Abstract
Exposure to stressors has been shown to change atrial responsiveness to catecholamines, but it is not clear yet how it affects the ventricular myocardium, which plays a major role in the catecholamine-stimulated increase in cardiac output. Adult male rats were submitted to restraint (RST) or footshock (FS) sessions for 3 days. Reactivity to agonists of the β-adrenergic pathway was analyzed in left ventricular myocytes isolated from stressed and control rats (CTR). Whereas no significant changes were detected after RST, enhancement of catecholamine-induced spontaneous activity, accompanied by decrease in inotropic maximal response, was observed in myocytes from FS rats. Changes were reversed by β1-, but not by α1- or β2-adrenoceptor (AR) blockade. Similar alterations were seen in response to forskolin. However, responsiveness to 3-isobutyl-1-methylxanthine and CaCl2 was comparable in control and FS groups. A significant negative correlation was observed between the maximally stimulated spontaneous activity rate and contraction amplitude. Results indicate that: (a) enhanced automatism during adrenergic stimulation of myocytes from FS rats is mediated by β1-ARs and seems to involve post-receptor mechanisms, probably decreased cAMP degradation; (b) the exaggerated spontaneous activity, which may contribute to generation of catecholaminergic arrhythmias, might limit the development of the inotropic response.
Introduction
The hallmarks of the physiological response known as the stress reaction are enhanced sympathetic outflow and activation of the hypothalamic-hypophysial-adrenal axis, resulting in increased catecholamine release and glucocorticoid secretion. Both types of chemical signal may affect the function of several peripheral tissues, thus evoking visceral, metabolic and behavioral changes that may enable the individual to cope with the stressor (Charmandari et al. Citation2005).
Sympathetic efferent neural activity is of paramount importance for regulation of cardiovascular function, by both the short-term effects of adrenergic mediators on vascular tonus, heart rate and myocardial inotropism, and the long-term effects of adrenoceptor (AR) stimulation on gene transcription (Müller et al. Citation2000). Norepinephrine (NE) and epinephrine, released from both sympathetic nerve terminals and adrenal medulla, play a role in the sympathetic regulation of cardiovascular function. Under challenging conditions (e.g., exercise, stress), catecholamines from the adrenal gland (mostly epinephrine) seem to be particularly important for enhancement of cardiac output via increase in stroke volume, apparently due to augmenting venous return and stimulating ventricular inotropic function, whereas heart rate is increased mainly by locally released NE (e.g., Donald and Shepherd Citation1963; Fuji and Vatner Citation1985; Bao et al. Citation2007).
Several studies have reported changes in the responsiveness of atrial tissue to catecholamines after repeated/continuous exposure to stressors such as FS, immobilization/RST, forced swimming, parasitosis and cold (Callia and De Moraes Citation1984; Bassani and De Moraes Citation1987, Citation1988; Spadari and De Moraes Citation1988; Bassani and Bassani Citation1993; Silveira et al. Citation2003; Brotto Citation2003; Santos and Spadari-Bratfisch Citation2006). Nevertheless, in spite of the primary importance of the ventricular inotropic response for the increase in cardiac output during sympathetic activation, it has not yet been investigated how stress may affect the adrenergic reactivity of ventricular myocardium. Changes in myocardial responsiveness to catecholamines might modify the effectiveness of visceral reflexes (e.g., baroceptor reflex) in which the sympathetic efferent branch modulates cardiovascular function, which may either provide adaptive support for physiological changes associated with the stress condition, or contribute to circulatory dysfunction.
In the present study, we examined the inotropic responsiveness to catecholamines in ventricular myocytes isolated from rats repeatedly exposed to two different stress-inducing procedures: RST and FS. Stress-inducing protocols were similar to those used in previous studies in which stress was shown to modify the reactivity of atrial tissue to catecholamines (Bassani and De Moraes Citation1987, Citation1988; Bassani and Bassani Citation1993; Silveira et al. Citation2003). Isolated myocytes are especially suitable for assessment of the direct effect of adrenergic agonists on the myocardium because, by contrast with what occurs with multicellular preparations, neurotransmitter release is absent and the influence of catecholamine uptake and metabolism is negligible. Our results point out stressor-dependent changes not only in the inotropic response to stimulation of the β-AR signaling pathway, but mainly in the induction of spontaneous activity, which may have important implications in the generation of ventricular arrhythmias (ter Keurs and Boyden Citation2007).
Methods
Stress induction
Adult male Wistar rats (4–5 months old) were maintained under controlled temperature (23 ± 2°C) and light–dark cycle (12:12 h; lights on at 6 AM), receiving pelleted chow and water ad libitum. Rats were randomly assigned to one of the following groups: (a) controls (75 rats): rats were not manipulated, except for the routine care procedures; (b) RST stress (31 rats): rats were physically restrained in a conical plexiglas tube (75 and 25 mm major and minor inner diameters) for 1 h (Silveira et al. Citation2003); (c) FS stress (FS; 57 rats): rats received electric shocks (1 mA, 1 s duration) applied through the cage floor at pseudo-random intervals (15 s average) for 30 min (Bassani and De Moraes Citation1987, Citation1988; Bassani and Bassani Citation1993).
RST and FS sessions started at 8:30–9:30 AM and were applied once a day for 3 days. Approximately 30 min after the last session, the rat was euthanized, and the heart was excised for myocyte isolation. Animal care and stress-inducing protocols were approved by the Committee of Ethics in Animal Research of the University of Campinas (CEEA/IB/UNICAMP. docs. No. 636-1, 799-1, 951-1, and 1184-1).
