Abstract
There is growing evidence linking caregiver stress with an increased risk for morbidity and mortality. While the emotional and practical burden experienced by caregivers is well established, the physiological changes that may affect the caregiver’s health are less understood. This study sought to compare self-reported stress, anxiety and depression along with neuroendocrine and immune markers of stress among adult caregivers of allogeneic hematopoietic stem cell transplantation patients during the acute transplant recovery period to matched non-caregivers controls. Biomarkers and self-reported data were collected at three points during the patient’s HSCT: (1) before transplant, (2) after initial transplantation discharge (±7 days) and (3) 6 weeks after initial transplantation discharge. Mixed linear modeling was used to examine differences by group and time. Twenty-one caregivers and 20 controls completed all study procedures. The majority of caregivers were female (57% or 57.1%) and married (95.2%), with a mean age of 52 ± 11.4 years. Caregiver perceived stress, anxiety and depression scores were significantly higher than controls (p < 0.001) with effect sizes (ES) ranging from 1.37 to 1.80 and they did not change over time (p > 0.05) for either group. Caregivers had significantly lower serum cortisol levels than controls at both discharge (p = 0.013; ES = 0.81) and 6 weeks after discharge (p = 0.028; ES = 0.72) but exhibited no significant relationship between self-reported stress and serum cortisol. In addition, caregivers showed a significant inverse relationship between stress and epinephrine levels (rs=−0.654, p = 0.021). These findings support the evidence of the caregiving experience being stressful. The counter-intuitive relationship between cortisol and epinephrine might suggest dysregulation of the HPA axis and central nervous system but additional research on the physiological impact of caregiving is warranted.
Introduction
Providing care to a family member is a common yet challenging experience (NAC, Citation2009). An estimated 65.7 million people in the US serve as unpaid family caregivers, affecting approximately 36.5 million households. Cancer, a diagnosis that affects 16.6 million people in the US (ACS, Citation2015), is the third leading reason for needing a caregiver. Although cancer caregivers have reported positive effects from their experience (Bishop et al., Citation2011), the emotional and practical burden associated with caring for a person with cancer can be greater than those associated with the care of an elderly family member or one with Alzheimer’s disease (NAC, Citation2009).
The burden of caring for a cancer patient is complex and often greatest when the patient’s burden from the illness or treatment is high (Burton et al., Citation1997). Allogeneic hematopoietic stem cell transplantation (HSCT) is arguably the most intense of all cancer treatments, creating serious and sometimes life-threatening toxicities. Reduced intensity conditioning (RIC) regimens are often provided to patients with pre-existing conditions that preclude them from receiving a standard myeloablative HSCT. Although RIC regimens might decrease the symptom profile and impact on quality of life in patients (Andersson et al., Citation2009), patients are often discharged to the outpatient setting more quickly, shifting responsibility from healthcare providers to the family caregiver. As a result, a dedicated caregiver is required following all types of allogeneic transplantation. This intense support, often referred to as burden, includes caring for the transplant recipient’s physical and emotional wellbeing as well as increasing household duties (Beattie & Lebel, Citation2011). This burden often forces a shift in roles and responsibilities for the caregiver, frequently requiring them to alter their work schedule and relocate to a temporary residence near the transplant center. Although the burden of care begins during the inpatient phase of the HSCT, the initial discharge following transplantation represents a time when the caregiver assumes responsibility for care, around the clock, for weeks if not months after transplantation. Seldom a choice, a caregiver is a critical partner in HSCT recovery and may be a factor if a patient receives and even survives the treatment (Foster et al., Citation2004; Frey et al., Citation2002).
When the burden of an experience exceeds the available resources it is perceived as stressful and physiological changes occur (Chrousos, Citation2009; McEwen & Seeman, Citation1999). These include a cascade of physiological and hormonal reactions (Koolhaas et al., Citation2011) that in the presence of an acute stressor, can improve physical function (Chrousos & Gold, Citation1992). However, in the context of a sustained stressor, the effects may be more harmful (Dallman, Citation2010; Kassel et al., Citation2003; McEwen, Citation2000; McReynolds et al., Citation2014; Rose et al., Citation2008; Sterling, Citation2004; Van Reeth et al., Citation2000).
While evidence exists linking the chronic stress of caregiving to an increased risk for illness, (Vitaliano et al., Citation2003), little research has been published documenting the stress response from cancer caregiving. The majority of published research focuses on caregivers of individuals with dementia (Lovell & Wetherell, Citation2011). In caregivers of individuals with cancer, examination of the autonomic nervous system and immune system revealed that salivary cortisol (Lucini et al., Citation2008; Rohleder et al., Citation2009) and IL-6 (Rohleder et al., Citation2009) do not differ between caregiver and control subjects while levels of salivary alpha-amylase and hs-CRP do, suggesting increased sympathetic nervous system activity and inflammation in caregivers (Rohleder et al., Citation2009). Only one published study has examined the physiological changes that occur in caregivers of HSCT recipients, reporting that salivary cortisol and DHEA, as well as NK cells, IL-6 and hs-CRP, did not differ between HSCT caregivers and non-caregiver controls (Laudenslager et al., Citation2015).
