Abstract
Acute psychological stress has primarily been investigated regarding its effects on conventional lymphocytes such as natural killer (NK) cells and CD4+ and CD8+ T cells. However, it might be important to focus on more “specialized” lymphocyte subsets, playing a role, for instance, in allergic conditions and autoimmunity, to identify links between stress, the immune system and somatic diseases. Using flow cytometry we determined frequencies of circulating T helper (Th)1-type (CD226+) and Th2-type (CRTH2+) T cells, γδ T cells, conventional CD56+ natural killer T (NKT) cells and invariant NKT cells (iNKT) in healthy young males (N = 31; median age 26 years) undergoing a laboratory computer-based stressor lasting 12 min. We found that acute psychological stress induced a prolonged increase in CD4+ and CD8+ T cells expressing a Th2 phenotype. We also detected an acute increase in CD4− and CD8− double negative γδ T cells. Finally, we found that the well-known increase in NK cells under stressful conditions was paralleled by a significant increase in numbers of conventional CD56+ NKT cells. In contrast, numbers of iNKT was not altered by stress. This study adds further evidence to a psychoneuroimmunological model proposing that under stressful conditions certain lymphocyte subsets, including iNKT and less mature T cells, are retained in lymphoid tissues while antigen-experienced effector-type T cells with a Th2 phenotype, γδ T cells and conventional CD56+ NKT cells are mobilized into the peripheral blood. We suggest that in the case of frequent stress exposure, this might result in the promotion of, for example, allergic conditions.
Introduction
That acute psychological stress exerts a number of effects on the human cellular immune system has firmly been established. In particular, acute stress leads to a marked increase in natural killer (NK) cells, as components of the innate immune system, in the peripheral blood (Haczku & Panettieri, Citation2010; Segerstrom & Miller, Citation2004). Furthermore, numbers of circulating CD8+ T cells, but not their CD4+ counterparts, increase in healthy subjects who undergo a brief laboratory stressor (Haczku & Panettieri, Citation2010; Segerstrom & Miller, Citation2004). We have previously shown that this redistribution of T cells, which belong to the adaptive immune system, is based on an increase in T cells with a memory effector phenotype while naïve and less differentiated T cells as well as immunosuppressive CD4+FOXP3+ regulatory T cells (Tregs) decrease (Atanackovic et al., Citation2006; Freier et al., Citation2010).
The development and/or progression of immune-mediated conditions including allergies or autoimmune diseases such as asthma, rheumatoid arthritis or multiple sclerosis is suspected to be exacerbated or even induced by psychological stress (Anane et al., Citation2009; Bonneville et al., Citation2010; Buske-Kirschbaum et al., Citation2001; Haczku & Panettieri, Citation2010; Hassett & Clauw, Citation2010; Iwamura & Nakayama, Citation2010; Kilpelainen et al., Citation2002; Kodama et al., Citation1999). Based on our experimental data outlined above, we have hypothesized that the stress-induced shift into the direction of the effector arm of the adaptive immune system might eventually result in the promotion of inflammatory processes in patients with autoimmune and/or allergic diseases if they are repeatedly exposed toward stressful conditions. However, before such a link can be established, further basic studies need to be undertaken in order to delineate the exact effect of acute psychological stress on other components of T cellular immunity relevant for the development and/or progression of immune-mediated diseases. In particular, more specialized T cell subsets such as T-helper (Th) 1 and Th2 cells, gamma delta (γδ) T cells and NKT cells should be investigated.
CD4+ T-helper cells have been divided into functionally distinct subsets based on the cytokines they secrete. Th1 cells produce Interleukin (IL)-2 and Interferon (IFN)-γ while Th2 cells produce IL-4, IL-5, IL-6, IL-10 and IL-13 (Mosmann et al., Citation1986). Th1 cells seem to play a role in the induction and promotion of autoimmune diseases such as type 1 diabetes mellitus and experimental autoimmune encephalomyelitis. Th2 cells, however, play a decisive role in promoting allergic conditions such as atopic dermatitis and asthma (Shyr et al., Citation2003). Despite their relevance for immune-mediated disease, little is known regarding the effect of acute psychological stress on the distribution and function of Th1 versus Th2 cells.
Conventional T cells have the typical αβ T cell receptor (TCR) and generally express lineage markers CD4 or CD8. In contrast, T cells expressing the alternate gamma γδ TCR are usually CD4/CD8-negative and are specific for non-peptide antigens which they recognize outside the human leukocyte antigen (HLA) context. γδ T cells are capable of exerting a large number of functions, providing protective immunity against pathogens, tissue healing and epithelial cell maintenance. However, under certain circumstances they also seem to promote autoimmune processes as well as allergy and asthma (Bonneville et al., Citation2010). Interestingly, it has recently been suggested that acute psychological stress might mobilize γδ T cells into the peripheral blood (Anane et al., Citation2009).
