Abstract
The purpose of the present research was to develop bioadhesive buccal tablets for Felodipine (FDP) and Pioglitazone (PIO), low bioavailability drugs, in a combined dosage form for the management of diabetes and hypertension. Buccal tablets were prepared by direct compression method using bioadhesive polymers hydroxypropyl methyl cellulose, sodium carboxymethyl cellulose, and carbopol, alone or in combination of two polymers, and were evaluated for physicochemical properties, swelling index, in vitro bioadhesion, in vivo residence time, in vitro drug release, and ex vivo permeation through porcine buccal membrane. Formulation (PF6) showed peak detachment force (3.12 N), work of adhesion (0.72 mJ), swelling index (196%), erosion (10.8%), in vivo residence time of 280 min, in vitro drug release (99.65% and 98.96% in 6 h for FDP and PIO, respectively) with higuchi model release profile and permeated 66.1 and 64.6 % with a flux of 0.118 and 0.331 mg/h/cm2 of FDP and PIO through porcine buccal membrane. The bioavailability study for optimized formulation (PF6) in pigs showed 2.05- and 2.13-times statistically significant (p < 0.05) improvement in bioavailability for FDP and PIO, respectively, after administration of buccal tablets compared to oral suspension. The ex vivo–in vivo correlation was found to have a biphasic pattern and followed type A correlation. The stability of the PF6 was studied and no significant changes were detected in drug content and in vitro release and ex vivo permeation through porcine buccal membrane after 6 months.
Introduction
The buccal administration of drugs is drawing considerable attention since it has excellent accessibility, an expanse of smooth muscle, robustness of the epithelium, relatively immobile mucosa, and comparatively less susceptibility to enzymatic activity, hence it is suitable for administration of retentive dosage forms (CitationRathbone et al., 1996). Direct access to systemic circulation through the internal jugular vein bypasses drugs from the first-pass metabolism leading to high bioavailability (CitationSudhakar et al., 2006).
Buccal mucosa makes a more appropriate choice of route if prolonged drug delivery is desired, because the buccal route is less permeable than the sub-lingual route. Buccal bioadhesive drug devices designed to remain in contact with buccal mucosa and release the drug over a longer period of time in a controlled fashion overcomes drug degradation in the GI tract, active drug loss due to first pass metabolism, and inconvenience of parenteral administration (CitationNarendra et al., 2005). Buccal delivery has high patient acceptability compared to other alternative routes of drug administration and controls plasma concentrations of potent drugs and can interrupt drug input quickly in the case of toxicity. Buccal adhesive dosage forms were prepared for oral delivery, in the form of adhesive tablets (CitationJafar et al., 2004; CitationOwens et al., 2005), adhesive patches (CitationAnders & Merkle., 1989; CitationVamshi Vishnu et al., 2007a; CitationChinna Reddy et al., 2010), and adhesive gels (CitationIshida et al., 1983).
Felodipine (FDP), a calcium channel blocker belonging to dihydropyridines, is used as a potent peripheral vasodilator, which effectively reduces blood pressure when given at doses of 5–20 mg per day. After a single, 20 mg oral dose of FDP, peak plasma concentrations are achieved within 2.5–5 h (CitationEdgar et al., 1987; CitationChinna Reddy et al., 2010). It was reported to be well absorbed following oral administration, but undergoes extensive first pass metabolism; leading to poor bioavailability (CitationKaravas et al., 2005). Pioglitazone is a thiazolidinedione compound used in the treatment of type 2 diabetes. It is an insulin sensitizer that acts as an agonist of the peroxisome proliferators activated receptor sub-type gamma (PPAR-γ) (CitationJaakkola et al., 2005). Pioglitazone is rapidly absorbed, its oral bioavailability 80%, and it is extensively metabolized by hydroxylation and oxidation to active and inactive metabolites in the liver (CitationEckland & Danhof., 2000; CitationActos, 2004). Patients with hyperglycemia also suffered from hypertension, therefore a combination of two drugs is prescribed to patients. Currently there is no combined dosage form available and they are available only as individual tablets FDP (Plendil) and PIO (Actos) (CitationActos, 2004). Since FDP suffers from extensive first pass hepatic metabolism and an alternative mode of delivery system like buccal delivery system is desired.