The purpose of this study was to compare HSCT caregiver’s self-reported stress, anxiety, and depression, along with neuroendocrine and immune markers of stress, to those of non-caregiver controls. It was hypothesized that transplant caregivers would report greater stress, anxiety and depression, and display differences in their biomarkers. A second objective was to explore the relationships among the caregiver’s burden, perceived stress, anxiety and depression with their neuroendocrine and immune biomarkers. It was hypothesized that caregivers with higher levels of psychological distress and burden would have increased levels of biomarkers that suggest a risk for impaired health.
Methods
Study design and participants
This study applied a prospective repeated measure design to examine changes in perceived stress, anxiety and depression, along with neuroendocrine and immune biomarkers of stress in allogeneic HSCT caregivers at pre-transplantation (pre-HSCT), the time of initial hospital discharge (DC) and 6 weeks following hospital discharge (6-week), compared with age, gender and race/ethnicity-matched controls. This study was approved by the National Heart, Lung and Blood Institute intramural Institutional Review Board, and all participants provided written informed consent before participation. HSCT caregivers were eligible if they were an adult, English or Spanish speaking and planning to serve as an active caregiver for an individual planning to undergo their first allogeneic HSCT at the NIH Clinical Center. Caregivers were excluded from participating if they had been treated with glucocorticosteroids in the past 2 months, were pregnant or lactating, diagnosed with Cushing’s, Addison’s, or Parkinson’s disease, a history of a heart transplant, pacemaker, problems with orthostatic hypotension or diagnosed with autonomic dysfunction, unwilling to refrain from smoking for 12 h or from drinking alcohol for 24 h prior to blood sampling, serving as a paid caregiver, serving or had previously served as a stem cell transplant donor, or were taking medicines that interfered with immune system functioning. The caregivers were approached for participation before the HSCT recipients’ day of transplant.
The NIH Clinical Center Clinical Research Volunteer Program registry was used to identify age (±10 years), sex, race and ethnicity matched individuals for each caregiver. Matched controls were contacted by phone and screened for eligibility. They were excluded from participating if they were: pregnant/lactating, treated with glucocorticosteroids in the past 2 months, diagnosed with Cushing’s, Addison’s or Parkinson’s disease, had a history of a heart transplant, pacemaker, problems with orthostatic hypotension or diagnosed with autonomic dysfunction, unwilling to refrain from smoking for 12 h or from drinking alcohol for 24 h prior to blood sampling, serving as an informal unpaid or were a professional paid caregiver, receiving mental health services and/or taking psychiatric medications. The non-caregiver control subject study visits were scheduled within 1 week of the caregiver’s visit.
Procedures
After enrollment, participants were provided with instructions regarding study procedures. Each clinic visit included a history and physical, assessment of serious life stressors over the prior 3 months, intravenous line placement, fasting blood draw, vital signs, anthropometric measures, return of the saliva specimen and questionnaire completion. Participants were provided with verbal and written instructions to avoid all products that included acetaminophen for 5 days prior to the study visit, abstain from eating, drinking and smoking for 12 h and avoid alcohol consumption for 24 h prior to the study visit. Participants were instructed to not eat, drink or brush their teeth for at least 15 min prior to saliva collection. A saliva sample was collected 30 min after waking the morning of the study visit. A diary was completed by the participants to document adherence to study procedures.
Clinic appointment times were consistent for each case across study time points, typically in the early- to mid-morning. Height and weight were obtained while standing without shoes in normal clothes, using a digital height and weight scales. A peripheral intravenous catheter was placed and blood samples were collected after a 15-min rest period in a quiet, dark room.
Measures
Demographic factors were self-reported and included age, gender, marital status, race, ethnicity and education. The history and physical, including height and weight, were collected by a nurse practitioner and clinical research nurse.
Perceived stress was measured with the Perceived Stress Scale-14 (PSS-14). The PSS-14 is a 14-item questionnaire that captures how stressed and overwhelmed subjects felt in the last month. Items were designed to ascertain how unpredictable, uncontrollable and overloaded respondents find their lives, as well as examine current levels of experienced stress (Cohen et al., Citation1983). Responses are scaled from “never” (0) to “very often” (4) and total scores can range from 0 to 54, where higher scores indicate more perceived stress. The Cronbach’s alpha for the PSS-14 in this study sample ranged from 0.88 to 0.91.