Isolated ventricular myocytes
Rats were rendered unconscious by cerebral concussion, and euthanized by rapid exsanguination, after which the heart was immediately removed. Myocytes were isolated from the left ventricle by coronary perfusion at 37°C with collagenase (Carvalho et al. Citation2006). Briefly, hearts were perfused with modified Krebs-Henseleit solution (composition in mM: 115 NaCl; 4.6 KCl; 1.2 KH2PO4; 25 NaHCO3; 2.4 MgSO4; 11 glucose; pH 7.4, gassed with 95%O2/5% CO2) for 5 min, and then with the same solution containing 0.8–1 mg/ml collagenase I (Worthington Biochem., Lakewood, NJ, USA) for approximately 15 min, and again with the enzyme-free solution for 5 min. After the left ventricle was excised, cells were dissociated and kept at 4°C in the storage solution (composition in mM: 30 KCl; 70 glutamic acid; 10 KH2PO4; 5 4-(2-hydroxyethyl)-l-piperazine-ethanesulfonic acid (HEPES); 1 MgCl2; glucose 11; taurine 20; pH 7.4).
Cells were plated on a collagen-coated perfusion chamber, and perfused at 23–24°C with modified Tyrode's solution with the following composition (mM): 140 NaCl, 6 KCl, 1.5 MgCl2, 1 CaCl2; 5 HEPES; 11 glucose; pH 7.4. Myocytes were field-stimulated at 1 Hz (biphasic voltage pulses, amplitude 1.2X the threshold, 5 ms duration).
Cell shortening was recorded with a video-edge detector (Crescent Instruments, Sandy, UT, USA; and Center for Biomedical Engineering/UNICAMP, Campinas, SP, Brazil). Twitch amplitude (ΔL), considered as the peak cell shortening evoked by an electrical stimulus, was averaged from three twitches not preceded by extrasystolic contractions (ES), and expressed as percentage of the resting cell length (RL).
Some cells developed ES during electric stimulation when exposed to high catecholamine concentrations (>0.1 μM), but not in the absence of the agonists. In all in vitro protocols, spontaneous activity (automatism) was quantitated as the rate of spontaneous contractions (SC) at rest (Boer and Bassani Citation2004; Carvalho et al. Citation2006), which significantly correlates with the rate of extrasystolic activity during electrical stimulation (Pearson correlation coefficient = 0.701; N = 261; p < 0.001). The total number of SCs was computed during two 30 s-long rest intervals separated by a 30 s electrical stimulation. In some experiments, the rate of ES developed under pacing was computed during 20–40 s periods at each agonist concentration. Irreversible inhibition of the sarcoplasmic reticulum (SR) Ca2+ ATPase by treatment with 10 μM thapsigargin for 5 min (Bassani et al. Citation1995a,Citationb) totally abolished spontaneous activity during both rest and electrical stimulation, but not ΔL increase, in the presence of catecholamines (not shown).
In vitro experiments
Concentration-effect curves were determined for isoproterenol (ISO) and NE, as well as for 3-isobutyl-1-methylxanthine (IBMX) and CaCl2. Some cells were challenged with 3 μM forskolin (FSK). For each cell, SC rate and ΔL were determined at baseline and at different agonist concentrations. Inotropic and automatic responses were recorded after ΔL stabilization following agonist addition (2–4 min for catecholamines and CaCl2; 10–15 min for IBMX and FSK). When used, competitive AR antagonists were present in the perfusate for at least 30 min before the agonist was added. At the concentrations employed in this study, none of the antagonists produced significant change in ΔL or SC rate (not shown). In some experiments with control myocytes, concentration-effect curves to ISO were determined also in the presence of 1 mM caffeine.
The pA2 value of the selective β1-AR antagonist metoprolol (MET; 30, 60, 100, and 300 nM; N = 5–9 for each MET concentration) was determined according to Arunlakshana and Schild (Citation1959), using ISO as the agonist.
Except for FSK and thapsigargin (Calbiochem, La Jolla, CA, USA), all chemicals were from Sigma Chem Co. (St Louis, MO, USA). Most stock solutions were kept at − 20°C, and work solutions were prepared daily. Dimethylsulfoxide was used as the solvent for hydrophobic drugs, and its concentration in the perfusate was ≤ 0.1%. Tyrode's solution was prepared with salts of analytical grade and type I water.
Data analysis
The concentration-effect curve parameters maximum response (Rmax) and pD2 (the negative logarithm of the agonist molar concentration that produces half-maximum response) were obtained by non-linear curve fitting, except for CaCl2, in which case fitting of a sigmoid function was not reliable. Linear regression was used for pA2 determination.
Data are expressed as mean ± SEM. N refers to the number of studied cells, isolated from 4 to 16 different rats of a given group, for each in vitro protocol. Statistical comparisons were made by one or two-way analysis of variance followed by post-hoc Bonferroni t-test, or by Student's t-test for paired or unpaired samples, taking p = 0.05 as the limit of statistical significance. pA2 values were compared by superposition of the 95% confidence intervals. Prism (4.0, GraphPad Software) was used for curve fitting and statistical analysis.
Results
Stressor-dependent changes in ventricular myocyte responsiveness to catecholamines
RL was comparable in all groups (control: 130 ± 2, N = 91; RST: 133 ± 3, N = 28; FS: 126 ± 2 μm, N = 85; p>0.14). Under electric stimulation at 1 Hz, ΔL and SC rate were similar for control, RST and FS groups (ΔL: 8.2 ± 0.7, 7.2 ± 0.6, and 8.0 ± 0.6% of RL, respectively, p>0.55; SC rate: 0.6 ± 0.1, 0.2 ± 0.1, and 0.7 ± 0.2 SC/min, respectively; p>0.10).