NKT cells are phenotypically and functionally diverse (Hammond et al., Citation1999). Conventional NKT cells represent αβ TCR cells which express the NK cell marker CD56. Conventional CD56+ NKT cells contain high amounts of granzymes and perforin (Bade et al., Citation2005) and, accordingly, have been shown to mediate very potent HLA-restricted (Baxevanis et al., Citation2002; Saeterdal et al., Citation1998; Vergelli et al., Citation1996) and, at least when generated in vitro by cytokine stimulation, HLA-unrestricted (Lanier et al., Citation1986; Ohkawa et al., Citation2001; Phillips & Lanier, Citation1986) cytotoxicity. Invariant natural killer T (iNKT) cells, however, are characterized by the expression of a restricted repertoire of T cell receptors (TCRs) consisting of a Vα24Jα18 chain combined with a variable TCR Vβ11 chain in humans (Linsen et al., Citation2005). The most potent ligand for the iNKT antigen receptor is the glycolipid α-galactosylceramide (α-GalCer), which is exclusively presented by the monomorphic class Ib molecule CD1d. It has repeatedly been suggested that iNKT cells might promote the development and progression of allergic asthma (Iwamura & Nakayama, Citation2010). Accordingly, animal studies have revealed that iNKT promote Th2-type cytokine production and allergen-induced airway inflammation through a CD1d-dependent mechanism (Chuang et al., Citation2011; Lisbonne et al., Citation2003). Recently, a CD1d-dependent antagonist has been shown to inhibit the activation of iNKT Cells and to prevent development of allergen-induced airway hyper-reactivity (Lombardi et al., Citation2010).
In our current study we have evaluated our hypothesis that acute psychological stress might lead to a shift of the T cell-mediated immunity toward a Th2 phenotype and a mobilization of CD56+ NKT cells, and may also be invariant NKT cells, into the peripheral blood.
Methods
Test subjects
Most subjects were medical students who were recruited through a public announcement at the Benjamin Franklin University Hospital, Berlin, Germany. A total of 31 healthy young men (median age 26 years, range: 21–41 years) were included in the study and completed the protocol. Before inclusion, participants underwent a standard physical examination. All test subjects were nonsmokers and were not on any medication. Subjects with alcohol abuse, history of chronic physical (e.g. cardiac, pulmonary, renal, gastrointestinal or endocrine illnesses) or psychiatric diseases, recent surgery, mental stress on the test day or needle phobia were excluded. Test subjects were not required to fast, however, they were asked to refrain from drinking coffee or black tea after 20:00 h the night before the test. In addition, they were told not to perform any type of strenuous physical exercise during the 24 h preceding the stress test. All participants provided written informed consent in accordance with the revised version of the Declaration of Helsinki. The study protocol had received approval by the institutional review board of the Charité Campus Benjamin Franklin at the Free and Humboldt University.
Test procedure and cardiovascular parameters
The stress test took place at the Benjamin Franklin hospital, Berlin, Germany. The experimental procedure started at about 09:00 h. After written consent was obtained, an 18G catheter was placed into a lower arm vein. The first blood sample was obtained at the end of a first “resting period” lasting for 25 min and served as the baseline value. Next, our computer-based mental stressor, which has previously been described in detail (Atanackovic et al., Citation2003) was initiated. The stressor consisted of a standardized computer-based information-processing task to be completed using a tracking ball. It creates a purely mental stress situation and requires no major physical activity. During the stress test, which usually takes approximately 12 min, an increasing number of 3 to 11 clocks is presented to the test subjects who have to decide whether one or more hands of the clocks deviate by more than 90 degrees from the direction of an arrow shown on top of the screen. Wrong answers are indicated by a loud acoustic signal. At the end of the stress phase, which has a mean duration of about 12 min, a second blood sample was collected (“stress” value). Following a second “resting” period, which lasts for another 25 min, the third blood sample was collected. Blood samples of 10 ml each, obtained within 1 min after the end of each study phase were placed in tubes, supplied pre-coated with lithium heparin to inhibit clotting. Heart rate was continuously recorded by a transducer (TG424; Schwarzer, Munich, Germany). For blood pressure measurements, the cuff of a blood pressure oscillometer (DINAMAP 1846SX; Critikon, Tampa, FL) was placed around the left arm. Cardiovascular data were recorded for 5 min during each trial phase and averages + standard error of mean (SEM) were calculated.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) of all 31 test subjects were stained using monoclonal antibodies against CD3 (Caltag, San Francisco, CA), CD4, CD8, CD226, CD56, 6B11, TCRγδ (BD Biosciences, San Jose, CA), CRTH2 (Miltenyi Biotec, Bergisch Gladbach, Germany), CCR7 (R&D Systems, Minneapolis, MN) and appropriate IgG isotype controls. A total of 1 × 106 PBMC was washed in phosphate-buffered saline (PBS; Life Technologies, Paisley, UK) and resuspended in 80 µl PBS with 10 µl of each antibody according to the manufacturer’s instructions. After incubation on ice for 30 min, the cells were washed and resuspended in 500 µl PBS. Gating was performed using a morphologic lymphocyte gate and different combinations of CD3, CD4 and CD8 staining to define the given lymphocyte subtype. Samples were analyzed using a FACSCalibur cytometer and CELLQuest software (BD Biosciences).