Caregiver burden was assessed with the Caregiving Reaction Assessment (CRA) in caregiver participants only. The CRA is designed to assess the positive and negative effects of caregiving for persons providing care to patients with chronic illnesses. The measure consists of 24 items, with responses on a 5-point Likert Scale ranging from “strongly disagree” (1) to “strongly agree” (5). The five subscales (caregiver esteem, lack of family support, impact on finances, impact on schedule and impact on health) are scored by calculating the mean of the subscale’s items after appropriate reversals. Higher subscale scores indicate more burden, except caregiver esteem, where a higher score indicates less burden (Given et al., Citation1992). A total score, representing overall caregiver burden, is obtained by calculating the mean of all 24 items, after appropriate reversals; a higher total score denotes greater burden for the caregiver (Grov et al., Citation2006). The Cronbach’s alpha for the CRA total score in this study sample ranged from 0.79 to 0.90. The alpha coefficients by subscale ranged from 0.60 to 0.73, 0.81 to 0.93, 0.63 to 0.77, 0.65 to 0.83 and 0.65 to 0.77 (esteem, support, finance, schedule and health, respectively).
Anxiety and Depression were assessed with the Patient-Reported Outcomes Measurement Information System (PROMIS®) Anxiety Short Form-1 and Depression Short Form-8b. The PROMIS item databanks have well established validity and reliability across multiple studies (PROMIS, Citation2011). A total raw score for each measure (anxiety and depression) is calculated summing each item. The total raw score is converted into a T-score with a mean of 50 and standard deviation of 10; a higher score indicating more symptoms of anxiety or depression.
Assays were selected to assess HPA, autonomic nervous system (ANS) and immune system activation including salivary and serum cortisol, epinephrine, norepinephrine, catecholamine turnover, IL-6 and TNF-α. As described previously, plasma catecholamines were measured using standard high-performance liquid chromatography (HPLC) assays (Eisenhofer et al., Citation1986; Lenders et al., Citation1993). Catecholamine concentrations were determined through reverse phase liquid chromatography with electrochemical detection on alumina (Eisenhofer et al., Citation1986). However, concentrations of catecholamines in the blood do not fully capture the dynamic equilibrium in which these molecules live (Eisenhofer et al., Citation2004). Thus, catecholamine turnover rates were calculated as combined levels of dihydroxyphenylalanine (DOPA), dopamine (DA), dihydroxyphenylacetic acid (DOPAC), norepinephrine (NE) and dihydroxyphenylglycol (DHPG) (Pacak et al., Citation1993). As such, these turnover rates reflect the continual process of catecholamine synthesis and metabolism, which involve the dynamic mechanisms of leakage of catecholamines from storage vesicles, sequestration of the catecholamines back into the vesicles, exocytotic release from the vesicles and reuptake (Eisenhofer et al., Citation1986). Deviation of turnover rate from those of steady-state conditions can be indicative of a stress response (Shah & Donald, Citation1984).
The salivary cortisol awaking rise was obtained with a single specimen (30 min after awakening) to decrease burden for the caregiver participants. Salivary cortisol samples were collected using the Salivette container (Sarstedt Inc., Newton, NC) which required subjects to roll the cotton swabs in their mouth for approximately 2 min and then return them to the Salivette container. Upon receipt of the samples, the Salivette tubes were centrifuged for 10 min at 4000 rpm and then immediately stored in a −20 °C freezer. Samples were thawed and analyzed at study completion using tandem mass spectrometry (LC-MS/MS) using an IMMULITE® 1000 autoanalyzer (Siemens, Germany). The limit of detection for this assay is 60 ng/dL and the within-run precision is 4.7–12.2%, while the run-to-run precision is 11–16%.
Free serum cortisol values were obtained from subjects during their clinic visits. Recognizing a single morning salivary measure of cortisol might be limiting, serum cortisol was also obtained. Blood was centrifuged for 10 min at 2500 rpm, after which the serum layer was transferred out into separate vials and stored in a −20 °C freezer. Serum samples were thawed and evaluated for free cortisol using a Chemiluminescence Immunoassay, run on an IMMULITE® 2000 XPI (Siemens, Germany). The limit of detection for this assay is 1 mcg/dL but quality checks were performed using mass spectrometry for all values less than 5 mcg/dL (n = 5) to ensure accuracy. The within-run precision is 4.6–8.4% and the run-to-run precision is 6.4–13.5%. There were no significant differences between the methods. Levels of inflammatory cytokines TNF-α and IL-6 were analyzed with Quantikine® HS enzyme-linked immunosorbent assay (ELISA) kits SS600B and SSTA00D (R&D Systems, Inc., Minneapolis, MN) using a VICTOR3® 1420 Multilabel Readder (PerkinElmer, Inc., Waltham, MA).
Catecholamine, cytokine and both salivary and serum cortisol bio-specimens were each batch-analyzed so that samples from caregivers and their matched controls were processed together. In addition, samples from all time points were batched for each subject to maintain analysis consistency for longitudinal evaluations.
Analysis
Initial data analysis consisted of examining the frequency distributions for all variables at each time point and computing descriptive statistics appropriate for the level of measurement (e.g. mean and standard deviation for interval level data, median for ordinal level data). Identified outliers were either removed or winsorized. Variables were natural log or square root transformed in the final model if necessary to meet normality assumption for the analyses. Relationships among perceived stress, anxiety, depression, caregiver burden (caregivers only) and neuroendocrine and immune biomarkers were examined using Spearman rho due to non-normality of most variables.