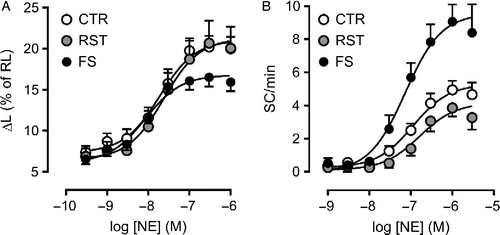
The analysis of variance revealed a significant (p ≤ 0.01) group-dependent influence on both inotropic and automatic Rmax to NE, the main sympathetic neurotransmitter. Whereas responsiveness to the agonist was comparable in RST and control groups, a significant decrease in inotropic Rmax, accompanied by marked increase in automatic Rmax, was observed in myocytes from the FS group (, ). Accordingly, 62% of the FS group myocytes developed ES (vs. 24% in the control group) when exposed to high NE concentrations. NE pD2 values for both responses were also affected by the in vivo experimental protocol (p < 0.03), as they were significantly increased in the FS group versus RST and/or control groups (). For ISO, the responsiveness pattern was similar to that observed with NE, except for the change in the pD2 value for the inotropic response. In short, these results indicate that FS, but not RST, was effective at modifying the responsiveness of ventricular myocytes to catecholamines, decreasing the apparent inotropic efficacy of the agonists, while enhancing catecholamine-evoked automatism.
Table I. Inotropic and automatic responses to isoproterenol (ISO) and norepinephrine (NE) in ventricular myocytes from control rats (CTR) and rats subjected to restraint (RST) and footshock (FS) for 3 days.
Figure 1 Concentration-effect curves for norepinephrine (NE) in ventricular myocytes isolated from control rats (CTR; N = 27), as well as rats submitted to restraint (RST; N = 15) and footshock (FS; N = 27) sessions for 3 days. Inotropic response (panel A) was taken as the increase in peak twitch cell shortening (ΔL), expressed as percent of the resting cell length (RL). Automatic response (panel B) was taken as the increase in the rate of spontaneous contractions (SC) in the absence of electric stimulation. Symbols and bars indicate mean and SEM values, respectively. Curve parameters are presented in .
The AR signaling pathway and FS-induced increase in spontaneous activity
The selective α1-AR blocker prazosin (PRZ, 0.1 μM) did not affect significantly inotropic or automatic response to NE (which acts at both α1- and β1-AR) in either control or FS group (). The lack of significant effects of PRZ on the responses of control cells is consistent with previous observation that α1-AR contribution to NE effects seems to be small in rat atrium and ventricular myocytes (Boer et al. Citation2004). On the other hand, the persistence of the FS-induced alterations in the presence of PRZ indicates that the α1-AR signaling cascade does not seem to play an important role in such alterations.
Table II. Inotropic and automatic responses in ventricular myocytes from control (CTR) and footshock-stressed rats (FS): influence of β-adrenoceptor antagonists and response to 3-isobutyl-1-methylxanthine (IBMX).
To identify the role of β-AR subtypes involved in the altered responsiveness to ISO seen in FS group myocytes, curves for the agonist were determined during blockade of β1-AR (MET, 0.1 μM) or β2-AR (butoxamine, BUT, 0.3 μM; and ICI118551, 0.1 μM). β1-AR antagonism by MET markedly decreased ISO pD2 for both responses in control cells (p < 0.001), without affecting significantly Rmax values. In FS group myocytes, however, the rightward shift in both curves was accompanied by a paradoxical enhancement of the inotropic Rmax and concomitant depression of the automatic Rmax (p < 0.04 for group × MET interaction), so that both Rmax values were comparable with those in the control group (). Blockade of β2-AR by BUT or ICI118551 did not significantly change Rmax values to ISO in FS cells. In control myocytes, however, the automatic Rmax was significantly elevated by both antagonists (p < 0.05; ; ), whereas the inotropic Rmax was ∼30% lower, although this difference did not reach statistical significance (p>0.05 for antagonist and group × antagonist interaction). A trend to lower pD2 values was observed, which was marginally significant for ICI118551 (automatic response only, 0.05>p>0.04), but not for BUT (0.08>p>0.05). Thus, the changes in ISO responsiveness evoked in control cells by β2-AR blockade resembled those of FS stress, while β1-AR antagonism abolished the alterations in ISO apparent efficacy observed in FS myocytes.
Figure 2 Concentration-effect curves for isoproterenol (ISO) in ventricular myocytes isolated from control (CTR) and footshock-stressed rats (FS), determined in the presence of 0.1 μM ICI118551 (ICI). Dashed gray and black lines indicate the curve to ISO obtained in the absence of the antagonist in CTR and FS, respectively. Twitch shortening peak (panel A) and rate of spontaneous contractions during rest (panel B) are expressed as in Figure 1. Panel C shows the rate of extrasystolic contractions (ES) recorded during pacing as a function of ISO concentration. Symbols and bars indicate mean and SEM values, respectively. Curve parameters are presented in .
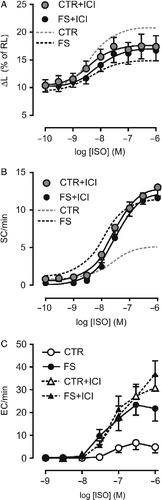
shows that ES induction by ISO in control myocytes requires high agonist concentrations ( ≥ 0.1 μM), with attainment of a maximum average rate of ∼5 ES/min. This was partially due to the low incidence of ES in these cells (36% at 0.3–1 μM ISO). In contrast, in cells from the FS group, ES could be observed at a lower agonist concentration (30 nM), and reached a rate fourfold higher at 0.3–1 μM ISO, at which ES occurred in 89% of the cells. Similarly to SC, ES rate in FS in myocytes was little affected by β2-AR blockade by ICI118551, which, however, greatly enhanced the response in control cells, reproducing the pattern observed in FS myocytes. Comparison of ES rates at 1 μM ISO by two-way analysis of variance revealed significant influence of group and ICI118551 (p < 0.04), while the post-hoc test detected significant effect of the antagonist only in the control group (p < 0.01).