Statistical analysis
Based on the finding that most test results were not normally distributed, we used the non-parametric Friedman’s test for related samples in order to find out whether any significant changes occurred throughout the course of the experiment for a given parameter. Only if this was the case, Wilcoxon’s rank sum test for related samples was applied to compare baseline values to results from later timepoints. If not stated otherwise, results of Friedman’s test are given within the main text and results of the Wilcoxon test for individual timepoints are shown within figures and tables. Results were considered significant if p < 0.05. If not stated otherwise, values given represent mean and standard error of mean (SEM).
Results
Acute psychological stress induces a prolonged increase in CD3+CD4+ T cells expressing a Th2 phenotype
We have previously demonstrated that our laboratory stressor is effective in causing cardiovascular activation as a sign of psychophysiological arousal (Atanackovic et al., Citation2002, Citation2003, Citation2006; Freier et al., Citation2010). Accordingly, we also observed in our current study a highly significant increase in heart rate (Friedman test, p = 0.000) as well as systolic (Friedman test, p = 0.000) and diastolic (Friedman test, p = 0.000) blood pressure in our 31 healthy subjects undergoing the brief stressor ().
Table 1. Influence of psychological stress on cardiovascular parameters in healthy male volunteers (N = 31).
Analyzing percentages of total CD3+CD4+ T-helper cells in the peripheral blood of our test subjects, we observed significant variations over time (Friedman test, p = 0.000) with a significant (Wilcoxon test, p = 0.0005) decrease immediately after the stress procedure. Percentages of circulating CD4+ T cells were still reduced (Wilcoxon test, p = 0.003) after the final resting period (pre: 34.3% ± 1.4%; stress: 27.9% ± 1.3%; post: 31.2% ± 1.4%). In contrast, CD3+CD8+ cytotoxic T cells (Friedman test, p = 0.006) remained stable shortly after exposure to the stressor and had declined only slightly but significantly (Wilcoxon test, p = 0.001) after the resting period (pre: 21.9 ± 1.1%; stress: 21.4 ± 1.3%; post: 19.4 ± 1.3%).
Next, we further differentiated CD4+ and CD8+ T cells according to their Th1 or Th2 characteristics as identified by the expression of surface markers CD226 and CRTH2, respectively. When we looked at T cells expressing CRTH2, which has been confirmed to be exclusively expressed by Th2-type T cells (Cosmi et al., Citation2000), we found a highly significant increase (Friedman test, p = 0.000) in peripheral proportions of CD4+CRTH2+ T cells at the end of the study period when compared to baseline levels (). We detected comparably fewer CD8+ T cells in the peripheral blood of our test subjects; however, cytotoxic T cells expressing a Th2 phenotype also showed a stress-induced increase (Friedman test, p = 0.001), which was already significant shortly after the completion of the stress test and reached its maximum at the end of the final resting period ().
Figure 1. Acute psychological stress influences T cells expressing Th1 or Th2 phenotypes. The stress-induced redistribution of CRTH2+ and CD226+ T cell subsets was analyzed in healthy male subjects (N = 31) after resting phase 1 (pre), immediately after completion of the stress test (stress), and following the final resting phase (post) using four-color flow cytometry. Analysis was performed using a combination of a morphological lymphocyte gate and gates for CD4+ T cells (CD3+CD4+) and CD8+ T cells (CD3+CD8+), respectively. Asterisks indicate significant (**p < 0.01, ***p < 0.001) differences compared to baseline values as determined by Wilcoxon’s rank sum test (A). Dot plots representative for CCR7 versus CD226 staining of CD4+ and CD8+ T cells are shown for one test subject (B).