Mixed model repeated measure analyses were used to determine whether there was a change in the perceived stress, anxiety, depression, neuroendocrine and immune measures of stress (salivary and serum cortisol, norepinephrine, epinephrine, CAT-turnover, IL-6 and TNF-α) over three study time points between groups. The model included fixed effects of visit (time), group and visit by group interaction. Visit was treated as a categorical variable. Since caregiver and non-caregiver subjects were matched for age and gender, these variables were not included in the model. Any other demographic variables which are significantly different between groups and are significant predictors to any of the outcomes will be entered in the model as the covariates. Restricted maximum likelihood (REML) procedure was used for model parameter estimation. Aikake information criterion (AIC) and Bayesian information criterion were used to compare and select final models. The model selection process was to compare different covariance structures. All fixed effect terms would be in the final models.
Standardized Cohen’s d effect size (ES) was calculated using model estimated means and standard errors to characterize the magnitude of changes between groups and changes over time. All data analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). A p < 0.05 was considered significant.
Results
Participants
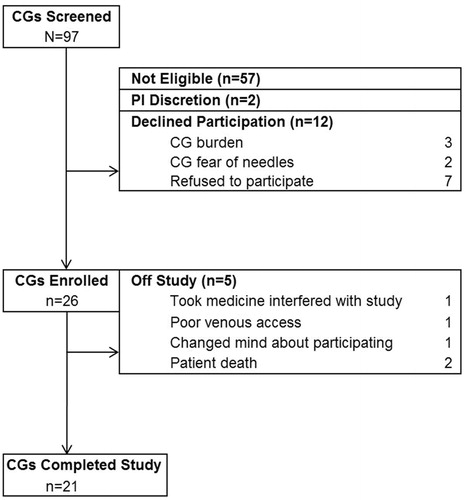
Participants were accrued from a single center between November 2011 and April 2013. Ninety-seven caregivers were screened for participation. Fifty-seven (59%) were not eligible, 12 (12%) declined participation and two (2%) were not enrolled at the discretion of the Principal Investigator (e.g. compliance barriers). Twenty-six (27%) caregiver subjects were enrolled along with 24 matched non-caregiver controls ().
Forty-one participants (21 caregivers and 20 matching normal volunteers) completed all study procedures. A total of nine subjects were removed from study (n = 5 caregiver subjects and n = 4 matching normal volunteers; ). Two caregivers were removed when the care-recipient died since their responses might reflect the grief process as opposed to caregiving. When a caregiver was removed from study, the matched control subject was also removed except in the case of one caregiver who was permitted to stay on study despite the matched non-caregiver control coming off study due to sudden family crisis. This caregiver matched a control subject already enrolled who had completed all study procedures and was therefore included in the analyses. The average time from pre-HSCT time point to the 6-week post-HSCT time point was 83.8 days (SD = 3.1; range = 54–170 days).
The majority of caregivers were female (57%), and married (95.2%), with a mean age of 52 ± 11.4 years (). The caregiver sample was primarily white, non-Hispanic (57%) with eight (38%) self-reporting as Hispanic. Over half of the caregivers were spouses to the related HSCT patient (57.1%), three of whom had two active caregivers (2CG/patient) who participated in the study (n = 6).
Table 1. Demographic characteristics (N = 41).
The transplant recipients’ characteristics are displayed in . The majority of transplant recipients received a reduced intensity conditioning regimen (n = 12; 57.1%), with peripheral blood stem cells (n = 20; 95.2%). Stem cells were often from an unrelated donor (n = 17; 81.0%) for an underlying life-threatening condition such as leukemia (n = 7; 33.3%), lymphoma (n = 7; 33.3%) or other non-malignant diseases (n = 7; 33.3%).
Table 2. Transplant information of HSCT patient.
Psychological and biomarker outcomes
presents the descriptive values for burden, stress, anxiety, and depression in caregiver and non-caregiver participants at all study time-points. Caregiver Reaction Assessment total scores [mean (SD)] ranged from 2.40 (±0.48) to 2.43 (±0.64) and remained stable across all time points. Caregiver PSS scores were significantly higher than non-caregiver controls (p < 0.001), with a large ES at each time point > 1.0). The trajectory from pre-HSCT to 6 weeks post-discharge did not change (p > 0.05) for either group ().
Table 3. Observed psychological and burden outcomes values for caregiver and non-caregiver participants.
Table 4. Test results and estimated mean differences from mixed model repeated measures analysis for study outcomes.