The aim of the present investigation is to develop bioadhesive tablets for buccal sustained release of FDP and PIO in combined dosage form using different mixtures of cellulose derivates (HPMC K4M, Sodium CMC) and polyacrylic acid derivatives (Carbopol 971) as bioadhesive polymers, pearlitol SD 200 as filler, sodium stearyl fumerate (SSF) as lubricant and hydroxypropyl β-cyclodextrin (HPβCD) as solubility and permeability enhancer. The present investigation consisted of the following steps: (a) preparation of drug-free tablets by direct compression, (b) physicochemical, ex vivo, and in vivo studies, (c) choice of bioadhesive tablets showing the best performances, (d) preparation of tablets with PIO and FDP combined dosage form, (e) evaluation of in vitro drug release, (f) evaluation of in vivo bioavailability, (g) investigation of the release modality from the matrix, (h) ex vivo and in vivo correlation, and (i) statistical analysis.
Materials and methods
Materials
Felodipine was gifted by Sun pharmaceuticals (Baroda, India). Pioglitazone, HPMC K4M, Carbopol 971P, sodium CMC, Pearlitol SD200, SSF, and HPβCD were gifted by Dr Reddys Laboratories (Hyderabad, India). Polyester backing membrane was gifted by 3M (St. Paul, MI). Mucin (Crude Type II) was purchased from Sigma-Aldrich (Germany) and was used without further purification. Phenol red was purchased from Hi Media (Mumbai, India). All reagents used were of analytical grade.
Ex vivo drug permeation studies through porcine buccal membrane
Porcine buccal mucosa was used as it resembles the human buccal mucosa regarding permeability, barrier lipid composition, thickness, and histology (CitationWertz & Squier., 1991; CitationLangoth et al., 2005). Buccal tissue from pigs was obtained from a local slaughterhouse and used within 2 h of slaughter. The tissue was stored in Krebs buffer (CitationChandra Sekhar et al., 2008) (sodium chloride (118 mm), potassium chloride (5.4 mm), sodium hydrogen phosphate (1 mm), magnesium sulfate (1.2 mm), calcium chloride (1.9 mm), sodium hydrogen carbonate (25 mm), and dextrose (11.1 mm)) at 4°C after collection, and separated from the underlying connective tissue with surgical technique. Franz diffusion cell with an internal diameter of 2.1 cm (3.46 cm2 area) with a receptor compartment volume of 25 mL was used for this study. The buccal epithelium was carefully mounted between donor and receptor compartment. Phosphate buffer saline (PBS) pH 7.4 containing polyethylene glycol (PEG 400) and 5% v/v alcohol was placed in the receptor compartment. PBS pH 7.4 containing FDP (4 mg), PIO (4 mg), and a marker compound, phenol red (20 μg/mL) was placed in the donor compartment. The entire set-up was placed over a magnetic stirrer and temperature was maintained at 37°C (CitationTucker., 1988). Samples of 1 mL were collected at pre-determined time points from the receptor compartment and replaced with an equal volume of fresh solution and analyzed utilizing high performance liquid chromatography (HPLC).
Estimation of drug content in the samples by HPLC
The HPLC system (Shimadzu, Kyoto, Japan) consisting of a LC-10AT solvent module, SPD10A UV–visible detector with LC10 software. The analytical column used was C18 (Inertsil, 150 × 4.6 mm i.d., particle size 5 µm) at room temperature. The mobile phase used was acetonitrile and 50 mM ammonium acetate buffer (pH 5.0, adjusted with glacial acetic acid) (67:33%, v/v) at a flow rate of 1.0 mL/min. The linearity range of proposed method was 1–5000 ng/mL for each analyte with regression coefficient greater than 0.999. The retention times for PIO, FDP, and nitrendipine (NTDP) were found to be 5.32, 10.68, and 7.26 min, respectively. The required studies were carried out to estimate the precision and accuracy of the HPLC method.
Preparation of bioadhesive buccal tablets
Buccoadhesive flat-faced tablets (110 mg, 8 mm in diameter, 1.4 mm mean thickness) of combined dosage form were prepared by direct compression using a rotary tabletting machine (Cadmach, Mumbai, India). HPMC, sodium CMC, and Carbopol were used as bioadhesive polymers, sodium stearyl fumerate (SSF) as a lubricant, pearlitol SD200 as a filler, and HPβCD as a solubility and permeability enhancer (). FDP and PIO were first mixed with the bioadhesive polymeric mixture for 10 min in a mixer (VJ Instruments Ltd., Mumbai, India). Pearlitol and SSF were then added, and mixing continued for another 10 min. The machine was adjusted to produce tablets with a weight of 110 mg. The drug-free tablets were prepared by replacing the drugs with filler (Pearlitol SD 200).