Concentration-effect curves for the partial β-AR agonist CGP12177 were determined in a few cells. In both control and FS groups, inotropic Rmax was very low ( < 20% of that to NE), and CGP12177 concentrations up to 10 μM were unable to evoke significant spontaneous activity (data not shown).
Schild plot for the antagonism of ISO inotropic effect by MET in control ventricular myocytes resulted in a line with slope not significantly different from 1.0 (mean and 95% confidence interval were 0.90 ± 0.06; R2 = 0.99). MET pA2 value (mean and 95% confidence interval: 8.71 [8.34–9.08]) was not statistically different from that previously determined in rat atria (Bassani and De Moraes Citation1988; Bassani and Bassani Citation1993), in which the contribution of the β2 subtype to the response to ISO is undetectable (Juberg et al. Citation1985; Bassani and De Moraes Citation1988). The increase in contractile Rmax observed during MET exposure precluded the pA2 determination in FS cells.
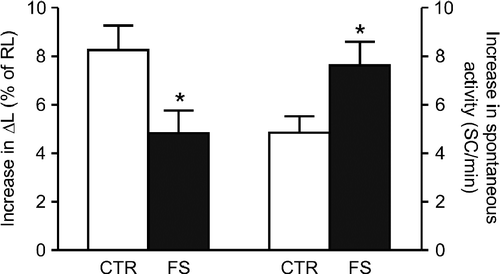
To investigate post-receptor mechanisms, we initially determined the response to the adenylyl-cyclase stimulator FSK (3 μM). The positive inotropic effect of FSK on rat ventricular myocytes is completely abolished by H-89 (Bassani et al. Citation1995b), which indicates a crucial role of cAMP-dependent protein kinase (PKA) activation in its induction. The inotropic response to FSK was comparable to the Rmax to ISO, although the increase in spontaneous activity was 25% lower than the automatic Rmax to ISO in control cells. As shown in , FS group cells developed greater automatic response and lower inotropic response to FSK in comparison with control (p < 0.03), which resembled the changes in the Rmax to catecholamines. This result could not be explained if changes in β-AR coupling to Gαs proteins and adenylyl-cyclase stimulation upon receptor occupation were relevant in the determination of the altered responsiveness to catecholamines in FS group myocytes.
Figure 3 Inotropic (left bars: increase in twitch peak shortening, ΔL, as percent of resting cell length, RL) and automatic (right bars: rate of spontaneous contractions, SC, at rest) responses to 3 μM forskolin (FSK) in ventricular myocytes isolated from control (CTR) and footshock-stressed (FS) rats. Bars are mean and SEM. Baseline ΔL was 8.0 ± 1.1 in CTR (N = 19) and 8.9 ± 0.8% of RL in FS (N = 20). Baseline SC rate was 0.3 ± 0.2 and 0.8 ± 0.3 SC/min in CTR and FS, respectively (p>0.10). *p < 0.05 vs CTR (t-test for unpaired samples).
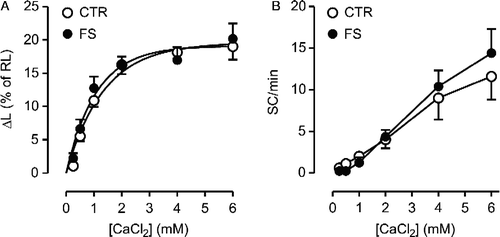
The Rmax and pD2 values for the non-selective phosphodiesterase (PDE) inhibitor IBMX in producing positive inotropic and automatic responses were similar in control and FS group cells (p>0.22; , ). Likewise, comparable responses (p>0.38) to the increase in extracellular Ca2+ concentration ([Ca2+]o (from 0.25 to 6 mM) were observed in control and FS groups ().
Figure 4 Concentration-effect curves for 3-isobutyl-1-methylxanthine (IBMX) in ventricular myocytes isolated from control (CTR; N = 9) and footshock-stressed rats (FS; N = 12). Inotropic (panel A) and automatic (panel B) responses are expressed as in Figure 1. Symbols and bars indicate mean and SEM values, respectively. Baseline ΔL and SC rate were not significantly different in CTR and FS (p>0.36). Curve parameters are presented in .
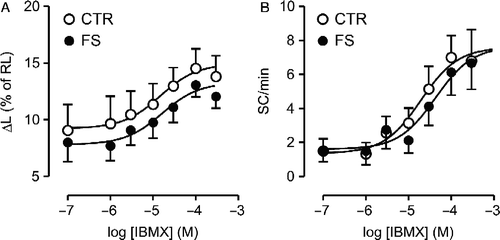
Figure 5 Inotropic (panel A) and automatic (panel B) responses to the increase in extracellular CaCl2 in isolated ventricular myocytes from control (CTR; N = 8) and footshock-stressed rats (FS; N = 8). Symbols and bars indicate mean and SEM values, respectively.