The trajectory for the anxiety and depression scores from pre-HSCT to 6 weeks post-discharge also did not change (p > 0.05) for either group. Caregiver anxiety scores (standardized) were significantly higher than non-caregiver controls (p < 0.001). Caregiver depression scores (standardized) were significantly higher than non-caregiver controls (p < 0.001). Similar to perceived stress, the magnitude of difference (ES) between groups for anxiety and depression was large at all study time points ().
presents the descriptive statistics for the observed biomarkers in caregiver and non-caregiver participants at all study time-points. The mean (SD) time of collection for the morning salivary cortisol samples was 31.9 (±10.1), 28.17 (±7.08) and 30.32 (±7.45) min after awakening (pre-HSCT, DC, 6-week, respectively). The caregiver morning salivary cortisol levels were not significantly different from the non-caregiver controls and did not change significantly over time in either group ().
Table 5. Observed biomarker values for caregiver and non-caregiver participants.
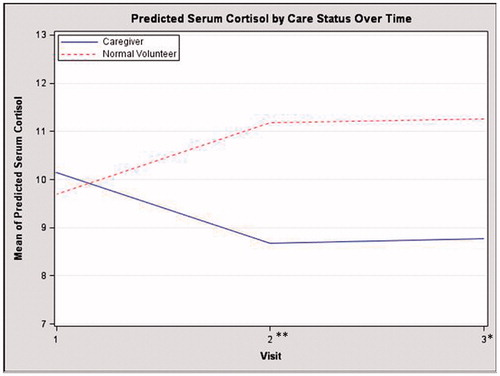
The mean time of serum cortisol collection ranged from 8:45 a.m. (±1:29 h) to 8:57 a.m. (±1:13 h) across the three study time points. The serum cortisol model revealed a significant group by time interaction effect (p = 0.003; ). The caregiver serum cortisol levels decreased over time while the levels for non-caregiver control subjects increased (). Caregivers had significantly lower predicted mean (SE) scores than non-caregiver controls at discharge (t39 = −2.60, p = 0.013; ES = 0.81] and 6 weeks after discharge (t39 = −2.29, p = 0.028; ES = 0.71] (). The serum cortisol model was computed with and without the outliers (serum drawn after 10:00 a.m.; n = 21) and the results of the model did not change.
Figure 2. Estimated means for serum cortisol from mixed model. TP, Time-point. Significant interaction (p = 0.0031); Individual time point differences **p = 0.013, *p = 0.0275.
Twenty-six (65%) cases had valid catecholamine samples at all study time points (13 caregiver/non-caregiver dyads). Catecholamine missing data were systemic and due to processing of specimens, not subject factors. The caregiver epinephrine, norepinephrine and CAT-turnover levels were not significantly different than the non-caregiver controls. Only the caregiver norepinephrine levels were significantly lower at time point 2 compared with time point 1 ().
The caregiver IL-6 model had a significant group by time interaction revealing that while the caregiver’s IL-6 levels remained stable over time, IL-6 levels for non-caregiver control subjects increased. Caregiver and control IL-6 levels did not differ significantly at any study time-point. Caregiver TNF-α levels were not significantly different from those of the non-caregiver controls. Only the caregiver TNF-α levels were significantly lower at time point 2 compared with time point 1 ().
Correlational analyses
Caregiver burden, perceived stress, anxiety and depression did not significantly change over time, therefore, baseline values were used to evaluate the relationship between these factors and their neuroendocrine and immune biomarkers (). Caregiver CRA scores were significantly (rs = 0.587, p < 0.005) related to PSS scores with higher burden scores related to higher perceived stress scores. Higher CRA burden, specifically the caregiver schedule (rs = 0.672, p = 0.0008), esteem (rs = −0.664, p = 0.001) and health (rs = 0.592, p = 0.005) correlated with higher PSS scores.
Table 6. Baseline correlations (rs) between biomarkers of stress and inflammation and perceived stress and burden in caregivers.
Caregiver subjects exhibited no significant relationship between perceived stress and serum cortisol (rs = −0.095, p = 0.681). However, there was a significant inverse relationship between perceived stress scores and epinephrine levels (rs = −0.654; p = 0.021), with higher caregiver stress scores related to lower levels of epinephrine.
Neither perceived stress (caregiver and non-caregiver controls) nor caregiver burden (caregiver only) was significantly related to IL-6 or TNF-α. However, inverse relationships were present in non-caregiver controls between epinephrine and IL6 (rs = −0.711; p = 0.0095). This relationship was not seen in the caregiver sample.
Discussion
The findings of this study support the literature suggesting that the burden and stress of caring for someone receiving intense cancer therapy, such as an allogeneic HSCT, is substantial (Beattie & Lebel, Citation2011; Girgis et al., Citation2013). This study expands the current evidence by demonstrating that despite transitions in the treatment trajectory or intensity of conditioning regimen, caregiver burden remains high and is associated with self-reported stress, anxiety and depression during the acute phase post-HSCT. Caregivers in this study reported a level of burden, specifically around schedule and finances, comparable to caregivers of patients undergoing treatment for lung cancer (Milbury et al., Citation2013) and those with advanced cancers (Utne et al., Citation2013). Perceived stress, anxiety and depression start high and appear to be unrelenting across many months. The scores for perceived stress in caregivers was remarkable and higher than those reported in the general population (Simoneau et al., Citation2013), caregivers of individuals with Alzheimer’s disease (Li et al., Citation2007) and another HSCT sample where the percentage of those receiving standard myeloablative HSCT was similar to this study (Laudenslager et al., Citation2015). While the anxiety and depression scores were higher than those of the general population, the anxiety scores specifically exceeded a common standard for minimally important difference (greater than 0.5 SD difference), suggesting clinical relevance.