Table 1. Composition of FDP-PIO bioadhesive buccal tablets.
Swelling and erosion studies
Swelling and erosion studies of the tablets were determined in phosphate buffer, pH 6.6 (CitationShanker et al., 2009). The tablets were attached to pre-weighed glass supports using a cyanoacrylate adhesive sealant. The supports with tablets were immersed into the phosphate buffer at 37°C. At pre-determined time intervals (1, 2, 3, and 4 h), the devices were removed from the media, blotted with tissue paper to remove excess surface water, and weighed. After determining the wet weight, the tablets were dried at 40°C until constant mass. Swelling index (S.I) and mass loss were determined gravimetrically according to the following equations:
In vitro bioadhesion measurement
The adhesive binding of the tablets containing FDP and PIO to porcine buccal mucosa was studied in triplicate using a microprocessor based advanced force gauze with a motorized test stand (Ultra Test, Mecmesin, West Sussex, UK) and fitted with a 5 kg load cell (CitationVamshi Vishnu et al., 2007b). In this test, porcine buccal membrane was secured tightly to a circular stainless steel adaptor and the buccal tablet to be tested was adhered to another cylindrical stainless steel adaptor similar in diameter using a cyanoacrylate adhesive. During the test, 100 μL of 1% w/v mucin solution was spread over the surface of the buccal mucosa and the tablet was immediately brought into contact. A force of 0.5 N was applied for 180 s to enhance the contact of the tablet with the mucosa. At the end of the contact time, upper support was withdrawn at 0.5 mm/s until the tablet was completely detached from the mucosa. The work of adhesion and the peak detachment force was calculated using data plot software.
Evaluation of in vivo residence time for buccal tablets
Six healthy volunteers aged between 24–28 years participated in the study. Volunteers were instructed not to eat during the study and drinking was allowed after 1 h of the application of buccoadhesive drug-free tablet. Tablets were applied manually by pressing them against the cheek for ~ 30 s, without moistening before application (CitationBoukaert et al., 1993; CitationKhanna et al., 1997). Volunteers were instructed to record the time of the tablet application, and the time and circumstances of the end of adhesion (erosion or detachment of the tablet).
In vitro drug release studies
In vitro release studies were conducted using USP type II dissolution test apparatus (Electrolab, TDT-08L, Mumbai, India). Tablets were supposed to release the drug from one side only; therefore, an impermeable backing membrane was placed on the other side of the tablet. The tablet was then fixed to a 2 × 2 cm glass slide with a solution of cyanoacrylate adhesive. The dissolution medium was 500 mL of 0.5% w/v of sodium lauryl sulfate solution at 25 rpm and temperature was maintained at 37 ± 0.5°C. Samples of 5 mL were collected at pre-determined time intervals and replenished with fresh media. Appropriate dilutions were made with blank solution and analyzed utilizing HPLC.
Ex vivo permeation studies from buccal tablets
Ex vivo permeation of the optimized formulations through porcine buccal membrane was studied. The porcine buccal membrane was mounted over a Franz diffusion cell with an internal diameter is 2.1 cm. The acceptor compartment was continuously stirred at 600 rpm using a magnetic stirrer. The tablet was placed into the donor compartment, and was wetted with 1 mL of phosphate buffer. The amount of drug permeated through the porcine buccal membrane was determined by removing aliquots from the receptor compartment, and by replacing the same volume of fresh solution. The samples were analyzed by HPLC. The FDP and PIO flux through the membrane was calculated using equation (3):
where J is the steady-state flux, dQ/dt is the permeation rate, and A is the diffusion area.
In vivo bioavailability study in pigs
The animal study protocol was reviewed and approved by the institutional animal ethical committee, University College of Pharmaceutical Sciences, Kakatiya University, India. White pigs weighing 30 ± 5 kg were selected for the study. The bioavailability of optimized bioadhesive buccal tablet (PF6) was compared with an oral suspension. The suspension was prepared by suspending 15 mg of PIO and 5 mg of FDP in 10 mL of 0.5% (w/v) sodium carboxy methyl cellulose. They were allowed free access to food and water, until the night prior to dosing, and were fasted for 10 h. Latin square cross-over design was followed; the animals were divided into two groups, each consisting of six pigs. To one group, oral suspension was administered through feeding tube followed by rinsing with 10 mL of water and bioadhesive buccal tablet to another group in the first phase. The pigs were anesthetized during sample collection until the third hour sample. In the second phase vice versa was followed and was conducted after 15 days of wash out period. Blood samples (5 mL) from the tail vein were collected at pre-set time intervals of 0, 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h. All blood samples were allowed to clot and centrifuged for 10 min at 5000 rpm (MIKRO 220R, Hettich, Germany). The serum was separated and transferred into clean micro-centrifuge tubes and stored at −20°C until HPLC analysis. The amount of PIO and FDP in the samples was estimated using HPLC.