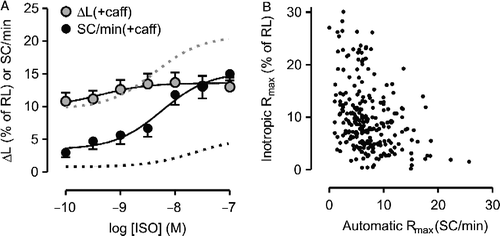
Because FS-dependent differences were not present when IBMX was the agonist, we further investigated whether the altered responsiveness to ISO observed in FS group cells could be reproduced in control myocytes exposed to 1 mM caffeine, which causes both PDE inhibition and facilitation of diastolic SR Ca2+ release (Daly Citation2000). In the absence of ISO, caffeine did not change ΔL significantly (10.8 ± 1.3 vs. 10.8 ± 1.4% of RL, p>0.98). Basal SC rate was increased, but the difference did not achieve statistical significance (3.8 ± 0.9 vs. 1.0 ± 0.7 SC/min, p>0.05). With caffeine in the perfusate, the inotropic curve was markedly flattened, as shown in (Rmax = 3.9 ± 0.8 vs. 11.2 ± 1.1% of RL in the absence of caffeine, p < 0.01), whereas the automatic Rmax was nearly threefold greater (13.0 ± 1.2 vs. 4.4 ± 0.1 SC/min in the absence of caffeine; p < 0.01). Caffeine also enhanced the sensitivity to ISO (pD2 for inotropic effect: 9.10 ± 0.15 vs. 8.37 ± 0.07 in the absence of caffeine; pD2 for automatic effect: 8.74 ± 0.33 vs. 7.64 ± 0.08 in the absence of caffeine, p < 0.001), probably due to PDE inhibition, which is expected to potentiate ISO-stimulated cAMP accumulation. Thus, regarding the Rmax values, caffeine reproduced in control cells the same qualitative changes in the responsiveness to ISO seen in FS group myocytes, i.e., an enhanced automatic response paralleled by decreased positive inotropic response. The possibility of a causal relationship between these alterations is suggested by the significant negative correlation between inotropic and automatic Rmax values (Pearson correlation coefficient = − 0.355; N = 205; p < 0.001), when considering the data of the curves in all three experimental groups ().
Figure 6 A: Concentration-effect curve for isoproterenol (ISO) determined in the presence of 1 mM caffeine (caff). Inotropic and automatic responses were determined in the same set of control ventricular myocytes (N = 8) and are expressed as in Figure 1. Data are mean and SEM. Gray and black dashed lines indicate the curves for the inotropic and automatic effects of ISO, respectively, in the absence of caffeine. B: Relationship between automatic and inotropic maximum responses (Rmax) to agonists determined in a set of 205 myocytes.
Discussion
This study reports for the first time that short, intermittent exposure to a noxious stressor FS may enhance catecholaminergic stimulation of spontaneous activity in rat ventricular myocytes. This change was significantly correlated with decreased inotropic response to stimulation of the β1-AR signaling pathway. The results indicate involvement of post-receptor mechanisms in the genesis of this alteration, which may limit inotropic responsiveness at high catecholamine levels and increase the susceptibility to catecholamine-dependent ventricular arrhythmias.
In mammalian ventricle, most of the contraction-activating Ca2+ pool is released from the SR due to activation of SR Ca2+ channels (ryanodine receptors, RyR) by Ca2+ that enters the cell during the action potential. It is generally accepted that the positive inotropic effect of β-AR stimulation relies on increased transmembrane Ca2+ influx and Ca2+ uptake by the SR Ca2+-ATPase due to PKA phosphorylation of sarcolemmal L-type Ca2+ channels and phospholamban (PLB, a negative regulator of the SR Ca2+-ATPase), respectively (ter Keurs and Boyden Citation2007). The resulting elevation in SR Ca2+ content and in L-type Ca2+ current peak contributes to increase the fraction of the SR Ca2+ content released at a twitch, thus enhancing Ca2+ transient and contraction amplitude (Bassani et al. Citation1995a; Ginsburg and Bers Citation2004). In addition, positive RyR regulation by PKA and/or Ca2+-calmodulin-dependent protein kinase II has also been reported (e.g., Zalk et al. Citation2007). On the other hand, SR Ca2+ overload and RyR hyperphosphorylation also enhance the rate of diastolic SR Ca2+ release, which may give rise to spontaneous Ca2+ transients and contractions (Bassani et al. Citation1997; Lukyanenko et al. Citation1999; ter Keurs and Boyden Citation2007; Zalk et al. Citation2007). Because SR Ca2+ loading seems to underlie both effects of catecholamines, it is plausible to consider that stimulation of inotropism and spontaneous activity might be mechanistically related. The potency of catecholamines for enhancing spontaneous activity is lower than for increasing inotropism (Boer and Bassani Citation2004; Carvalho et al. Citation2006; present results), which suggests that manifestation of automatism requires a certain degree of inotropic stimulation. Accordingly, we observed that the partial β-AR agonist CGP12177 failed at both evoking a substantial inotropic response and stimulating automatism. The crucial role of enhanced SR-cytosol Ca2+ cycling in the development of spontaneous activity was confirmed by total ES and SC suppression after the SR function was disabled by thapsigargin, whereas increase of ΔL by catecholamines, albeit smaller, was still visible.