The relationship between caregiver burden and emotional distress (perceived stress, anxiety and depression) supports previously published findings. The findings that higher scores for burden or negative impact on the caregiver’s life, were positively related to anxiety and depression supports previous research (Petruzzi et al., Citation2013), with caregivers reporting scores not only significantly greater than the matched non-caregiver controls, but also well above the scores reported in the general population and are considered clinically meaningful (>2 or 2.5 points; respectively) (Kroenke et al., Citation2014). The positive relationship between burden and stress seen in this sample of HSCT caregivers supports previous work in cancer caregivers (Cohen, Citation1988; Li et al., Citation2007; Simoneau et al., Citation2013).
Relative to physiological biomarkers, only the serum cortisol and epinephrine analyses were statistically remarkable. The serum cortisol finding reveals a different trajectory in caregivers compared with non-caregiver controls. Despite substantial self-reported burden, and perceived stress that far exceeded the controls, the caregiver levels of cortisol were decreasing over time while the levels in the control group were increasing; a descriptive increase in the non-caregivers which may be due to the experience of coming to the hospital to participate in the study noting that the change from baseline to the end of the study was not significant. In healthy systems, perceived stress and cortisol would be positively correlated, with higher stress associated with high levels of cortisol. This finding might suggest dysregulation of the HPA axis in the caregivers.
In other populations characterized by substantial stress, anxiety or depression, a blunted HPA axis response or hypocortisolemia has been reported. In women with chronic stress and premenopausal dysphoric disorder, higher stress was related to lower levels of cortisol (Girdler et al., Citation1998; Klatzkin et al., Citation2010) in individuals with depression, decreased levels of cortisol were also reported (Ahrens et al., Citation2008; Burke et al., Citation2005; Wu et al., Citation2014). Similarly, individuals who have reported “burn-out” in their work environments also had lower levels of cortisol (Juster et al., Citation2010; Pruessner et al., Citation1999). While some studies have reported dysregulation of the HPA axis in individuals with PTSD, the reports are not consistent (Meewisse et al., Citation2007). The decreasing levels of serum cortisol seen over time in this sample of caregivers, coupled with their substantial unrelenting stress, potentially could be a real-time sign that the HPA systems of these caregivers are beginning to show the wear and tear of chronic stress. However, further research is needed to validate these findings.
The second interesting finding which may supports the dysregulation of the stress system, is the relationship between perceived stress and epinephrine, an area not previously studied in cancer caregivers. Unlike the control subjects, caregiver’s perceived stress was significantly related to epinephrine levels; however, like the relationship between perceived stress and serum cortisol, epinephrine levels decreased with higher stress in the caregivers. This finding, however, should be interpreted with caution. The sample size for this analysis was small and additionally limited by processing errors and therefore, should be validated in future research.
An alternative hypothesis for the lower levels of cortisol and epinephrine in caregivers reporting higher stress is that caregivers of patients who receive a transplant will display signs of HPA adaptation or habituation to this chronic stressor. Although the evidence is still unclear how to differentiate the two responses to a chronic, primarily psychological, stressor (Rabasa et al., Citation2015), there is evidence that changes can occur suggesting a tolerance or de-sensitization to a repeated stressor (Grissom & Bhatnagar, Citation2009; Pervanidou et al., Citation2007; Rosmond et al., Citation1998). Further research with more extensive assessments including longitudinal samples, may be needed to test this hypothesis in a clinical setting.
A number of limitations exist in this study related to the physiological biomarker assessment. Documenting abnormalities of the central nervous system can be difficult and accurately interpreting the results can be challenging. The experience of caregiving for an individual undergoing HSCT is likely very individual, and a number of factors could have interfered with the physiological outcome variables that are not included as covariates in the analyses (e.g. pre-existing health conditions and outside stressors). Unhealthy caregivers may also be more vulnerable to changes in health during the chronic stress of the experience. These concurrent issues contribute to the difficulty in interpreting these study findings. In addition, with the small sample size, the study is underpowered for the biomarker effects, which are likely to be more subtle. These factors coupled with the short time frame for data collection and the possible confounding effect of the individual caregiver’s health, e.g. diabetes are limitations to be considered in interpreting these findings. Moreover, future research should extend the follow-up through 100 days post-HSCT, a watershed point in transplant recovery. However, having an age, gender and ethnicity matched sample strengthens the design and allows for a unique perspective not seen in previous studies.