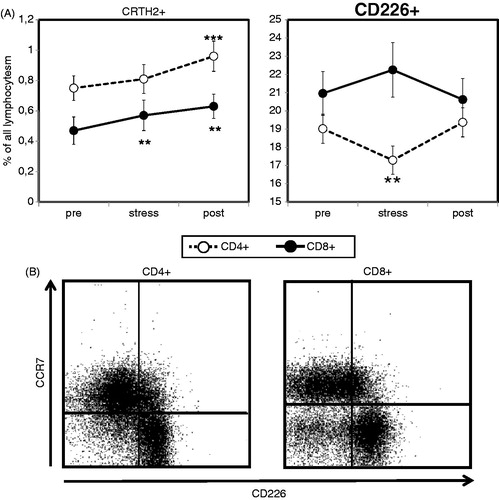
Determining the effect of acute psychological stress on numbers of circulating CD4+ and CD8+ T cells expressing surface marker CD226, which has been described as being selectively expressed on Th1-type T cells, we observed a significant stress-induced decrease in CD4+CD226+ T cells (Friedman test, p = 0.003) which was paralleled by a trend (Wilcoxon test, p = 0.08) toward an increase in CD8+CD226+ T cells (). Both, CD4+CD226+ and CD8+CD226+ T cell numbers had returned to baseline levels after the final resting period.
Based on our observation that percentages of CD4+ and CD8+ T cells expressing CD226 were much higher (∼20% of all lymphocytes) than what might be expected for fully differentiated Th1-type T cells, we suspected that the expression of CD226 was not as specific as previously described. Co-staining with an antibody directed against chemokine receptor CCR7, a surface molecule which is absent from memory/effector T cells, we indeed found that the CD4+/CD226+ T cell population was nearly congruent with the CD4+/CCR7− subgroup (). In the case of CD8+ T cells, we commonly observed a small CD226-/CCR7-subpopulation (); however, the vast majority of CCR7-negative CD8+ T cells showed an exclusive expression of CD226. These collected findings indicate that CD226 expression might indeed not be restricted to Th1-type T cells, but is detectable on all T cells with a memory/effector phenotype including Th1-type T cells.
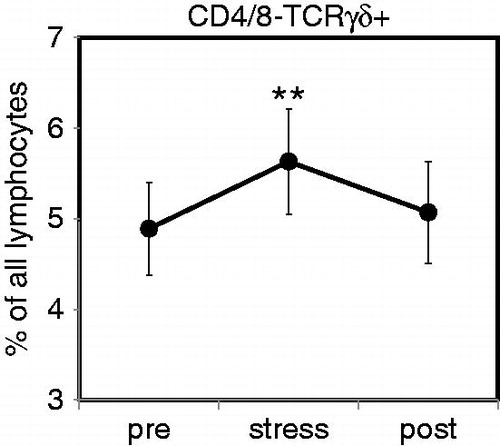
Acute psychological stress causes a short-lasting increase in lymphocytes expressing the γδ T cell receptor
Analyzing percentages of CD3+ T cells expressing the γδ T cell receptor, we found that the presence of the this type of TCR was, as expected, restricted to T cells with a CD4− and CD8-negative phenotype (data not shown). We observed a significant (Friedman test, p = 0.036) increase in the percentage of CD4−CD8−TCRγδ+ T cells after the stress procedure (). Numbers of CD4−CD8−TCRγδ+ T cells had returned to baseline level at the end of the final resting period.
Figure 2. Acute psychological stress causes a short-lasting increase in circulating γδ T cells. Percentages of circulating T cells expressing the γδ T cell receptor (TCR) were determined in healthy male subjects (N = 31) after resting phase 1 (pre), immediately after completion of the stress test (stress), and following the final resting phase (post) using four-color flow cytometry. Analysis was performed using a combination of a morphological lymphocyte gate and gates for CD4+ T cells (CD3+CD4+) and CD8+ T cells (CD3+CD8+). Percentages of CD4 and CD8 double-negative T cells expressing the γδ TCR were then measured using an appropriate isotype control. Asterisks indicate significant (**p < 0.01) differences compared to baseline values as determined by Wilcoxon’s rank sum test.
Acute psychological stress induces an increase in conventional CD3+CD56+ NKT cells but not invariant chain NKT cells
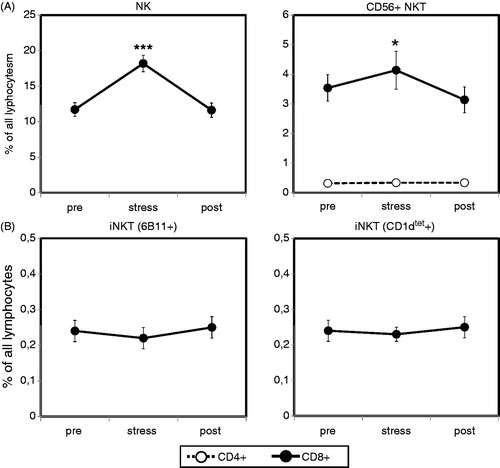
A marked increase in NK cells in the peripheral blood is a well-known effect of acute psychological stress (Haczku & Panettieri, Citation2010; Segerstrom & Miller, Citation2004). Analyzing percentages of CD3−CD56+ NK cells, as parts of the innate immune system, in the peripheral blood of our test subjects (Friedman test, p = 0.000), we indeed observed a highly significant increase followed by a return to baseline levels at the end of the final resting period (). This further demonstrated that our stressor indeed caused sufficient psychophysiological arousal to influence the distribution of lymphocyte subsets in the periphery.