Pharmacokinetic analysis
Pharmacokinetic parameters of PIO and FDP after administration of bioadhesive buccal tablet and oral suspension were estimated for each pig by using a computer program, KINETICA 2000 (Version 3.0, Innaphase Corporation, Philadelphia, PA). Non-compartmental analysis was used to calculate the pharmacokinetic parameters, Cmax, Tmax, and area under the curve (AUC). Cmax (ng/mL) and Tmax (h) were the observed maximal drug concentration and its time, respectively. The relative bioavailability (F) for buccal delivery was calculated using equation (4):
Statistical analysis
Statistical comparisons were made using Student’s t-test using the Sigmastat software package (Jandel Corp., CA). Results were considered significant at 95% confidence interval (p < 0.05) and results were expressed as mean ± SD.
Ex vivo–in vivo correlation
The cumulative amount of PIO and FDP permeated across the porcine buccal membrane ex vivo from the optimized buccal tablet was compared against the extent of absorption, i.e. cumulative AUC values for a possible ex vivo–in vivo correlation.
Stability studies
Stability studies were carried out for optimized formulation PF6 according to the International Conference on Harmonization (ICH) guidelines (CitationKhanna et al., 1997). The samples were stored at 40 ± 2°C/75 ± 5% R.H. (Skylab Instruments and Engineering Pvt Ltd., Mumbai, India) for 6 months. Samples were withdrawn after 1, 2, 3, and 6 months, and were evaluated for drug content, in vitro percentage drug release, and cumulative percentage of drug permeated through porcine buccal membrane.
Results and discussion
Ex vivo permeation studies
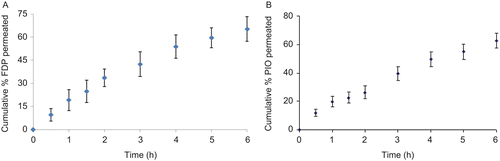
The permeation of drugs through porcine buccal membrane is shown in and . The tissue was isolated successfully as there was no evidence of detectable levels of phenol red in the receiver compartment, whereas FDP and PIO could permeate freely. Cumulative percentage amount permeated in 6 h for FDP and PIO was found to be 65.2, 62.7%, flux, and permeability coefficient were calculated to be 0.154, 0.122 mg/h/cm2 and 0.031, 0.024 cm/h for FDP and PIO, respectively.
Evaluation of buccoadhesive tablets
The prepared bioadhesive buccal tablet formulations were evaluated for physicochemical properties such as thickness, weight variation, friability, drug content, and surface pH. The values of thickness (1.3–1.6 mm), weight variation (106–114 mg), and friability (0.05–0.23%) were found to be within the limits of conventional oral tablets stated in the Indian Pharmacopoeia 1996. All the formulations demonstrated uniform assay and content uniformity, with a mean drug content of ~ 98% and relative standard deviation of less than 2% for both the drugs. The surface pH of the buccal tablets was determined in order to investigate the possibility of any irritation effects in vivo, as acidic or alkaline pH may cause irritation to the buccal mucosa. Surface pH of the optimized formulation PF6 was found to be 6.50 (near to neutral pH). It was inferred that neutral pH of the formulation does not cause any irritation to the mucosa and it is in agreement with the responses of healthy human male volunteers who participated during the in vivo residence time study.
Swelling and erosion studies
The results of swelling and erosion studies for all of the formulations at the end of 4 h were presented in . Maximum swelling index for the formulations PF3 (278%) and PF6 (196%) was found at the end of 4 h. Erosion was investigated by comparing the initial and final tablet weight after immersion in phosphate buffer. Erosion values for the formulations were found to be 17.5% (PF3) and 10.8% (PF6) at the end of 4 h. The swelling of the bioadhesive buccal formulations was dependant on both the type and concentration of the polymer included in the formulations. The swelling indices of the prepared buccoadhesive tablets were in the order: PF4 < PF5 < PF1 < PF2 < PF6 < PF3. The highest swelling index was observed in the case of the formulation PF3; this may be due to the faster hydration rate of polymers in the presence of the HPβCD.