Conclusion
The caregivers of HSCT patients consistently describe a level of burden and emotional distress that serve as a “call to action” for health care providers. Although interventions to reduce caregiver burden, improve caregivers’ ability to cope, increase their self-efficacy and enhance their quality of life (Hurley et al., Citation2014; Lavretsky et al., Citation2013; Northouse et al., Citation2010) exist, identifying objective ways to document their impact on the physical health of the caregiver is likely required for the systematic uptake into policy and practice. The purpose of this study was to correlate the self-reported burden and distress with objective neuroendocrine and immune measures of the stress response to operationalize the impact on the caregiver’s health. Overall, this study offers initial evidence that may help to guide future research highlighting the clinical and methodological limitations in this area. Accepting the complex nature of our neuroendocrine response is critical to the development of successful interventions for caregivers (Laudenslager, Citation2014). He states in “Anatomy of Illness”: Control from a Caregiver’s Perspective. “…one must be cautious in identifying any single outcome measure as presumed to reflect stress” (Laudenslager, Citation2014). To build on the results of this study and explore the dysregulation of the neuroendocrine stress response and impact on a caregiver’s health, a broad, biopsychosocial perspective needs to be embraced.
Acknowledgements
The authors acknowledge Thanh Huynh, Nonniekaye Shelburne, Maureen Sampson and the staff of the NIH Clinical Center Nursing Department for their contributions. In addition, we thank the patients and caregivers who participated in this study.
Declaration of interest
The authors declare no conflicts of interest. This research was supported by the Intramural Research Program of the NIH.
References
- ACS. American Cancer Society. Cancer Facts & Figures 2015 [Online]. American Cancer Society, available at http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index (accessed 21 August 2015)
- Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F. (2008). Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosom Med 70:461–7
- Andersson I, Ahlberg K, Stockelberg D, Brune M, Persson LO. (2009). Health-related quality of life in patients undergoing allogeneic stem cell transplantation after reduced intensity conditioning versus myeloablative conditioning. Cancer Nurs 32:325–34
- Beattie S, Lebel S. (2011). The experience of caregivers of hematological cancer patients undergoing a hematopoietic stem cell transplant: a comprehensive literature review. Psychooncology 20:1137–50
- Bishop MM, Curbow BA, Springer SH, Lee JA, Wingard JR. (2011). Comparison of lasting life changes after cancer and BMT: perspectives of long-term survivors and spouses. Psychooncology 20:926–34
- Burke HM, Davis MC, Otte C, Mohr DC. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology 30:846–56
- Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. (1997). Preventive health behaviors among spousal caregivers. Prev Med 26:162–9
- Chrousos GP. (2009). Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–81
- Chrousos GP, Gold PW. (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–52
- Cohen S. (1988). Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S editors. The social psychology of health. Thousand Oaks, CA: Sage Publications, Inc. p 31–67
- Cohen S, Kamarck T, Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav 24:385–96
- Dallman MF. (2010). Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21:159–65
- Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. (1986). Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 32:2030–3
- Eisenhofer G, Kopin IJ, Goldstein DS. (2004). Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–49
- Foster LW, McLellan LJ, Rybicki LA, Sassano DA, Hsu A, Bolwell BJ. (2004). Survival of patients who have undergone allogeneic bone marrow transplantation: the relative importance of in-hospital lay care-partner support. J Psychosoc Oncol 22:1–20
- Frey P, Stinson T, Siston A, Knight SJ, Ferdman E, Traynor A, O'Gara K, et al. (2002). Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplant 30:741–8
- Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. (1998). Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res 81:163–78
- Girgis A, Lambert S, Johnson C, Waller A, Currow D. (2013). Physical, psychosocial, relationship, and economic burden of caring for people with cancer: a review. J Oncol Pract 9:197–202
- Given CW, Given B, Stommel M, Collins C, King S, Franklin S. (1992). The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health 15:271–83
- Grissom N, Bhatnagar S. (2009). Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92:215–24
- Grov EK, Fossa SD, Tønnessen A, Dahl AA. (2006). The caregiver reaction assessment: psychometrics, and temporal stability in primary caregivers of Norwegian cancer patients in late palliative phase 29. Psycho-Oncol 15:517–27
- Hurley RV, Patterson TG, Cooley SJ. (2014). Meditation-based interventions for family caregivers of people with dementia: a review of the empirical literature. Aging Ment Health 18:281–8
- Juster RP, McEwen BS, Lupien SJ. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 35:2–16
- Kassel JD, Stroud LR, Paronis CA. (2003). Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull 129:270–304
- Klatzkin RR, Lindgren ME, Forneris CA, Girdler SS. (2010). Histories of major depression and premenstrual dysphoric disorder: evidence for phenotypic differences. Biol Psychol 84:235–47