When we analyzed the numbers of circulating CD3+CD56+ conventional NKT cells (Friedman test, p = 0.001), as parts of the adaptive immune system, we observed stress-induced increase, similar to that for NK cells, although to a lesser degree (). As in the case of NK cells, percentages of CD3+CD56+ conventional NKT cells returned to baseline levels at the end of the resting period.
Finally, we asked for the first time whether invariant NKT cells would show stress-induced changes comparable to conventional NKT and NK cells. To this end, we used two different methods to identify iNKT cells in the peripheral blood of our male test subjects by flow cytometry. First, we stained the PBMC with an α-GalCer-loaded CD1d-tetramer which allows reliable identification of iNKT cells (Karadimitris et al., Citation2001). In addition, we applied in a separate analysis an anti-6B11 monoclonal antibody which has also been used to determine the iNKT cell population (Thomas et al., Citation2003). Both approaches revealed that, in contrast to NK cells and conventional NKT cells, acute psychological stress did not have an effect of the distribution of iNKT cells in the peripheral blood of healthy human subjects ().
Figure 3. Peripheral numbers of conventional CD56+ NKT cells, but not invariant chain NKT (iNKT) cells, increase after acute psychological stress. Percentages of circulating NK cells, CD56+ NKT cells (A) and iNKT cells (B) were determined in healthy male subjects (N = 31) after resting phase 1 (pre), immediately after completion of the stress test (stress) and following the final resting phase (post) using four-color flow cytometry. Analysis was performed using a combination of a morphological lymphocyte gate and gates for NK cells (CD3−CD56+), conventional NKT cells (CD3+CD8+CD56+ or CD3+CD4+CD56+) and iNKT (CD3+6B11+ or CD3+CD1d-tetramer+). Percentages of the respective lymphocyte subsets were measured using appropriate isotype controls. Asterisks indicate significant (*p < 0.05, ***p < 0.001) differences compared to baseline values as determined by Wilcoxon’s rank sum test.
Discussion
In our current study we evaluated the hypothesis that acute psychological stress might lead to a shift of the T cell-mediated immunity toward a Th2 phenotype and a mobilization of CD56+ NKT cells, and may also be invariant NKT cells, into the peripheral blood. Indeed, we found acute psychological stress to induce a prolonged increase in CD4+ and CD8+ T cells expressing a Th2 phenotype and an increase in the numbers of conventional CD56+ NKT cells but not in iNKT cells. In addition, we detected a stress-induced increase in γδ T cells.
Previously, acute psychological stress has primarily been examined regarding its effects on conventional lymphocyte subsets such as NK cells as well as CD4+ and CD8+ T cells (Segerstrom & Miller, Citation2004). We consider, however, that it will be important to focus on more “specialized” types of lymphocytes in order to reliably identify links between psychological stress, the immune system, and the development and/or exacerbation of somatic diseases.
Based on findings derived from animal models, it has been suggested that acute psychological stress might skew immunity from a Th1 into a Th2-type of response (Costa-Pinto & Palermo-Neto, Citation2010; Curtin et al., Citation2009a,Citationb). Surprisingly, few reliable data are available on the effect of acute psychological stress on the numbers of human Th1 and Th2 cells in the peripheral blood and on possible stress-induced changes in the cytokine patterns they produce (Ackerman et al., Citation1998; Hoglund et al., Citation2006; Kaufmann et al., Citation2007).
Surface molecule CD226 was initially described as being selectively expressed on human Th1-type T cells and its function was defined as promoting Th1-type immune responses and related autoimmune diseases (Dardalhon et al., Citation2005). Based on these findings, we had chosen this marker to examine possible stress-induced changes in the distribution of circulating T cells with a Th1 phenotype. We indeed observed a stress-induced decrease in CD226-positive CD4+ T cells, indicating at first sight a suppression of Th1-type immunity under stressful conditions. However, additional analyses revealed that CD226 is broadly expressed on all effector type T cells, most likely regardless of their Th1/Th2 differentiation. This finding would be in line with studies of other groups questioning the specificity of CD226 expression for a Th1 phenotype (Shibuya et al., Citation2006).