The β1 subtype comprises 90–95% of the total β-AR population in rat ventricular myocytes (Kitagawa et al. Citation1995; Ranu et al. Citation2000). β1-ARs mediate most of the catecholamine-stimulated cAMP generation in rodent ventricle, producing global increase in cytosolic cAMP concentration, whereas the cAMP elevation evoked by β2-AR stimulation, when detectable, is much smaller and spatially confined to the subsarcolemmal region (Kitagawa et al. Citation1995; Nikolaev et al. Citation2006; Xiao et al. Citation2006). Accordingly, PLB phosphorylation by PKA at Ser-16 takes place during β1-AR but not β2-AR stimulation of mammalian ventricle (Jo et al. Citation2002; Xiao et al. Citation2006; DeSantiago et al. Citation2008). PLB phosphorylation results in enhanced SR Ca2+ uptake and consequent increase in SR Ca2+ content, which plays an important role in the inotropic response (Li et al. Citation2002) and seems to be essential for the development of spontaneous activity. Galindo-Tovar and Kaumann (Citation2008) recently showed that the β1-AR antagonist CGP20712A could suppress extrasystolic activity induced by epinephrine in mouse ventricular myocardium. In line with these observations, our present results show that β1-AR blockade was able to reverse the increase in automatic Rmax to ISO in FS cells. It should be observed that the latter effect was accompanied by elevation of the inotropic Rmax, so that, in the presence of MET, inotropic and automatic responsiveness to ISO was comparable in control and FS group myocytes. These results not only indicate that altered responses in FS myocytes are mediated by β1-ARs, but also imply that these cells are able to develop normal contractile response to catecholamines, provided that the exaggerated spontaneous activity is suppressed.
Depressed inotropic stimulation associated with enhanced spontaneous activity by catecholamines was previously observed by Steinberg et al. (Citation2002) in ventricular myocytes from arrhythmia-prone canines. Venetucci et al. (Citation2006) reported that partial RyR inhibition by tetracaine suppresses spontaneous Ca2+ transients at high ISO concentrations, while increasing the amplitude of systolic transients. This may occur because spontaneous release causes transient, partial SR Ca2+ depletion (Díaz et al. Citation1997), which would contribute to decrease systolic SR Ca2+ release. In the present study, a significant, negative correlation was revealed between inotropic and automatic maximal responses. It should be noticed that depressed inotropic responses and enhanced automatism were also seen in FS myocytes challenged with FSK, even though these cells could produce “normal” (i.e., similar to control) responses under partial β1-AR blockade. An implication of these findings is that, if spontaneous activity may limit the inotropic response, the function of the β-AR signaling pathway may be underestimated in conditions associated with enhanced diastolic SR Ca2+ leak (e.g., Ca2+ overload, ventricular hypertrophy, heart failure; Bassani et al. Citation1997; Pogwizd et al. Citation2001; Carvalho et al. Citation2006; ter Keurs and Boyden Citation2007; Zalk et al. Citation2007). Additionally, these results suggest that the depressed contractile response could not be attributed to impaired coupling between β1-AR and Gs, or of the latter to adenylyl-cyclase.
It has been reported that FS enhances functional β2-AR expression in the mediation of positive chronotropic and inotropic responses to ISO and epinephrine in rat atria (Bassani and De Moraes Citation1988; Bassani and Bassani Citation1993; Santos and Spadari-Bratfisch Citation2006; A.L. Moura and R.C. Spadari, personal communication). However, we could not detect major effects of β2-AR blockade on the inotropic or automatic responsiveness to ISO in ventricular myocytes from rats submitted to the same FS protocol as in these previous studies. It thus appears that the FS-induced functional changes in the myocardial β-AR population are chamber-specific. This also seems to be the case for repeated RST, as similar experimental protocols were reported to cause supersensitivity and subsensitivity to chronotropic and inotropic effects of catecholamines, respectively, in rat atria (Brotto Citation2003; Silveira et al. Citation2003), but no detectable effects on ventricular myocytes (present results).
An intriguing result was observed in control myocytes exposed to BUT and ICI118551: automatic Rmax to ISO was markedly increased, whereas inotropic Rmax was decreased by 30%, suggesting that β2-AR blockade may unmask a strong β1-AR facilitation of spontaneous activity. If so, β2-AR occupation might activate signaling pathways functionally antagonistic to the β1-AR-cAMP-PKA cascade. We have indeed observed attenuation of NE-stimulated spontaneous activity by exposure to a low concentration of β2-AR agonist (D.C. Boer, J.W.M. Bassani, R.A. Bassani, unpublished observations). It has been proposed that, in addition to the classical Gαs-cAMP-PKA pathway, myocardial β2-ARs (and possibly β1-ARs) may be coupled to alternative signaling pathways that lead to phosphatidylinositol-3-kinase (PI3K) activation. Evidence is available that PI3K is involved in the spatial restriction of β2-AR-PKA signaling to the subsarcolemmal region (which precludes PLB phosphorylation) and inhibition of β1-AR stimulated enhancement of L-type Ca2+ current (Jo et al. Citation2002; Leblais et al. Citation2004). Moreover, cAMP compartmentalization and PI3K activation have been associated with cAMP degradation by PDEs, particularly PDE4 with regard to catecholamine inotropic effect and regulation of the SR Ca2+-ATPase function (e.g., Patrucco et al. Citation2004; Xiao et al. Citation2006; Kerfant et al. Citation2007; Christ et al. Citation2009).
FS group myocytes showed altered automatic and inotropic responses to FSK and catecholamines, but not to the non-selective PDE inhibitor IBMX or to increasing [Ca2+]o, which suggests that PKA activation by cAMP, myofilament responsiveness to Ca2+ and intrinsic cellular mechanisms involved in enhanced inotropism and spontaneous activity during increased Ca2+ availability (e.g., SR Ca2+ uptake and release) do not seem to be markedly changed by FS. It is tempting to speculate that FS stress might cause decrease in myocardial PDE activity, possibly involving loss of a putative β2-AR-mediated PDE stimulation, particularly in the SR Ca2+ pump compartment. This would potentiate local cAMP accumulation during β1-AR stimulation. As a result of greater PKA activation and PLB phosphorylation, increased SR Ca2+ loading and leak would lead to heightened spontaneous activity, which would limit further increase in inotropism. This speculation is based on the observation that the development of catecholamine-dependent automatism at both rest and pacing in FS myocytes was similar to that observed in control cells exposed to β2-AR blockers, and that β2-AR antagonism failed to further enhance automatic responses in the FS group. It has been shown that PDE4 strongly modulates the inotropic response to β1-AR activation in rodent ventricle, and that its inhibition potentiates catecholamine-induced contractile and spontaneous activity (Vargas et al. Citation2006; Galindo-Tovar and Kaumann Citation2008). Moreover, we were able to reproduce in control cells the qualitative changes in the magnitude of the responses to ISO seen in FS group myocytes by either β2-AR blockade or PDE inhibition with caffeine. Nevertheless, further experiments are required to investigate this possibility, as well as the possible involvement of PI3K.