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, et al. (2011). Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35:1291–301
- Kroenke K, Yu ZS, Wu JW, Kean J, Monahan PO. (2014). Operating characteristics of PROMIS four-item depression and anxiety scales in primary care patients with chronic pain. Pain Med 15:1892–901
- Laudenslager ML. (2014). “Anatomy of an Illness”: control from a caregiver's perspective. Brain Behav Immun 36:1–8
- Laudenslager ML, Simoneau TL, Kilbourn K, Natvig C, Philips S, Spradley J, Benitez P, et al. (2015). A randomized control trial of a psychosocial intervention for caregivers of allogeneic hematopoietic stem cell transplant patients: effects on distress. Bone Marrow Transplant 50:1110–18
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, et al. (2013). A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry 28:57–65
- Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. (1993). Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem 39:97–103
- Li J, Cowden LG, King JD, Briles DA, Schroeder HW, Jr Stevens AB, Perry RT, et al. (2007). Effects of chronic stress and interleukin-10 gene polymorphisms on antibody response to tetanus vaccine in family caregivers of patients with Alzheimer's disease. Psychosom Med 69:551–9
- Lovell B, Wetherell MA. (2011). The cost of caregiving: endocrine and immune implications in elderly and non elderly caregivers. Neurosci Biobehav Rev 35:1342–52
- Lucini D, Cannone V, Malacarne M, Bruno D, Beltrami S, Pizzinelli P, Piazza E, et al. (2008). Evidence of autonomic dysregulation in otherwise healthy cancer caregivers: a possible link with health hazard. Eur J Cancer 44:2437–43
- McEwen BS. (2000). Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22:108–24
- McEwen BS, Seeman T. (1999). Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann NY Acad Sci 896:30–47
- McReynolds JR, Pena DF, Blacktop JM, Mantsch JR. (2014). Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress 17:22–38
- Meewisse ML, Reitsma JB, De Vries GJ, Gersons BPR, Olff M. (2007). Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry 191:387–92
- Milbury K, Badr H, Fossella F, Pisters KM, Carmack CL. (2013). Longitudinal associations between caregiver burden and patient and spouse distress in couples coping with lung cancer. Support Care Cancer 21:2371–9
- NAC. National Alliance for Caregiving. (2009). Caregiving in the U.S. [Online]. National Alliance for Caregiving and American Association of Retired Persons, available at http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf (accessed 20 August 2010)
- Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. (2010). Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin 60:317–39
- Pacak K, Kvetnansky R, Palkovits M, Fukuhara K, Yadid G, Kopin IJ, Goldstein DS. (1993). Adrenalectomy augments in vivo release of norepinephrine in the paraventricular nucleus during immobilization stress. Endocrinology 133:1404–10
- Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Papassotiriou I, Hindmarsh P, Bakoula C, et al. (2007). The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: progressive divergence of noradrenaline and cortisol concentrations over time. Biol Psychiatry 62:1095–102
- Petruzzi A, Finocchiaro CY, Lamperti E, Salmaggi A. (2013). Living with a brain tumor : reaction profiles in patients and their caregivers. Support Care Cancer 21:1105–11
- PROMIS. (2011). Patient Reported Outcomes Measurement Information System (PROMIS) [Online]. NIH, available at http://www.nihpromis.org/ (accessed 20 May 2011)
- Pruessner JC, Hellhammer DH, Kirschbaum C. (1999). Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med 61:197–204
- Rabasa C, Gagliano H, Pastor-Ciurana J, Fuentes S, Belda X, Nadal R, Armario A. (2015). Adaptation of the hypothalamus-pituitary-adrenal axis to daily repeated stress does not follow the rules of habituation: a new perspective. Neurosci Biobehav Rev 56:35–49
- Rohleder N, Marin TJ, Ma R, Miller GE. (2009). Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol 27:2909–15
- Rose M, Bjomer JB, Becker J, Fries JF, Ware JE. (2008). Evaluation of a preliminary physical function item bank supported the expected advantages of the patient-reported outcomes measurement information system (PROMIS). J Clin Epidemiol 61:17–33
- Rosmond R, Dallman MF, Bjorntorp P. (1998). Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 83:1853–9
- Shah NS, Donald AG. (1984). Psychoneuroendocrine dysfunction. New York, NY: Springer US/Plenum Publishing Corporation
- Simoneau TL, Mikulich-Gilbertson SK, Natvig C, Kilbourn K, Spradley J, Grzywa-Cobb R, Philips S, et al. (2013). Elevated peri-transplant distress in caregivers of allogeneic blood or marrow transplant patients. Psycho-Oncol 22:2064–70
- Sterling P. (2004). Principals of allostasis: optimal design, predictive regulation, pathophysiology, and rational therapeutics. Cambridge, UK: Cambridge University Press
- Utne I, Miaskowski C, Paul SM, Rustoen T. (2013). Association between hope and burden reported by family caregivers of patients with advanced cancer. Support Care Cancer 21:2527–35
- Van Reeth O, Weibel L, Spiegel K, Leproult R, Dugovic C, Maccari S. (2000). Interactions between stress and sleep: from basic research to clinical situations. Sleep Med Rev 4:201–19
- Vitaliano PP, Zhang J, Scanlan JM. (2003). Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull 129:946–72
- Wu SM, Yang HC, Thayer JF, Andersen BL. (2014). Association of the physiological stress response with depressive symptoms in patients with breast cancer. Psychosom Med 76:252–6