In contrast to CD226, surface molecule CRTH2 has repeatedly been proven to reliably identify CD4+ and CD8+ T cells with a Th2 phenotype (Cosmi et al., Citation2000). CRTH2, also called CD294, is a receptor for allergy-promoting prostaglandin D2 (Hirai et al., Citation2001) and was first described more than 10 years ago as a surface marker selectively expressed on human Th2 cells (Nagata et al., Citation1999). Currently, CRTH2 is even being evaluated in clinical trials as a therapeutic target for allergic diseases and asthma (Schuligoi et al., Citation2010). Our finding of a prolonged increase in CRTH2-expressing CD4+ and CD8+ T cells under stressful conditions would support the view that psychological stress induces a shift towards Th2-type immune reactions. Future studies should delineate in detail whether this stress-induced redistribution of T cell subsets has functional consequences for the human immune system and whether it is indeed related to the promotion of allergic conditions such as atopic dermatitis and asthma (Shyr et al., Citation2003).
In addition to the prolonged stress-induced increase in Th2-type T cells, we also found a short-lasting increase in T cells expressing the γδ T cell receptor under psychological stress. This observation is in agreement with findings by Anane et al. (Citation2009) who have recently shown that γδ T cells are mobilized into the peripheral blood during exercise, β-agonist infusion and acute psychological stress (Anane et al., Citation2009). Therefore, γδ T cells, like NK cells and effector-type memory T cells, evidently belong to those cell types that are redistributed into the peripheral blood in situations representing a potential threat to the human body.
We also found a stress-induced increase in CD3+CD56+ NKT cells comparable to the well-known rise in NK cells under stressful conditions. This finding is in line with an observation made by us in a previous study (Atanackovic et al., Citation2006). The stress-induced increase in CD4+ and CD8+ T cells expressing CD56 might be of clinical relevance, as it has been proposed that CD56-positive CD8+ T cells represent circulating effector lymphocytes (Pittet et al., Citation2000) contributing to the immune defense against pathogens, i.e. viral antigens (Kambayashi et al., Citation2000; Slifka et al., Citation2000). In addition, CD56 has been shown to be expressed by peripheral (Ou et al., Citation2002; Satoh et al., Citation1996) and tissue-infiltrating (Barnaba et al., Citation1994) CD4+ T helper cells with a very strong cytotoxic potential (Baxevanis et al., Citation2002; Lanier et al., Citation1986; Mendes et al., Citation2000; Ohkawa et al., Citation2001; Ortaldo et al., Citation1991; Saeterdal et al., Citation1998; Vergelli et al., Citation1996). Interestingly, it has recently been shown that numbers of conventional NKT cells, and particularly CD8+CD56+ NKT cells, are markedly increased in the sputum of patients with severe asthma (Hamzaoui et al., Citation2006; Koh & Shim, Citation2010). Hence, we consider it is possible that repeated mobilization of conventional NKT cells under stressful conditions might mediate the exacerbating effects of psychological stress on allergic conditions such as asthma or atopic dermatitis.
In contrast to our findings regarding conventional CD56+ NKT cells, we found the levels of invariant NKT cells to remain uninfluenced by acute psychological stress. Interestingly, it has been suggested that conventional NKT and iNKT cells reside in differential compartments of the body. At least in mice, thymus and liver primarily contain iNKT cells while spleen and bone marrow are enriched for CD1d-independent conventional NKT cells (Eberl et al., Citation1999). The redistribution of circulating lymphocytes under acute psychological stress is mainly mediated by catecholamines, which trigger β2-adrenergic receptors on the cell surface (Benschop et al., Citation1996; Kohm & Sanders, Citation2001; Schedlowski et al., Citation1996). We consider that when influenced by acute stress, CD56+ NKT cells are mobilized from the bone marrow and spleen into the peripheral blood, while iNKT cells remain in the liver. Studies are underway in our laboratory investigating the molecular mechanisms mediating the differing effects of psychological stress on the two NKT cell populations. However, we consider it most likely that the mobilization of CD56+ NKT cells is catecholamine-dependent as is the case for the stress-induced increase in the numbers of NK cells and γδ T cells (Anane et al., Citation2009; Benschop et al., Citation1996; Kohm & Sanders, Citation2001; Schedlowski et al., Citation1996).
Limitations of our current study include the lack of measurements of “stress hormones”, such as cortisol and catecholamines, and the lack of an unstressed control group. However, our collected findings presented here do add further support to the hypothesis that in humans certain lymphocyte subsets, such as iNKT cells and less mature T cells are kept in lymphoid tissues during acute stress. At the same time, antigen-experienced effector-type T cells with a Th2 phenotype, γδ T cells, and conventional CD56+ NKT cells are mobilized into the peripheral blood, probably providing a pool of immune cells which can more rapidly reach target tissues in case of injury or local inflammation. However, CD4+FOXP3+ regulatory T cells are retracted from the peripheral blood (Freier et al., Citation2010), thus allowing more pronounced effector T cell responses in “fight or flight” situations. This well-organized T cell redistribution might benefit immune defense against microbial infections. However, the disturbed balance of effector versus immunosuppressive cells might also promote allergic and autoimmune diseases if stressors occur intensely and frequently. Future studies should delineate in detail the molecular mechanisms behind this psychoneuroimmunological model and should examine whether the phenomena we observed in our subjects’ blood are also of relevance for peripheral tissues (e.g. the gastrointestinal tract and the lungs) where the respective T cell subtypes typically exert their function.