Table 2. Physicomechanical and bioadhesive properties of buccal tablets.
During development of bioadhesive formulation, tablet hydration capacity is very important to be considered because the medium penetration is responsible for drug release. However, since swelling and gel formation can make tablets erodible, it is very important to know when the formulation loses its integrity. For this purpose erosion was investigated by comparing the initial and final tablet weight after immersion in phosphate buffer. The negative erosion values after 1–2 h confirmed the good hydration of all tablets, and positive erosion values for all the formulations were observed at the end of 4 h because of the erosion effect.
Measurement of in vitro bioadhesion and in vivo residence time
In vitro bioadhesion and in vivo residence time for all the formulations were conducted, and results were presented in . The formulations PF3 and PF6 displayed maximum bioadhesion, i.e. work of adhesion, peak detachment force, and in vivo residence time compared with other formulations developed. Formulations PF3 and PF6 showed work of adhesion, peak detachment force, and in vivo residence time, respectively, 2.86 N, 0.64 mJ, and 208 min and 3.12 N, 0.72 mJ, and 292 min. After completion of the in vivo residence time study, volunteers were asked to score the parameters such as irritancy, comfort, dry mouth, salivation, dislodgment of the buccal tablet during study, and heaviness of the buccal tablet at the place of attachment. No volunteer reported irritancy and heaviness during the study; only two volunteers felt slightly uncomfortable and slight salivary secretion during study. No volunteer felt heaviness of the buccal tablet at the place of attachment because of the moderate thickness and light weight (110 mg) of the tablet.
In vitro drug release studies
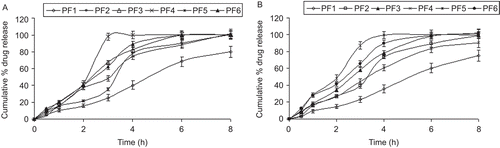
In vitro dissolution profiles of various formulations prepared are shown in and . The differences in drug release patterns from different tablet formulations were related to the differences observed in the swelling indices and erosion behavior of the tablets as a function of the polymer type and concentration. The tablets formulated with HPMC and Carbopol mixture (PF6) exhibited almost 100% FDP and 99% PIO release, whereas the drug release from the tablets formulated with Carbopol and sodium CMC mixture (PF1) was slower, with ~ 68% FDP and 60% PIO released in 6 h. Dissolution rate of the FDP and PIO from the PF6 and PF3 was significantly higher than the corresponding tablet formulations with no HPβCD (PF5 and PF2). Due to very low aqueous solubility of the FDP and PIO, only a limited amount of drug could dissolve inside the hydrated polymeric matrices. Incorporation of the HPβCD in the matrix improved the drug solubility in the polymeric gel matrix by forming an in situ complex, thereby enhancing the dissolution (CitationMario & Mira., 2004).
Drug release kinetics
To examine the release mechanism of FDP and PIO from the prepared buccoadhesive tablets, the results were analyzed using the equation proposed by CitationKorsmeyer et al. (1983).
where Mt/Mα is the fractional amount of drug release at any time t, K is the release rate constant, and n is the diffusional exponent that characterizes the type of the release mechanism during the dissolution process. The value of n was estimated by linear regression of log (Mt/M∞) vs log t, and is listed in . For non-Fickian release, the n value falls between 0.5–1.0, while in the case of Fickian diffusion, n = 0.5; for zero-order release (case II transport), n = 1; and for supercase II transport n > 1.
Table 3. FDP and PIO estimated values of release exponent (n) and correlation coefficients (r2), in combined dosage form for all of the formulations.
The values of the release exponents, however, indicated that the release mechanism changed with the type of the polymer used. Formulations PF1, PF2, and PF5 (r2 > 0.9) followed zero order release kinetics, whereas PF3, PF4, and PF6 (r2 > 0.9) showed Higuchi release kinetics for both the drugs, as was evidenced from correlation coefficients. All the formulations showed a non-Fickian release pattern, as was evidenced from the release exponent (n > 0.6), i.e. the drug release proceeded by both diffusion as well as erosion of the polymer. Therefore, the release of the drug from the prepared tablets is controlled by the initial swelling of the polymer, followed by drug diffusion through the swollen polymer, and slow erosion of the polymer (CitationParodi et al., 1999).
