cortisol. Lancet. 1:628–631.
- Clements AD, Parker CRJr., Dixon WEJr., Salley B. 2007. Marshmallows used as saliva stimulant do not affect cortisol concentrations: Finally a palatable alternative for toddler saliva collection. Dev Psychobiol. 49:702–707.
- D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. 2011. Hair cortisol levels as a retrospective marker of hypothalamic–pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiol Behav. 104:348–353.
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 147:255–261.
- del Arbol JL, Munoz JR, Ojeda L, Cascales AL, Irles JR, Miranda MT, Ruiz Requena ME, Aguirre JC. 2000. Aguirre Plasma concentrations of beta-endorphin in smokers who consume different numbers of cigarettes per day. Pharmacol Biochem Behav. 67:25–28.
- Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. Introducing a novel method to assess cumulative steroid levels: Increased hair cortisol levels over six months in medicated patients with depression. Stress. 2011 Nov 1 (Epub ahead of print).
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. 2010. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 35:1404–1409.
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. 1997. With aging in humans the activity of the hypothalamus–pituitary–adrenal system increases and its diurnal amplitude flattens. Life Sci. 61:2239–2246.
- Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, Van Uum S, Koren G, Lanctot KL. 2010. Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatr Dis Treat. 6:393–400.
- Eser HP, Potsch L, Skopp G, Moeller MR. 1997. Influence of sample preparation on analytical results: Drug analysis [GC/MS] on hair snippets versus hair powder using various extraction methods. Forensic Sci Int. 84:271–279.
- Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. 2011. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology. 36:1201–1208.
- Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte SB, Fioravanti M, Cuzzoni G, Pontiggia B, Magri F. 2001. Age-related changes of the hypothalamic–pituitary–adrenal axis: Pathophysiological correlates. Eur J Endocrinol. 144:319–329.
- Friedman AJ, Ravnikar VA, Barbieri RL. 1987. Serum steroid hormone profiles in postmenopausal smokers and nonsmokers. Fertil Steril. 47:398–401.
- Fujimoto VY, Villanueva AL, Hopper B, Moscinski M, Rebar RW. 1986. Increased adrenocortical responsiveness to exogenous ACTH in oral contraceptive users. Adv Contracept. 2:343–353.
- Gao W, Xie Q, Jin J, Qiao T, Wang H, Chen L, Deng H, Lu Z. 2010. HPLC-FLU detection of cortisol distribution in human hair. Clin Biochem. 43:677–682.
- Goldzieher JW, Fotherby K. 1994. Pharmacology of the contraceptive steroids. New York: Raven Press.
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G. 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 196:32–37.
- Groschl M, Rauh M, Dorr HG. 2003. Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clin Chem. 49:1688–1691.
- Gusenoff JA, Harman SM, Veldhuis JD, Jayme JJ, St Clair C, Munzer T, Christmas C, O'Connor KG, Stevens TE, Bellantoni MF, Pabst K, Blackman MR. 2001. Cortisol and GH secretory dynamics, and their interrelationships, in healthy aged women and men. Am J Physiol Endocrinol Metab. 280:E616–E625.
- Gygi SP, Wilkins DG, Rollins DE. 1997. A comparison of phenobarbital and codeine incorporation into pigmented and nonpigmented rat hair. J Pharm Sci. 86:209–214.
- Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA. 2011. Effects of shampoo and water washing on hair cortisol concentrations. Clin Chim Acta. 412:382–385.
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. 2007. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 30:E103–E107.
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. 1995. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatr Res. 37:502–506.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 61:154–162.
- Kirschbaum C, Scherer G, Strasburger CJ. 1994. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin Investig. 72:804–810.
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. 2009. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 34:32–37.
- Kirschbaum C, Wust S, Strasburger CJ. 1992. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 50:435–442.
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, Albertsson-Wikland K. 1997. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab. 82:536–540.
- Kudielka BM, Broderick JE, Kirschbaum C. 2003. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 65:313–319.
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. 2004. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 29:83–98.
- Larsson CA, Gullberg B, Rastam L, Lindblad U. 2009. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: A cross-sectional study. BMC Endocr Disord. 9:16.
- Laudenslager ML, Jorgensen MJ, Grzywa R, Fairbanks LA. 2011. A novelty seeking phenotype is related to chronic hypothalamic–pituitary–adrenal activity reflected by hair cortisol. Physiol Behav. 104:291–295.
- Laughlin GA, Barrett-Connor E. 2000. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: The Rancho Bernardo Study. J Clin Endocrinol Metab. 85:3561–3568.
- Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. 2011. Evaluation of a method to measure long term cortisol levels. Steroids. 76:1032–1036.
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. 2008. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic–pituitary–adrenal axis hormones and mood in men. Neuropsychopharmacology. 33:749–760.
- Nakahara Y, Takahashi K, Kikura R. 1995. Hair analysis for drugs of abuse. X. Effect of physicochemical properties of drugs on the incorporation rates into hair. Biol Pharm Bull. 18:1223–1227.
- Netherton C, Goodyer I, Tamplin A, Herbert J. 2004. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 29:125–140.
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. 2009. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 34:307–316.
- Ouellet-Morin I, Tremblay RE, Boivin M, Meaney M, Kramer M, Cote SM. 2010. Diurnal cortisol secretion at home and in child care: A prospective study of 2-year-old toddlers. J Child Psychol Psychiatry. 51:295–303.
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. 2011. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress. 14:73–81.
- Pragst F, Balikova MA. 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 370:17–49.
- Raul JS, Cirimele V, Ludes B, Kintz P. 2004. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 37:1105–1111.
- Reinberg AE, Touitou Y, Soudant E, Bernard D, Bazin R, Mechkouri M. 1996. Oral contraceptives alter circadian rhythm parameters of cortisol, melatonin, blood pressure, heart rate, skin blood flow, transepidermal water loss, and skin amino acids of healthy young women. Chronobiol Int. 13:199–211.
- Rohleder N, Kirschbaum C. 2006. The hypothalamic–pituitary–adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 59:236–243.
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. 2005. Determinants of salivary cortisol levels in 10-12 year old children: A population-based study of individual differences. Psychoneuroendocrinology. 30:483–495.
- Rothe M, Pragst F, Thor S, Hunger J. 1997. Effect of pigmentation on the drug deposition in hair of grey-haired subjects. Forensic Sci Int. 84:53–60.
- Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 30:E183–E191.
- Seeman TE, Singer B, Wilkinson CW, McEwen B. 2001. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 26:225–240.
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. 2010. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol. 85:357–360.
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology. 2011 2001 Sep 12 (Epub ahead of print).
- Steptoe A, Ussher M. 2006. Smoking, cortisol and nicotine. Int J Psychophysiol. 59:228–235.
- Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology. 2011a; 36:1193–1200.
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011b; 186:310–314.
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SH. 2010. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocrinol Diab. 118:133–138 (official journal, German Society of Endocrinology [and] German Diabetes Association).
- Tornhage CJ. 2002. Reference values for morning salivary cortisol concentrations in healthy school-aged children. J Pediatr Endocrinol Metab. 15:197–204.
- Tornhage CJ, Alfven G. 2006. Diurnal salivary cortisol concentration in school-aged children: Increased morning cortisol concentration and total cortisol concentration negatively correlated to body mass index in children with recurrent abdominal pain of psychosomatic origin. J Pediatr Endocrinol Metab. 19:843–854.
- Touitou Y, Haus E. 2000. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol Int. 17:369–390.
- Van Cauter E, Leproult R, Kupfer DJ. 1996. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 81:2468–2473.
- Van Neste D. 2004. Thickness, medullation and growth rate of female scalp hair are subject to significant variation according to pigmentation and scalp location during ageing. Eur J Dermatol. 14:28–32.
- Vermeer HJ, van Ijzendoorn MH. 2006. Children's elevated cortisol levels at daycare: A review and meta-analysis. Early Childhood Res Quart. 21:390–401.
- Wennig R. 2000. Potential problems with the interpretation of hair analysis results. Forensic Sci Int. 107:5–12.
- Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. 1982. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl). 78:305–308.
- Xue Y, Morris M, Ni L, Guthrie SK, Zubieta JK, Gonzalez K, McConnell DS, Domino EF. 2010. Venous plasma nicotine correlates of hormonal effects of tobacco smoking. Pharmacol Biochem Behav. 95:209–215.
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. 2007. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 92:42–49.
- Yeh J, Barbieri RL. 1989. Twenty-four-hour urinary-free cortisol in premenopausal cigarette smokers and nonsmokers. Fertil Steril. 52:1067–1069.
- Zhao ZY, Lu FH, Xie Y, Fu YR, Bogdan A, Touitou Y. 2003. Cortisol secretion in the elderly. Influence of age, sex and cardiovascular disease in a Chinese population. Steroids. 68:551–555.