Declaration of interest
The authors do not have any conflicts of interests to declare.
References
- Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS. (1998). Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom Med 60:484–91
- Anane LH, Edwards KM, Burns VE, Drayson MT, Riddell NE, van Zanten JJ, Wallace GR, et al. (2009). Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and beta-agonist infusion. Brain Behav Immun 23:823–9
- Atanackovic D, Brunner-Weinzierl MC, Kroger H, Serke S, Deter HC. (2002). Acute psychological stress simultaneously alters hormone levels, recruitment of lymphocyte subsets, and production of reactive oxygen species. Immunol Invest 31:73–91
- Atanackovic D, Schnee B, Schuch G, Faltz C, Schulze J, Weber CS, Schafhausen P, et al. (2006). Acute psychological stress alerts the adaptive immune response: stress-induced mobilization of effector T cells. J Neuroimmunol 176:141–52
- Atanackovic D, Schulze J, Kroger H, Brunner-Weinzierl MC, Deter HC. (2003). Acute psychological stress induces a prolonged suppression of the production of reactive oxygen species by phagocytes. J Neuroimmunol 142:159–65
- Bade B, Boettcher HE, Lohrmann J, Hink-Schauer C, Bratke K, Jenne DE, Virchow JC Jr, Luttmann W. (2005). Differential expression of the granzymes A, K and M and perforin in human peripheral blood lymphocytes. Int Immunol 17:1419–28
- Barnaba V, Franco A, Paroli M, Benvenuto R, De Petrillo G, Burgio VL, Santilio I, et al. (1994). Selective expansion of cytotoxic T lymphocytes with a CD4+CD56+ surface phenotype and a T helper type 1 profile of cytokine secretion in the liver of patients chronically infected with Hepatitis B virus. J Immunol 152:3074–87
- Baxevanis CN, Gritzapis AD, Tsitsilonis OE, Katsoulas HL, Papamichail M. (2002). HER-2/neu-derived peptide epitopes are also recognized by cytotoxic CD3(+)CD56(+) (natural killer T) lymphocytes. Int J Cancer 98:864–72
- Benschop RJ, Jacobs R, Sommer B, Schurmeyer TH, Raab JR, Schmidt RE, Schedlowski M. (1996). Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. Faseb J 10:517–24
- Bonneville M, O'Brien RL, Born WK. (2010). Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 10:467–78
- Buske-Kirschbaum A, Geiben A, Hellhammer D. (2001). Psychobiological aspects of atopic dermatitis: an overview. Psychother Psychosom 70:6–16
- Chuang YH, Wang TC, Jen HY, Yu AL, Chiang BL. (2011). {alpha}-Galactosylceramide-induced airway eosinophilia is mediated through the activation of NKT cells. J Immunol 186:4687–92
- Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. (2000). CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol 30:2972–9
- Costa-Pinto FA, Palermo-Neto J. (2010). Neuroimmune interactions in stress. Neuroimmunomodulation 17:196–9
- Curtin NM, Boyle NT, Mills KH, Connor TJ. (2009a). Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun 23:535–47
- Curtin NM, Mills KH, Connor TJ. (2009b). Psychological stress increases expression of IL-10 and its homolog IL-19 via beta-adrenoceptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun 23:371–9
- Dardalhon V, Schubart AS, Reddy J, Meyers JH, Monney L, Sabatos CA, Ahuja R, et al. (2005). CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J Immunol 175:1558–65
- Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. (1999). Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol 162:6410–19
- Freier E, Weber CS, Nowottne U, Horn C, Bartels K, Meyer S, Hildebrandt Y, et al. (2010). Decrease of CD4(+)FOXP3(+) T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology 35:663–73
- Haczku A, Panettieri RA Jr. (2010). Social stress and asthma: the role of corticosteroid insensitivity. J Allergy Clin Immunol 125:550–8
- Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, Smyth MJ, et al. (1999). NKT cells are phenotypically and functionally diverse. Eur J Immunol 29:3768–81
- Hamzaoui A, Cheik Rouhou S, Graïri H, Abid H, Ammar J, Chelbi H, Hamzaoui K. (2006). NKT cells in the induced sputum of severe asthmatics. Mediators Inflamm 2006:71214
- Hassett AL, Clauw DJ. (2010). The role of stress in rheumatic diseases. Arthritis Res Ther 12:123
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, et al. (2001). Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 193:255–61
- Hoglund CO, Axen J, Kemi C, Jernelov S, Grunewald J, Muller-Suur C, Smith Y, et al. (2006). Changes in immune regulation in response to examination stress in atopic and healthy individuals. Clin Exp Allergy 36:982–92
- Iwamura C, Nakayama T. (2010). Role of NKT cells in allergic asthma. Curr Opin Immunol 22:807–13