Ex vivo permeation studies from buccal tablets
Based on the in vitro drug release, in vitro bioadhesion, and in vivo residence time results, two formulations (PF3 and PF6) were selected for the ex vivo permeation study. The ex vivo permeation studies of FDP and PIO from the optimized bioadhesive buccal tablets were carried out and the flux values were determined from the steady state region of the diffusion profiles obtained using the linear regression analysis. The cumulative percentage of drug permeated in 6 h, flux and permeation coefficient from formulation PF3 were found to be 53.8%, 0.712 mg/h/cm2, 0.142 cm/h, and 48.3%, 1.54 mg/h/cm2, 0.102 cm/h, for FDP and PIO, respectively. Formulation PF6 showed cumulative percentage of drug permeated in 6 h, flux and permeation coefficient for FDP and PIO were found to be 66.1%, 0.816 mg/h/cm2, 0.163 cm/h, and 64.6%, 2.34 mg/h/cm2, 0.156 cm/h, respectively. The differences in the flux values of FDP and PIO from the tablets could be attributed to the polymers used, and the presence of HPβCD. Both the formulations showed good cumulative percentage of drug permeated; the reasons might be due to the presence of HPβCD. Addition of HPβCD to the matrix increased the flux by increasing the solubility of FDP and PIO, thus improving the diffusible form of the drug species at the tablet membrane interface. Cyclodextrins have been suggested to act as penetration enhancers (CitationFigueiras et al., 2009). They enhance the drug permeation by carrying the actives through the aqueous barrier towards the surface of the membrane, where the drug passes from the complex into the membrane.
In vivo bioavailability studies
The mean plasma concentration–time curves of FDP and PIO following the application of the bioadhesive buccal tablet and oral suspension to pigs are shown in and . The mean peak plasma concentrations (Cmax) and time to reach peak plasma concentration (Tmax) for FDP were calculated to be 61.3 and 62.6 ng/mL; 2.7 and 6.7 h, respectively, after administration of oral suspension and bioadhesive buccal tablet. The mean Cmax and Tmax for PIO were calculated to be 358.9 and 404.6 ng/mL; 2.4 and 6.4 h, respectively, after administration of oral suspension and bioadhesive buccal tablet. The AUC0–n and AUC0–α for FDP were found to be, respectively, 212.03 and 435.92 ng/h/mL; 212.24 and 440.66 ng/h/mL after administration of oral suspension and bioadhesive buccal tablet. The AUC0–n and AUC0–α for PIO were found to be, respectively, 1351.7 and 2884.28 ng/h/mL; 1358.7 and 2908.9 ng/h/mL after administration of oral suspension and bioadhesive buccal tablet.
Figure 3. Mean serum profiles of (a) FDP and (b) PIO in pigs, after administration of oral solution and buccal tablet, values represented are mean ± SD (n = 6).
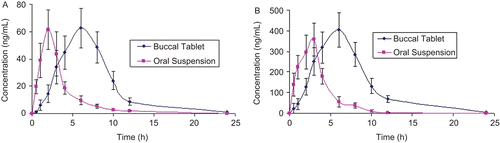
The results of bioavailability study () reveal that FDP and PIO were released and permeated well from the bioadhesive buccal tablet, as compared with oral suspension. The Cmax, Tmax, and AUC profiles were compared in all the pigs, Cmax was found to be higher for both drugs by the buccal route than oral route, greater Cmax values could be attributed due to avoidance of first pass hepatic metabolism after buccal administration. The Tmax values for both FDP and PIO in all pigs were higher for buccal administration than oral administration, and the difference was statistically significant (p < 0.05). This difference was because of the mucosal membrane that could delay the permeation of drugs from buccal tablet in contrast to the suspension administered by the oral route which is an immediate release dosage form. The overall mean value of AUC0−24 by the buccal route was 2.05- and 2.13-times higher than that of the oral route for FDP and PIO, respectively, and the difference was found to be statistically significant (p < 0.05), demonstrating improved bioavailability of both drugs from bioadhesive buccal tablets. This is due to avoidance of first pass hepatic metabolism by the buccal route. Therefore, for the effective management of diabetes and hypertension, FDP and PIO in combined dosage form of bioadhesive buccal tablet could provide an effective treatment.
Table 4. Pharmacokinetic parameters of FDP and PIO in pigs after administration of oral suspension and bioadhesive buccal tablet, values represented are mean ± SD (n = 6).
Stability studies
Drug content and in vitro percentage drug release and cumulative percentage of drug permeated through porcine buccal membrane results () reveal that after 6 months of the stability studies there was no significant difference in drug content and in vitro drug release and cumulative percentage of drug permeation.