Increased diastolic SR Ca2+ release during catecholamine stimulation may activate depolarizing Ca2+-dependent currents, especially via the Na+/Ca2+ exchanger, generating abnormal electrical activity that may be propagated and lead to arrhythmias (Fujiwara et al. Citation2008). This mechanism has been considered to underlie stress- and/or exercise-triggered catecholaminergic polymorphic ventricular tachycardia of genetic etiology, as well as increased susceptibility to sudden death in heart failure and other cardiovascular pathophysiological conditions (Pogwizd et al. Citation2001; Kontula et al. Citation2005; ter Keurs and Boyden Citation2007; Zalk et al. Citation2007). However, regardless of genetic alterations, a strong association between stress and cardiac arrhythmias has been also recognized under less severe conditions (e.g., Brodsky et al. Citation1987; Lane et al. Citation2005). Although more investigation is required to elucidate the underlying mechanisms, our present results are suggestive that the increased automatism in FS myocytes during β-adrenergic stimulation may affect negatively ventricular pumping function by both blunting the inotropic response to catecholamines and increasing the susceptibility to ventricular arrhythmias.
Acknowledgements
This work received partial support from CNPq (Proc N. 300632/2005-3 and 141175/2002-8). We are grateful to Ms. Elizângela S. Oliveira for excellent technical support, to Dr. Pedro X. Oliveira for developing the software used for analysis of cell shortening data, to Dr. Regina C. Spadari for kindly providing CGP12177, and to Dr. José W.M. Bassani for his critical comments on the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arunlakshana O, Schild HO. 1959. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 14:48–58.
- Bao X, Lu CM, Liu F, Gu Y, Dalton ND, Zhu B, Foster E, Chen J, Karliner JS, Ross JJr, . 2007. Epinephrine is required for normal cardiovascular responses to stress in the phenylethanolamine N-methyltransferase knockout mouse. Circulation. 116:1024–1031.
- Bassani RA, Bassani JWM. 1993. Effects of escapable and inescapable foot-shock on rat atrial β-adrenoceptors. Pharmacol Biochem Behav. 44:869–875.
- Bassani RA, De Moraes S. 1987. Subsensitivity to β-adrenoceptor agonists in right atria isolated from footshock-stressed rats. Gen Pharmacol. 18:473–477.
- Bassani RA, De Moraes S. 1988. Effects of repeated footshock stress on the chronotropic responsiveness of the isolated pacemaker of the rat: Role of beta-2 adrenoceptors. J Pharmacol Exp Ther. 246:316–321.
- Bassani JWM, Yuan W, Bers DM. Fractional Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol. 1995a; 268:C1313–C1319.
- Bassani RA, Mattiazzi A, Bers DM. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 1995b; 268:H703–H713.
- Bassani RA, Bassani JWM, Lipsius SL, Bers DM. 1997. Diastolic Ca efflux in atrial pacemaker cells and Ca-overloaded myocytes. Am J Physiol Heart Circ Physiol. 273:H886–HH892.
- Boer DC, Bassani RA. 2004. Quantitation of the dose-response relationship for arrhythmogenic agents in isolated cardiac tissue. Braz J Biomed Eng. 20:3–10.
- Boer DC, Bassani JWM, Bassani RA. 2004. Catecholamine-dependent spontaneous activity is mediated by β1-adrenoceptors in rat myocardium. J Mol Cell Cardiol. 37:149 (abstr).
- Brodsky MA, Sato DA, Iseri LT, Wolff LJ, Allen BJ. 1987. Ventricular tachyarrhythmia associated with psychological stress: The role of sympathetic nervous system. JAMA. 257:2064–2067.
- Brotto MAP. 2003. Temporal effects of stress by immobilization and sensitivity of the isolated rat pacemaker to isoproterenol: Roles of corticosterone, neuronal uptake, and β-adrenergic homogeneity. J Pharmacol Exp Ther. 306:1152–1158.
- Callia ML, De Moraes S. 1984. Heterogeneity of beta-adrenoceptors in right atria isolated from cold-exposed rats. J Pharmacol Exp Ther. 230:450–454.
- Carvalho MB, Bassani RA, Franchini KG, Bassani JWM. 2006. Enhanced calcium mobilization in rat ventricular myocytes during the onset of pressure overload-induced hypertrophy. Am J Physiol Heart Circ Physiol. 291:H1803–H1813.
- Charmandari E, Tsigos C, Chrousos G. 2005. Endocrinology of the stress response. Annu Rev Physiol. 67:259–284.
- Christ T, Galindo-Tovar A, Thoms M, Ravens U, Kaumann AJ. 2009. Inotropy and L-type Ca2+ current, activated by β1- and β2-adrenoceptors, are differently controlled y phosphodiesterases 3 and 4 in rat heart. Br J Pharmacol. 156:62–85.
- Daly JW. 2000. Alkylxanthines as research tools. J Auton Nervous Syst. 81:44–52.
- DeSantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM, Pogwizd SM. 2008. Arrhythmogenic effects of β2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ Res. 102:1389–1397.