- Kambayashi T, Assarsson E, Michaelsson J, Berglund P, Diehl AD, Chambers BJ, Ljunggren HG. (2000). Emergence of CD8+ T cells expressing NK cell receptors in influenza A virus-infected mice. J Immunol 165:4964–9
- Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, Chen JL, et al. (2001). Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci USA 98:3294–8
- Kaufmann I, Eisner C, Richter P, Huge V, Beyer A, Chouker A, Schelling G, Thiel M. (2007). Lymphocyte subsets and the role of TH1/TH2 balance in stressed chronic pain patients. Neuroimmunomodulation 14:272–80
- Kilpelainen M, Koskenvuo M, Helenius H, Terho EO. (2002). Stressful life events promote the manifestation of asthma and atopic diseases. Clin Exp Allergy 32:256–63
- Kodama A, Horikawa T, Suzuki T, Ajiki W, Takashima T, Harada S, Ichihashi M. (1999). Effect of stress on atopic dermatitis: investigation in patients after the great hanshin earthquake. J Allergy Clin Immunol 104:173–6
- Koh YI, Shim JU. (2010). Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol 153:239–48
- Kohm AP, Sanders VM. (2001). Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev 53:487–525
- Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. (1986). The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 136:4480–6
- Linsen L, Somers V, Stinissen P. (2005). Immunoregulation of autoimmunity by natural killer T cells. Hum Immunol 66:1193–202
- Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, et al. (2003). Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol 171:1637–41
- Lombardi V, Stock P, Singh AK, Kerzerho J, Yang W, Sullivan BA, Li X, et al. (2010). A CD1d-dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen-induced airway hyperreactivity. J Immunol 184:2107–15
- Mendes R, Bromelow KV, Westby M, Galea-Lauri J, Smith IE, O'Brien ME, Souberbielle BE. (2000). Flow cytometric visualisation of cytokine production by CD3−CD56+ NK cells and CD3+CD56+ NK-T cells in whole blood. Cytometry 39:72–8
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. (1986). Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136:2348–57
- Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, Abe H, et al. (1999). Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol 162:1278–86
- Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, Hiraide H, Sekine I. (2001). Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology 103:281–90
- Ortaldo JR, Winkler-Pickett RT, Yagita H, Young HA. (1991). Comparative studies of CD3− and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol 136:486–95
- Ou D, Metzger DL, Wang X, Pozzilli P, Tingle AJ. (2002). Beta-cell antigen-specific CD56(+) NKT cells from type 1 diabetic patients: autoaggressive effector T cells damage human CD56(+) beta cells by HLA-restricted and non-HLA-restricted pathways. Hum Immunol 63:256–70
- Phillips JH, Lanier LL. (1986). Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med 164:814–25
- Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. (2000). Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol 164:1148–52
- Saeterdal I, thor Straten P, Myklebust JH, Kirkin AF, Gjertsen MK, Gaudernack G. (1998). Generation and characterization of gp100 peptide-specific NK-T cell clones. Int J Cancer 75:794–803
- Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. (1996). Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol 157:3886–92
- Schedlowski M, Hosch W, Oberbeck R, Benschop RJ, Jacobs R, Raab HR, Schmidt RE. (1996). Catecholamines modulate human NK cell circulation and function via spleen-independent beta 2-adrenergic mechanisms. J Immunol 156:93–9
- Schuligoi R, Sturm E, Luschnig P, Konya V, Philipose S, Sedej M, Waldhoer M, et al. (2010). CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology 85:372–82
- Segerstrom SC, Miller GE. (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130:601–30
- Shibuya K, Shibata K, Tahara-Hanaoka S, Shibuya A. (2006). Comment on “CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions”. J Immunol 176:3855–6
- Shyr YM, Su CH, Wu CW, Lui WY. (2003). Does drainage fluid amylase reflect pancreatic leakage after pancreaticoduodenectomy? World J Surg 27:606–10
- Slifka MK, Pagarigan RR, Whitton JL. (2000). NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol 164:2009–15
- Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, Strominger JL, et al. (2003). CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol 171:2571–80
- Vergelli M, Le H, van Noort JM, Dhib-Jalbut S, McFarland H, Martin R. (1996). A novel population of CD4+CD56+ myelin-reactive T cells lyses target cells expressing CD56/neural cell adhesion molecule. J Immunol 157:679–88