Table 5. Stability study of the optimized formulation (PF6) for 3 months.
Ex vivo–in vivo correlation
Ex vivo–in vivo correlation between cumulative amount of drugs permeated through porcine buccal membrane and AUC showed a biphasic curve pattern ( and ), which can be distinguished into two regions for the buccal tablet. Good linear correlation was observed with correlation coefficients for FDP and PIO, r2 = 0.999 and 0.995 during the lag phase and r2 = 0.994 and 0.988 during the absorption phase, respectively.
Figure 4. Ex vivo–n vivo correlation of cumulative amount of (a) FDP and (b) PIO permeated ex vivo vs AUC.
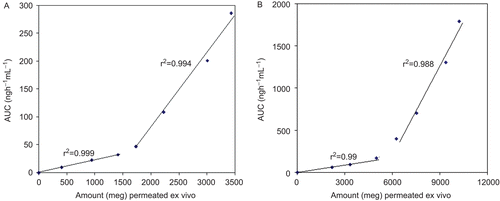
Point-to-point correlation of ex vivo permeation of drug to in vivo performance was observed, indicating that it follows type A correlation (CitationEmami, 2006). The slow permeation of FDP and PIO through the buccal membrane in initial stages is explained as follows; in the first phase FDP and PIO was released and permeated through the mucosa and deposition of drug took place in the buccal membrane, and concentration build up was maintained. Permeation and concentration build up at the buccal membrane is the lag phase observed in the first region. Concentration built up resulted in flux establishment and AUC increased at a rapid rate in the second phase. This indicates that initially drug permeated into buccal membrane rapidly, but it takes some time for permeation and absorption. Once the necessary flux is established, absorption was rapid as a large amount of drug is deposited in the layers of the buccal membrane.
Conclusions
Bioadhesive buccal tablets for felodipine and pioglitazone in combined dosage form were developed and evaluated. The results demonstrated that the optimized formulation showed good in vitro drug release, ex vivo permeation through porcine buccal membrane, and bioadhesive properties. In vitro residence time in human volunteer study results revealed that the optimized formulation showed good retentive property and no irritation. Results of the bioavailability study showed improved permeation of both drugs from the buccal tablet compared with oral suspension. An improvement of bioavailability by the buccal tablet to the extent of 2.05- and 2.13-times higher than that of the oral route for FDP and PIO, respectively, was obtained. Hence, the development of a bioadhesive buccal tablet in combined dosage form for FDP and PIO may be a promising one, as the necessary dose of individual drugs may be decreased, and thus side-effects may be reduced. Good ex vivo–in vivo correlation was obtained for both drugs.
Declaration of Interest
The authors thank University Grant Commission (UGC), New Delhi for providing UGC major research project [F.No: 32–134/2006(SR)]. The authors also acknowledge the liberal help of Dr. Reddys Laboratories, Hyderabad, India for providing Pioglitazone as gift sample. The authors report no conflicts of interest.
References
- Actos. (2004). Product information for Actos. Available online at: http://www.actos.com/pi.pdf, accessed 20 December 2004.
- Anders, R., Merkle, H.P. (1989). Evaluation of laminated mucoadhesive patches for buccal drug delivery. Int J Pharm. 49:231–40.
- Boukaert, S., Lefebvre, R.A., Remon, J.P. (1993). In vitro/in vivo correlation of the bioadhesive properties of a buccal bioadhesive miconazole slow-release tablet. Pharm Res. 10:853–60.
- Chandra Sekhar, K., Naidu, K.V.S., Vamshi Vishnu, Y., Ramesh, G., Kishan, V., Madhusudan Rao, Y. (2008). Transbuccal delivery of chlorpheniramine maleate from mucoadhesive buccal patches. Drug Delivery. 15:195–201.
- Chinna Reddy, P., Ramesh, G., Vamshi Vishnu, Y., Shravan Kumar, Y., Madhusudan Rao, Y. (2010). Development of bilayered mucoadhesive patches for buccal delivery of felodipine: in vitro and ex vivo characterization. Curr Trends Biotech Pharm. 4:673–83.
- Eckland, D.A., Danhof, M. (2000). Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes. 108:234–42.
- Edgar, B., Collste, P., Haglund, K., Regardh, C.G. (1987). Pharmacokinetics and haemodynamic effects of felodipine as monotherapy in hypertensive patients. Clin Invest Med. 10:388–94.