- Díaz ME, Trafford AW, O'Neill SC, Eisner DA. 1997. A measurable reduction of s.r. Ca content follows spontaneous Ca release in rat ventricular myocytes. Pflügers Arch Eur J Physiol. 434:852–854.
- Donald DE, Shepherd JT. 1963. Response to exercise in dogs with cardiac denervation. Am J Physiol. 205:393–400.
- Fuji AM, Vatner SF. 1985. Autonomic mechanisms regulating myocardial contractility in conscious animals. Pharmacol Ther. 29:221–238.
- Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. 2008. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger. Circ Res. 103:509–518.
- Galindo-Tovar A, Kaumann AJ. 2008. Phosphodiesterase-4 blunts inotropism and arrhythmias but not sinoatrial tachycardia of (-)-adrenaline mediated through mouse cardiac β1-adrenoceptors. Br J Pharmacol. 153:710–720.
- Ginsburg KS, Bers DM. 2004. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol. 556:463–480.
- Jo SH, Leblais V, Wang PH, Crow MT, Xiao RP. 2002. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent Gs signaling during β2-adrenergic stimulation. Circ Res. 91:46–53.
- Juberg EN, Minneman KP, Abel PW. 1985. Beta-1 and beta-2 adrenoceptor binding and functional response in right and left atria of the rat heart. Naunyn Schmiedeberg Arch Pharmacol. 330:193–202.
- Kerfant BG, Zhao D, Lorenzen-Schmidt I, Wilson LS, Cai S, Chen SRW, Maurice DH, Backx PH. 2007. PI3Kγ is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticulum calcium ATPase in cardiomyocytes. Circ Res. 101:400–408.
- ter Keurs HEDJ, Boyden PA. 2007. Calcium and arrhythmogenesis. Physiol Rev. 87:457–586.
- Kitagawa Y, Adachi-Akahane S, Nagao T. 1995. Determination of β-adrenoceptor subtype on rat isolated ventricular myocytes by use of highly-selective β-antagonists. Br J Pharmacol. 116:1635–1643.
- Kontula K, Laitinen PJ, Lehtonen A, Toivonen L, Viitasalo M, Swan H. 2005. Catecholaminergic polymorphic ventricular tachycardia: Recent mechanistic insights. Cardiovasc Res. 67:379–387.
- Lane RD, Laukes C, Marcus FI, Chesney MA, Sechrest L, Gear K, Fort CL, Priori SG, Schwartz PJ, Steptoe A. 2005. Psychological stress preceding idiopathic ventricular fibrillation. Psychosom Med. 67:359–365.
- Leblais V, Jo SH, Chakir K, Maltsev V, Zheng M, Crow MT, Wang W, Lakatta EG, Xiao RP. 2004. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyoyctes. Circ Res. 95:1183–1190.
- Li Y, Kranias EG, Mignery GA, Bers DM. 2002. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 90:309–316.
- Lukyanenko V, Subramanian S, Györke I, Wiesner TF, Györke S. 1999. The role of luminal Ca in the generation of Ca waves in rat ventricular myocytes. J Physiol. 518:173–186.
- Müller FU, Neumann J, Schmitz W. 2000. Transcriptional regulation by cAMP in the heart. Mol Cell Biochem. 212:11–17.
- Nikolaev VO, Bünemann M, Schmitteckert E, Lohse MJ, Engelhardt D. 2006. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching β1-adrenergic but locally confined β2-adrenergic receptor-mediated signaling. Circ Res. 99:1084–1091.
- Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, . 2004. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and independent effects. Cell. 118:375–387.
- Pogwizd SM, Schlottauer L, Li L, Weilong Y, Bers DM. 2001. Arrhythmogenesis and contractile dysfunction in heart failure. Circ Res. 88:1150–1167.
- Ranu HK, Mak JCW, Barnes PJ, Harding SE. 2000. Gi-dependent suppression of β1-adrenoceptor effects in ventricular myocytes from NE-treated guinea-pigs. Am J Physiol Heart Circ Physiol. 278:H1807–H1814.
- Santos IN, Spadari-Bratfisch RC. 2006. Stress and cardiac beta-adrenoceptors. Stress. 9:69–84.
- Silveira AC, Gilioli R, Oliveira ES, Bassani RA. 2003. Subsensitivity to beta-adrenergic stimulation in atria from rats infested with Syphacia sp. Lab Anim. 37:63–67.
- Spadari RC, De Moraes S. 1988. Repeated swimming stress and responsiveness of the isolated rat pacemaker to the chronotropic effect of noradrenaline and isoprenaline: Role of adrenal corticosteroids. Gen Pharmacol. 19:553–557.
- Steinberg SF, Alcott S, Pak E, Hu D, Protas L, Möise NS, Robinson RB, Rosen MR. 2002. β1-receptors increase cAMP and induce abnormal Ca cycling in the German shepherd sudden death model. Am J Physiol Heart Circ Physiol. 282:H1181–H1188.
- Vargas ML, Hernandez J, Kaumann AJ. 2006. Phosphodiesterase PDE3 blunts the positive inotropic and cyclic AMP enhancing effects of CGP12177 but not of noradrenaline in rat ventricle. Br J Pharmacol. 147:158–163.
- Venetucci LA, Trafford AE, Díaz ME, O'Neill SC, Eisner DA. 2006. Reducing ryanodine receptor open probability as a means to abolish spontaneous Ca2+ release and increase Ca2+ transient amplitude in adult ventricular myocytes. Circ Res. 98:1299–1305.
- Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. 2006. Subtype-specific α1- and β-adrenoceptor signaling in the heart. TIPS. 27:330–337.
- Zalk R, Lehnart SE, Marks AS. 2007. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 76:367–385.