- Emami, J. (2006). In vitro-in vivo correlations: from theory to applications. J Pharm Pharm Sci. 9:31–51.
- Figueiras, A., Hombach, J., Veiga, F., Bernkop-Schnürch, A. (2009). In vitro evaluation of natural and methylated cyclodextrins as buccal permeation enhancing system for omeprazole delivery. Eur J Pharm Biopharm. 71:339–45.
- Ishida, M., Nambu, N., Nagai, T. (1983). Highly viscous gel ointment containing Carbopol for application to the oral mucosa. Chem Pharm Bull. 31:4561–4.
- Jaakkola, T., Backman, J.T., Neuvonen, M., Laitila, J., Neuvonen, P.J. (2005). Effect of rifampicin on the pharmacokinetics of Pioglitazone. Br J Clin Pharmacol. 61:70–8.
- Jafar, A., Ali, N., Djavad, F., Massoud, A., Mohammad, R.S.S., Majid, S. (2004). Development and evaluation of buccoadhesive propranolol hydrochloride tablet formulations: effect of fillers. Farmaco. 59:155–61.
- Karavas, E., Ktistis, G., Xenakis, A., Georgarakis, E. (2005). Miscibility behaviour and formation mechanism of stabilized felodipine polyvinyl pyrrolidone amorphous nanodispersions. Drug Dev Ind Pharm. 31:473–89.
- Khanna, R., Agarwal, S.P., Ahuja, A. (1997). Mucoadhesive buccal tablets of clotrimazol for oral candidiasis. Drug Dev Ind Pharm. 23:831–7.
- Korsmeyer, R.W., Guruy, R., Doelker, E., Buri, P., Peppas, N.A. (1983). Mechanism of solute release from porous polymers. Int J Pharm. 15:25–35.
- Langoth, N., Bernkop-Schnürch, A., Kurka, P. (2005). In vitro evaluation of various buccal permeation enhancing systems for PACAP (pituitary adenylate cyclose activating polypeptide). Pharm Res. 22:2045–50.
- Mario, J., Mira, B.L. (2004). Influence of hydroxypropyl-β-cyclodextrin complexation on piroxicam release from buccoadhesive tablets. Eur J Pharm Sci. 21:251–60.
- Narendra, C., Srinath, M.S., Prakash Rao, B. (2005). Development of three layered buccal compact containing metoprolol tartrate by statistical optimization technique. Int J Pharm. 304:102–14.
- Owens, T.S., Dansereau, R.J., Sakr, A. (2005). Development and evaluation of extended release bioadhesive sodium fluoride tablets. Int J Pharm. 288:109–22.
- Parodi, B., Russo, E., Gatti, P., Cafaggi, S., Bignardi, G. (1999). Development and in vitro evaluation of buccoadhesive tablets using a new model substrate for bioadhesion measures: the eggshell membrane. Drug Dev Ind Pharm. 25:289–95.
- Rathbone, M.J., Ponchel, G., Chazali, F.A. (1996). Systemic oral mucosal drug delivery and delivery systems. New York: Marcel Dekker.
- Shanker, G., Kumar, C.K., Chandra Sekhara Rao, G., Vijaya Kumar, B., Prabhakar Reddy, V. (2009). Formulation and evaluation of bioadhesive buccal drug delivery of tizanidine hydrochloride tablets. AAPS PharmSciTech. 10:530–9.
- Sudhakar, Y., Kuotsu, K., Bandyopadhyay, A.K. (2006). Buccal bioadhesive drug delivery - A promising option for orally less efficient drugs. J Contr Rel. 114:15–40.
- Tucker, I.G. (1988). A method to study the kinetics of oral mucosal drug absorption from solutions. J Pharm Pharmacol. 40:679–83.
- Vamshi Vishnu, Y., Chandrasekhar, K., Ramesh, G., Madhusudan Rao, Y. (2007a). Development of mucoadhesive patches for buccal administration of carvedilol. Curr Drug Del. 4:27–39.
- Vamshi Vishnu, Y., Ramesh, G., Chandrasekhar, K., Bhanoji Rao, M.E., Madhusudan Rao, Y. (2007b). Development and in vitro evaluation of buccoadhesive carvedilol tablets. Acta Pharm. 57:185–97.
- Wertz, P.W., Squier, C.A. (1991). Cellular and molecular basis of barrier function in oral epithelium. Crit Rev Ther Drug Carrier Syst. 8:237–69.